Abstract
Background
The objectives of this phase I study were to determine the maximum tolerated dose (MTD), toxicity profile and pharmacokinetics of a 24-hour continuous intravenous infusion of trabectedin administered to children and adolescents with refractory or relapsed solid tumors.
Procedure
Patients between the ages of 4 and 16 years old with refractory solid tumors received trabectedin as a 24-hour infusion every 21 days. Dexamethasone and prophylactic growth factor support were administered with each cycle. Pharmacokinetic studies were conducted during cycle 1.
Results
Patients (n=12) median (range) age 14.5 (8–16) years received trabectedin at 1.1 (n=3), 1.5 (n=6) or 1.7 (n=3) mg/m2. At the 1.5 mg/m2 dose level, one patient had dose limiting anorexia and fatigue. At 1.7 mg/m2, 2 patients experienced dose limiting toxicity, dehydration and gamma-glutamyl transpeptidase (GGT) elevation. Non-dose limiting toxicities included elevated serum transaminases, myelosuppression, nausea, emesis and fatigue. Plasma pharmacokinetic parameters were similar to historical data in adults. One partial response (PR) was observed in a patient with neuroendocrine carcinoma. Stable disease (SD) (≥6 cycles) was achieved in 3 patients (osteosarcoma n=2, desmoplastic small round cell tumor n=1).
Conclusions
The MTD of trabectedin in pediatric patients with refractory solid tumors is 1.5 mg/m2 IV over 24 hours every 21 days. Dexamethasone to ameliorate hepatic toxicity and prophylactic growth factor support are required.
Keywords: trabectedin, phase I clinical trial, pediatrics, pharmacokinetics
Introduction
Trabectedin (ET-743, Yondelis®; J&J PRD, LLC, Titusville NJ) is a potent synthetic alkylating agent originally isolated from the marine tunicate Ecteinascidia turbinata [1]. It binds to the minor groove of DNA in a sequence specific manner and its cytotoxicity is mediated through interfering with transcription coupled nucleotide excision repair and homologous recombination DNA repair pathways as well as inhibition of transcriptional activation [2–6]. Trabectedin is cytotoxic at nanomolar concentrations in in vitro and in vivo preclinical tumor models [7]. Several doses and schedules have been studied in adults, and the recommended dose and schedule for sarcomas in adults is 1.5 mg/m2 infused over 24 h every 21 days [8,9]. Frequently reported adverse events included serum transaminase elevations, myelosuppression, nausea, emesis and fatigue. Dose-limiting toxicities (DLTs) were myelosuppression (neutropenia and thrombocytopenia), fatigue and rhabdomyolysis.
In clinical trials in adults, the definition of hepatic DLT was grade 3 or 4 hepatic dysfunction that did not recover by day 28 of the treatment cycle. Although serum transaminase elevations were common, they did not fulfill this DLT definition. Data from more than 5000 adults treated with multiple cycles of trabectedin indicate that these transient elevations in serum transaminases are not indicative of cumulative hepatotoxicity. Liver biopsies from patients treated with trabectedin in combination with liposomal doxorubicin did not demonstrate permanent hepatic damage [10]. In addition, dexamethasone mitigates trabectedin-related, transient hepatic toxicity [11–14].
The anti-tumor activity of trabectedin appears to be dose and schedule dependent. Objective responses on the initial adult phase I study were achieved in patients with liposarcoma, breast cancer and osteosarcoma treated at doses of 1.5 mg/m2 and above, however, doses higher than 1.5 mg/m2 were not tolerable [9]. In a study in patients with lipo and leiomyosarcoma randomized to receive either the weekly 3-hour or every 3 week 24-hour infusion schedule, the 24-hour infusion was associated with an increased time to progression [15]. Based on modest response rates and duration of stable disease in adults with sarcomas, particularly liposarcoma and leiomyosarcoma [16–21], trabectedin 1.5 mg/m2 IV over 24 h has been approved for refractory sarcomas by the European Medicines Agency (EMA).
A prior pediatric phase I trial utilizing a 3-hour infusion schedule defined a maximum tolerated dose (MTD) of 1.1 mg/m2 every 21 days [22]. Dexamethasone was administered to patients receiving doses ≥ 1.3 mg/m2, but prophylactic growth factor support was not allowed during cycle 1. The definition of hepatic DLT was any grade 4 elevation in serum ALT/AST. Toxicities included dose-limiting serum ALT/AST elevations and neutropenia. Non-dose limiting toxicities were nausea, emesis, fatigue and anemia. A complete response was observed in a patient with Ewing sarcoma.
We conducted a limited dose escalation phase I trial of trabectedin in children age 4–16 years with refractory or recurrent solid tumors to establish the safety, toxicity profile, and pharmacokinetics of a 24-hour infusion schedule of trabectedin in children.
Patients and Methods
Patient Eligibility
Patients were eligible if they were between 4 and 16 years of age, had a measurable or evaluable solid tumor that relapsed after or was refractory to standard therapy, had a Lansky (≤10 yrs) or Karnofsky (>10 yrs) performance ≥ status 60%, were recovered to grade ≤ 1 toxicity from prior therapy and were ≥3 weeks from prior myelosuppressive chemotherapy, ≥1 week from growth factors (e.g., filgrastim, epoetin), ≥2 weeks from pegfilgrastim, ≥30 days from an investigational agent, ≥7 days from a biologic agent, ≥4 weeks from radiation therapy to greater than 25% marrow-containing bones, ≥2 weeks from palliative radiation, and ≥2 months from autologous transplant, and had an absolute neutrophil count (ANC) ≥ 1500/mcL, platelet count ≥ 75,000/mcL (transfusion independent), hemoglobin ≥ 8 g/dL (transfusion permitted), AST/ALT ≤ 2.5 × upper limit of normal (ULN), bilirubin ≤ ULN, alkaline phosphatase ≤ ULN (or if above ULN, 5′ nucleotidase ≤ ULN or gamma-glutamyl transpeptidase [GGT] ≤ 2.5 × ULN), normal age-adjusted serum creatinine or creatinine clearance ≥ 60mL/min/1.73m2 and creatine kinase ≤2.5 × ULN.
Exclusion criteria included a history of xeroderma pigmentosum or other diseases with impaired DNA repair, pregnancy or breast-feeding, severe or uncontrolled infections, other investigational agents, allogeneic stem cell transplants or prior therapy with trabectedin. This trial was performed under an investigator IND (held by EF) and was approved by the institutional review board at the National Cancer Institute. Informed consent and assent, as appropriate, were obtained from patients and their guardians prior to enrollment.
Treatment Regimen and Dose Escalation
Trabectedin (supplied by J&J PRD, LLC, Titusville, NJ) was administered via a central venous catheter by continuous infusion over 24 h every 21 days. The starting dose was 1.1 mg/m2 with escalation to 1.5 and 1.7 mg/m2 in subsequent cohorts of 3–6 patients. For patients enrolled at the 1.1 mg/m2 dose level, intrapatient dose-escalation to 1.5 mg/m2 was permitted in subsequent cycles in patients who did not experience DLT and had recovered from toxicity by day 21 of the prior cycle. All patients received dexamethasone pre-treatment (2.5 mg/m2 every 12 h for 8 doses starting 12 h prior to the trabectedin infusion) and ondansetron. Other antiemetics, except aprepitant, were administered as needed. Filgrastim or pegfilgrastim was administered 24–48 hours after the end of the trabectedin infusion; daily filgrastim was continued until the post-nadir ANC was >2500/mcL.
Toxicities were graded according to NCI CTCAE v.3. Patients were monitored with twice weekly complete blood counts and liver function tests as well as weekly physical exams during the first cycle. Subsequent cycles were administered if the patient’s drug related toxicities recovered to baseline or grade 1 by cycle day 21 to 28 and there was no evidence of tumor progression.
Baseline radiographic disease evaluation was performed within 2 weeks prior to enrollment and follow-up scans were obtained after every 2 treatment cycles. Measurable tumor response to therapy was assessed with 2-dimensional WHO criteria.
Definition of Dose Limiting Toxicity (DLT)
Hematological DLT was defined as ANC <500/mcL (grade 4) on 2 or more consecutive measurements performed at least 72 h apart or a platelet count <25,000/mcL (grade 4) on 2 separate days of a treatment cycle. A platelet transfusion given for a platelet count ≤50,000/mcL was considered grade 4 thrombocytopenia. In addition, failure to recover neutrophil count to 1000/mcL or platelets to 75,000/mcL by day 28 of a treatment cycle was considered a DLT. Hepatic DLT was defined as serum AST or ALT >10 × ULN for >7 days or failure to recover to grade ≤1 by day 28 of the treatment cycle. Other non-hematological DLT was defined as any grade 3 or higher toxicity with the exception of grade 3 nausea, emesis or diarrhea that could be controlled with appropriate therapy within 72 h or grade 3 fatigue that resolved within 72 hours.
Definition of Maximum Tolerated Dose (MTD) and Dose Modifications for Toxicity
The MTD was determined from toxicity during cycle 1 and was defined as the dose level immediately below the dose at which 2 or more of a cohort of up to 6 patients experienced a DLT. Patients experiencing DLT had subsequent doses reduced by 30%. Two dose reductions for DLT were permitted if the patient was experiencing benefit from trabectedin.
Pharmacokinetics
Plasma pharmacokinetic sampling was performed during cycle 1. Blood samples, drawn from a site separate from the drug infusion site, were obtained prior to the infusion, 4, 8–16, 23.9 (end of infusion), 24.5, 25, 27, 30, 48, 96, 120, and 168 h after the start of the infusion. Plasma concentrations of trabectedin were measured by Johnson & Johnson Pharmaceutical Research and Development using a validated liquid chromatography, tandem mass spectrometry assay [23] with a lower limit of quantification of 0.025 ng/mL. Pharmacokinetic parameters were calculated using non-compartmental methods [24].
Results
Patient characteristics
Twelve patients were enrolled on the trial at the Pediatric Oncology Branch, NCI between June 2008 and December 2009. All were evaluable for toxicity and response. Patient characteristics and treatment course are presented in Table I. A total of 58 cycles of trabectedin were administered. The median (range) number of cycles administered per patient was 2.5 (1–18).
Table I.
Patient characteristics and treatment outcomes
| Dose level (mg/m2) | Patient | Diagnosis | Age (y) | Gender | # of prior chemo regimens | Prior radiation therapy | Performance status | Total # cycles received | Cycle 1 DLT | Best response |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 1 | Desmoplastic small round cell tumor | 15 | F | 1 | No | 100 | 6 | - | SD |
| 2 | Embryonal liver sarcoma | 11 | M | 4 | No | 90 | 1 | - | PD | |
| 3 | Synovial sarcoma | 15 | M | 2 | Yes | 90 | 2 | - | PD | |
| 1.5 | 4 | Neuroendocrine carcinoma | 16 | F | 2 | No | 100 | 12 | - | PR |
| 5 | Nasopharyngeal carcinoma | 15 | M | 1 | Yes | 90 | 3 | Anorexia, fatigue | SD | |
| 6 | Osteosarcoma | 13 | F | 2 | No | 90 | 6 | - | SD | |
| 7 | Osteosarcoma | 15 | F | 2 | No | 100 | 18 | - | SD | |
| 8 | Ewing’s sarcoma | 11 | M | 4 | No | 80 | 1 | - | PD | |
| 9 | Malignant peripheral nerve sheath tumor | 14 | F | 3 | Yes | 90 | 2 | - | PD | |
| 1.7 | 10 | Osteosarcoma | 11 | M | 7 | Yes | 80 | 4 | Dehydration | SD |
| 11 | Diffuse intrinsic pontine glioma | 8 | M | 1 | Yes | 80 | 2 | - | PD | |
| 12 | Mesenchymal chondrosarcoma | 15 | F | 5 | Yes | 60 | 1 | Elevated GGT | PD |
PR=partial response; SD=stable disease; PD=progressive disease,
Toxicity and MTD
Three patients were enrolled on the starting dose level of 1.1 mg/m2. No DLTs attributable to trabectedin were observed. One patient had progressive disease prior to cycle 2 and was removed from protocol therapy. Two patients were eligible for intra-patient dose-escalation and had their dose increased to 1.5 mg/m2 for all subsequent cycles.
Six patients enrolled on the second dose level (1.5 mg/m2). One patient had dose-limiting (grade 3) anorexia and fatigue during cycle 1.
Three patients enrolled on the 1.7mg/m2 dose level. Two had a DLT during cycle one. The first patient experienced dose-limiting (grade 3) dehydration, and several non-dose-limiting toxicities, including grade 2 elevation of creatinine (3.5-fold higher than baseline), grade 3 elevation of serum transaminases and grade 2 fatigue. The second patient tolerated 2 cycles of trabectedin (1.7 mg/m2) without DLT, however, discontinued protocol therapy due to progressive disease. The third patient had a dose-limiting elevation of gamma-glutamyl transferase (GGT, grade 3) and grade 4 alanine aminotransferse (ALT) and aspartate aminotransferase (AST) elevation that did not meet the protocol specified criteria for DLT. Therefore, the maximum tolerated dose was 1.5 mg/m2 IV over 24 hours.
Table II presents all toxicities at least possibly attributed to trabectedin during cycle 1. The most common toxicities during cycle 1 were elevation in serum transaminases, alkaline phosphatase and GGT, anemia, thrombocytopenia, fatigue, nausea and emesis.
Table II.
Trabectedin-associated toxicity during cycle 1.
| Toxicity During Cycle 1 | Dose Level (mg/m2/dose) and CTCAE grade toxicity
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 (n=3) | 1.5 (n=6) | 1.7 (n=3) | ||||||||||
|
| ||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Hematologic | ||||||||||||
| Leukopenia | 1 | 1 | 1 | 1 | 1 | |||||||
| Anemia | 2 | 1 | 3 | 1 | ||||||||
| Thrombocytopenia | 1 | 2 | 1 | |||||||||
| Neutropenia | 1 | 1 | ||||||||||
|
| ||||||||||||
| Gastrointestinal | ||||||||||||
|
| ||||||||||||
| Increased ALT | 2 | 1 | 1 | 1 | 2 | 1 | 3 | |||||
|
| ||||||||||||
| Increased AST | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | ||||
|
| ||||||||||||
| Increased GGT | 2 | 2 | 1 | 1 | 1* | |||||||
|
| ||||||||||||
| Nausea | 3 | 1 | 2 | 1 | ||||||||
|
| ||||||||||||
| Vomiting | 2 | 1 | ||||||||||
|
| ||||||||||||
| Anorexia | 1* | 1 | ||||||||||
|
| ||||||||||||
| Constipation | 1 | 1 | ||||||||||
|
| ||||||||||||
| Constitutional | ||||||||||||
| Fatigue | 1 | 1 | 1 | 1* | 2 | |||||||
| Dehydration | 1 | 1* | ||||||||||
| Generalized pain | 1 | |||||||||||
| Dizziness | 1 | |||||||||||
|
| ||||||||||||
| Metabolic/Laboratory | ||||||||||||
|
| ||||||||||||
| Increased alkaline phosphatase | 1 | 3 | ||||||||||
|
| ||||||||||||
| Hypoalbuminemia | 2 | |||||||||||
|
| ||||||||||||
| Renal | ||||||||||||
| Creatinine elevation | 1 | |||||||||||
Dose limiting toxicity
In subsequent cycles, 2 patients required trabectedin dose-reductions for dose-limiting hematologic toxicity. Patient 6 enrolled at the 1.5 mg/m2 dose level, developed thrombocytopenia and prolonged neutropenia during cycle 2. After recovery of her blood counts, the trabectedin dose was reduced to 1.1 mg/m2 and she remained on study for 4 additional cycles without significant toxicity. She discontinued protocol therapy for progressive disease after 6 cycles. Patient 4 enrolled at the 1.5 mg/m2 dose level, experienced delayed neutrophil count recovery during cycle 6. She continued on study at a reduced dose of 1.1 mg/m2. She did not experience additional DLTs but elected to discontinue therapy after 12 cycles.
Pharmacokinetics
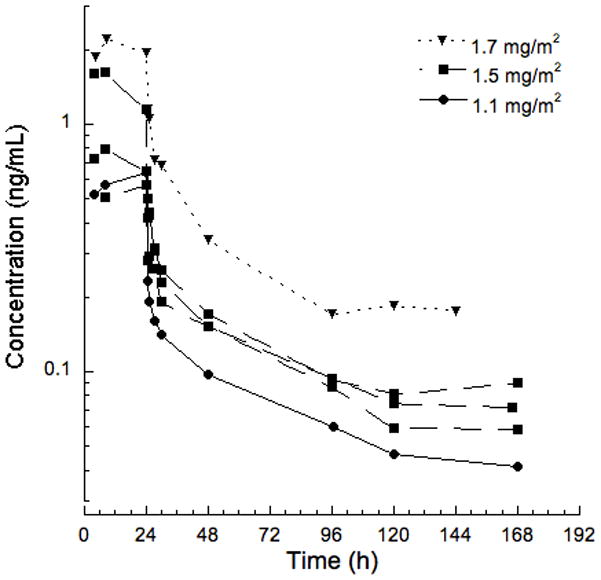
One patient at the 1.1 mg/m2 dose level, three patients at 1.5 mg/m2 and one patient at 1.7 mg/m2 consented to pharmacokinetic sampling during cycle 1. Pharmacokinetic parameters for each patient with a comparison to prior pediatric and adult studies are presented in Table III, and plasma trabectedin concentration-time profiles are presented in Figure 1. As shown in the Table III, the disposition of trabectedin in children was similar to adults.
Table III.
Plasma pharmacokinetic parameters of trabectedin in pediatric patients on this study and comparison to other pediatric and adult studies.
| Patient Population | Dose level (mg/m2) | Infusion Duration (h) | Pt No. | Dose (mg) | EOI Conc. (ng/ml) | AUC0-8d (ng•h/ml) | AUC0-∞ (ng•h/ml) | t1/2 (h) | Clearance (L/h/m2) | Vdss (L/m2) |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| NCI Pediatric | 1.1 | 24 | No. 3 | 1.7 | 0.64 | 23.5 | 28.8 | 94.8 | 38 | 3010 |
|
| ||||||||||
| 1.5 | 24 | No. 4 | 2.2 | 0.57 | 29.3 | 36.5 | 83.2 | 40.5 | 2460 | |
| No. 6 | 2.6 | 0.65 | 34.6 | 50.8 | 129.3 | 29.9 | 4020 | |||
| No. 9 | 1.8 | 1.15 | 48.9 | 60.4 | 104.3 | 24.8 | 1880 | |||
|
| ||||||||||
| Mean ± SD (n=3) | 0.79 ± 0.31 | 37.6 ± 10.2 | 49.2 ± 12 | 105.6 ±2 3.1 |
31.7 ± 8 | 2790 ± 1100 | ||||
|
| ||||||||||
| 1.7 | 24 | No. 12 | 3.1 | 1.94 | 80.2 | 105.5 | 101.8 | 16.3 | 1400 | |
|
| ||||||||||
| Pediatric22 | 1.1 | 3 | Mean ± SD (n=4) | - | 6.02 ± 3.28 | - | 38.9 ± 24.2 | 40.8 ±24.9 | 29.1 ± 11.9 | 1023 ± 1115 |
|
| ||||||||||
| Adult8 | 1.5 | 24 | Mean ± SD (n=25) | - | 1.8 ± 1.1 | - | 55 ± 25 | 89 ± 41 | 33 ± 17 | 2170 ± 1060 |
EOI= end of infusion; AUC=area under the curve; t1/2=half life; Vdss=volume of distribution at steady state; SD=standard deviation
Figure 1.
Response
One patient achieved a partial response, and 3 patients (desmoplastic small round cell tumor n=1 and osteosarcoma n=2) had stable disease for 6 or more cycles (Table I). There were no complete responses. A 16 year old female with neuroendocrine carcinoma (patient 4) with extensive hepatic, pulmonary and bone marrow metastases had a partial response after 4 cycles of 1.5 mg/m2 trabectedin. After cycle 6, she received 1.1 mg/m2 due to hematological toxicity. She electively discontinued protocol therapy with stable disease after 12 cycles. Four months after her last dose of trabectedin, she had widespread progression of disease.
Discussion
The recommended dose of trabectedin in children is 1.5 mg/m2 infused IV over 24 h every 21 days, which is identical to the recommended dose and schedule for adults. Our study included mostly adolescents, but the toxicity profile in the 4 patients under 13 years of age was not different than that of the older children. A prior pediatric phase I trial of trabectedin defined a MTD of 1.1 mg/m2 infused IV over 3 h using a more conservative definition of hepatic dose-limiting toxicity. Preclinical and adult toxicity data indicate that trabectedin-related hepatic toxicity is transient and is not cumulative. Therefore, in this trial, we used less conservative definitions of dose-limiting serum AST and ALT elevations, which were similar to criteria used in prior phase 1 trials in adults, and ALT/AST elevation was not dose-limiting on this trial.
The toxicity profile, which included myelosuppression, elevated serum transaminases, fatigue, nausea and emesis, in children receiving trabectedin on this trial is similar to that reported in adults. Myelosuppression (neutropenia and to a lesser extent thrombocytopenia) was the DLT in adults receiving 1.8 mg/m2 as a 24 h IV infusion without growth factor support [9]. In our study in children with refractory or recurrent solid tumors, neutropenia was not dose limiting during cycle 1. However, two patients required trabectedin dose reductions on treatment cycles 2 and 6 for prolonged myelosuppression that delayed the subsequent treatment cycle by >7 days. Therefore, growth factor support should be administered to children receiving 1.5 mg/m2 of trabectedin as a 24 h infusion. Cumulative bone marrow toxicity from trabectedin has not been reported in the adults, and delays in later treatment cycles on our trial may be a consequence of limited bone marrow reserve from prior treatment or the progression of bone marrow metastases.
The Children’s Oncology Group has recently completed a phase 2 trial of trabectedin in children with recurrent or refractory sarcomas [25]. There was a partial response in a patient with rhabdomyosarcoma and stable disease for >2 cycles in one patient each with rhabdomyosarcoma, spindle cell and Ewing sarcoma. The objective response in neuroendocrine carcinoma observed in this phase I trial suggests that clinical trials to determine the activity of trabectedin in other childhood solid tumors and or in combination with non-myleosuppressive chemotherapy may be warranted.
In summary, the recommended dose of trabectedin in children with refractory or recurrent solid tumors is 1.5 mg/m2 IV infused over 24 hours every 21 days with dexamethasone pre-treatment and neutrophil growth factor support.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
The views presented in this manuscript do not necessarily represent the views of the Federal government.
Conflict of interest statement: Authors PZ and EB are employed by Johnson & Johnson Pharmaceutical R&D, LLC
References
- 1.Martinez EJ, Corey EJ. A new, more efficient, and effective process for the synthesis of a key pentacyclic intermediate for production of ecteinascidin and phthalascidin antitumor agents. Org Lett. 2000;2:993–996. doi: 10.1021/ol0056729. [DOI] [PubMed] [Google Scholar]
- 2.Pommier Y, Kohlhagen G, Bailly C, Waring M, Mazumder A, Kohn KW. DNA sequence- and structure-selective alkylation of guanine N2 in the DNA minor groove by ecteinascidin 743, a potent antitumor compound from the Caribbean tunicate Ecteinascidia turbinata. Biochemistry. 1996;35:13303–13309. doi: 10.1021/bi960306b. [DOI] [PubMed] [Google Scholar]
- 3.Friedman D, Hu Z, Kolb EA, Gorfajn B, Scotto KW. Ecteinascidin-743 inhibits activated but not constitutive transcription. Cancer Res. 2002;62:3377–3381. [PubMed] [Google Scholar]
- 4.Takebayashi Y, Pourquier P, Zimonjic DB, et al. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repair. Nat Med. 2001;7:961–966. doi: 10.1038/91008. [DOI] [PubMed] [Google Scholar]
- 5.Tavecchio M, Simone M, Erba E, et al. Role of homologous recombination in trabectedin-induced DNA damage. Eur J Cancer. 2008;44:609–618. doi: 10.1016/j.ejca.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Herrero AB, Martin-Castellanos C, Marco E, Gago F, Moreno S. Cross-talk between nucleotide excision and homologous recombination DNA repair pathways in the mechanism of action of antitumor trabectedin. Cancer Res. 2006;66:8155–8162. doi: 10.1158/0008-5472.CAN-06-0179. [DOI] [PubMed] [Google Scholar]
- 7.D’Incalci M, Jimeno J. Preclinical and clinical results with the natural marine product ET-743. Expert Opin Investig Drugs. 2003;12:1843–1853. [PubMed] [Google Scholar]
- 8.van Kesteren C, Cvitkovic E, Taamma A, et al. Pharmacokinetics and pharmacodynamics of the novel marine-derived anticancer agent ecteinascidin 743 in a phase I dose-finding study. Clin Cancer Res. 2000;6:4725–4732. [PubMed] [Google Scholar]
- 9.Taamma A, Misset JL, Riofrio M, et al. Phase I and pharmacokinetic study of ecteinascidin-743, a new marine compound, administered as a 24-hour continuous infusion in patients with solid tumors. J Clin Oncol. 2001;19:1256–1265. doi: 10.1200/JCO.2001.19.5.1256. [DOI] [PubMed] [Google Scholar]
- 10.Yver A, Cohen R, Williams D, von Mehren M. Assessment of trabectedin (T) induced liver toxicity with correlation to liver morphology in a phase I study of T + pegylated liposomal doxorubicin (PLD). Journal of Clinical Oncology; 2006 ASCO Annual Meeting Proceedings Part 1; Jun 20, 2006. p. 9568. Supplement. [Google Scholar]
- 11.Beumer JH, Schellens JH, Beijnen JH. Hepatotoxicity and metabolism of trabectedin: a literature review. Pharmacol Res. 2005;51:391–398. doi: 10.1016/j.phrs.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Donald S, Verschoyle RD, Greaves P, et al. Complete protection by high-dose dexamethasone against the hepatotoxicity of the novel antitumor drug yondelis (ET-743) in the rat. Cancer Res. 2003;63:5902–5908. [PubMed] [Google Scholar]
- 13.Fetterly GJ, Owen JS, Stuyckens K, et al. Semimechanistic pharmacokinetic/pharmacodynamic model for hepatoprotective effect of dexamethasone on transient transaminitis after trabectedin (ET-743) treatment. Cancer Chemother Pharmacol. 2008;62:135–147. doi: 10.1007/s00280-007-0583-8. [DOI] [PubMed] [Google Scholar]
- 14.Grosso F, Dileo P, Sanfilippo R, et al. Steroid premedication markedly reduces liver and bone marrow toxicity of trabectedin in advanced sarcoma. Eur J Cancer. 2006;42:1484–1490. doi: 10.1016/j.ejca.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–4196. doi: 10.1200/JCO.2008.21.0088. [DOI] [PubMed] [Google Scholar]
- 16.Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23:576–584. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 17.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 18.Morgan JA, Le Cesne A, Chawla S, et al. Randomized phase II study of trabectedin in patients with liposarcoma and leiomyosarcoma (L-sarcomas) after failure of prior anthracyclines (A) and ifosfamide (I). Journal of Clinical Oncology; 2007 ASCO Annual Meeting Proceedings Part 1; Jun 20, 2007. p. 10060. Supplement. [Google Scholar]
- 19.Yovine A, Riofrio M, Blay JY, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol. 2004;22:890–899. doi: 10.1200/JCO.2004.05.210. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Carbonero R, Supko JG, Manola J, et al. Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol. 2004;22:1480–1490. doi: 10.1200/JCO.2004.02.098. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Carbonero R, Supko JG, Maki RG, et al. Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol. 2005;23:5484–5492. doi: 10.1200/JCO.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Lau L, Supko JG, Blaney S, et al. A phase I and pharmacokinetic study of ecteinascidin-743 (Yondelis) in children with refractory solid tumors. A Children’s Oncology Group study. Clin Cancer Res. 2005;11:672–677. [PubMed] [Google Scholar]
- 23.Stokvis E, Rosing H, Lopez-Lazaro L, Beijnen JH. Simple and sensitive liquid chromatographic quantitative analysis of the novel marine anticancer drug Yondelis (ET-743, trabectedin) in human plasma using column switching and tandem mass spectrometric detection. J Mass Spectrom. 2004;39:431–436. doi: 10.1002/jms.608. [DOI] [PubMed] [Google Scholar]
- 24.Collection of terms, symbols, equations, and explanations of common pharmacokinetic and pharmacodynamic parameters and some statistical functions. AHAH Working Group PK-PD Modeling. 2004 Available at: http://www.agah.info/en/the-agah/publications.html.
- 25.Baruchel S, Pappo A, Krailo M, et al. A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: A report from the Children’s Oncology Group. European J of Cancer. 2012;48:579–585. doi: 10.1016/j.ejca.2011.09.027. [DOI] [PubMed] [Google Scholar]


