Abstract
Background
The rhesus enteric caliciviruses (ReCVs) were recently described.
Methods
Prevalence of ReCV antibodies was tested in six species of captive non-human primates.
Results
High ReCV seroprevalence was revealed in rhesus and cynomolgus macaques.
Conclusions
High rates of ReCV seroprevalence and diarrhea in juvenile macaques suggest that ReCVs may play a role in morbidity.
Keywords: rhesus, calicivirus, enteritis, diarrhea
Introduction
Caliciviruses (CV) are small, non-enveloped, icosahedral viruses with a ~7.5–8.5 kb positive sense, single stranded, polyadenylated RNA genome. Noroviruses (NoVs) are important members of Caliciviridae family that cause acute gastroenteritis in humans, including 80–90% of nonbacterial gastroenteritis outbreaks and >50% of all food-related outbreaks. No robust in vivo or in vitro model exists to study human NoVs. Our group recently discovered and characterized a new group of enteric CVs of rhesus monkey (Macaca mulatta) host origin with the proposed name Recovirus [3, 5, 13]. ReCVs are closely related to human NoVs in their biology and in contrast to human NoVs, ReCVs can be grown in cell culture. At least four ReCV genotypes within two genogroups (G1.1–3 and G2.1) with phylogenetic distances comparable to those of human NoV genotypes have been described (5). Recent data also suggest the existence of ReCV serotypes (unpublished data). It was demonstrated that, in susceptible macaques, ReCVs induce symptomatic infection characterized by diarrhea, virus shedding in stools, fever and inflammation of the small intestine [10]. Many features of ReCV infection still remain to be elucidated. Moreover, there are underlying aspects of enteric CV infection and epidemiology that are understudied in all primate species including humans.
The main objective of this study was to estimate the prevalence of ReCV infections in six species of captive non-human primates (NHP) at three different U.S. National Primate Research Centers (NPRC). In addition, epidemiological analysis concerning the contribution of dehydrating diarrhea to overall mortality of rhesus macaques at the Tulane NPRC was performed, in order to estimate the impact of diarrhea-associated disease.
Materials and Methods
Serum samples and virus-neutralization (VN) test
VN test was used to measure ReCV-specific antibodies in 180 serum samples collected in 2011 from six different species of NHPs [Macaca mulatta (Rhesus macaque), Macaca fascicularis (Cynomolgus or crab-eating monkey), Macaca nemestrina (pig-tailed macaque), Callithrix jacchus (common marmoset), Papio hamadryas (Hamadrayas baboon) and Pan troglodytes (common chimpanzee)] from Tulane (TNPRC), New England (NENPRC) and Southwest Foundation (SNPRC) as indicated (Figure 1). Only samples from clinically healthy, specific pathogen-free [SPF: simian immunodeficiency virus (SIV), simian retrovirus (SRV), simian T cell leukemia virus (STLV), herpes B-virus (Macacine herpesvirus 1 or B virus) and Mycobacterium tuberculosis] animals were used. Approval for veterinary procedures in this study had been obtained from the Tulane University Animal Care and Use Committee. Animals were under the full care of veterinarians with the National Standards incorporated in the Guide to the Care and Use of Laboratory Animals (NIH) 78-23 (revised 1996). Samples were tested at a single dilution (1:20) for the presence of anti-Tulane virus (ReCV serotype 1, TV) antibodies in a cytopathic effect-based assay as described in detail elsewhere [4].
Fig. 1.
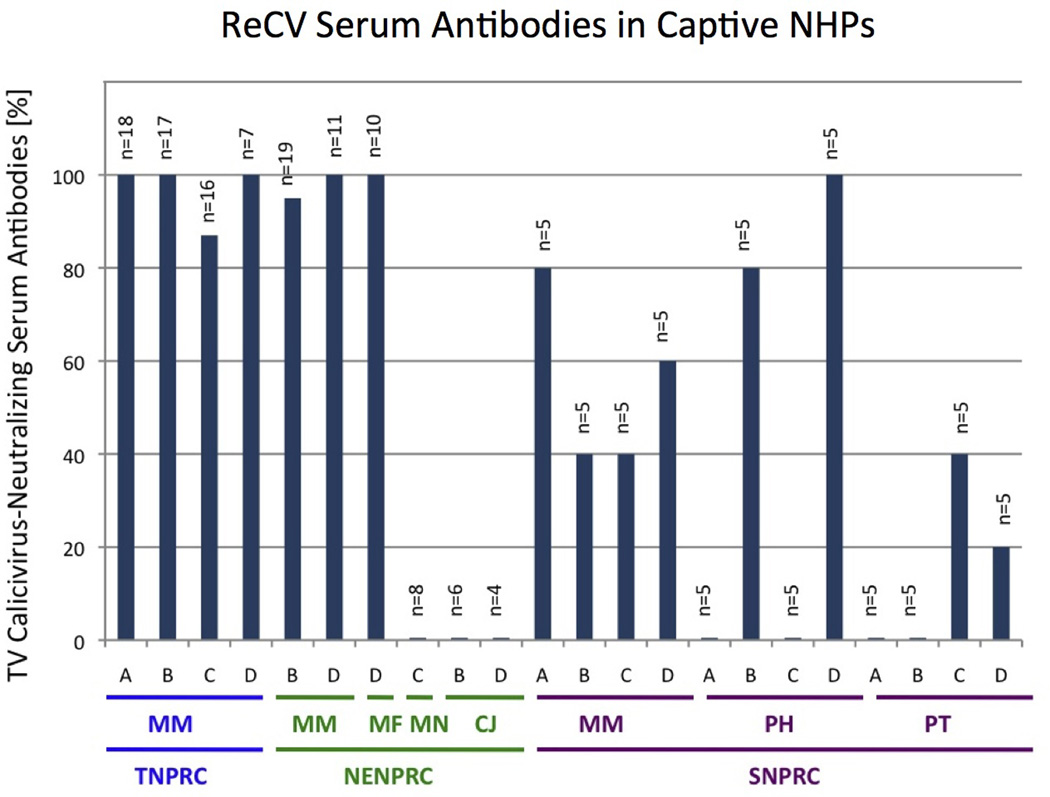
Virus-neutralizing antibody test was used to determine the proportion (%) of ReCV-positive serum samples in six different species of NHPs housed at three different NPRCs: Tulane (TNPRC), New England (NENPRC) and South-West Foundation (SNPRC). Serum samples were tested at a single dilution (1:20) for their ability to neutralize the 100 TCID50 of the Tulane virus, serotype 1. Capital letter acronyms below X-axis indicate the species: MM = Macaca mulatta (Rhesus macaque), MF = Macaca fascicularis (Cynomolgus or crab-eating monkey), MN = Macaca nemestrina (Pig-tailed macaque), CJ = Callithrix jacchus (common marmoset), PH = Papio hamadryas (Hamadrayas baboon) and PT = Pan troglodytes (common chimpanzee). Capital single letters stand for different categories of animals: A = juvenile (<3 years of age) female, B = adult female, C = juvenile male and D = adult male.
Epidemiological data
Contribution of dehydrating diarrhea to overall mortality (%) of 3,675 Indian and Chinese subspecies of rhesus macaques was determined based on epidemiological records from the Tulane NPRC database. Presented data correspond to a seven-year period between 2002 and 2009. Animals were subdivided based on viral screening for four viruses: Conventional animals are SIV and SRV negative but may be positive for STLV and/or B virus while SPF animals are negative for all four viruses (Table 1). Each category was further subdivided into nine age groups.
Table 1.
Mortality (%) due to dehydrating diarrhea in conventional and SPF colony macaques
| Age [Years] |
M. mulatta, Indian Conventional n = 1,144 |
M. mulatta, Indian SPF n = 1,558 |
M. mulatta, Chinese Conventional n = 190 |
M. mulatta, Chinese SPF n = 138 |
|---|---|---|---|---|
| 0 – 0.99 | 2.4 | 4.8 | 1.7 | 6.8 |
| 1 – 1.99 | 3.4 | 4 | 3.5 | 2.4 |
| 2 – 3.99 | 2 | 2.9 | 1.1 | 1.8 |
| 4 – 5.99 | 1.2 | 1.2 | 0.9 | 1.3 |
| 6 – 7.99 | 0.9 | 0.6 | 3.2 | 1.8 |
| 8 – 9.99 | 0.7 | 0.8 | 1.7 | NA |
| 10 – 11.99 | 0.7 | 3.8 | NA | NA |
| 12 – 13.99 | 1.9 | NA | NA | NA |
| 14 – 15.99 | 2.9 | NA | NA | NA |
NA – not applicable; no animals of this age group were available for sampling.
Results
Data collected between 2002–2009 indicate that highest mortality due to diarrhea occurs in juvenile rhesus macaques between 0–2 years of age regardless of subspecies affiliation or animal category (Table 1). Although other causes of mortality in macaques were not investigated in this study, presented data indicate that diarrhea is one of the leading causes of mortality in captive juvenile macaques.
ReCV seroprevalence data generated with serum samples from rhesus macaques as well as from five other nonhuman primate species housed at three separate NPRCs revealed up to 100% seroconversion in rhesus and cynomolgus macaques, while pig-tailed macaques, common marmosets, baboons and chimpanzees showed only moderate or low seroconversion (Figure 1). In addition to species-related differences, a trend for differences in ReCV seroconversion between TNPRC and NENPRC versus SNPRC rhesus macaques, as well as age- (baboons) and sex-related (chimpanzees) differences, was observed. Since Tulane virus represents only one ReCV serotype (prototype strain), the prevalence of other ReCV strains may be different. Due to the relatively low number of samples analyzed for some of the species or categories (Figure 1), statistical analysis of observed differences was not performed.
Discussion
While several infectious agents and syndromes have been suggested to cause diarrhea and gastroenteritis in captive NHPs [1–3, 6–9, 12], the high incidence of diarrhea in juvenile rhesus macaques suggests that there might be a mechanism linked to transition of maternal to active immunity with corresponding onset of enteric viral infections, as seen in human infants [11]. Since ReCVs represent one of the most rapidly emerging [3–5] and still understudied group of enteric viruses in captive NHPs, the main objective of this study was to determine how prevalent serum antibodies against ReCVs are in different species of NHPs and if the prevalence varies at different NPRCs, or among species, ages and sexes. The secondary objective was to illustrate the contribution of diarrhea to the overall mortality of captive rhesus macaques. It is hypothesized, but not yet proven, that a significant portion of diarrhea-associated morbidity and mortality is linked to ReCV infections. Our group recently completed a study which suggests that ReCVs induce symptomatic disease, including diarrhea, in seronegative macaques [10]. Therefore, it is important to determine at which age ReCV infection first takes place in these animals.
The approximately equal incidence of diarrhea-associated mortality found in conventional and SPF macaques likely reflects that current preventive measures at NPRCs do not target enteric pathogens. Instead, the focus of SPF breeding programs has been on important blood-borne pathogens such as SIV, SRV, STLV and B virus. However, considering that the “diarrhea-associated loss of production” impact was estimated at ~$700,000 for the TNPRC SPF rhesus macaque colony in 2010 alone, it is becoming evident that strategies that would help to reduce such losses, not only at Tulane, but also on a national scale would be of great economic benefit. In summary, high ReCV seroprevalence rates together with the high diarrhea rate found in this study in juvenile rhesus macaques plus the capability of ReCVs to induce symptomatic infection in seronegative animals suggest that new hypothesis-driven studies should be pursued in order to address the economically relevant issues of disease control and prevention in NHP research colonies.
Acknowledgements
We thank Amanda Tardo, Stephanie Feely and Brittney Fey for their technical assistance. We also thank Drs. Thomas M. Folks and Keith Mansfield for their help and support. Grants from the National Institutes of Health P51RR000164, U24RR018111, P01HD013021 and the Infectious Disease Scholar Fund of CCHMC were used to support this study.
References
- 1.Bethune MT, Borda JT, Ribka E, Liu M, Falkenstein K, Jandacek RJ, Doxiadis GGM, Gray GM, Khosla C, Sestak K. A non-human primate model for gluten sensitivity. PLoS ONE. 2008;3:e1614. doi: 10.1371/journal.pone.0001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dassanayake RP, Zhou Y, Hinkley S, Stryker CJ, Plauche G, Borda JT, Sestak K, Duhamel GE. Characterization of cytolethal distending toxin of Campylobacter species isolated from captive macaque monkeys. J Clin Microbiol. 2005;43:641–649. doi: 10.1128/JCM.43.2.641-649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farkas T, Sestak K, Wei C, Jiang X. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J Virol. 2008;82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farkas T, Dufour J, Jiang X, Sestak K. Detection of norovirus-, sapovirus- and rhesus enteric calicivirus-specific antibodies in captive juvenile macaques. J Gen Virol. 2010;91:734–738. doi: 10.1099/vir.0.015263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkas T, Cross RW, Hargitt E, Lerche NW, Morrow AL, Sestak K. Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J Virol. 2010;84:8617–8625. doi: 10.1128/JVI.00630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang B, McClure HM, Fankhauser RL, Monroe SS, Glass RI. Prevalence of rotavirus and norovirus antibodies in non-human primates. J Med Primatol. 2004;33:30–33. doi: 10.1111/j.1600-0684.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 7.McNeal MM, Sestak K, Choi A, Basu M, Cole MJ, Aye PP, Bohm RP, Ward RL. Development of a rotavirus-shedding model in rhesus macaques, using a homologous wild-type rotavirus of a new P genotype. J Virol. 2005;79:944–954. doi: 10.1128/JVI.79.2.944-954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F, Didier ES, Didier PJ, Plauche G, Bohm RP, Aye PP, Alexa P, Ward RL, Lackner AA. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect & Immun. 2003;71:4079–4086. doi: 10.1128/IAI.71.7.4079-4086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sestak K, McNeal MM, Choi A, Cole MJ, Ramesh G, Alvarez X, Aye PP, Bohm RP, Mohamadzadeh M, Ward RL. Defining T-cell-mediated immune responses in rotavirus-infected juvenile rhesus macaques. J Virol. 2004;78:10258–10264. doi: 10.1128/JVI.78.19.10258-10264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sestak K, Feely S, Fey B, Dufour J, Hargitt E, Alvarez X, Pahar B, Gregoricus N, Vinje J, Farkas T. Experimental inoculation of juvenile rhesus macaques with primate enteric caliciviruses. PLoS ONE. 2012 doi: 10.1371/journal.pone.0037973. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss JH, Strauss EG. Viruses and Human Disease. San Diego: Academic Press; 2002. [Google Scholar]
- 12.Wang Y, Tu X, Humphrey C, McClure H, Jiang X, Qin C, Glass RI, Jiang B. Detection of viral agents in fecal specimens of monkeys with diarrhea. J Med Primatol. 2007;36:101–107. doi: 10.1111/j.1600-0684.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei C, Farkas T, Sestak K, Jiang X. Recovery of infectious virus by transfection of in vitro-generated RNA from tulane calicivirus cDNA. J Virol. 2008;82:11429–11436. doi: 10.1128/JVI.00696-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


