Abstract
We report evidence that the CotC polypeptide, a previously identified component of the Bacillus subtilis spore coat, is assembled into at least four distinct forms. Two of these, having molecular masses of 12 and 21 kDa, appeared 8 h after the onset of sporulation and were probably assembled on the forming spore immediately after their synthesis, since no accumulation of either of them was detected in the mother cell compartment, where their synthesis occurs. The other two components, 12.5 and 30 kDa, were generated 2 h later and were probably the products of posttranslational modifications of the two early forms occurring directly on the coat surface during spore maturation. None of the CotC forms was found either on the spore coat or in the mother cell compartment of a cotH mutant. This indicates that CotH serves a dual role of stabilizing the early forms of CotC and promoting the assembly of both early and late forms on the spore surface.
The Bacillus subtilis spore is encased within a complex multilayered protein structure known as the coat, whose role is to protect the spore against bactericidal enzymes and chemicals, such as lysozyme and chloroform, and to influence the spore's ability to germinate in response to appropriate germinants. However, the recent finding that a component of the B. subtilis coat has laccase activity (20) suggests that the coat may have other, so far unexplored, roles. The coat is composed of a heterogeneous group of over 25 polypeptides arranged into three main structural layers: a diffuse undercoat, a laminated lightly staining inner layer, and a thick electron-dense outer coat. Several of these polypeptides have been studied, and their structural genes (cot genes) have been identified. Expression of all cot genes is governed by a cascade of four transcription factors, acting specifically in the mother cell compartment of the sporangium in the sequence sigma E-SpoIIID-sigma K-GerE, with sigma E and sigma K being RNA polymerase sigma factors and SpoIIID and GerE being DNA-binding proteins acting in conjunction with sigma E- and sigma K-driven RNA polymerase (5, 11).
In addition to the transcriptional control, a variety of posttranslational modifications have been shown to occur during coat formation. At least two coat-associated polypeptides (of about 8 and 9 kDa) appear to be glycosylated (11), while others are derived from proteolytic processing of larger precursors (1, 3, 27). Cross-linking of structural proteins is also believed to occur and result in the insolubilization of specific components. Since several coat proteins are tyrosine rich and since dityrosine bonds are present in purified coat material, it is believed that this type of modification may contribute to the assembly and function of the coat (13). Also (γ-glutamyl)lysine cross-links are found in purified spores, and a coat-associated transglutaminase has been identified (17). The occurrence of transglutaminase-dependent cross-linking of the outermost coat layer has been suggested (12).
The initial stages in coat assembly occur early after the onset of sporulation and involve functional interactions among at least two morphogenetic proteins, both made under sigma E control. First, the SpoIVA protein localizes at the outer forespore membrane. Second, SpoIVA directs the assembly of CotE in a ring-like structure that surrounds the forespore at a distance of about 75 nm from it (6). The gap generated by the localization of SpoIVA and CotE is thought to be the site of assembly of the inner coat components. Within this region, the inner coat may correspond to the more internal sector, adjacent to the SpoIVA protein. In contrast, the outer coat proteins are assembled on the outside of the CotE structure (5, 11). Additional proteins with morphogenetic functions are needed for coat formation. SpoVID and SafA are made under sigma E control; SpoVID interacts with SafA and directs it to the forming spore and is also required to maintain the CotE ring around the forespore (5, 11, 23). In contrast CotH is a morphogenetic protein produced under sigma K control that plays a role in outer coat assembly and the lysozyme resistance of the spore and that, in conjunction with CotE, is also responsible for efficient spore germination (21, 33).
Most coat components are produced at late stages of sporulation, with some proteins, such as CotD, CotT, and CotS, targeted to the inner coat, and others, such as CotB, CotC, and CotG, directed to the outer coat (11). Of these outer coat components, CotB has been recently identified as exposed on the spore surface (7, 15).
This study focuses on the incorporation of CotC into the coat structure and on how this event is controlled by the morphogenetic protein CotH. CotC is a coat component initially identified by a reverse genetic approach (4) and later associated with the outer coat layer (32). Together with CotD and CotG, CotC represents about 50% of the total solubilized coat proteins and, being alkali soluble, can be selectively extracted from purified spores by an NaOH treatment (11). CotC is highly similar to the protein encoded by ynzH, an open reading frame identified during the analysis of the B. subtilis genome (18), and has recently been proposed as a new coat component and renamed CotU (19). CotC and CotU have almost identical N-terminal regions, diverging in only 1 out of 24 amino acid residues. In addition, CotC is also relatively similar to CotG, and, intriguingly, assembly of both CotC and CotG proteins is under the control of the morphogenetic protein CotH (21).
MATERIALS AND METHODS
Bacterial strains and transformation.
B. subtilis strains utilized are listed in Table 1. Plasmid amplification for nucleotide sequencing, subcloning experiments, and transformation of Escherichia coli competent cells were performed with E. coli strain DH5α (26). Bacterial strains were transformed by previously described procedures for CaCl2-mediated transformation of E. coli competent cells (26) and two-step transformation of B. subtilis (2).
TABLE 1.
B. subtilis strains
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| PY79 | Wild type | 30 |
| BD063 | cotA::cat | 4 |
| BD071 | cotC::cat | 4 |
| BZ213 | cotE::cat | 31 |
| ER203 | cotG::erm | 24 |
| ER209 | cotH::cat | 21 |
| RH101 | cotC::spc | This work |
| RH201 | cotB::spc | 15 |
| RH211 | cotE::spc | This work |
| RH202 | cotU::neo | This work |
| RH214 | cotA::cat cotU::neo | This work |
| RH203 | cotC::cat cotU::neo | This work |
| RH213 | cotB::spc cotU::neo | This work |
| RH216 | cotH::cm cotU::neo | This work |
| RH217 | cotG::erm cotU::neo | This work |
| RH218 | cotE::spc cotU::neo | This work |
Genetic and molecular procedures.
Isolation of plasmids, restriction digestion, and ligation of DNA were carried out by standard methods (26). Chromosomal DNA from B. subtilis was isolated as described elsewhere (2). Fragments of cotC and cotU DNA were amplified by PCR from the B. subtilis chromosome, and the amplification was primed with the synthetic oligonucleotides listed in Table 2. The PCR products were visualized on ethidium bromide-stained agarose gels and gel purified by the QIAquick gel extraction kit (Qiagen) as specified by the manufacturer.
TABLE 2.
Synthetic oligonucleotides
| Oligonucleotide | Sequence (5′-3′)a | Restriction site | Positions of annealingb |
|---|---|---|---|
| Ys1 | gcggccgcACTAATCTCTCTATACAC | NotI | −200/−182 |
| Ya1 | gaattcCCAAGTATAATACTCTTC | EcoRI | +42/+25 |
| Ys2 | gtcgacGATCACGATTATCATTAC | SalI | +175/+192 |
| Ya2 | gcatgcTTATAAATAGGGGAAGGC | SphI | +449/+430 |
| CotCcoding | ctcgagATGGGTTATTATTACAAA | XhoI | +1/+15 |
| CotCSTOP | gtcgacTTATTAGTAGTGTTTTTTATGC | SalI | +357/+338 |
| C/5/Nco | gagccatggATATGGGTTATTACAAA | NcoI | −11/+15 |
| C2 | ccggaattcTGGCGTTTAGTAGTGTTT | EcoRI | +188/+205 |
Capital and lowercase letters indicate nucleotides complementary to corresponding cotU or cotC DNA and unpaired flanking sequences carrying a restriction site, respectively.
Referred to cotU or cotC sequences, taking the initiation codon as +1.
A cotU null mutant was obtained by transforming competent cells of the B. subtilis strain PY79 with plasmid pRH41, carrying a neomycin resistance (neo) cassette in the cotU coding region. A purified 274-bp DNA fragment originating from the amplification of B. subtilis chromosomal DNA with Ys2 and Ya2 oligonucleotides (Table 2) was digested with NotI and EcoRI and cloned into plasmid pBEST501 (16). The plasmid obtained was cleaved with SphI and SalI and used to clone, to the 5′ end of the neo cassette, a second PCR fragment of 242 bp, originating from amplification of the B. subtilis chromosome with Ys1 and Ya1 oligonucleotides (Table 2). Neor clones were the result of double-crossover recombination, resulting in the interruption of the cotU gene on the B. subtilis chromosome. Several Neor clones were analyzed by PCR, and one of them, RH202, was used for further studies. The cotU null mutation was then moved by chromosomal DNA-mediated transformation to the following isogenic strains (Table 1): BD063 (cotA), generating RH214; BD071 (cotC), generating RH203; ER203, (cotG), generating RH217; ER209 (cotH), generating RH216; RH211, (cotE) generating RH218; and RH201 (cotB), generating RH213.
Strain RH211 was obtained by transforming strain BZ213 (cotE::cat) with a linearized form of plasmid pJL62 (cat::spc) (a gift from A. Grossman) to inactivate cat and introduce a spectinomycin resistance gene. Several clones resistant to spectinomycin but sensitive to chloramphenicol were isolated, and one of them, RH211, was used for further studies.
cotC expression in E. coli.
The cotC coding region was amplified by PCR from B. subtilis chromosomal DNA with primers CotCcoding and CotCSTOP (Table 2). The 210-bp PCR product was cleaved with XhoI and SalI and ligated into XhoI-digested expression vector pRSETA (Invitrogen). The recombinant plasmid carrying an in-frame fusion of the 5′ end of the cotC coding region to six histidine codons under the transcriptional control of a T7 promoter was used to transform competent cells of E. coli BL21(DE3) (Invitrogen), yielding strain RH52. This strain was grown in ampicillin-supplemented (50 μg/ml) TY medium (26) to an optical density of 0.7 at 600 nm. The T7 promoter was then induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG; final concentration, 0.5 mM) to the culture, which was incubated for 2 h at 37°C. The six-His-tagged CotC protein was purified under denaturing conditions via Ni-nitrilotriacetic acid affinity chromatography as recommended by the manufacturer (Qiagen, Inc.).
Western blotting.
Sporulation of wild-type and recombinant strains was induced by the exhaustion method (2, 22). After a 30-h incubation at 37°C, spores were collected, washed four times, and purified by lysozyme treatment as previously described (2, 22). The number of purified spores obtained was measured by direct counting with a Bürker chamber under an optical microscope (Olympus; BH-2 with 40× lenses). Aliquots of 1010 spores suspended in 0.3 ml of distilled water were used to extract coat proteins by 0.1 N NaOH treatment at 4°C as previously reported (2). The concentration of the extracted coat proteins was determined by the Bio-Rad DC (detergent-compatible) protein assay to avoid potential interference by the NaOH present (final concentration, 0.2 to 0.6 mN) in the extraction buffer and 15 μg of total proteins fractionated on 18% denaturing poly-acrylamide gels. Proteins were then electrotransferred to nitrocellulose filters (Bio-Rad) and used for Western blot analysis by standard procedures. For the analysis of sporulating cells samples were harvested at various times during sporulation and disrupted by sonication in 25 mM Tris (pH 7.5)-0.1 M NaCl-1 mM EDTA-15% (vol/vol) glycerol-0.1 mg of phenylmethylsulfonyl fluoride/ml. Sonicated material was then fractionated by centrifugation at 12,000 × g for 20 min. The pellet, containing the forming spores resistant to the sonication treatment, was solubilized by 0.1 N NaOH treatment at 4°C, and the total protein concentration was determined as described above. Fifty (mother cell extract) or 15 μg (forespore extract) of total proteins was fractionated on 18% denaturing polyacrylamide gels. Western blot filters were visualized by the SuperSignal West Pico chemiluminescence (Pierce) method as specified by the manufacturer.
CotC-specific antibodies were raised in rabbits immunized with a 14-amino-acid synthetic peptide (NH2-YDYVVEYKKHKKHY-COOH) designed on the base of the C-terminal region of CotC (IGtech, Salerno, Italy).
Yeast two-hybrid system.
The Matchmaker two-hybrid system (Clontech) was used as described by Ozin et al. (23), with only minor modifications. The cotC coding region was amplified by PCR using primer pair C/5/Nco and C2 (Table 2). The PCR product was digested with NcoI and EcoRI and inserted between the same sites of plasmids pAS2-1 and pACT2 (Clontech) to create fusions to the Gal4 DNA binding or activation domains, yielding plasmids pRZ99 and pRZ98, respectively. Saccharomyces cerevisiae strains Y187 (MATα ura3-52 his3-200 ade2-101 trp1-901 leu2-3,112 gal4Δ met− gal80Δ URA3::GAL1UAS-GAL1TATA-HIS3) and Y190 (MATa ura3-52 his3-200 ade2-101 lys2-801 trp1-901 leu2-3,112 gal4Δ gal80Δ cyhr2 LYS2::URA::GAL1UAS-HIS3TATA-HIS3 URA3::GAL1UAS-GAL1TATA-HIS3) (Clontech) were independently transformed with the pAS2-1 or pACT-2 vector and/or each of the cotC constructs according to the protocols suggested by the manufacturer. The resulting clones were used in pairwise matings selecting for Leu and Trp. Colony lift assays for detection of β-galactosidase activity were as described by the manufacturer (Clontech).
RESULTS
Multiple cotC-dependent polypeptides are present in the spore coat.
The cotC gene of B. subtilis encodes a 66-amino-acid polypeptide (CotC) having a deduced molecular mass of 8.8 kDa but migrating on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel as a 12-kDa polypeptide (4). CotC has been identified as a component of the outer layer of the coat (32), and its assembly requires the action of the morphogenetic protein CotH (21). To study the mechanism of CotC assembly in more detail, we raised specific antibodies against a 14-amino-acid synthetic peptide designed on the basis of the CotC C-terminal sequence and performed a Western blot analysis on the coat protein fraction extracted from purified spores of wild-type and isogenic cotC mutant strains (4). Anti-CotC antibodies recognized six polypeptides in the coat proteins of wild-type spores, five of which were absent in the coat protein fraction of cotC null mutant spores (Fig. 1, lanes 1 and 2). The remaining 17-kDa polypeptide, present also in cotC mutant spores, is evidently encoded by a different gene and is, therefore, not directly dependent on cotC expression (Fig. 1, lanes 1 and 2). Only two of the five cotC-dependent polypeptides (12 and 21 kDa) were extracted from wild-type spores in amounts large enough to be observed by Coomassie blue staining of the SDS-PAGE gel (data not shown).
FIG. 1.
Western blot analysis of proteins extracted from spores of the wild type (lane 1), cotC (lane 2) and cotU (lane 3) null mutants, and a double cotC cotU null mutant (lane 4). Proteins were fractionated on 18% polyacrylamide gel and, upon electrotransfer on nitrocellulose membranes, were reacted with CotC-specific rabbit antibodies and then with peroxidase-conjugated secondary antibodies and visualized by the Pierce method. Molecular mass markers are indicated on the right. The estimated sizes of the polypeptides recognized by the CotC-specific antibody are also indicated.
Due to the high level of sequence similarity between CotC and the putative product of ynzH, an open reading frame identified in the analysis of the B. subtilis genome (18), recently identified as the coat component CotU (19), it was possible that at least some of the polypeptides recognized by our anti-CotC antibodies were the product(s) of cotU expression. To test this possibility, we constructed a cotU null mutant (see Materials and Methods) and, by chromosomal-DNA-mediated transformation, a double cotC cotU null mutant. Coat proteins were extracted from purified spores and analyzed by Western blotting with anti-CotC antibodies. In addition to the 17-kDa cotC-independent polypeptide (see above), another polypeptide (23 kDa) was dependent on the expression of both cotC and cotU (Fig. 1). We decided to focus our study on the four polypeptides exclusively dependent on cotC expression, and, to optimize definition of these four bands, we used the cotU null mutant in most of the Western blot experiments of this study.
Expression of cotC in E. coli produces two polypeptides.
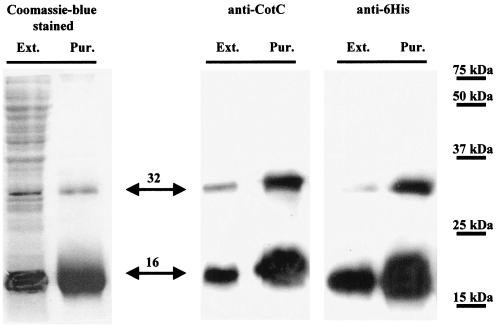
The cotC gene was fused to codons for a six-His tag at its 5′ end, placed under the control of the T7lac promoter, and introduced into cells of the E. coli host BL21(DE3) (Novagen). Cells of the recombinant strain obtained, RH52, were induced with IPTG and lysed as described in Materials and Methods, and total proteins were purified by affinity chromatography on Ni2+ columns. As shown in Fig. 2, two polypeptides of 16 and 32 kDa were purified and both were recognized by CotC-specific and six-His tag-specific antibodies. We identify the 16-kDa polypeptide as the six-His-CotC fusion product (4.5 and 8.8 kDa, respectively, with the latter migrating with an apparent mass of 12 kDa; Fig. 1), whose expected apparent mass is 16.5 kDa. The slower-migrating CotC polypeptide, with an apparent mass of 32 kDa (Fig. 2), is most easily explained if two CotC molecules are bound together. Based on this, we suggest that the faster-migrating protein corresponds to the monomeric form of CotC, whereas the slower one results from the assembly of CotC monomers into a homodimer. Cases of dimers and also oligomers resistant to detergent treatment and strong reducing conditions have been reported previously and are rather frequent (25, 28, 29).
FIG. 2.
Coomassie-blue stained SDS-PAGE gel and Western blots of crude extracts (Ext.) and Ni-nitrilotriacetic acid agarose column-purified proteins (Pur.) of the IPTG-induced E. coli RH52 strain. Proteins were fractionated on 18% polyacrylamide gel and visualized by Coomassie blue staining of the gel or used to perform Western blot analysis with anti-CotC- or anti-six-His-specific antibodies, as indicated. Molecular mass markers are indicated on the right. The estimated sizes of CotC-dependent polypeptides are also reported.
CotC-CotC interaction in a yeast two-hybrid system.
The results shown in Fig. 2 suggested that the 12-kDa polypeptide is most likely a CotC monomer able to self-interact to originate a homodimer in E. coli without the action of specific B. subtilis factors. We used a yeast two-hybrid system (8, 9) to confirm that CotC monomers can spontaneously self-interact. The cotC coding sequence was fused to either the activation domain or the DNA-binding domain of the yeast transcriptional activator GAL4, and the gene fusions were introduced into yeast reporter strains Y187 and Y190 (see Materials and Methods). Interaction of the fusion proteins within yeast cells results in the expression of a lacZ reporter gene (10). No β-galactosidase activity was detected when the individual fusion proteins were expressed with either control vector (data not shown). In contrast, an interaction between CotC and itself was detected, thus confirming the results of Fig. 2 and indicating that CotC molecules have the potential to self-interact.
Assembly of all CotC-dependent polypeptides depends on cotH and cotE expression.
Spores of strains carrying a cotU null mutation along with a null mutation in one other cot gene were solubilized by NaOH treatment, and the released proteins were compared with those released by a strain with mutations only in cotU (Fig. 3). This analysis showed that CotC-dependent polypeptides of 30, 21, 12.5, and 12 kDa were all present in the cotU single mutant as well as in the cotU cotA, cotU cotB, and cotU cotG double mutants. All four cotC-dependent polypeptides were absent in strains with double-null mutation cotU cotH or cotU cotE (Fig. 3, lanes 5 and 7, respectively). Moreover, the amount of the 30-kDa polypeptide extracted from cotU cotB mutant spores (Fig. 3, lane 6) was repeatedly less than that extracted from spores with cotU mutations only (Fig. 3, lane 2). Identical results were obtained in the single null mutant strains with mutations in cotA, cotB, cotG, cotH, or cotE (not shown), thus suggesting that assembly of all four cotC-dependent polypeptides in the spore coat strictly requires cotE and cotH expression and that, in addition, assembly of the 30-kDa polypeptide is partially dependent on cotB expression.
FIG. 3.
Western blot of proteins extracted from spores of cotC cotU (lane 1), cotU (lane 2), cotA cotU (lane 3), cotG cotU (lane 4), cotH cotU (lane 5), cotB cotU (lane 6), and cotE cotU (lane 7) mutants. Proteins were fractionated on 18% polyacrylamide gel and, upon electrotransfer on nitrocellulose membranes, were reacted with CotC-specific rabbit antibodies and then with peroxidase-conjugated secondary antibodies and visualized by the Pierce method. Molecular mass markers are indicated on the right. The estimated sizes of CotC-dependent polypeptides are also reported.
CotC 12.5- and 30-kDa polypeptides appear late during spore formation.
Sporulating cells of a cotU null mutant were harvested at various times during sporulation and lysed by sonication as described in Materials and Methods, and the forming spores surviving the treatment were separated by centrifugation. The forming spores were then extracted by alkali treatment, and the released proteins were compared with those present in the mother cell cytoplasm. For each time point both protein fractions were analyzed by Western blotting with CotC-specific antibodies. At all time points analyzed we detected no CotC-specific polypeptides in the mother cell fraction (Fig. 4). In the forespore fraction, CotC monomeric (12-kDa) and homodimeric (21-kDa) forms were observed starting 8 h after the onset of sporulation (T8), while the other two cotC-dependent polypeptides of 12.5 and 30 kDa appeared 2 h later (Fig. 4). Appearance of CotC-specific polypeptides starting from T8 was in perfect agreement with the previously described cotC expression pattern (14, 31). Identical results were obtained with a wild-type strain (not shown). While 15 μg of total proteins was used to detect CotC from purified spores and forespore fractions, 50 μg of total proteins was analyzed in the case of mother cell samples. This amount of total protein allowed visualization of CotB (15) and CotA (see below) in the mother cell fraction of sporulating cells. Therefore, absence of CotC-specific polypeptides in the mother cell fraction together with the appearance of the 12.5- and 30-kDa polypeptides 2 h later than the 12- and 21-kDa forms of CotC (Fig. 4) suggests that (i) CotC does not accumulate in the mother cell, probably as a consequence of its immediate assembly on the forming spore, and (ii) the 12.5- and 30-kDa polypeptides are most likely generated on the forming spore coat by specific posttranslational modifications of the previously assembled forms of 12 and 21 kDa.
FIG. 4.
Western blot of proteins extracted at various times after the onset of sporulation from the mother cell or forespore of sporulating cells of a cotU null mutant strain. Fifty (mother cell extract) or 15 μg (forespore extract) of total proteins was fractionated on 18% polyacrylamide gel, and upon electrotransfer on nitrocellulose membranes, proteins were reacted with CotC-specific rabbit antibodies and then with peroxidase-conjugated secondary antibodies and visualized by the Pierce method. Molecular mass markers are indicated. The estimated sizes of CotC-dependent polypeptides are also reported.
CotH stabilizes CotC in the mother cell compartment of sporulating cells.
As a consequence of our hypothesis that CotC assembles on the forming spore immediately after its synthesis in the mother cell compartment and that the 12.5- and 30-kDa polypeptides form only on the forespore, we expected to find only the 12- and 21-kDa CotC polypeptides in the mother cells of mutants that fail to assemble CotC. To test this prediction, we performed a Western blot analysis of CotC polypeptides from sporulating cells of cotU cotE and cotU cotH null mutant strains that, as shown in Fig. 3, do not assemble CotC. Cells were collected at various times during sporulation and lysed by sonication, and forespore and mother cell fractions were separated as described above. The forming spores were then extracted by alkali treatment, and the released proteins were compared with those present in the mother cell cytoplasm by using CotC-specific antibodies. In agreement with our prediction, accumulation of only two CotC-specific polypeptides, of 12 and 21 kDa, could be observed in the mother cell fraction of a cotU cotE null mutant (Fig. 5A). The intensities of the signals observed for the 12- and 21-kDa polypeptides, compared to those obtained for the same polypeptides from wild-type spores, make it extremely unlikely that the absence of the other two forms of CotC could be due to their low concentrations. To our surprise, we repeatedly failed to detect any CotC-specific polypeptide in the mother cell protein fraction of a cotH mutant at all time points analyzed (Fig. 5B), as well as in the forespore fraction (not shown). The same mother cell extracts were reacted with CotA-specific antibodies (L. Martins and A. O. Henriques, unpublished results); this revealed the presence of CotA-specific polypeptides of the expected sizes (Fig. 5C), thus suggesting that the phenomenon observed in the cotH mutant is restricted to CotC forms and is not due to aspecific protein degradation.
FIG. 5.
(A) Western blot of proteins extracted from mature spores of a cotU null mutant strain and from the mother cell fraction of sporulating cells of a cotE mutant strain, 8 (t8) and 10 h after the onset of sporulation. (B) Western blot of proteins extracted 8, 9, 10, and 11 h after the onset of sporulation from the mother cell of sporulating cells of a cotH null mutant strain. (C) Western blot of proteins extracted 10 h after the onset of sporulation from the mother cell of sporulating cells of a cotH null mutant strain. Fifty (mother cell extract) or 15 μg (forespore extract) of total proteins was fractionated on 18% polyacrylamide gel and, upon electrotransfer onto nitrocellulose membranes, proteins were reacted with CotC-specific (A and B) or CotA-specific (C) rabbit antibodies and then with peroxidase-conjugated secondary antibodies and visualized by the Pierce method. Molecular mass markers are indicated. The estimated sizes of CotC-dependent polypeptides are also reported.
DISCUSSION
In the present work we report evidence that the CotC component of the B. subtilis spore coat is assembled into at least four forms, all dependent on the expression of the cotH gene. Expression of the CotC structural gene in E. coli produced two polypeptides, most likely corresponding to a CotC monomer and a homodimer resistant to the detergent treatment and the reducing conditions used. Since it is unlikely that an E. coli enzyme forms specific covalent bonds between CotC molecules and since CotC has the potential to self-interact, as shown by our experiment with yeast cells (data not shown), we believe that the CotC homodimer is most probably formed by spontaneous, noncovalent assembly of monomers. Additional biochemical experiments, out of the scope of this study, are needed to understand the nature of this interaction.
CotC assembly depends on cotE and cotH expression. Dependency on cotE was expected, since it has been previously shown that cotE null mutant spores do not assemble the outer coat (31). Dependency on cotH expression was previously reported for the most abundant 12-kDa CotC form (21). Assembly of two other coat components, CotB and CotG, also depends on cotH expression. Since assembly of CotB, in turn, depends on cotG expression (24), hierarchical control CotH-CotG-CotB was proposed (21). Here we show (Fig. 3) that all four CotC polypeptides strictly require cotE and cotH expression and do not depend on the expression of the cotA, cotB, or cotG gene and that only the 30-kDa form has a partial requirement for cotB expression.
Western blotting performed at various times during sporulation with the wild type and cotH and cotE mutants allowed three conclusions. (i) The 12- and 21-kDa CotC forms are assembled on the forming spore immediately after their synthesis in the mother cell compartment. This is based on the observation that the 12- and 21-kDa forms accumulate in the mother cell of a cotE mutant (unable to assemble them) but not in the mother cell of wild-type spores. (ii) The 12.5- and 30-kDa forms of CotC are generated on the forming spore coat, since they are never found in the mother cell of wild-type or cotE mutant cells. Their formation is most likely due to specific posttranslational modifications of the previously assembled forms of 12 and 21 kDa. The nature of such modifications has not been clarified. However, since CotC contains several tyrosines (30.3% of total residues) and since dityrosine bond formation may be involved in coat assembly (reference 13 and references therein), it is possible that this type of cross-link is, at least in part, responsible for the formation of the 12.5- and 30-kDa forms of CotC. (iii) CotH or a cotH-controlled factor allows assembly of the 12- and 21-kDa forms of CotC on the spore surface. This is based on the observation that the 12- and 21-kDa forms do not accumulate in the mother cell of a cotH mutant. Since CotC is present in the cytoplasm of a cotE mutant (Fig. 5A) as well as in E. coli (Fig. 2) but is not found in the cytoplasm of a cotH mutant (Fig. 5B), its absence cannot be due to the low stability of the protein. We believe it more likely that a specific factor (possibly a protease) degrades CotC in the absence of CotH (or a cotH-dependent protein). According to this model, in a wild-type strain CotH (or a cotH-dependent protein) would prevent CotC degradation either by interacting in a chaperone-like manner with CotC or its specific protease in the mother cell or by immediately recruiting CotC into the coat of the forming spore. Either way, CotC is not present in the mother cell or on the forespore of a cotH mutant. Our data do not allow us to establish whether CotC is degraded or is only cleaved near its C-terminal end, making it undetectable for our antibodies. However, such hypothetical cleavage does not occur in a wild-type strain and would in any case lead to a nonphysiological situation (i.e., assembly of a shorter form of CotC).
Acknowledgments
This work was supported by the European Union grant no. QLK5-CT-2001-01729 to E.R. and A.O.H. and by MIUR (Cofin 2002; FIRB 2002) grants to E.R.
REFERENCES
- 1.Aronson, A. I., H.-Y. Song, and N. Bourne. 1988. Gene structure and precursor processing of a novel Bacillus subtilis spore coat protein. Mol. Microbiol. 3:437-444. [DOI] [PubMed] [Google Scholar]
- 2.Cutting, S., and P. B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C. Harwood and S. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 3.Cutting, S., L. Zheng, and R. Losick. 1991. Gene encoding two alkali-soluble components of the spore coat from Bacillus subtilis. J. Bacteriol. 173:2915-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donovan, W., L. Zheng, K. Sandman, and R. Losick. 1987. Genes encoding spore coat polypeptides from Bacillus subtilis. J. Mol. Biol. 196:1-10. [DOI] [PubMed] [Google Scholar]
- 5.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driks, A., S. Roels, B. Beall, C. P. Moran, Jr., and R. Losick. 1994. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 8:234-244. [DOI] [PubMed] [Google Scholar]
- 7.Duc, L. H., H. A. Hong, N. Fairweather, E. Ricca, and S. M. Cutting. 2003. Bacterial spores as vaccine vehicles. Infect. Immun. 71:2810-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-246. [DOI] [PubMed] [Google Scholar]
- 9.Fields, S., and R. Sternglanz. 1994. The two-hybrid system: an assay for protein-protein interactions. Trends Genet. 10:286-292. [DOI] [PubMed] [Google Scholar]
- 10.Guarente, L. 1993. Strategies for the identification of interacting proteins. Proc. Natl. Acad. Sci. USA 90:1639-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriques, A., and C. P. Moran, Jr. 2000. Structure and assembly of the bacterial endospore coat. Methods 20:95-110. [DOI] [PubMed] [Google Scholar]
- 12.Henriques, A. O., B. W. Beall, and C. P. J. Moran. 1997. CotM of Bacillus subtilis, a member of the α-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J. Bacteriol. 179:1887-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henriques, A. O., L. R. Melsen, and C. P. Moran, Jr. 1998. Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis. J. Bacteriol. 180:2285-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichikawa, H., and L. Kroos. 2000. Combined action of two transcription factors regulates genes encoding spore coat proteins of Bacillus subtilis. J. Biol. Chem. 275:13849-13855. [DOI] [PubMed] [Google Scholar]
- 15.Isticato, R., G. Cangiano, T.-H. Tran, A. Ciabattini, D. Medaglini, M. R. Oggioni, M. De Felice, G. Pozzi, and E. Ricca. 2001. Surface display of recombinant proteins on Bacillus subtilis spores. J. Bacteriol. 183:6294-6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itaya, M., K. Kondo, and T. Tanaka. 1989. A neomycin resistance cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acid Res. 17:4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, K., Y. Kumazawa, K. Miwa, and S. Yamanaka. 1994. ɛ-(γ-Glutamyl)lysine cross-links of spore coat proteins and transglutaminase activity in Bacillus subtilis. FEMS Microbiol. Lett. 144:157-160. [Google Scholar]
- 18.Kunst, F., N. Ogasawara, I. Moszer, H. Yoshikawa, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 19.Lai, E.-M., N. D. Phadke, M. T. Kachman, R. Giorno, S. Vazquez, A. J. Vazquez, J. R. Maddock, and A. Driks. 2003. Proteomic analysis of the spore coats of Bacillus subtilis and Bacillus anthracis. J. Bacteriol. 185:1443-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins, L. O., C. M. Soares, M. M. Pereira, M. Teixeira, T. Costa, G. H. Jones, and A. O. Henriques. 2002. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 277:18849-18859. [DOI] [PubMed] [Google Scholar]
- 21.Naclerio, G., L. Baccigalupi, R. Zilhao, M. De Felice, and E. Ricca. 1996. Bacillus subtilis spore coat assembly requires cotH gene expression. J. Bacteriol. 178:4375-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. Harwood and S. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, United Kingdom.
- 23.Ozin, A. J., C. S. Samford, A. O. Henriques, and C. P. Moran, Jr. 2001. SpoVID guides SafA to the spore coat in Bacillus subtilis. J. Bacteriol. 183:3041-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacco, M., E. Ricca, R. Losick, and S. Cutting. 1995. An additional GerE-controlled gene encoding an abundant spore coat protein from Bacillus subtilis. J. Bacteriol. 177:372-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salahpour, A., H. Bonin, S. Bhalla, U. Petaja-Repo, and M. Bouvier. 2003. Biochemical characterization of β2-adrenergic receptor dimers and oligomers. Biol. Chem. 384:117-123. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Serrano, M., R. Zilhao, E. Ricca, A. Ozin, C. Moran, and A. Henriques. 1999. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J. Bacteriol. 181:3632-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenney, K., I. Hunt, J. Sweigard, J. I. Pounder, C. McClain, E. J. Bowman, and B. J. Bowman. 2000. Hex-1, a gene unique to filamentous fungi, encodes the major protein of the Woronin body and functions as a plug for septal pores. Fungal Genet. Biol. 31:205-217. [DOI] [PubMed] [Google Scholar]
- 29.Tosi, G., R. Meazza, A. De Lerma Barbaro, A. D'Agostino, S. Mazza, G. Corradin, A. Albini, D. M. Noonan, S. Ferrini, and R. S. Accolla. 2000. Highly stable oligomerization forms of HIV-1 Tat detected by monoclonal antibodies and requirement of monomeric forms for the transactivating function on the HIV-1 LTR. Eur. J. Immunol. 30:1120-1126. [DOI] [PubMed] [Google Scholar]
- 30.Youngman, P., J. B. Perkins, and R. Losick. 1984. A novel method for the rapid cloning in Escherichia coli of Bacillus subtilis chromosomal DNA adjacent to Tn917 insertion. Mol. Gen. Genet. 195:424-433. [DOI] [PubMed] [Google Scholar]
- 31.Zheng, L., W. P. Donovan, P. C. Fitz-James, and R. Losick. 1988. Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev. 2:1047-1054. [DOI] [PubMed] [Google Scholar]
- 32.Zheng, L. B., and R. Losick. 1990. Cascade regulation of spore coat gene expression in Bacillus subtilis. J. Mol. Biol. 212:645-660. [DOI] [PubMed] [Google Scholar]
- 33.Zilhao, R., G. Naclerio, L. Baccigalupi, A. Henriques, C. Moran, and E. Ricca. 1999. Assembly requirements and role of CotH during spore coat formation in Bacillus subtilis. J. Bacteriol. 181:2631-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]