Abstract
Depression has been characterized as involving altered appetitive motivation and emotional reactivity. Yet no study has examined objective indices of emotional reactivity when the appetitive/approach system is suppressed in response to failure to attain a self-relevant goal and desired reward. Three groups of youth (N = 98, ages 9–15; remitted depressed, n = 34; externalizing disordered without depression, n = 30, and healthy controls, n = 34) participated in a novel reward striving task designed to activate the appetitive/approach motivation system. Objective facial expressions of emotion were videotaped and coded throughout both failure (i.e., nonreward) and control (success and reward) conditions. Observational coding of facial expressions as well as youths’ subjective emotion reports showed that the remitted depressed youth specifically exhibited more negative emotional reactivity to failure in the reward striving task, but not the control condition. Neither externalizing disordered (i.e., ADHD, CD, and/ or ODD) nor control youth displayed greater negative emotional reactivity in either the failure or control condition. Findings suggest that depression among youth is related to dysregulated appetitive motivation and associated negative emotional reactivity after failing to achieve an important, self-relevant goal and not attaining reward. These deficits in reward processing appear to be specific to depression as externalizing disordered youth did not display negative emotional reactivity to failure after their appetitive motivation system was activated.
Various theoretical models converge on the notion that depression may be characterized by deficits in appetitive motivation, in particular dysregulation in the behavioral activation system (BAS), also sometimes referred to as approach, but not necessarily by specific deficits in the behavioral inhibition system (BIS), also termed withdrawal (Davidson, 1998; Davey, Yucel, & Allen, 2008; Depue & Iacono, 1989; Forbes & Dahl, 2005, 2012). The appetitive system aids in regulating and guiding behavior toward rewards, and positive affect is generated in the anticipation of successful receipt of reward, whereas negative affect (e.g., sadness, frustration) can be engendered when striving for a desired goal is thwarted (Carver, 2004). Moreover, the appetitive/approach system is suppressed when pursuit of goals and attainment of rewards are blocked, and extensive suppression of the reward system has been proposed to result in depression (Davey et al., 2008). Symptoms of depression, such as anhedonia, withdrawal, and psychomotor retardation, may be understood as reflecting a perturbed appetitive motivational system and reward processing. These affective and motivational symptoms have been explained from an evolutionary perspective as an adaptive response signaling the need to desist from unsuccessful efforts to attain an unreachable goal or reward (Allen & Badcock, 2003).
Despite the prominence of emotion-based motivational theories of depression, however, no research has examined emotional reactivity to nonreward and lack of goal attainment in depression among youth. Relatively more research has investigated lowered positive affect and altered reward processing among currently depressed individuals (Forbes & Dahl, 2005, 2012; Forbes, 2009). The present study sought to examine negative emotion reactivity in response to failing to attain a desired goal and reward. We studied whether negative emotional reactivity to failure was elevated specifically among remitted depressed youth compared to a psychiatric control group of youth with externalizing disorders and healthy controls as the extant research has used currently depressed youth (e.g., Forbes, Shaw, & Dahl, 2007). Overall, the present research aimed to advance knowledge on whether altered appetitive motivation and approach processes are linked with negative emotional reactivity in response to failure and whether such negative emotions specifically characterize youth whose depression has remitted.
Considerable research shows that dysregulation of the appetitive/approach system is associated with reductions in the experience of positive affect (Forbes & Dahl, 2005, 2012). Most of the support for this proposal comes from research in which depressed individuals self-report low levels of positive affect (e.g., Clark, 2005; Lonigan et al., 2003). In addition, a few non-self-report studies have shown altered approach processes using tasks with reward-related choices and behavior paradigms (e.g., Forbes et al., 2007; Henriques & Davidson, 2000) or attenuated emotional response to positive affective stimuli (e.g., Sloan, Strauss, Quirk, & Sajatovic, 1997; Sloan, Strauss, Wisner, 2001). Forbes and colleagues found that under conditions of a high probability of reward, depressed boys did not choose high-magnitude reward options more often than low-magnitude reward options. This is in contrast to boys with anxiety and externalizing disorders, who did not exhibit unusual reward-related choices. Clearly, studying reduction in positive affect as an emotional response to dysregulated approach motivation and reward processing in depression is important.
However, less research has examined whether negative emotional responses are altered when the appetitive/approach system is thwarted in pursuit of a self-relevant goal and failing to attain a desired reward. This lack of research investigating negative emotional reactivity to appetitive/approach processes may be because appetitive motivation is frequently equated with increases in positive affect only, and BIS activity tends to be equated with negative affect only (see Carver, 1994, for review). Yet, Carver (2004) clearly articulates the links between negative affect (e.g., sadness, frustration) and doing poorly at approach, especially when the goal is self-relevant and the appetitive system is activated. Still, much of this evidence derives from social psychological research with healthy adults (e.g., Higgins, Shah, & Friedman, 1997; Shah & Higgins, 2001). To our knowledge, no research has extended this line of research indicating that disturbed appetitive/ approach motivation (i.e., prevention of attainment of maximum rewards) is linked with negative affect to clinical populations, particularly youth with clinical depression.
The present study tested this key hypothesis from emotion-based motivation depression theories that failure to attain a desired, self-relevant goal would suppress the reward/ approach system and produce negative affect. We examined whether the appetitive motivational system and negative emotional reactivity, that is expected to result from failure to attain a self-relevant goal and reward, are disturbed in youth who have experienced an episode of depression. We addressed this question by using a novel, ecologically valid reward striving task that was designed to activate the appetitive/ approach system as youth sought to obtain a self-relevant goal (i.e., achievement by succeeding at completing a very difficult puzzle) and attain reward (i.e., receive money). We designed this task to activate the BAS over time and with sustained effort necessary to achieve the goal and reward. We made this choice based on research demonstrating that actively working to achieve a goal and anticipating reward most activate the dopaminergic reward system (Berridge & Robinson, 1998) and are most relevant to depression (Davey et al., 2008). We examined objective facial expressions of emotion throughout this reward striving task as youth failed to achieve their desired goal.
We chose to study these emotion-based motivational processes among remitted depressed youth because depression is a recurrent and chronic condition (Rutter, Kim-Cohen, & Maughan, 2006). Most individuals experience their first onset of depression in childhood or adolescence (Hankin et al., 1998), and after an initial depressive episode, previously depressed youth are 2–7 times more likely to experience a recurrence later in life compared with nondepressed youth (Rutter et al., 2006). Depressed individuals exhibit reduced frequency of experiencing positive reinforcement (Lewinsohn & Gotlib, 1985), and behavioral activation interventions can ameliorate depression (Dimidjian et al., 2006). Examining disturbed appetitive motivation and emotion response after failing to achieve success and reward among remitted depressed youth could inform processes that contribute to the increased risk for recurrence over the lifespan (Rutter et al., 2006).
In addition, we investigated whether perturbed appetitive motivation and emotion reactivity to failing during the reward striving task was specifically associated with remitted depression compared to healthy normal and psychiatric control groups, specifically youth diagnosed with externalizing disorders (Attention Deficit Hyperactivity Disorder (ADHD), Conduct Disorder (CD), or Oppositional Defiant Disorder (ODD)). We elected externalizing disorders as the relevant psychiatric control group given prior proposals that externalizing symptoms, such as impulsivity, are the result of excessive activity in reward systems (Beauchaine, 2001).
In sum, our primary aim was to examine how emotion response in the pursuit of a desired goal would be affected specifically by an episode of depression. We used a novel, self-relevant, reward striving task to elicit approach, appetitive motivational processes, as these have been hypothesized to be disrupted in and by clinical depression. We hypothesized that objective facial expressions of negative emotions would be exhibited more during the failure condition of the reward striving task. We further hypothesized that this effect would be observed specifically among remitted depressed adolescents as these previously depressed adolescents’ inability to achieve their desired goal should suppress their appetitive motivational reward system. Inhibition of this motivational system has been hypothesized to occur in depression (Davey et al., 2008).
Method
Participants and Procedures
Recruitment targeted children who: (1) had a past diagnosis of depression, (2) had a diagnosis of ADHD, or (3) had no history of psychiatric diagnosis. Multiple recruitment methods were used, including flyers posted in community locations and medical/psychiatric clinics and advertisements placed in local newspapers and distributed through University list-serves.
Parents who were interested in having their child participate in the study called the laboratory. A brief screening was conducted with parents to determine the eligibility of their child. Youth were excluded if they had a severe learning or psychiatric problem (e.g., autism, psychosis) that was likely to interfere with completion of the extensive laboratory protocol; only one child met this criterion and was excluded. Each eligible youth, along with one parent (mothers in most cases), was scheduled for a laboratory visit. Parents of participating youth reported an average educational attainment of “some college or a 2-year degree” and an average yearly household income of $41,000 to $60,000. Children and parents were reimbursed for their participation.
The final sample consisted of 98 youth (56% female) between the ages of 9 and 15 (M age = 12.68, SD = 2.16). The sample was 67% Caucasian, 32% African-American, and 1% multi-racial. In our sample, 34 adolescents (55% female) received a pure past diagnosis of depression (i.e., no comorbid externalizing disorder), 30 adolescents (33% female) received a pure current or past diagnosis of ADHD or another externalizing disorder (i.e., no mood disorder), and 34 adolescents (50% female) received no diagnosis. Adolescents with comorbid depression and externalizing diagnoses (n = 12) were excluded from the present study in order to examine whether emotion reactivity to the reward striving task was associated specifically with pure remitted depression. Formerly depressed adolescents had to be 6 month symptom-free in order to receive a diagnosis of past depression (Frank et al., 1991). We requested that parents withhold stimulant medication on the day of the study for those youth with a diagnosis of ADHD.
The parent and child came to the laboratory, provided informed consent, and completed a battery of questionnaires, diagnostic interviews, and tasks. Measures relevant to the current study are described below. Youth and parents first completed some questionnaires, then the child completed the first reward striving task intended to end in failure, followed by diagnostic interviews and additional questionnaires, and finally the second, control reward striving task. Debriefing occurred at study end. The Institutional Review Board approved all procedures.
Clinical Diagnoses
The Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997) was used to assess DSM-IV (American Psychiatric Association, 1994) diagnoses of major (MDD) or minor (mDD) depressive disorder or an externalizing disorder (ADHD, CD, or ODD). The psychotic screen was used to rule out psychotic disorder. Adolescents and parents completed the KSADS interview separately. Discrepancies were resolved through consensus meetings and best estimate procedures. Inter-rater reliability for the K-SADS based on 20% of the interviews (n=20) was good (kappa = .87).
Reward Striving Task
To test how the appetitive/approach system and associated emotional reactivity are related to depression, we developed a reward striving task that was designed to activate self-relevant goal seeking and reward striving behavior. To this end, we utilized a puzzle box task modeled after a similar task used with younger children (2nd–5th graders) (Eisenberg et al., 2005). This puzzle box task was used in two conditions, both of which were designed to activate the appetitive/ approach system. In one condition, the reward striving task was modified to make it very difficult for the children to attain the self-relevant goal of demonstrating achievement and success and then to obtain monetary reward. As a result, this task was expected to elicit negative emotions in response to the failure to attain the desired goal and reward. Observational coding included a variable regarding persistence on the task. Youth were allotted five minutes to complete the task and receive a reward. Although several youth became very frustrated and stopped trying to complete the puzzle for several seconds during the failure condition, they continued to persist throughout the task. This level of persistence can be interpreted as an index of interest and that the task elicited self-relevant goals and appetitive/approach behaviors in youth. The second condition was designed as a control task and was not expected to substantially elicit positive or negative emotion. Youth were videotaped from behind a one-way mirror while completing both conditions of the task, and sound was recorded using a small, unobtrusive microphone placed next to the adolescent. They were not aware they were being videotaped or observed. Before and after each condition, the child completed a visual analogue scale (VAS) using an emotion thermometer (range 0–100) to assess particular emotions (sad, frustrated, angry, happy, alert, and interested) as a manipulation check that each condition was eliciting the expected emotional valence.
Failure condition
This condition was designed to increase the likelihood that youth would fail while striving to attain a self-relevant goal and desired reward, and as a result, was intended to elicit negative emotion (Carver, 2004). In this condition adolescents sat alone in a room at a table in front of a 16” × 18” × 7 ½” wooden box with armholes cut into one side and an open top. Inside the box were pieces of a magnetic puzzle. A photograph of the completed 3-dimensional puzzle was clipped to the outside of the box so that the adolescents could easily see what the final, completed puzzle should look like. The puzzle was difficult to complete. A loudly ticking kitchen timer was placed next to the box. The box was covered with a blanket, and adolescents were given 5 minutes to try to solve the puzzle in the box without being able to see the pieces they were manipulating. Adolescents were left alone in the room and told that if they solved the puzzle within the 5 minutes they would receive a prize—a $5 gift certificate. After 5 minutes the experimenter returned to the room and removed the box.
Control condition
This condition was intended to be emotionally neutral. We wanted to compare youths’ facial displays of emotion after failing on the first reward striving task to their facial displays of emotion after succeeding during this control task. For this part of the experiment, youth were given the same puzzle task. The blanket was removed from the puzzle box, and youth were given as much time as needed to finish the puzzle. The control condition was expected to be more emotionally neutral (i.e., less emotional reactivity) in comparison to the initial failure condition. In addition, youth were not offered the monetary reward if they completed the puzzle in the control condition. Thus, in this condition they were not striving as actively to attain self-relevant goal or desired reward, making BAS activation less likely.
Coding Emotion Reactivity
We used facial displays of emotion to index emotion response because emotions often involve micro-momentary changes and can be difficult to measure through questionnaires alone (Mauss & Robinson, 2009; Cole, Martin, & Dennis, 2004). Data derived from observational studies can better assess the activation and experience of emotion, especially emotional responses that are believed to reflect affective components of the BAS, such as positive affect when anticipating and successfully pursuing a goal, as well as negative affect when reward striving is thwarted or unsuccessful (Carver, 2004). One of the primary components of an emotion response is behavioral expression (facial, vocal, or bodily indicators; Keltner & Ekman, 2004). Of the three indices of emotion state, facial expression is particularly sensitive to the valence of an emotion (Ekman, Friesen, & Ancoli, 1980; Mauss & Robinson, 2009). Therefore, we used facial displays of emotion to assess emotional reactivity.
Two trained coders, blind to child diagnosis and task condition (i.e., failure or control), independently watched videotapes of youths’ facial displays of emotion in the failure and control conditions. Positive and negative facial displays of emotion reactivity were assessed across both conditions. Examples of facial displays of negative emotion reactivity included: brows sharply lowered and drawn together, vertical wrinkle or bulge between brows, nasal root broadened, eyes squinted, and mouth straight, angular, or drawn tightly shut. Examples of facial displays of positive emotion reactivity included: smooth forehead, crow’s feet apparent, mouth smiling or eyes squinted or narrowed, and cheeks raised from smiling.
To assess interrater reliability, we calculated intra-class correlation coefficients (ICCs) within the failure and control conditions for positive and negative emotional displays, separately, on a subset of 11 cases (11% of the sample). Coders demonstrated adequate reliability: Positive (ICC = .65) and negative (ICC = .92) emotion to failure, positive (ICC = .90) and negative (ICC = .84) emotion in the control condition.
In addition, coders used an 11-point Likert scale, ranging from −5 (‘extremely negative’) to 5 (‘extremely positive’), with 0 being ‘neutral,’ to provide ratings of the average emotion expression across the 5 minutes for each of the two conditions. During the failure condition 43% of youth displayed a negative emotional expression, 51% displayed a neutral expression, and 6% displayed a positive expression. During the control condition, 8% of youth displayed a negative emotional expression, 86% displayed a neutral expression, and 6% displayed a positive expression. These descriptive results provide initial validity data suggesting that the control condition was emotionally neutral, and the failure condition produced relatively more negative emotional displays, as expected.
Emotion was conceptualized along a dimensional perspective, which uses a two-factor circumplex model in which positive emotion states and negative emotion states are largely independent of each other and can be experienced simultaneously (Watson & Tellegen, 1985). According to the dimensional perspective, valence, which contrasts states of positive emotions with negative emotions, is considered a basic property of emotion experience (for a review, see Barrett, 2006). Because valence is considered a fundamental component of emotion responding, it has been suggested that valence be examined first before discrete categories of emotion specificity be investigated (Mauss & Robinson, 2009). In accordance with the dimensional perspective and given our primary aim to examine emotional reactivity to activation of the appetitive/approach system after failing to achieve a self-relevant goal and desired reward, we elected to dichotomize the valence of facial displays of emotion. This was done in order to examine specifically negative emotional response in the failure condition when the youth were unable to achieve their goal and the appetitive/approach system was blocked when striving for a desired reward. Prior research (Carver, 2004) suggests that failure to attain the desired goal would elicit predominantly negative emotional responses (e.g., frustration, sadness, anger) as opposed to neutral or positive emotion. Thus, for the main analyses in this study, coders’ ratings of adolescents’ average emotional reactivity was dichotomized into typically negative (ranging from −5 to −1) versus typically neutral or positive (ranging from 0 to 5). Coder agreement for this dichotomous global rating was 90%; kappa was .83.
Self-Report Measures
Depressive symptoms
Youth completed the 27-item Children’s Depression Inventory (CDI, Kovacs, 1985) to assess current levels of depressive symptoms. The CDI has demonstrated reliability and validity (Kovacs, 1985). Internal consistency was good, α = .83.
Trait positive and negative affect
The 21-item Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) was used to assess youths’ self-reported general emotional distress (i.e., trait negative affect) and well-being (i.e., trait positive affect). This instrument asks individuals to rate their degree of various feelings and emotions (e.g., interested, excited, irritable, distressed) experienced during the past week, using a 1–5 likert-scale. Youths’ total scores for 10 positive emotions (α = .85) and 11 negative emotions (α = .86) were utilized.
Results
Demographic and Clinical Characteristics
Table 1 shows associations among diagnostic groups, demographics, and clinical characteristics. The formerly depressed group did not significantly differ from the externalizing group or the no disorder control group on current depressive symptoms or positive affect. However, remitted depressed and externalizing adolescents reported significantly greater levels of negative affect than control adolescents. No significant age or sex differences among groups were found.
Table 1.
Demographic and Clinical Characteristics
| Characteristics | Group | |||
|---|---|---|---|---|
| Formerly Depressed (n = 34) |
Externalizing Disorder (n = 30) |
No Diagnosis (n = 34) |
||
| Age: | 13.65 (1.95) | 12.79 (.87) | 12.84 (1.44) | |
| Sex: female: n (%) | 16 (48%) | 10 (33%) | 19 (57%) | |
| Ethnicity | ||||
| n, (%Caucasian) | 26 (74%) | 18 (60%) | 21 (64%) | |
| n, (% African American) | 8 (26%) | 12 (40%) | 11 (33%) | |
| n, (% Other) | 0 (0%) | 0 (0%) | 1 (3%) | |
| CDI | 9.74 (6.8) | 6.32 (5.32) | 5.86 (4.32) | |
| PANAS NA | 22.60 (12.95)a | 21.32 (7.66)a | 13.54 (7.44)b | |
| PANAS PA | 29.81 (8.43) | 34.82 (8.73) | 30.42 (7.83) | |
| # MD episodes | 1.42 (.67) | 0 | 0 | |
| Age onset first MDD | 11.48 (.38) | 0 | 0 | |
| ADHD (%, age onset) | 0 | 83%; 6.5 yrs | 0 | |
| CD (%, age onset) | 0 | 33%; 12.34 yrs | 0 | |
| ODD (%, age onset) | 0 | 40%; 11.35 yrs | 0 | |
Note. Different subscripts within rows indicate significant group differences at p < .05.
Manipulation Check: Emotion Response
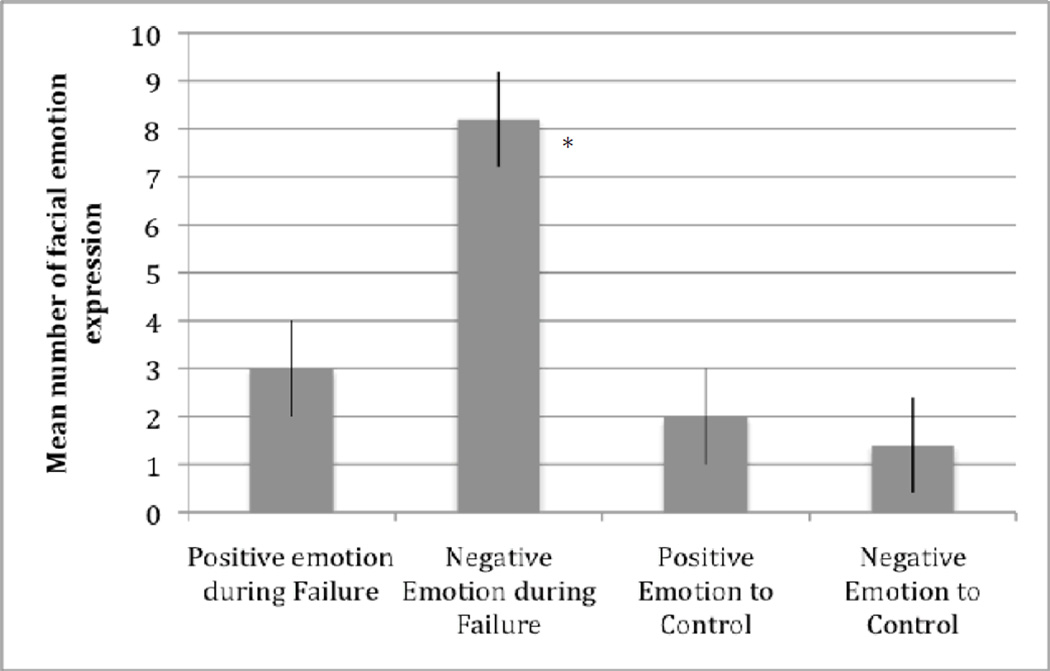
To determine if the failure condition of the reward striving task induced negative emotions, as anticipated given that the appetitive/approach system would be blocked when individuals strove for, but were unable to attain, reward, we first examined the mean number of discrete facial displays of positive and negative emotion across the failure and control conditions. As Figure 1 shows, the failure condition elicited significantly more negative facial expressions. The mean number of positive and negative emotions displayed in the failure condition significantly differed from 0, t(97) = 5.58, p < .001, d = 1.13; t(97) = 9.76, p < .001, d = 1.99, respectively. In addition, the mean number of positive and negative emotions displayed in the control condition significantly differed from 0, t(97) = 3.56, p < .01, d = .73; t(97) = 3.25, p < .01, d = .65, respectively. Importantly and consistent with expectation, significantly more negative emotions than positive emotions were displayed during the failure condition, t(97) = 8.87, p < .001, d = 1.8. Likewise, significantly more negative emotions were displayed during the failure condition compared to negative emotion in the control condition (t(97) = 8.68, p < .001, d = 1.76) or positive emotions in control condition (t(97) = 8.25, p < .001, d = 1.66). Second, using the 11-point Likert scale, there was a significant difference between the average emotion reactivity in the failure condition (M = -.43, SD = .76) versus the control condition (M = 0, SD= .32), t (97) = −3.17, p < .01, d = .77, showing that youth displayed more negative emotions during the failure condition and neutral emotion during the control condition. Last, results from changes on the VAS, emotion thermometer for the failure task showed significant differences for the combined negative emotions (i.e., sad, frustrated, angry) between pre- (M = 10.11, SD = 17.1) and post- (M = 51.67, SD = 61.6) task: t (97) = 6.37, p < .01, d = .93. No significant difference was observed for positive emotions (i.e., interested, alert, and happy) between pre- (M = 30, SD = 4) and post- (M = 30, SD = 3) task: t (97) = .16 ns, d = .03.
Figure 1.
Positive and Negative Emotion Reactivity in the Failure and Control Conditions in the Reward Striving Task.
In sum, these results from different methods and sources of assessing emotional reactivity, including observational coding of discrete facial displays of emotion, average observed emotional response, and self-report on the VAS/ emotion thermometer supported the hypothesis that youth exhibited greater negative emotion in response to failing to attain the desired goal during the reward striving task.
Emotion Reactivity in Formerly Depressed and Non-Depressed Control Participants
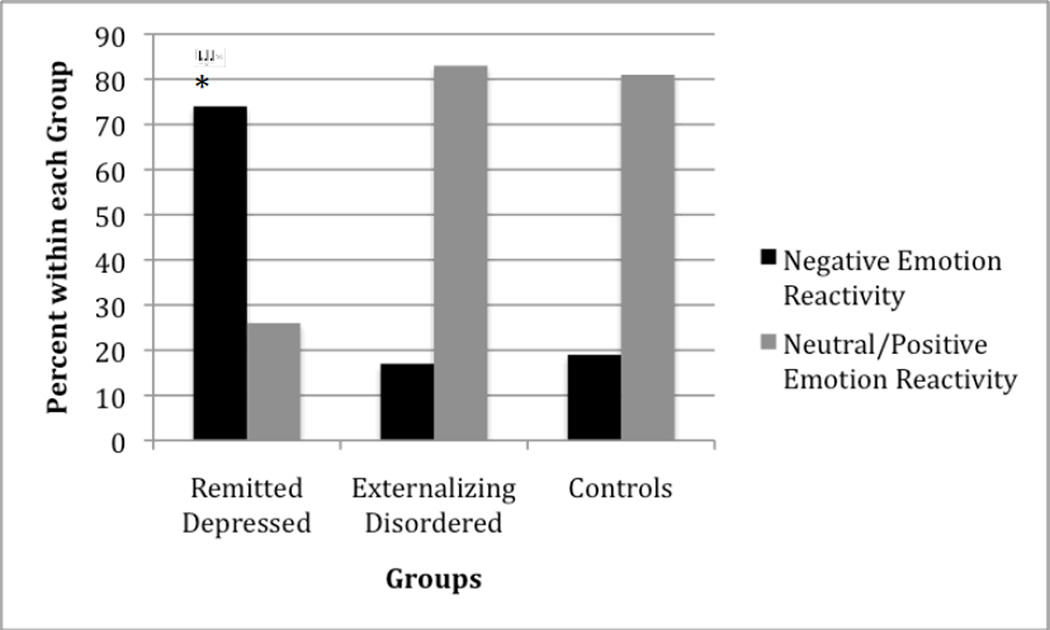
Our primary hypothesis was that formerly depressed youth would show significantly more negative emotion reactivity during the failure condition of the reward striving task compared to control youth. Chi square tests were used to compare diagnostic status (formerly depressed vs. non-depressed controls) with average emotion reactivity (negative emotion vs. neutral/positive emotion) observed throughout the failure condition. Results revealed significant differences between youths’ emotion reactivity and diagnostic status. Specifically, 74% of formerly depressed youth reacted with a negative emotional response in the failure condition of the reward striving task, whereas only 19% of the non-depressed youth displayed negative emotion responses, χ2(1, N = 68) = 3.98, p < .05. Figure 2 shows this effect. There was no significant difference in emotion response during the control condition between formerly depressed and non-depressed youth, χ2(1, N = 64) = .45, ns.
Figure 2.
Remitted Depressed Youth Displayed More Negative Emotion Reactivity in Failure Condition of Reward Striving Task
In addition to the significant difference for objective facial displays of negative emotion, a 2 (group) × 2 (time) repeated measures ANOVA revealed a significant group × time interaction for the VAS ratings on negative emotion (F(2, 97) = 3.13, p < .05) but not positive emotion (F(2, 97) = 1.16, ns). Follow-up analyses showed that the previously depressed group reported subjectively more negative emotional response on the VAS between pre-failure (M = 10.58, SD = 18.2) and post-failure (M = 73.23, SD = 52.2): t(33) = 6.61, p < .01, d = 1.6. The control group also reported subjectively more negative emotional response on the VAS between pre-failure (M = 10.03, SD = 17.2) and post-failure (M = 43.54, SD = 62.2): t(33) = 2.98, p < .05, d = .73, yet this difference for the control group was not as large as that observed with the remitted depressed group: t (63) = 2.16, p < .05 between depressed and control youth for VAS post-failure. These results show that formerly depressed youth exhibited greater negative emotional response only when they were striving for, yet failed to attain, their goal and reward.
Additional analyses were conducted to determine whether negative emotion reactivity to the failure condition of the reward striving task was uniquely associated with a history of depression compared with externalizing disorders (ADHD, CD, and ODD). Results of chi-square analyses revealed that negative emotion reactivity was associated specifically with remitted depression but not with externalizing diagnoses. Specifically, 74% of formerly depressed youth displayed negative emotion reactivity to the failure condition, whereas only 17% of externalizing youth exhibited negative emotion response in this condition, χ2(1, N = 64) = 4.87, p < .05. Figure 2 shows this effect. There was no significant difference in emotion response during the control condition between formerly depressed and externalizing, non-depressed youth, χ2(1, N = 64) = .53, ns. In addition, there was no significant difference in emotion response between youth with externalizing diagnoses and controls with no psychiatric disorder in either the failure or the control condition, χ2(1, N = 64) = .47, ns, χ2(1, N = 64) = .01, ns, respectively.
Discussion
Clinical and affective scientists have emphasized the central role that disturbed emotion and alterations in the appetitive/approach motivational system may play in the ontogeny of depression (e.g., Davidson, 1998; Forbes & Dahl, 2005). More recently, depression among youth has been viewed as a disorder involving disrupted emotion (e.g., Cole, Luby, & Sullivan, 2008; Kovacs, Joormann, & Gotlib, 2008) and perturbed motivation (Ernst & Spear, 2009; Forbes, 2009; Forbes & Dahl, 2012; Pine, 2009). The findings from this study demonstrate that youth with remitted clinical depression exhibit disturbances in negative emotion reactivity specifically after failing to achieve a self-relevant goal and not attaining reward in a novel reward striving task (i.e., not completing a difficult puzzle to demonstrate achievement and receive monetary reward). These results were obtained using objective observational ratings of youths’ emotional response, particularly facial displays of emotion, which are considered the most valid (Ekman et al., 1980; Mauss & Robinson, 2009), as well as changes in youths’ subjective emotional reports before and after failure. Finally, the significant differences in negative emotional reactivity to failure were specific to remitted depressed youth compared with healthy controls as well as psychiatric controls (i.e., youth diagnosed with externalizing disorders).
This novel reward striving task was designed to activate youths’ appetitive/ approach system as they pursued a self-relevant goal and desired reward. The fact that remitted depressed youth exhibited significantly more negative emotional reactivity after failure in this reward striving task suggests that the appetitive/approach motivation system is altered by depression. These findings are consistent with results from research showing that remitted depressed adults demonstrate a hypoactive BAS compared to controls (Kasch et al., 2002; Pinto-Meza et al., 2005; Sigmon & Nelson-Grey, 1992). Clearly, disturbed emotional response to failure after activation of the appetitive system may be a consequence of depression, but this dysregulated process may be fairly traitlike (Hasler et al., 2009). Prior research suggests that altered reward processing in a decision-making task predicts future depression in adolescent boys (Forbes et al., 2007) and that disturbed reward processing may be present before the onset of depression and persist between episodes among adults (Hasler et al., 2009).
Results from the present analyses advance knowledge on the roles that perturbed appetitive motivation as well as altered emotion reactivity in response to failure have in depression specifically Negative emotion reactivity in the failure condition was uniquely related to depression, not externalizing disorders. These results are especially striking because, despite the high levels of comorbidity among internalizing and externalizing disorders (Angold, Costello, & Erkanli, 1999), results demonstrate the diagnostic specificity of negative emotion reactivity to depression, compared to externalizing disorders. Trait negative emotionality is elevated in depression and externalizing disorders (Tackett & Krueger, 2005). Yet, ADHD, CD, and ODD are typically not conceptualized as disorders with altered emotion or motivation as core disturbances (Lahey, Moffitt, & Caspi, 2003), whereas these are viewed as central deficits in depression (Davidson, Pizzagalli, & Nitschke, 2008). Thus, our findings are consistent with and support conceptualizations that disturbed emotion reactivity and motivational processes are uniquely associated with depression rather than externalizing disorders.
Particular strengths and limitations of the present research need to be considered. To our knowledge, no prior research examining potential deficits in motivation and emotional reactivity used groups of remitted depressed youth and a psychiatric control group to examine specificity of emotion-based motivational processes. Although the present design cannot disentangle altered negative emotion reactivity in response to failure as potential cause, consequence, or correlate, prior research found deficient reward processing related to both current and future depressive symptoms among youth (Forbes et al., 2007). Thus, these data with remitted depressed youth complement prior research in suggesting that such deficiencies may persist beyond depression remittance and that dysregulation in reward processing and affective response may be fairly trait-like (Forbes, 2009). Clearly, future research is needed to address these hypothesized processes as potential vulnerability to development of depression.
The use of multiple methods to assess emotional reactivity, including observational coding of facial expression, questionnaires, and subjective ratings, suggests that results were not due to a particular method or informant. Still, examining the convergence of these results with other methods intended to activate BAS and assess emotional reactivity, especially drawn from affective neuroscience (Davidson et al., 2008), would be informative. Despite theoretical motivation for the dichotomization of positive and negative emotion reactivity, variability in the data was reduced as a result. The use of continuous measures of emotion response (e.g., number of discrete displays of emotion, frequency or intensity of emotional response) would add richness to the data and could lead to important advances in this area of research.
Last, additional research that examines both positive and negative emotion reactivity in response to BAS activation could enhance knowledge in the field. Specifically, it would be interesting to contrast the valence of emotion expressions for psychiatric and control youth in tasks designed to thwart success versus tasks designed to optimize success. The task used in the present study was designed to result in failure and illicit negative emotion reactivity, thus youth demonstrated a relatively low rate of positive emotions. This low rate of positive emotion reactivity prevented further analyses of positive emotion in the current study. Future research is needed to use ecologically valid tasks designed to elicit positive affect among adolescent to further investigate whether positive emotions are diminished in adolescent depression.
Implications for Research, Policy, and Practice
Extensive research in affective neuroscience has demonstrated the importance of positive emotion for protecting against psychiatric disorders, especially depression, and building resiliency and healthy development (Davey et al., 2008; Davidson et al., 2009; Forbes & Dahl, 2005). Low levels of positive affect, and dysregulation in emotion regulation, especially difficulty upregulating positive emotion, have been directly implicated in risk to depression (Durbin et al., 2005; Feng et al., 2009; Forbes et al., 2009; Sheeber et al., 2009). Deficits in appetitive motivation, especially dysregulation in BAS, have been one of the primary ways in which investigators have tended to study low positive affect and emotion dysregulation. With this increasing body of research and evidence, translational clinical scientists have begun to develop and test emotion-focused interventions aimed at low positive affect, difficulty upregulating positive emotions, and dysregulation in BAS in an effort to ameliorate depression. For example, Kovacs and colleagues (2006) have provided initial evidence demonstrating that a contextually based emotion regulation therapy is effective at treating depression among youth. In addition to this intervention aimed directly at helping youth to upregulate positive emotions, other successful, evidence-based interventions also target deficits in positive affect and reward (e.g., behavioral activation; Dimidjian et al., 2006) in an effort to treat depression.
The findings of the present research, in which remitted depressed adolescents continue to exhibit deficits in appetitive motivation, suggest that interventions targeting youths’ dysregulated emotion systems, especially BAS, hold promise for reducing this burdensome, recurrent psychiatric disorder. Finally, recent calls for personalized interventions (e.g., Insel, 2009) emphasize the importance of considering and evaluating individual differences that may moderate and enhance the possible effects for interventions aimed at increasing positive emotion and reward for depressed individuals. Recent research has demonstrated a significant interaction between youths’ genetic susceptibility (serotonin transporter promoter, 5-HTTLPR) and positive parenting in the prediction of youths’ level of positive emotion (Hankin et al., 2011). Consistent with the differential susceptibility hypothesis (Belsky & Pluess, 2009), results showed that youth carrying short alleles of 5-HTTLPR exhibited lower positive emotion under environmental conditions of unsupportive parenting and higher positive emotion levels under supportive parenting environmental contexts. Taken together, 5-HTTLPR may be one salient individual difference, and level of positive/supportive parenting may be one important environmental context, that could be used in future intervention development to personalize treatments focused at upregulating positive emotion and enhancing appropriate experience of rewards in efforts to improve positive emotion and behavioral activation interventions to treat adolescent depression.
Acknowledgments
This work was supported, in part, by NIMH grant 5R01 MH077195 (awarded to Benjamin L. Hankin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or National Institutes of Health.
Contributor Information
Benjamin L. Hankin, University of Denver, (ben.hankin@psy.du.edu)
Emily K. Wetter, University of South Carolina, (ekw0003@auburn.edu)
Kate Flory, University of South Carolina, (floryk@gwm.sc.edu; floryk@mailbox.sc.edu).
References
- Allen NB, Badcock PB. The social risk hypothesis of depressed mood: Evolutionary, psychosocial, and neurobiological perspectives. Psychological Bulletin. 2003;129:887–913. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Angold A, Costello EJ, Erkanli AE. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- Barrett LF. Valence is a basic building block of emotional life. Journal of Research in Personality. 2006;40:35–55. [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis-stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward? Hedonic impact, reward learning, or incentive salience? Brain Research Review. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Carver CS. Negative affects deriving from the behavioral approach system. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Clark LA. Temperament as a unifying basis for psychopathology. Journal of Abnormal Psychology. 2005;114:505–521. doi: 10.1037/0021-843X.114.4.505. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion Regulation as a Scientific Construct: Methodological Challenges and Directions for Child Development Research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Cole PM, Luby J, Sullivan MW. Emotions and the development of childhood depression: Bridging the gap. Child Development Perspectives. 2008;2:141–148. doi: 10.1111/j.1750-8606.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- Davidson RJ, Pizzagalli DA, Nitschke JB. The representation and regulation of emotion in depression: Perspectives from affective neuroscience. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. 2nd. New York: Guilford Press; 2008. pp. 218–248. [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Review of Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, Gallop R, McGlinchey JB, Markley DK, Gollan JK, Atkins DC, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. Journal of Consulting and Clinical Psychology. 2006;74:658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC. Temperamental emotionality in preschoolers and parental mood disorders. J Abnorm Psychol. 2005;114:28–37. doi: 10.1037/0021-843X.114.1.28. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations among positive parenting, children's effortful control, and externalizing problems: A three-wave longitudinal study. Child Development. 2005;76:1055–1071. doi: 10.1111/j.1467-8624.2005.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Ancoli S. Facial signs of emotion experience. Journal of Personality and Social Psychology. 1980;39:1125–1134. [Google Scholar]
- Ernst M, Spear LP. Reward systems. In: deHaan M, Gunnar MR, editors. Handbook of Developmental Social Neuroscience. NY: Guilford; 2009. pp. 324–341. [Google Scholar]
- Feng X, Keenan K, Hipwell AE, Henneberger AK, Rischall MS, Butch J, et al. Longitudinal associations between emotion regulation and depression in preadolescent girls: moderation by caregiving environment. Dev Psychol. 2009;45:798–808. doi: 10.1037/a0014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE. Where’s the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry. 2009;66:199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research review: Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KA. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10 year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–141. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness JL, Young JF, Abela JRZ, Smolen A, Ormel J, Oldehinkel AJ. Differential susceptibility in youth: Evidence that 5-HTTLPR x positive parenting is associated with positive affect “for better and worse”. Translational Psychiatry. 2011;1:1–7. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, Roiser J, Knutson B, Charney DS, Drevets WC. Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biological Psychiatry. 2009;66:201–205. doi: 10.1016/j.biopsych.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreases responsiveness to reward in depression. Cognition and Emotion. 2000;14:711–724. [Google Scholar]
- Higgins ET, Shah J, Friedman R. Emotional responses to goal attainment: Strength of regulatory focus as moderator. Journal of Personality and Social Psychology. 1997;72:515–525. doi: 10.1037//0022-3514.72.3.515. [DOI] [PubMed] [Google Scholar]
- Insel TR. Translating scientific opportunity into public health impact. Archives of General Psychiatry. 2009;66:128–133. doi: 10.1001/archgenpsychiatry.2008.540. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL), initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keltner D, Ekman P. Facial expression of emotion. In: Lewis M, Haviland-Jones JM, editors. Handbook of Emotions. 2nd. New York: Guilford; 2004. pp. 236–249. [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacological Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kovacs M, Joorman J, Gotlib IH. Emotion (dys)régulation and links to dépressive disorder. Child Development Perspectives. 2008;2:149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Sherrill J, George CJ, Pollock M, Tumuluru RV, Ho V. Contextual emotion-regulation therapy for childhood depression: Description and pilot testing of a new intervention. Journal of the American Academy of Child Adolescent Psychiatry. 2006;45:892–903. doi: 10.1097/01.chi.0000222878.74162.5a. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Moffitt TE, Caspi A, editors. Causes of conduct disorder in juvenile delinquency. New York: Guilford Press; 2003. [Google Scholar]
- Lewinsohn PM, Goltib IH. Beckman EE, Leber WR. Handbook of depression. 2nd. New York: Guilford Press; 1995. Behavioral theory and treatment of depression; pp. 352–375. [Google Scholar]
- Lonigan CJ, Phillips BM, Hooe ES. Relations of positive and negative affectivity to anxiety and depression in children: Evidence from a latent variable longitudinal study. Journal of Consulting and Clinical Psychology. 2003;71:465–481. doi: 10.1037/0022-006x.71.3.465. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Robinson MD. Measures of Emotion: A Review. Cognition and Emotion. 2009;23:209–237. doi: 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland BR, Klein DK. Emotional reactivity in depression: Diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depression and Anxiety. 2009;26:117–122. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, Silva PA. Sex differences in antisocial behavior: Conduct disorder, delinquency, and violence in the Dunedin longitudinal study. New York: Cambridge University Press; 2001. [Google Scholar]
- Pine DS. A social neuroscience approach to adolescent depression. In: deHaan M, Gunnar MR, editors. Handbook of Developmental Social Neuroscience. NY: Guilford; 2009. pp. 399–418. [Google Scholar]
- Pinto-Meza A, Caseras X, Soler J, Puigdemont D, Perez V, Torrubia R. Behavioural inhibition and behavioural activation systems in current and recovered major depression participants. Personality and Individual Differences. 2006;40:215–226. [Google Scholar]
- Rutter M, Kim-Cohen J, Maughan B. Continuities and discontinuities in psychopathology between childhood and adult life. Journal of Child Psychology and Psychiatry. 2006;47:276–295. doi: 10.1111/j.1469-7610.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- Shah J, Higgins ET. Regulatory concerns and appraisal efficiency: The general impact of promotion and prevention. Journal of Personality and Social Psychology. 2001;80:693–705. [PubMed] [Google Scholar]
- Sheeber LB, Allen NB, Leve C, Davis B, Shortt JW, Katz LF. Dynamics of affective experience and behavior in depressed adolescents. J Child Psychol Psychiatry. 2009;50:1419–1427. doi: 10.1111/j.1469-7610.2009.02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon ST, Nelson-Gray RO. Sensitivity to aversive events in depression: Antecedents, concomitant, or consequent? Journal of Psychopathology and Behavioral Assessment. 1992;14:225–246. [Google Scholar]
- Sloan DM, Strauss ME, Quirk SW, Sajatovic M. Subjective and expressive emotional responses in depression. Journal of Affective Disorders. 1997;46:135–141. doi: 10.1016/s0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. Journal of Abnormal Psychology. 2001;110:488–493. doi: 10.1037//0021-843x.110.3.488. [DOI] [PubMed] [Google Scholar]
- Tackett JL, Krueger RK. Interpreting personality as a vulnerability for psychopathology: A developmental approach to the personality-psychopathology relationship. In: Hankin BL, Abela JRZ, editors. Development of Psychopathology: A Vulnerability-Stress Perspective. CA US: Sage; New York: Springer; 2005. pp. 199–214. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98:219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]