Abstract
Asian series have shown 5 year survival of ∼70% after resection of hepatocellular cancer (HCC) <2cm. Western outcomes with resection have not been as good. In addition ablation of HCC ≤ 2cm has been shown to achieve competitive results leaving the role of resection unclear in these patients. Records of patients undergoing resection at two Western centers between 1/1990 and 12/2009 were reviewed. Patients with a single HCC ≤ 2cm on pathology were included. Thirty clinical variables including demographics, liver function, tumor characteristics, nature of the surgery, and the surrounding liver were examined. An exploratory statistical analysis was conducted to determine variables associated with recurrence and survival. The study included 132 patients with a median follow-up of 37.5 months. There was 1 (<1%) 90-day mortality. There were 32 deaths with a median survival of 74.5 months and 5-year survival of 70% (63% in cirrhotics). Median time-to-recurrence was 31.6 months and 5-year recurrence rate was 68%. Presence of satellites (HR=2.46, p=0.031) and platelet count <150,000/μl (HR=2.37, p=0.026) were independently associated with survival. Presence of satellites (HR=2.79, p=0.003), cirrhosis (HR=2.3, p=0.010), and non-anatomic resection (HR=1.79, p=0.031) were independently associated with recurrence. Patients with a single HCC ≤ 2cm and platelet count ≥150,000/μl achieved median and 5-year survivals of 138 months and 81%, respectively.
Conclusion
Resection of HCC ≤ 2cm is safe and achieves excellent results in Western centers. Recurrence continues to be a significant problem. Presence of satellites, platelet count, anatomic resection and cirrhosis are associated with outcomes after resection even among such early tumors. Resection should continue to be considered a primary treatment modality in patients with small HCC and well preserved liver function.
Keywords: surgery, small, early, platelet, satellites, survival
Introductory Statement
Hepatocellular cancer (HCC) ≤ 2cm is regarded as a separate and distinct clinical sub-group by both Eastern and Western experts (1, 2). Detection of tumors at such an early stage has traditionally been rare in the West and as a result, clinicians have had to rely on data almost exclusively from the East. However, as a result of the increased awareness of the need for screening in patients with liver disease and validated criteria for accurate noninvasive diagnosis of such small tumors, the number of HCC being detected at an early stage will likely increase in North America and Europe(3-5).
Patients with such early HCC have a good likelihood of cure with resection, transplantation or ablation (6-11). While there have been a significant number of recent publications on the indications and outcomes of both transplantation and ablation in the treatment of early HCC, the literature on which the recommendations regarding the role surgical resection are based is more dated. A review of the data collected by the Liver Cancer Study Group of Japan demonstrated 5-year survival of 71% for the 1,318 patients with a single hepatocellular cancer <2cm undergoing surgical resection(12). In contrast, examination of the Surveillance, Epidemiology, and End Results (SEER) Program database identified only 154 patients with HCC ≤ 2cm undergoing resection in the United States over an 8 year period with 5-year survival of only 49%(13). Such differing results leave the role of surgical resection for such early tumors unclear. In addition, such poor results reported by Western series as well as the lack of well defined criteria for resection have lead some authors to suggest that radiofrequency ablation may be the treatment of choice for patients with HCC ≤ 2cm even when surgical resection is possible (10, 14).
The data presented in this study details the results from two Western centers performing a large volume of HCC resections. It represents the largest Western series to examine the outcomes of patients undergoing resection of single HCC ≤ 2cm. We also provide the results of our exploratory analyses to determine the clinical variables associated with survival and recurrence. These results will hopefully provide new and valuable insight into the role of resection for such early tumors.
Experimental Procedures
Prospectively collected data from two Western centers (Mount Sinai Medical Center, New York, USA and National Cancer Institute-Istituto Nazionale Tumori, Milan, Italy) was reviewed retrospectively. Patients undergoing resection of a single pathologically proven HCC ≤ 2cm in size between 1990 and 2009 were chosen. Approval for conducting the study was obtained from both the Institutional Review Boards (IRB).
Thirty variables including age, gender, and underlying liver disease were recorded. The anatomic location and distance of the recurrent tumor from the cut surface of the liver after resection was recorded by reviewing postoperative imaging studies. We also recorded preoperative platelet count, albumin level, total bilirubin, creatinine, INR (international normalized ratio), MELD (Model for End-Stage Liver Disease) score, AFP (α-feto protein) level, type of resection performed, need for transfusion, tumor size, and number of tumors. Pathological slides were systematically reviewed for tumor differentiation, extent of vascular invasion and degree of fibrosis in the surrounding liver using the Metavir staging system. Anatomic resection was defined as removal of the entire Couinaud segment(s) involved with the tumor. Patients without satellites and without vascular invasion were classified “very early” HCC: BCLC stage 0 or Japanese T1 based on pathology(1, 2).
Criteria for diagnosis and resection and follow-up protocol
Criteria for diagnosis and resection have evolved over time but have been similar in the two Centers, with only slight differences regarding preoperative diagnosis. Currently, in New York at least two contrast enhanced imaging studies (CT and MRI) are required. The tumor had to display arterial enhancement and venous washout. Currently, in Milan the diagnosis of HCC was made on sequential contrast enhanced imaging studies (CT, MRI or Ultrasound) unless one study conclusively demonstrated HCC with arterial enhancement and venous washout. Prior to the establishment of the criteria for non-invasive diagnosis of HCC at the European Association of the Study of the Liver (EASL) in 2000, the diagnosis was established by biopsy. Also, in cases without conclusive radiological diagnosis, ultrasound-guided biopsy was used in both centers. Fourteen (11%) of patients, eleven prior to 2000 and three after, required biopsy to confirm the diagnosis of HCC.
Only patients with a single tumor and no evidence of extrahepatic spread were included in the study. In New York only patients with Child's A liver disease or no cirrhosis were considered for resection. After 2002 patients with clinical portal hypertension or platelet count <100,000 /μl were excluded from resection. In Milan, platelet count was not the sole criteria defining portal hypertension: patients with Child's A liver disease without esophageal varices (≤F1 grade) and with indocyanine green retention (ICGR) < 20% at 15 minutes were allowed resection up to two segments even if they had platelet count <100,000/μl.
The patients were followed with either contrast enhanced CT or MRI scans of the abdomen as well as blood work including AFP. Contrast enhanced ultrasound was also used for surveillance in Milan. Non-contrast CT of the chest was used to detect lung recurrence irrespective of the modality used to screen for abdominal recurrence. The follow-up schedule consisted of scans every 3 (New York) or 4 (Milan) months for the first year, every 4 months for the second year, and subsequently every 6 months. No adjuvant therapy was used.
Treatment of recurrence
“Very early” recurrence was defined as recurrence within the first year after surgery based on previously published data showing this to be a clinically significant cut-off (15). Data on the more conventional cut-off at 2 years for “early” recurrence is also provided. Solitary recurrences were treated with resection. Patients with a solitary liver recurrence (New York) or multiple tumors within Milan criteria (Milan) and Child–Pugh A liver disease and no evidence of portal hypertension underwent a second hepatic resection. Patients with multiple intrahepatic recurrences or compromised hepatic function were treated with radiofrequency ablation and/or transarterial chemoembolization. Patients with recurrence confined to the liver and without significant comorbidities were also referred for liver transplantation. Patients undergoing liver transplantation were censored at the time of transplant for the purposes of this study. After 2008, patients not eligible for repeat resection, liver transplantation, or local-regional therapies were treated with sorafenib.
Statistical Analysis
The primary end-point analyzed was survival. Secondary end-points included overall, very early (< 1 year), and early (< 2 year) recurrence. Exploratory analyses were conducted to determine factors associated with survival and time-to-recurrence. Sub-groups analyzed included patients with cirrhosis, pathologically very early tumors (BCLC stage 0 / Japanese T1), satellites, and if surgery was based on the anatomical resection of all involved segments.
The primary end-points of survival and time-to-recurrence were calculated using the Kaplan–Meier method. An exploratory analysis was conducted to determine the variables associated with survival and recurrence. Univariate associations between clinical variables and survival as well as time-to-recurrence were conducted using the log-rank test. All variables found to be significant on univariate analysis (P <.05) were entered into a step-down Cox proportional hazard regression analysis.
Categorical data were compared using χ2 or Fisher exact test as indicated. Continuous variables were compared using Student t test or Mann–Whitney test for variables with an abnormal distribution. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-offs of continuous variables by choosing the point along the curve that maximized the sum of sensitivity and specificity. Platelet count was entered as a continuous variable into a Cox model, after checking the necessary assumptions (16). We also drew plots of the hazard function to describe the instantaneous rate of death and disease recurrence over the follow-up period. These plots were obtained with the Epanechnikov method (17). SPSS version 16.0 (SPSS, Inc, Chicago, IL) for Windows was used.
Results
During the study period, over 2000 hepatic resections were performed for HCC at the two centers. Out of this large group, 132 patients with pathologically proven single HCC ≤2cm (New York - 57, Milan - 75) were identified. There were no instances when a patient was explored for HCC ≤ 2cm without cancer being found in the specimen at either center. During the same period, 79 patients (New York - 36, Milan - 43) with HCC ≤2cm and Child's A liver disease underwent RFA at the two centers. These patients underwent RFA either as a bridge to liver transplantation because of the presence of significant portal hypertension or as definitive cancer therapy because of the presence of significant co-morbidities precluding safe resection. Patient demographics, tumor characteristics and details of the surgery are summarized in Table 1. All of the patients in the study were Child's A without history of decompensation. Median follow-up was 37.5 months. At the time of data collection, there had been 32 deaths including 1 (0.7%) perioperative death within 90 days of surgery. Median survival for the entire cohort was 74.5 months with a 5-year survival rate of 70% (Figure 1A). ROC curve analysis found an optimal cut-off of 148,000/μl for platelet count and 1.1 for INR in terms of predicting survival. Variables significantly associated with survival on univariate and multivariate analyses are listed in tables 2 and 4 respectively. The two variables independently associated with survival for the entire cohort included presence of satellites (HR=2.46, p=0.031) and platelet count <150,000/μl (HR=2.37, p=0.026).
Table1. Patient demographics, pathological characteristics and surgical details.
| Mean ± standard deviation or (n) | Range | |
|---|---|---|
| Age (years) | 63.1 ± 10.5 | 34-82 |
| Gender (M:F) | 95:37 | |
| Size of arterial enhancement on imaging (mm) | 19.5 ± 4.8 | 10-32 |
| Liver disease | ||
| HBV | 49(37%) | |
| HCV | 68(52%) | |
| Alcohol | 8(5%) | |
| Other | 7(6%) | |
| Platelet (×1000/μl) | 154±74I | 42 - 463 |
| INR | 1.1±0.1 | 0.9 - 1.6 |
| Total bilirubin (mg/dl) | 0.9±0.5 | 0.1 - 3.6 |
| Creatinine (mg/dl) | 0.9±2.6 | 0.4 - 2.8 |
| AFP (ng/ml) | 23* | 1.1 - 7,001 |
| <10 ng/ml (n) | 50 (38%) | |
| 10-100 ng/ml (n) | 48 (36%) | |
| 101-1000 ng/ml (n) | 20 (15%) | |
| >1000 ng/ml | 8 (6%) | |
| Missing AFP (n) | 6 (5%) | |
| Anatomic resection | 76(58%) | |
| Transfusion required | 7(5%) | |
| Margin (mm) | 9.8±8.6 | 0 - 60 |
| Margin positive | 4(3%) | |
| Tumor size (mm) | 16.4±3.6 | 7 - 20 |
| Vascular invasion | ||
| None | 95(72%) | |
| Micro | 35(27%) | |
| Macro | 2(2%) | |
| Differentiation | ||
| Well | 31(24%) | |
| Moderate | 73(55%) | |
| Poor | 21(16%) | |
| Missing | 7(5%) | |
| Satellites present | 16(12%) | |
| BCLC 0 / Japanese T1 | 85 (64%) | |
| Fibrosis score (mean)† | 3.5±0.9 | |
| 0-2 | 17(13%) | |
| 3 | 21(16%) | |
| 4 (cirrhosis) | 89(67%) | |
| Missing | 5(4%) | |
| Hepatitis score (mean)† | 1.5±0.7 | |
| 0-1 | 59(45%) | |
| 2-4 | 66(50%) | |
| Missing | 7(5%) |
Median,
Metavir score
Figure 1.
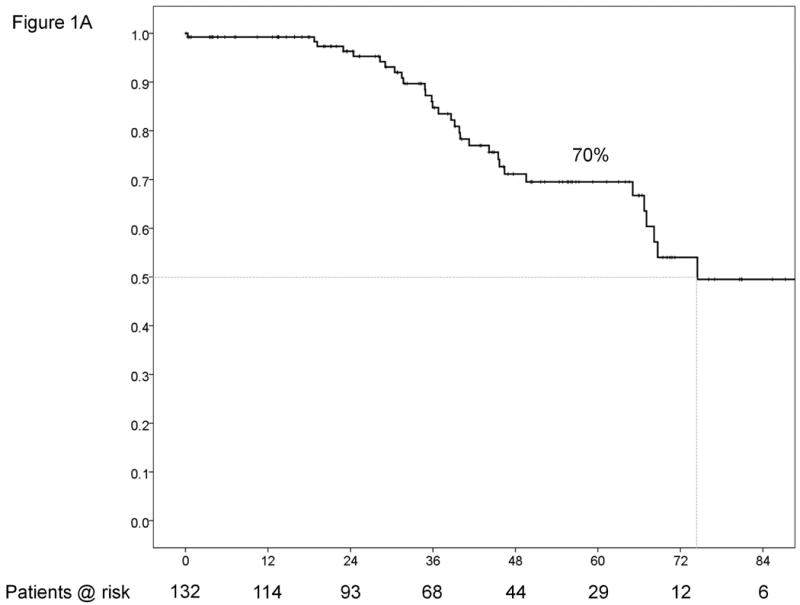
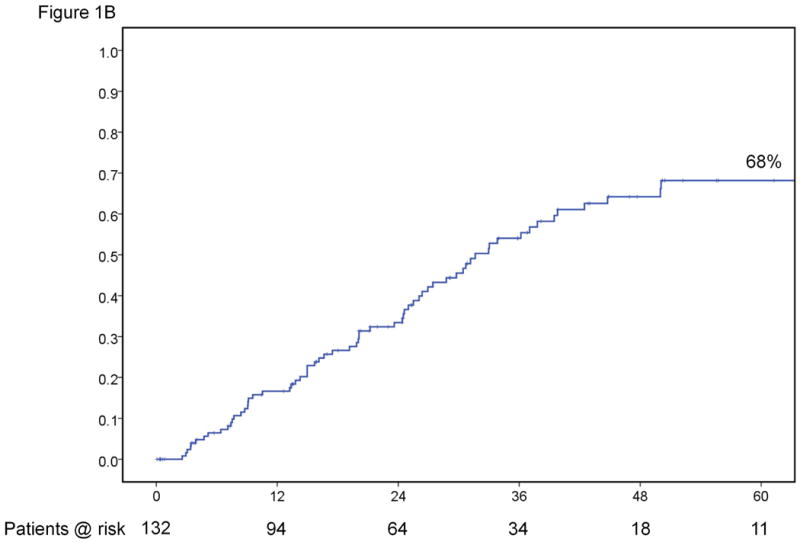
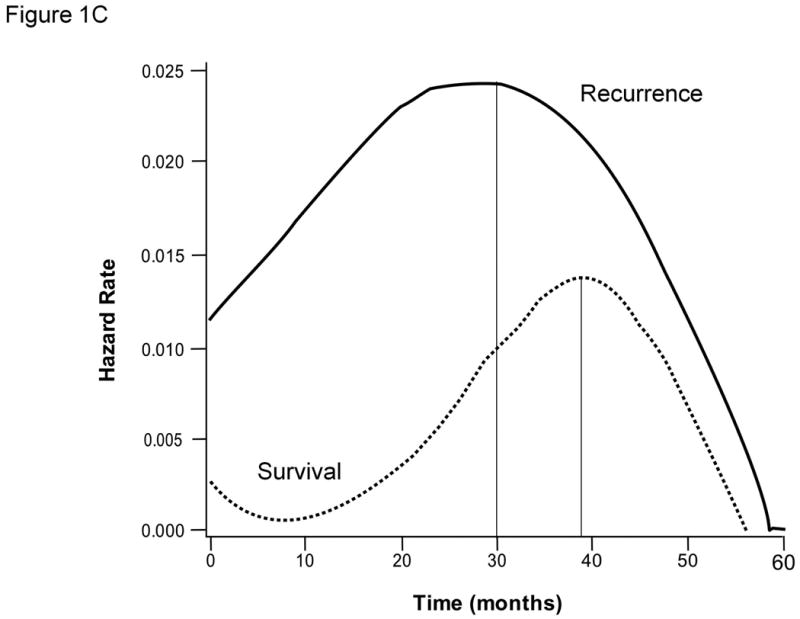
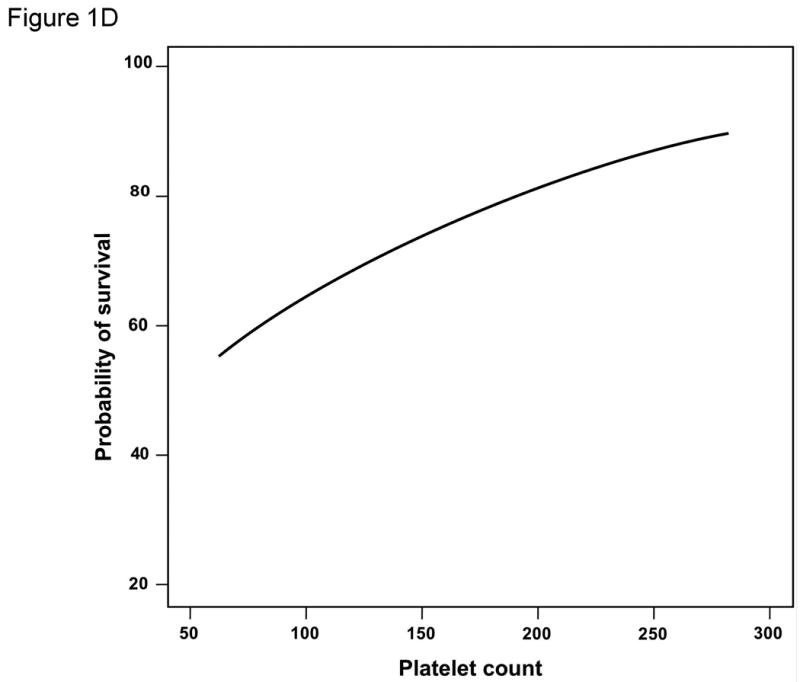
Results for the entire cohort. Overall survival (A) and time-to-recurrence (B) for 132 patients with HCC ≤2cm undergoing hepatic resection at two Western centers. The instantaneous risk of death and recurrence over time (C). Relationship between platelet count as a continuous variable and survival at 5 years (regression coefficient -0.00764 ±0.00373; p=0.0404) (D).
Table 2. Results of univariate analysis of survival and recurrence.
| Variable | n | Median time-to-event (mo)* | 95% CI (mo) | p | Outcome @ 3 years | Outcome @ 5 years |
|---|---|---|---|---|---|---|
| SURVIVAL | ||||||
| Platelets <150,000 /μl | 66 | 66.7 ± 9.7 | 47.7-85.8 | 0.010 | 84% | 60% |
| Platelets ≥ 150,000 /μl | 66 | 137.9 ± 0.0 | 87% | 81% | ||
| Platelets <100,000 /μl | 25 | 65.1 ± 11.1 | 41.7-88.5 | 0.019 | 83% | 59% |
| Platelets ≥ 100,000 /μl | 107 | 137.9 ± 0.0 | 86% | 72% | ||
| Satellites present | 16 | 39.9 ± 7.7 | 24.8-55.2 | 0.004 | 55% | 44% |
| Satellites absent | 116 | 137.9 ± 0.0 | 91% | 74% | ||
| INR ≤ 1.1 | 85 | Not reached | 0.032 | 92% | 74% | |
| INR > 1.1 | 47 | 68.2 ± 2.3 | 63.7-72.6 | 80% | 63% | |
| RECURRENCE | ||||||
| Satellites present | 16 | 14.3 ± 4.4 | 5.6-22.9 | 0.005 | 77% | 85% |
| Satellites absent | 116 | 36.2 ± 4.0 | 28.3-44.0 | 50% | 65% | |
| Fibrosis (0-3)† | 38 | 50.1± 2.9 | 44.3-55.9 | 0.007 | 32% | 54% |
| Fibrosis (4)† | 89 | 26.1± 3.2 | 19.7-32.4 | 65% | 75% | |
| Anatomic resection | 76 | 37.8± 5.8 | 26.4-49.2 | 0.032 | 48% | 60% |
| Non-anatomic resection | 56 | 26.9 ± 3.4 | 20.2-33.6 | 66% | 80% | |
| Hepatitis activity (0-1)† | 59 | 31.2 ± 2.8 | 25.6-36.8 | 0.047 | 51% | 62% |
| Hepatitis activity (2-4)† | 66 | 25.5 ± 2.3 | 20.9-30.1 | 63% | 78% | |
median ± standard error,
Metavir score
Table 4. Results of multivariate analysis of survival and recurrence.
| Variable | P | Hazard ratio | 95% Confidence interval |
|---|---|---|---|
| SURVIVAL | |||
| Satellites present | 0.031 | 2.46 | 1.08-5.62 |
| Platelet < 150,000 | 0.026 | 2.37 | 1.07-5.24 |
| OVERALL RECURRENCE | |||
| Satellites present | 0.003 | 2.79 | 1.42-5.46 |
| Cirrhosis (Fibrosis 4)* | 0.010 | 2.30 | 1.22-4.34 |
| Non-anatomic resection | 0.031 | 1.79 | 1.06-3.07 |
| VERY EARLY RECURRENCE (≤ 1yr) | |||
| Non-anatomic resection | 0.010 | 3.39 | 1.33-8.62 |
| Tumor size ≥15mm | 0.004 | 3.83 | 1.55-9.45 |
| Satellites present | 0.011 | 3.30 | 1.31-8.41 |
| EARLY RECURRENCE (≤ 2yr) | |||
| Tumor size ≥15mm | 0.021 | 2.39 | 1.14-5.01 |
| Poor differentiation | 0.030 | 2.40 | 1.09-5.29 |
| Cirrhosis (Fibrosis 4)* | 0.020 | 3.40 | 1.16-9.38 |
Metavir fibrosis score
Both the conventional platelet cut-off of 100,000/μl as well as that identified by ROC curve analysis (150,000/μl) were significantly associated with survival on univariate analysis (Table 2 and Figure 2A). In addition, platelet count used as a continuous variable was also significantly associated with survival at 5 years (regression coefficient -0.00764 ±0.00373; p=0.0404) (Figure 1D). Other relevant clinical variables that did not reach statistical significance on univariate analysis for survival are listed in Supplementary Table 1.
Figure 2.
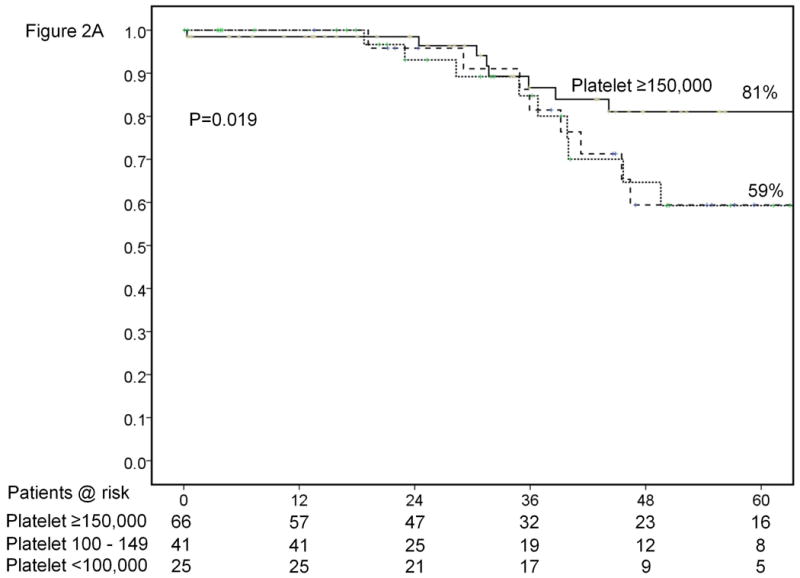
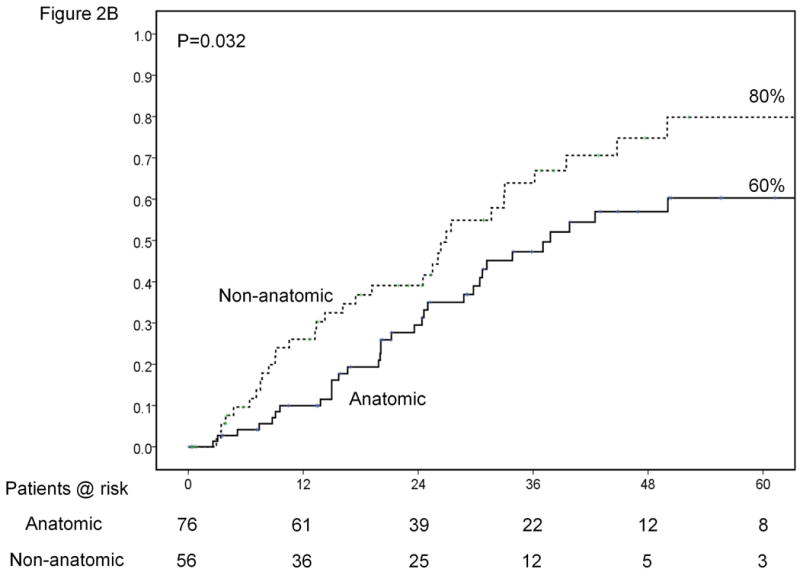
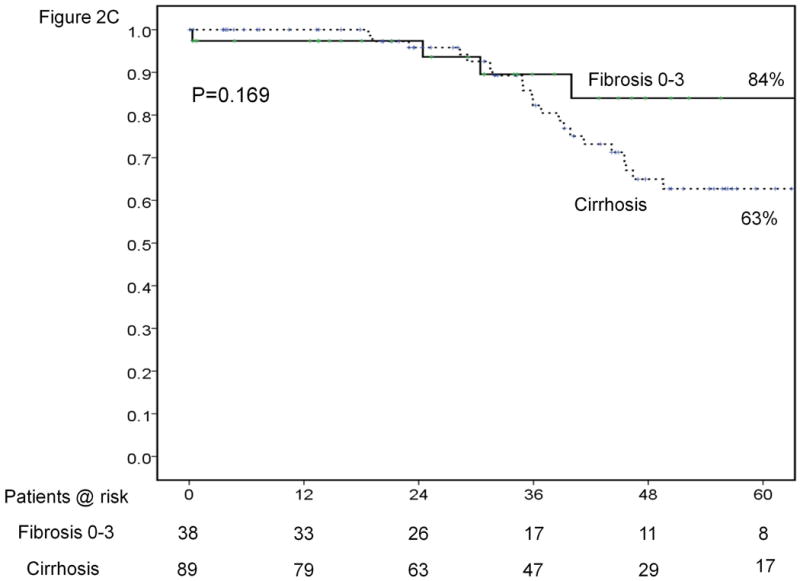
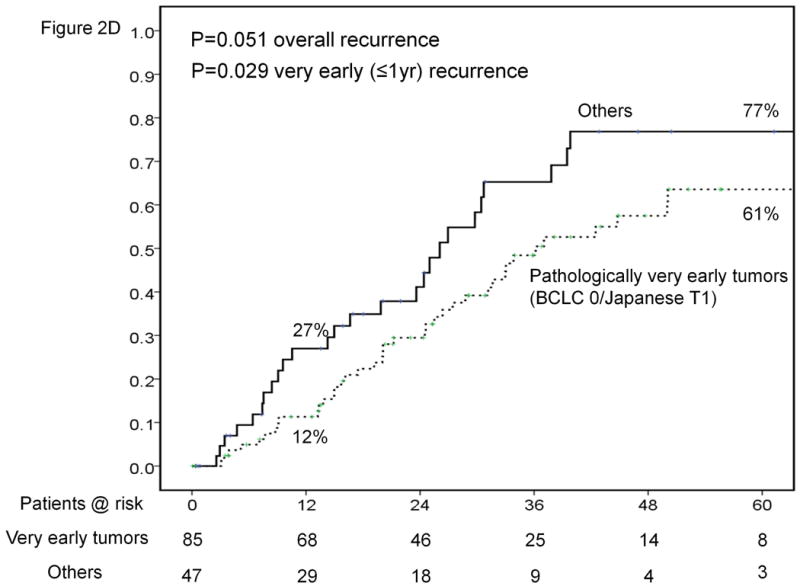

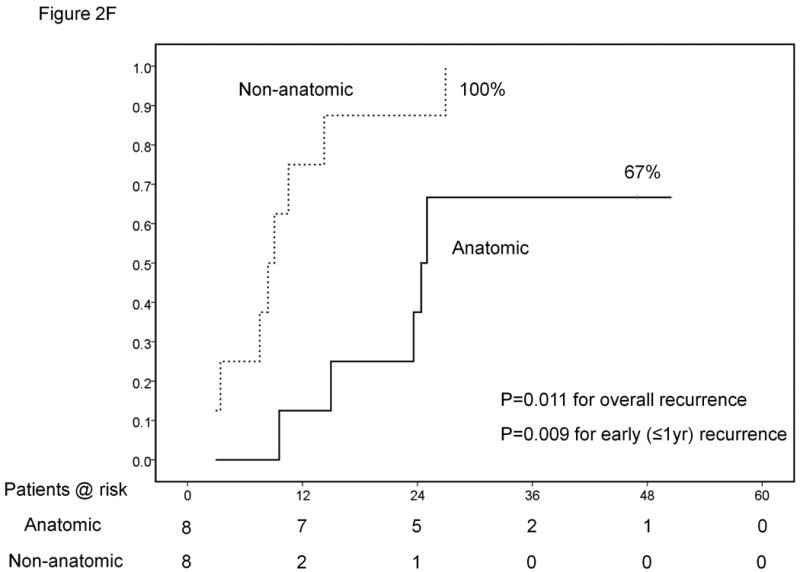
Results of sub-group analyses. Overall survival of patients stratified according to platelet count (<100,000, 100-149,000 and ≥150,000/μl) (A). Time-to-recurrence stratified by anatomic vs. nonanatomic resection (B). Overall survival of patients undergoing resection of HCC ≤ 2cm stratified according to presence of cirrhosis (C). Time-to-recurrence stratified according to presence of microvascular invasion and /or satellites – BCLC 0 / Japanese T1 versus others (D). Overall survival (E) and time-to-recurrence (F) for patients with satellite tumors stratified by anatomic vs. nonanatomic resection.
At the time of data collection, there had been 67 (50.7%) recurrences. Median time to recurrence was 31.6 months with a 5-year recurrence rate of 68% (Figure 1B). Approximately 80% of the deaths were preceded by recurrence. The instantaneous risk of death and cancer recurrence over time are demonstrated in Figures 1C. Variables significantly associated with recurrence on univariate and multivariate analyses are outlined in Tables 2 and 4. The variables significantly associated with time-to-recurrence for the entire cohort included presence of satellites (HR=2.79, p=0.003), cirrhosis (HR=2.3, p=0.010), and non-anatomic resection (HR=1.79, p=0.031). Other relevant clinical variables that did not reach statistical significance on univariate analysis for recurrence are listed in Supplementary Table 1.
At one year, there had been 20 instances of “very early” recurrence with a rate of 17%. At 2 years there had been 38 recurrences resulting in a rate of 29%. Variables significantly associated with recurrence at 1 and 2 years on univariate and multivariate analysis are listed in Tables 3 and 4.
Table 3. Results of univariate analysis of very early (<1yr) and early (<2yr) recurrence.
| Variable | Recurrence @ 1 year | N | P |
|---|---|---|---|
| Anatomic resection | 10% | 76 | 0.019 |
| Non-anatomic resection | 26% | 56 | |
| Tumor size <15mm | 12% | 27 | 0.002 |
| Tumor size ≥ 15mm | 35% | 105 | |
| Margin <10mm | 24% | 36 | 0.023 |
| Margin ≥ 10mm | 4% | 47 | |
| Satellites present | 45% | 16 | 0.002 |
| Satellites absent | 12% | 116 | |
| Very early tumors (BCLC 0/Japanese T1) | 12% | 85 | 0.029 |
| Others (BCLC A/Japanese T2) | 27% | 47 | |
| Recurrence @ 2 year | |||
| Tumor size <15mm | 27% | 27 | 0.017 |
| Tumor size ≥ 15mm | 49% | 105 | |
| Satellites present | 62% | 16 | 0.003 |
| Satellites absent | 28% | 116 | |
| Poor differentiation | 57% | 21 | 0.034 |
| Moderate or Well | 28% | 104 | |
| Fibrosis (0-3)* | 25% | 38 | 0.019 |
| Cirrhosis (Fibrosis 4)* | 42% | 89 |
Metavir fibrosis score
All but one of the 67 patients with recurrent tumor underwent treatment, either with a single modality or a combination of therapies. Treatments included trans-arterial chemo-embolization (n=38), percutaneous ethanol ablation (n=3), Yttrium90 radioembolization (n=1), liver transplantation (n=6), repeated hepatic resection (n=21), radiofrequency ablation (n=17), sorafenib (n=2), and resection of extrahepatic tumor (lung =1, omentum =1).
Several other findings deserve specific mention. Etiology of underlying liver disease did not correlate with survival or recurrence. Laparoscopic resection was performed in 15 (11%) of cases which also did not alter these end-points. There was no relationship between the location of the tumor and the type of resection (anatomic vs. nonanatomic) performed. However, we did find a correlation between non-anatomic resection and platelet count <100,000/μl and/or bilirubin >1 mg/dl most probably in an attempt to preserve functioning parenchyma.
Best candidates
Of the two variables found to be significantly associated with survival on multivariate analysis, only platelet count is available preoperatively to help guide patient selection. If resection was limited only to the 66 patients with platelet count ≥ 150,000/μl, as determined by ROC curve analysis, median survival increased to 138 months and 5 year survival to 81% (figure 2A).
Likewise, the only variable significantly associated with recurrence that can be controlled by the clinician is the type of resection that is performed. Performing anatomic resection was associated with a 20% decrease in the recurrence rate from 80% down to 60% at 5 years (Figure 2B).
Cirrhotic patients
Patients with Metavir stage 4 fibrosis were chosen in order to analyze results in the cirrhotic subgroup (n=89, 67% of the overall series). Median and 5-year survivals in this group were 67.1 months and 63%. There was no significant difference in survival between the cirrhotic and non-cirrhotic groups (Figure 2C). Recurrence rates at 1 and 5 years were 20% and 75%, respectively, for the cirrhotic subgroup. The overall rate of recurrence was significantly higher in the cirrhotic subgroup compared to the non-cirrhotic subgroup (Table 2).
The only variable associated with survival on univariate analysis in the cirrhotic population was platelet count. Both a cut-off of 100,000/μl (p=0.046) and 150,000/μl (p=0.039) were significantly associated with survival (Table 5). The only variables significantly associated with time-to-recurrence on univariate analysis among these patients with cirrhosis were performing a non-anatomic resection (p=0.017) and the presence of satellites (p=0.035) (Table 5). Mulitvariate analysis was not conducted in this subgroup.
Table 5.
Results of subgroup univariate analyses.
| Variable | n | Median time-to-event (mo)* | 95% CI (mo) | p | Outcome @ 3 years | Outcome @ 5 years |
|---|---|---|---|---|---|---|
| CIRRHOTIC PATIENTS (n=89) | ||||||
| SURVIVAL | ||||||
| Platelets <150,000 /μl | 59 | 65.7 ± 11.9 | 40.1-88.5 | 0.039 | 82% | 57% |
| Platelets ≥ 150,000 /μl | 30 | 137.9 ± 0.0 | 85% | 74% | ||
| Platelets <100,000 /μl | 23 | 65.1 ± 19.9 | 25.9-104.2 | 0.046 | 80% | 57% |
| Platelets ≥ 100,000 /μl | 66 | 137.9 ± 0.0 | 84% | 66% | ||
| RECURRENCE | ||||||
| Anatomic resection | 52 | 30.7± 5.0 | 20.8-40.6 | 0.017 | 58% | 64% |
| Non-anatomic resection | 37 | 24.5 ± 7.9 | 9.1-39.9 | 75% | 90% | |
| Satellites present | 12 | 10.5 ± 4.5 | 1.7-19.4 | 0.035 | 81% | N/A |
| Satellites absent | 77 | 30.7± 3.6 | 23.7-37.7 | 62% | 74% | |
| PATHOLOGICALLY VERY EARLY TUMORS (BCLC 0/JAPANESE T1)(n=85) | ||||||
| SURVIVAL | ||||||
| Platelets ≥150,000 /μl | 43 | 137.9 ± 0.0 | 0.011 | 91% | 87% | |
| Platelets <150,000 /μl | 42 | 67.1 ± 13.6 | 40.5-93.6 | 86% | 64% | |
| RECURRENCE | ||||||
| Fibrosis (0-3)† | 25 | 50.1± 3.8 | 42.5-57.8 | 0.012 | 21% | 51% |
| Cirrhosis (Fibrosis 4) † | 89 | 31.2 ± 4.9 | 21.4-40.3 | 64% | 72% | |
Pathologically very early HCC
Patients with no vascular invasion and no satellites (BCLC 0 / Japanese T1) on pathology were selected as “true” cases of very early HCC (n=85). These patients had median and 5-year survivals of 138 months and 76% compared to 65.1 months and 57% (p=0.137) for those with vascular invasion and/or satellites. Recurrence rates at 1 and 5 years were 12% and 61%, respectively, for this subgroup (Figure 2D). Recurrence at 1 year was significantly lower for patients with very early tumors and the difference was just at the cut-off for significance for overall recurrence.
The only variable significantly associated with survival on univariate analysis in this subgroup of patients was platelet count <150,000/μl (p=0.011) and the only variable associated with recurrence was cirrhosis (stage 4 fibrosis) (p=0.012)(Table 5). Again, multivariate analyses were not performed. Performing an anatomic resection in these patients with no vascular invasion and no satellites did not result in lower early or overall recurrence
However, for the remaining 47 patients with either vascular invasion or satellites, performing an anatomic resection was associated with a significant reduction in recurrence at 1 year from 50% down to 11% (p=0.008). While there was a clear trend toward better overall survival as well as lower overall recurrence with anatomic resection in these 47 patients with either vascular invasion or satellites, the p values did not reach significance.
Satellite tumors
There were 16 (12%) of patients who were found to have satellites on pathology. The presence of satellites was not recognized pre-operatively in any of the cases. As demonstrated in tables 2 and 4, the presence of satellites was an independent predictor of survival, overall recurrence and early recurrence at 1 year.
By coincidence, half (n=8) of the patients with satellites underwent anatomic liver resections while the other half did not. Despite the very small sample size, anatomic resection was associated with significantly better survival, lower overall recurrence and lower early recurrence at 1 year in these patients (Figures 2E and 2F). There were no other factors associated with mortality or recurrence in this sub-group of patients even on univariate analysis.
Discussion
The data from this study confirms that performing hepatic resection for HCC ≤ 2cm is safe with the occurrence of only one (0.8%) perioperative mortality. In addition, it demonstrates that the long term results are excellent with median and 5 year survivals of 75 months and 70%, respectively. These results from two high volume Western centers are much more compatible with those reported by the larger Japanese series showing 5 year survivals near 70%.
The presence of satellites and platelet count, with an optimal cut-off of 150,000/μl were the only the only variables independently associated with survival for the overall cohort on our exploratory analyses. Unfortunately, we were not able to detect the presence of satellites on imaging in any of the 16 cases making it impossible to use this variable preoperatively to select patients for resection. Portal hypertension has previously been shown to have a significant impact on survival after hepatic resection for HCC (18) and so it was not surprising that when resection was limited to patients with platelet count ≥ 150,000 /μl, survival significantly improved. Median survival in these patients without significant portal hypertension, as measured by platelet count, was 138 months with 5 year survival was 81%. Even patients with established cirrhosis and a platelet count ≥ 150,000 /μl achieved a 5 year survival rate of 74%. These outcomes certainly compare very favorably with the 68% survival at 5 years reported for “resectable” patients undergoing radio-frequency ablation of HCC ≤ 2cm(10). The inclusion of patients with platelet counts as low as 40,000 /μl in the randomized study by Chen et al, may be an explanation as to why no difference in survival was detected when comparing RFA with surgical resection for patients with HCC <5cm(19).
An interesting finding was that resection of patients with platelet count <150,000/μl or even <100,000/μl was not associated with an increased early perioperative mortality as we had expected. It seems that, in this particular scenario with small tumors, the influence of portal hypertension becomes evident only late after hepatic resection. Finally, we discovered a near linear relationship between platelet count and 5 year survival. While we identified a platelet count of 150,000/μl as the optimal cut-off in this cohort, there was no point along the curve in Figure 3B below which the survival at 5 years dropped precipitously. It would appear that incremental decreases in platelet count at the time of surgery will result in incremental decreases in long-term survival.
Prior Eastern reports have shown that even for tumors ≤2cm, approximately 10% of cases will have microvascular invasion of portal branches by tumor and that 3% will have satellite tumors(20-23). Pathological examination from our Western patients with HCC ≤ 2cm revealed a more aggressive picture with 27% of patients having microvascular portal invasion and, very surprisingly, 2% with gross invasion. In addition we found satellite lesions in 12% of our patients. Overall, 47 (36%) of the patients had either vascular invasion or satellite tumors and did not meet the pathological criteria for “very early” HCC defined as T1 by the Japanese Society of Hepatology or as BCLC stage 0.
The overall recurrence rate of 68% at 5 years and the one year recurrence rate of 17% seemed, at first, surprising high to us for such small cancers. A recurrence rate of 61% at 5 years for small tumors without vascular invasion or satellites was particularly unexpected. However, a Japanese study of 70 patients with HCC ≤ 2cm undergoing resection found an overall recurrence rate of 88% for the entire cohort (24). The same study demonstrated a 1 and 5 year recurrence rates of 8% and 53% for those found to have T1 tumors (24). These numbers are very similar to what we have reported, 12% at 1 year and 61% at 5 years, for our patients with pathologically proven very early tumors. Again, the recurrence rates for the entire cohort from our study (17% at 1 year and 68% at 5 years) compare favorably to the 1 and 5 year recurrence rates of 34% and 80% reported for RFA of similarly sized HCC(10).
The vast majority of the recurrences occurred within the first 3 years after surgery after which there were very few events. The pattern of the instantaneous risk of recurrence for these small tumors was also very different from that published for more advanced tumors (25, 26). Instead of the two peaks generally seen for larger tumors, one at approximately 12 months representing early metastatic recurrence and another at approximately 36 months representing late de novo recurrence, we see only a single and delayed peak at 30 months. This pattern may reflect a reduction in early metastatic recurrences given the early stage of the tumors but deserves further investigation.
The presence of satellites, underlying cirrhosis and non-anatomic resection were associated with time-to-recurrence. The presence of satellites has been found to be a significant predictor of outcome after resection of HCC in many other studies (27). Likewise, the nature of the non-tumoral liver around the HCC has also been shown to be a strong predictor of recurrence of HCC after resection(28). Generally, neither variable is known preoperatively to help guide patient selection or the selection of the most appropriate therapy.
The success of sorafenib in the treatment of advanced HCC has opened the door for the testing of targeted molecules in the adjuvant setting (29). The degree of fibrosis and the presence of satellites can help select or stratify patients who are most at risk for recurrence and who may benefit most from sorafenib after resection if the drug is eventually found to be an effective agent in the adjuvant setting. Alternatively, those with satellites who are at risk for early metastatic recurrence can be referred for salvage liver transplantation as has been proposed by the Barcelona group (30). Those with cirrhosis, who are at risk for later recurrence, can potentially benefit from anti-viral therapy which has been shown to decrease the incidence of late recurrence in one randomized trial (26). However, further studies are needed in this regard.
Anatomic resection was found to be independently associated with both overall and very early recurrence at 1 year. Other authors have also found a benefit from performing anatomic resection for small HCC (31,32). While anatomic resection was associated with a lower overall recurrence of 60% and very early (<1yr) recurrence of 10% for the entire cohort, it was associated with a very dramatic 40% lower rate of recurrence at 1 year for patients who were found to have either vascular invasion or satellites and thus did not meet the criteria for pathologically very early cancers. In addition, anatomic resection was associated with a very significant improvement in survival as well as lower rates of overall and early recurrence in patients with satellites. These findings support the theory that removing the entire segment supplied by a portal pedicle will result in lower recurrence and, hopefully, better survival, particularly in those patients where the tumor has gained access to the microcirculation or developed micro-metastases(33). These oncological benefits of an anatomical resection are something that is unique to hepatectomy and cannot be duplicated by percutaneous ablation.
Several shortcomings of this study must be made explicitly clear so that readers can exercise the appropriate level of caution when interpreting and extrapolating the results of this study. Perhaps the most significant limitation is the relatively small sample size. With only 32 events in terms of survival, the study lacked statistical power leading to an increased possibility of type II error. In addition, the low number of events may also result in type I error where some of the variables identified as significant on univariate analyses may have, in fact, not been truly associated with survival. The fact that two centers with such a large volume of hepatic resections for HCC were able to accrue only 132 cases of HCC ≤ 2cm over a 20 year period points out the exceeding rare nature of these small tumors in the Western experience. A sample size this small also prevented the ideal scenario of constructing and testing predictors of outcomes using separate training and validation cohorts. In addition, the limited number of events made robust multivariate analysis difficult. Consequently, the results of the multivariate analysis must be viewed as an exploratory analysis of this group of patients and clearly need validation in an independent cohort before clinical decisions can be made based on this data.
In summary, hepatic resection for HCC ≤2cm is safe and offers excellent long-term results. Platelet count ≥ 150,000 /μl was associated with survival on multivariate analysis. Recurrence remains a significant problem despite the small size of these tumors. Anatomic resection was associated with lower overall and one year recurrence. Even with such early tumors, approximately one third will present with either vascular invasion, satellite tumors or both. It is these patients in particular, who cannot be identified preoperatively by imaging, in whom anatomic resection is associated with a lower rate of recurrence.
Supplementary Material
Acknowledgments
Financial support: VM is partially supported by the Hepato-Oncology grant of the INT-Milan (5×1000), by the Italian Association for Cancer Research (AIRC) and by the Ministry of Health grants in Oncology and Transplantation
JML is supported by grants from the US National Institutes of Diabetes and Digestive and Kidney Diseases (1R01DK076986-01), European Comission-FP7 Framework (HEPTROMIC, proposal no: 259744), the Samuel Waxman Cancer Research Foundation and the Spanish National Health Institute (SAF-2007-61898 and SAF-2010-16055) and The Landon Foundation-American Association for Cancer Research Innovator Award for International Collaboration in Cancer Research.
Abbreviations
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- INR
International normalization ratio
- MRI
magnetic resonance imaging
- ICGR
indocyanine green retention rate
- AFP
alpha feto-protein
- CI
confidence interval
- RFA
radiofrequency ablation
- CT scan
computed tomography
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer. Kanehara. 2003 [Google Scholar]
- 3.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, et al. Clinical management of hepatocellular carcinoma. conclusions of the barcelona-2000 EASL conference. european association for the study of the liver. J Hepatol. 2001;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, Boix L, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47(1):97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 6.Liu JH, Chen PW, Asch SM, Busuttil RW, Ko CY. Surgery for hepatocellular carcinoma: Does it improve survival? Ann Surg Oncol. 2004;11(3):298–303. doi: 10.1245/aso.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto J, Iwatsuki S, Kosuge T, Dvorchik I, Shimada K, Marsh JW, Yamasaki S, et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer. 1999;86(7):1151–1158. doi: 10.1002/(sici)1097-0142(19991001)86:7<1151::aid-cncr8>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonas S, Herrmann M, Rayes N, Berg T, Radke C, Tullius S, Settmacher U, et al. Survival after liver transplantation for hepatocellular carcinoma in cirrhosis according to the underlying liver disease. Transplant Proc. 2001;33(7-8):3444–3445. doi: 10.1016/s0041-1345(01)02484-8. [DOI] [PubMed] [Google Scholar]
- 10.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47(1):82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25(2):181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 12.Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: A retrospective and nationwide survey in japan. the liver cancer study group of japan. Hepatology. 2000;32(6):1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 13.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249(5):799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 14.Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: A markov model analysis. Hepatology. 2010;51(4):1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 15.Roayaie S, Bassi D, Tarchi P, Labow D, Schwartz M. Second hepatic resection for recurrent hepatocellular cancer: A western experience. J Hepatol. 2011;55(2):346–350. doi: 10.1016/j.jhep.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life tables (with discussion) J Royal Stat Soc, Series B. 1972;34:187–220. [Google Scholar]
- 17.Epanechnikov VA. Non-parametric estimation of a multivariate probability density. Theory of Probability and its Applications. 1969;14:153–158. [Google Scholar]
- 18.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: Resection versus transplantation. Hepatology. 1999;30(6):1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 19.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojiro M, Nakashima O. Histopathologic evaluation of hepatocellular carcinoma with special reference to small early stage tumors. Semin Liver Dis. 1999;19(3):287–296. doi: 10.1055/s-2007-1007118. [DOI] [PubMed] [Google Scholar]
- 21.Maeda T, Takenaka K, Taguchi K, Kajiyama K, Shirabe K, Shimada M, Tsuneyoshi M, et al. Clinicopathological characteristics of surgically resected minute hepatocellular carcinomas. Hepatogastroenterology. 2000;47(32):498–503. [PubMed] [Google Scholar]
- 22.Nakashima Y, Nakashima O, Tanaka M, Okuda K, Nakashima M, Kojiro M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res. 2003;26(2):142–147. doi: 10.1016/s1386-6346(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 23.Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, Kosuge T, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95(9):1931–1937. doi: 10.1002/cncr.10892. [DOI] [PubMed] [Google Scholar]
- 24.Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, Kosuge T, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998;28(5):1241–1246. doi: 10.1002/hep.510280511. [DOI] [PubMed] [Google Scholar]
- 25.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatology. 2003;38(2):200–2007. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 26.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44(6):1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 27.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359(19):1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Sala M, Fuster J, Llovet JM, Navasa M, Sole M, Varela M, Pons F, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: An indication for salvage liver transplantation. Liver Transpl. 2004;10(10):1294–1300. doi: 10.1002/lt.20202. [DOI] [PubMed] [Google Scholar]
- 31.Wakai T, Shirai Y, Sakata J, Kaneko K, Cruz PV, Akazawa K, Hatakeyama K. Anatomic resection independently improves long-term survival in patients with T1-T2 hepatocellular carcinoma. Ann Surg Oncol. 2007;14(4):1356–1365. doi: 10.1245/s10434-006-9318-z. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242(2):252–259. doi: 10.1097/01.sla.0000171307.37401.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makuuchi M. Remodeling the surgical approach to hepatocellular carcinoma. Hepatogastroenterology. 2002;49(43):36–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


