Abstract
BACKGROUND
Treatments are being developed for an increasing number of mucopolysaccharidoses, and early diagnosis is expected to be necessary to maximize the benefits of therapy. Therefore, we developed an assay for N-acetylgalactosamine-6-sulfate sulfatase (GALNS), the enzyme deficient in mucopolysaccharidosis IVA (Morquio A syndrome), that is applicable for clinical diagnosis.
METHODS
A novel substrate for GALNS was synthesized for a new enzyme activity assay that is based on tandem mass spectrometry and uses dried blood spots (DBSs) as the enzyme source. We optimized the assay conditions, including the substrate concentration, reaction pH, lead formate concentration, incubation time, punch size of the DBS, and mass spectrometer conditions. We also assessed inter- and intraassay variation.
RESULTS
The assay uses either solid-phase or liquid-phase extraction before analysis by mass spectrometry. An evaluation of blood spots from 90 randomly chosen healthy newborns and 9 patients with Morquio A syndrome showed a well-defined interval between their respective enzyme activities. Inter- and intraassay imprecision was <10%.
CONCLUSIONS
This tandem mass spectrometry assay requires a minimal number of sample-preparation steps, thus making it easy to implement. The assay has the potential to be adopted for early diagnosis of Morquio A syndrome. We believe this assay could be performed in a multiplex fashion with assays for other lysosomal enzymes.
Morquio syndrome type A, or mucopolysaccharidosis IVA (MPS-IVA),4 is an autosomal recessive disorder that causes a deficiency of lysosomal N-acetylgalactosamine-6-sulfate sulfatase (GALNS) (EC 3.1.6.4) activity (1). The enzyme hydrolyzes the sulfate ester bond of galactose 6-sulfate in keratan sulfate and of N-acetylgalactosamine 6-sulfate in chondroitin C. Treatment for MPS-IVA is currently being evaluated in phase I/II clinical trials. Early detection of MPS-IVA seems prudent to maximize the potential benefit of treatment, and thus tests appropriate for early diagnosis and possibly newborn screening are needed. Fluorometric (2) and radiometric (3) GALNS enzyme assays have been reported. We have used electrospray ionization–tandem mass spectrometry (ESI-MS/MS) in the development of enzyme activity assays relevant to several lysosomal storage diseases (4, 5). ESI-MS/MS assays offer the capability of assaying the products of several enzymes by a single infusion into the mass spectrometer and may provide data that are more accurate than those produced by fluorometric assays. We describe the development of a specific enzyme activity assay that uses both a novel substrate in MPS-IVA and ESI-MS/MS.
All experiments were conducted in compliance with institutional review board guidelines. We obtained samples from affected patients who had their MPS-IVA disease previously diagnosed according to established clinical and biochemical procedures. Dried blood spots (DBSs) were kept at ambient temperature during shipment (<10 days) and then stored at −20 °C in zip-lock plastic bags (one bag sealed inside a second bag). The zip-lock bags were kept in a sealed plastic box that contained desiccant (anhydrous CaSO4 granules). The method used for the synthesis of the GALNS substrate (GALNS-S) and the GALNS internal standard (GALNS-IS) has recently been described (6).
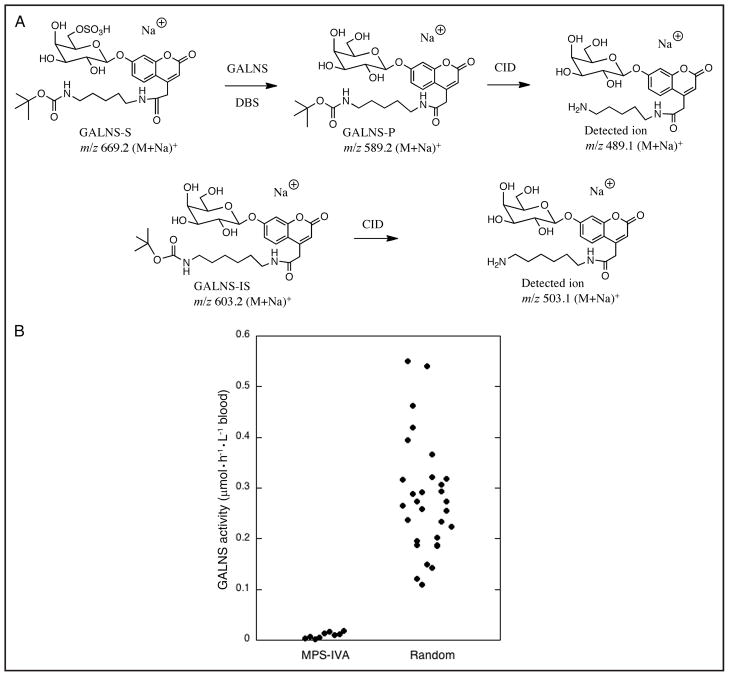
GALNS-S consists of umbelliferyl-β-D-galactose with a sulfate group at the 6-position of the sugar. To facilitate postassay purification, we incorporated a hydrophobic 5-carbon linker with a terminal t-butylcarbamate at the 4-position of the umbelliferyl unit. The presence of this linker increases the ESI-MS/MS signal (owing to a dominant fragmentation pathway involving cleavage of the t-butylcarbamate) (Fig. 1A). Incubation of the substrate with a DBS leads to enzymatic release of the sulfate group to produce the GALNS product (GALNS-P) (Fig. 1A). GALNS-IS is a GALNS-P homolog that possesses 1 additional methylene unit in the linker chain. ESI-MS/MS enables separate detection and quantification of GALNS-P and GALNS-IS by their fragment ions after collision-induced dissociation of their parent ions (Fig. 1A). The presence of the umbelliferyl moiety in GALNS-S permits fluorometric assay of GALNS (2) by laboratories that do not desire to use ESI-MS/MS; however, such an assay will require that a glycohydrolase be added to act after the sulfatase.
Fig. 1. Structures of GALNS-S, GALNS-P, and GALNS-IS for the GALNS reaction, and GALNS activities measured in this study.
(A), Shown are structures of GALNS-S, GALNS-P, and GALNS-IS for the GALNS reaction in this study and structures of the product ions from GALNS-P and GALNS-IS after collision-induced dissociation (CID) in the mass spectrometer. GALNS-P and GALNS-IS were quantified by ESI-MS/MS in positive-ion, multiple reaction monitoring mode as the sodiated species (M+Na)+. (B), GALNS activities were measured in DBSs by the standard assay with ethyl acetate extraction, as described in the text. The mean GALNS activities for each sample of 9 MPS-IVA patients and 30 healthy newborns are provided in Table 1 of the online Data Supplement.
A single DBS punch (2 mm in diameter, containing approximately 1.6 μL of blood) was obtained with a leather punch and placed in a 1.5-mL Eppendorf tube. A 20-μL aliquot of assay cocktail containing 100 mmol/L sodium formate buffer (pH 4.0), 30 mmol/L lead(II) formate, 1 mmol/L GALNS-S, and 0.5 μmol/L GALNS-IS was added to the tube (GALNS-S and GALNS-IS stock solutions were prepared in methanol and stored at −20 °C; the solvent was removed before adding buffer). The mixture was vortex mixed for 15 s and centrifuged for 15 min. A blank containing all assay components but with a 2-mm filter paper punch (no blood) was similarly prepared. The samples were incubated at 37 °C for 16 h in a thermostated air shaker. The reaction was quenched by adding 100 μL of 25 mmol/L Na2HPO4, pH 7.85. An analysis of the product before and after a 4-h postquench incubation confirmed that the quench stopped the reaction. To purify the product and internal standard, we carried out solid-phase extraction with the aid of a 20-position vacuum extraction manifold (Waters) connected to a water aspirator. We then pipetted 20 mg DEAE-cellulose resin suspended in 300 μL acetic acid into each well of a 96-well filter plate (Innovative Microplate) and washed each well twice with 0.5 mL methanol. A slurry of C18 silica gel (20 mg octadecyl-functionalized silica gel; Sigma-Aldrich) in 300 μL methanol was pipetted on top of the ion-exchange resin in each well. We sequentially washed the dual layer twice with 0.5 mL methanol and then twice with 1.0 mL deionized water. We centrifuged the quenched sample for 5 min, loaded the supernatant onto the column, and washed the column twice with 1.0 mL deionized water to remove salts. GALNS-P and GALNS-IS were eluted into a deep-well plate (Neptune) with two 0.5-mL additions of methanol. We used a SpeedVac vacuum concentrator (Thermo Scientific) to remove the methanol and reconstituted the residue in 30 μL of solvent (200 mL water containing 400 μL formic acid added to 800 mL acetonitrile).
For the recovery of GALNS-P and GALNS-IS by liquid extraction, we quenched the assay sample by adding a suspension of 64 mg DEAE-cellulose (DE52, preswollen; Whatman) in 250 μL water. We added 500 μL ethyl acetate to extract GALNS-P and GALNS-IS, vortex mixed the suspension for 15 s, and centrifuged the sample for 15 min. We then transferred the ethyl acetate portion (300 μL) to a new Eppendorf tube, removed the solvent with a stream of nitrogen gas (or filtered air), and reconstituted the residue in 30 μL of the acetonitrile/water/formic acid solvent described above.
The postassay purification step is imperative because the presence of buffer salts in high concentrations will interfere with the ESI and because the unreacted sulfated substrate can dissociate in the source of the mass spectrometer to form GALNS-P ions, thereby giving rise to false-positive GALNS activity. In the solid-phase extraction, the buffer salts are removed with water while the hydrophobic GALNS-S, GALNS-IS, and GALNS-P molecules are retained on the C18 resin. The anionic GALNS-S is retained on the DEAE-cellulose, and GALNS-P and GALNS-IS are passed through. In the liquid-phase extraction, GALNS-S is captured on the DEAE-cellulose, and GALNS-IS and GALNS-P are extracted into ethyl acetate.
ESI-MS/MS analysis was carried out on a Quattro Micro instrument (Waters) operating in the positive-ion, multiple reaction monitoring mode (see the Data Supplement that accompanies the online version of this Brief Communication at http://www.clinchem.org/content/vol57/issue1). A 10-μL aliquot of the 30-μL sample was infused into the mass spectrometer and analyzed within 1 min of infusion. The precursor ions for GALNS-P and GALNS-IS (m/z 589.2 and 603.2, respectively) were each isolated by mass and subjected to collision-induced dissociation. We then quantified the product ions m/z 489.1 and 503.1 derived from GALNS-P and GALNS-IS, respectively. The amount of GALNS-P was calculated by comparing the ion peak intensity of GALNS-P to that of GALNS-IS. The signal generated from the blank assay (no blood) was approximately 1% of that seen with the complete assay (see tables in the online Data Supplement).
Assay-optimization experiments revealed a maximum GALNS activity at pH 4.0 in formate buffer (see Fig. 1 in the online Data Supplement). Sulfate and phosphate ions are competitive inhibitors of sulfatases (7). We therefore used lead(II) formate (30 mmol/L) to suppress these inhibitors (see Fig. 2 in the online Data Supplement). The amount of GALNS-P increased with reaction time from 0 to 30 h (see Fig. 3 in the online Data Supplement). We chose a 16-h incubation time to allow overnight incubation and to simplify laboratory work schedules. The GALNS-P amount decreases when the DBS punch size is increased from 2 to 4 mm (see Fig. 4 in the online Data Supplement), presumably because of endogenous inhibitors in the DBS. We therefore chose a 2-mm DBS punch for the assays. The ESI-MS/MS response to GALNS-P was 1.10 times that of GALNS-IS (see Fig. 5 in the online Data Supplement). Assay imprecision was calculated from the results of replicate analyses of the DBS from a healthy control individual; the intra- and interassay CVs were 6.4% (n = 3) and 8.3% (n = 10), respectively. A control assay containing all assay components except GALNS-S was performed with 30 different DBSs, and the results indicated the signal to be approximately 1% of that obtained with the complete assay. This finding rules out any ESI-MS/MS signal coming from sources other than GALNS action (see Table 1 in the online Data Supplement). Enzyme-stability studies showed GALNS to be stable for at least 1 year in DBSs stored at −20 °C; however, about 50% of the activity is lost over 3 days at 37 °C.
Use of the GALNS assay with liquid-phase extraction demonstrated that the enzyme activity in DBSs from 9 MPS-IVA patients (range, 0.0019 – 0.018 μmol · h−1 · L−1 blood; mean, 0.0096 μmol · h−1 · L−1 blood) was well below the range of activities in blood samples obtained from 30 healthy newborns (range, 0.109 – 0.550 μmol · h−1 · L−1 blood; mean, 0.279 μmol · h−1 · L−1 blood) (Fig. 1B). ESI-MS/MS analysis measured the relative amounts of GALNS-P and GALNS-IS that extract into ethyl acetate after the extraction of buffer containing equivalent moles of analytes. As expected, the less hydrophobic GALNS-P is extracted less than GALNS-IS. The combined factor for relative extraction and ionization efficiency is 1.81. We multiplied the ESI-MS/MS signal for GALNS-P by this factor to obtain the specific enzymatic activities reported.
In addition, we used the solid-phase extraction method to evaluate the enzyme activities for 60 additional DBSs from healthy newborns, along with the DBSs from 9 MPS-IVA patients. These data are reported in the online Data Supplement. The values obtained with solid-phase extraction were in the same activity interval as those for liquid-phase extraction. We believe the latter is preferable for newborn-screening laboratories because it is easier and faster to execute.
Supplementary Material
Acknowledgments
Research Funding: M.H. Gelb, Genzyme. M.H. Gelb, F. Turecek, and C.R. Scott, BioMarin Pharmaceuticals Inc. and the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (grant R01 DK067859).
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
We thank BioMarin Pharmaceuticals Inc. and the NIH, National Institute of Diabetes and Digestive and Kidney Diseases for financial support of this research.
Footnotes
Nonstandard abbreviations: MPS-IVA, mucopolysaccharidosis type IVA; GALNS, N-acetylgalactosamine-6-sulfate sulfatase; ESI-MS/MS, electrospray ionization–tandem mass spectrometry; DBS, dried blood spot; GALNS-S, GALNS substrate; GALNS-IS, GALNS internal standard; GALNS-P, GALNS product.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: M.H. Gelb, Genzyme.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
References
- 1.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8. New York: McGraw-Hill; 2001. pp. 3421–52. [Google Scholar]
- 2.van Diggelen OP, Zhao H, Kleijer WJ, Janse HC, Poorthuis BJHM, van Pelt J, et al. A fluorimetric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A) Clin Chim Acta. 1990;187:131–40. doi: 10.1016/0009-8981(90)90339-t. [DOI] [PubMed] [Google Scholar]
- 3.Glossl J, Kresse H. A sensitive procedure for the diagnosis of N-acetylgalactosamine-6-sulphate sulphatase deficiency in classical Morquio’s disease. Clin Chim Acta. 1978;88:111–9. doi: 10.1016/0009-8981(78)90157-2. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, Gelb MH. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785–96. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin Chem. 2008;54:2067–70. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffey TA, Khaliq T, Scott CR, Turecek T, Gelb MH. Design and synthesis of substrates for newborn screening of MaroteauxLamy and Morquio A syndromes. Bioorg Med Chem Lett. 2010;20:5994–6. doi: 10.1016/j.bmcl.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masue M, Sukegawa K, Orii T, Hashimoto T. N-Acetylgalactosamine-6-sulfate sulfatase in human placenta: purification and characteristics. J Biochem. 1991;110:965–70. doi: 10.1093/oxfordjournals.jbchem.a123697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.