Abstract
Most human exposure to mercury (Hg) in the United States is from consuming marine fish and shellfish. The Gulf of Maine is a complex marine ecosystem comprised of twelve physioregions, including the Bay of Fundy, coastal shelf areas and deeper basins that contain highly productive fishing grounds. Here we review available data on spatial and temporal Hg trends to better understand the drivers of human and biological exposures. Atmospheric Hg deposition from U.S. and Canadian sources has declined since the mid-1990s in concert with emissions reductions but deposition from global sources has increased. Oceanographic circulation is the dominant source of total Hg inputs to the entire Gulf of Maine region (59%), followed by atmospheric deposition (28%), wastewater/industrial sources (8%), and rivers (5%). Resuspension of sediments increases MeHg inputs to overlying waters raising concerns about benthic trawling activities in shelf regions. In the near coastal areas, elevated sediment and mussel Hg levels are co-located in urban embayments and near large historical point sources. Temporal patterns in sentinel species (mussels and birds) have in some cases declined in response to localized point source mercury reductions but overall Hg trends do not show consistent declines. For example, levels of Hg have either declined or remained stable in eggs from four seabird species collected in the Bay of Fundy since 1972. Quantitatively linking Hg exposures from fish harvested from the Gulf of Maine to human health risks is challenging at this time because no data are available on the geographic origin of seafood consumed by coastal residents. In addition, there is virtually no information on Hg levels in commercial species for offshore regions of the Gulf of Maine where some of the most productive fisheries are located. Both of these data gaps should be priorities for future research.
Keywords: Gulf of Maine, North Atlantic, methylmercury, fish, risk
1.0 Introduction
High levels of methylmercury (MeHg) exposure through fish consumption cause a variety of adverse neurological and reproductive effects in humans and wildlife (Clarkson and Magos, 2006; Mahaffey et al., 2011; Scheuhammer et al., 2007). Coastal ecosystems provide foraging habitat for a variety of commercial and recreationally harvested fish species and through this pathway contribute substantially to human exposures to mercury (Hg). The Bay of Fundy/Gulf of Maine region, located on the east coasts of Canada and the United States, supports what has historically been some of the world’s most productive fisheries and provides important habitat for abundant whales, porpoises, seals and many bird species (Hildebrand et al., 1997; Sinclair et al., 1991; Thompson, 2010). Like many coastal regions of the United States and Canada, high Hg levels have been documented in fish and wildlife from this area since measurements began in the 1970s (Gaskin et al., 1972; Gaskin et al., 1979; Gaskin et al., 1973). Although anthropogenic Hg emissions within North America have declined substantially and many large point sources eliminated, the effectiveness of these and other management actions at reducing risks to human and ecological health is still poorly understood. Here we use data from Bay of Fundy/Gulf of Maine ecosystem to examine controls on Hg concentrations across a range of physical environments, industrial development and population densities. We use our synthesis to comment on the apparent effectiveness of various past and future management efforts for Hg and coastal water quality and to highlight gaps in monitoring data and research.
A key question often asked by environmental managers is how long will it take for an ecosystem to respond to changes in Hg inputs from human sources. Hg emissions have declined by over 90% in Canada since the 1970s and by over 60% in the United States since 1990 (Sunderland and Chmura, 2000a; 2000b; U.S. EPA, 2005). Coastal zones represent the transition between terrestrial environments and the ocean, and are often heavily influenced by watershed processes and human activities. Prior studies of freshwater ecosystems have shown that fish Hg levels often exhibit a rapid decline in response to decreased atmospheric deposition followed by a second, more gradual decline in response to reduced inputs from watershed sources (Harris et al., 2007; Knightes et al., 2009). Understanding the relative importance of direct atmospheric deposition, watershed inputs and other Hg sources to coastal ecosystems therefore provides insights on the expected temporal dynamics of Hg concentrations.
Here we review available information on total Hg and MeHg inputs to the Gulf of Maine from direct atmospheric deposition, wastewater and industry, rivers, and oceanographic influences. Comparing input sources to measured concentrations in water, sediments and biota of different physioregions reveals observable associations between concentrations and inputs, and helps to identify the likely drivers of human and biological Hg exposures in the different regions of the Gulf. We use this analysis to highlight key uncertainties for future research initiatives and conclude with recommendations for future management aimed at further reducing Hg exposures of both humans and the marine ecosystem.
2.0 Major Physioregions of the Gulf of Maine
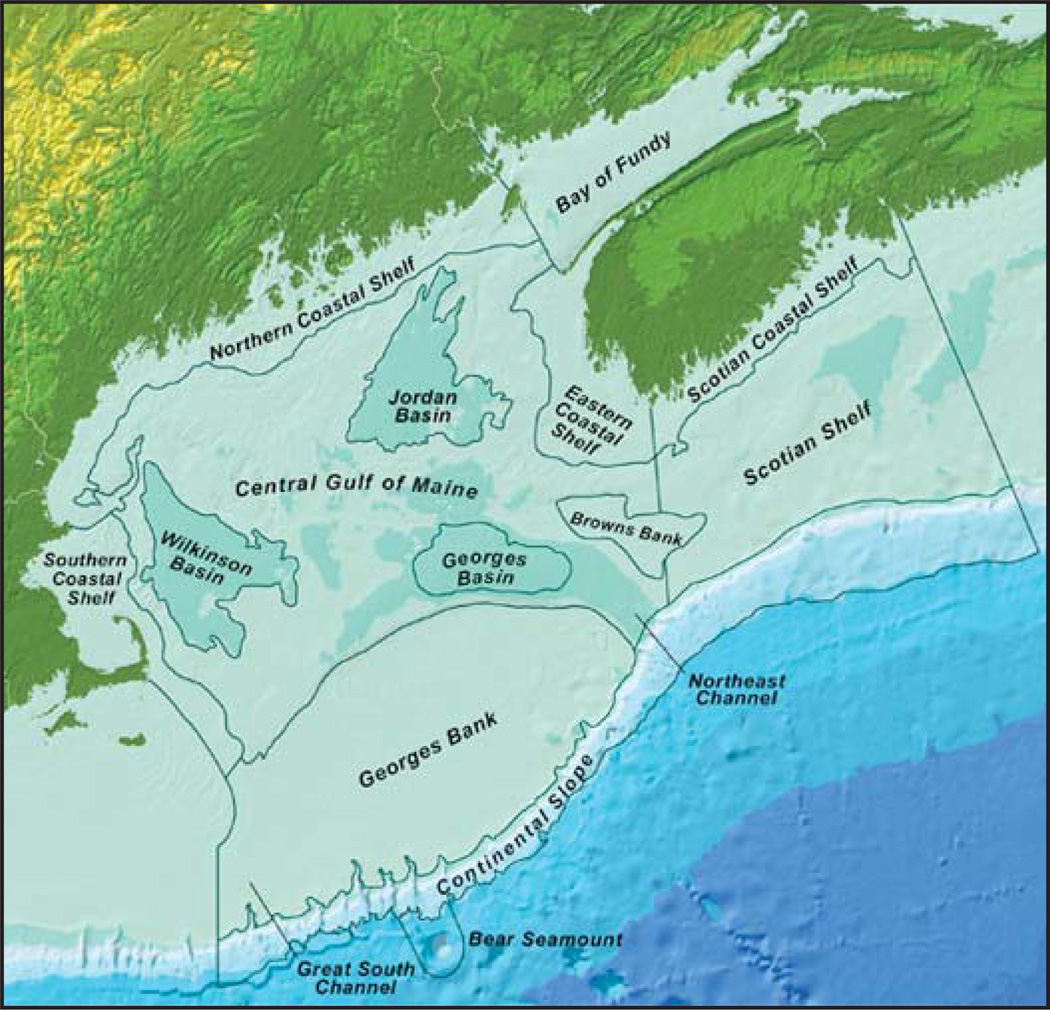
The Gulf of Maine is comprised of twelve major physioregions bounded inland by the Canadian provinces Nova Scotia and New Brunswick and the states of Maine, New Hampshire and Massachusetts, USA. It is frequently referred to as a “sea within a sea” because the only exchange with the North Atlantic is through the Northeast Channel and the mid-shelf channel entering the Scotian Shelf (Figure 1). Around 60% of the surface area and volume of the Gulf of Maine is found offshore in the central Gulf of Maine and Georges Bank (Table 1). Offshore regions do not receive any direct freshwater or point source Hg inputs from the watershed although freshwater inflows do exert a major influence on near surface waters and circulation (Geyer et al., 2004; Hetland and Signell, 2005). During spring freshet in particular, the St. John River and rivers along the Northern Coastal Shelf (Androscoggin, Penobscot, Merrimack, and Kennebec Rivers) contribute substantially to the upper 40 m of the water column and maintain the counterclockwise circulation of the Gulf (Xue et al., 2000).
Figure 1.
Map of study region (From: http://www.gommea.org/about-the-gulf/physical-characteristics/physioregions/). Note that the Bay of Fundy is part of the Gulf of Maine ecosystem.
Table 1.
Attributes of the major sub-regions of the Gulf of Maine shown in Figure 1. n/a = Not Applicable; WWTP = wastewater treatment plant. Surface areas and average water depths data are taken from the Gulf of Maine Education Association [Available: http://www.gommea.org/about-the-gulf/physical-characteristics/physioregions/].
| Region | Surface Area in km2 (% of total area) |
Volume in 105 m3 (% of total volume) |
Major Rivers (% of total discharge) |
aTop Ten Hg Effluent Point Sources (kg year−1) |
|---|---|---|---|---|
| Gulf of Maine | 170,860 | 217 | cAll rivers: 8.81 × 1010 m3 year−1 | Point Sources Total: 586 (ca. 1991) |
| Coastal Regions | ||||
| Bay of Fundy | 12,810 (7.5%) | 9.61 (4.4%) | St. John (33%) St. Croix (3%) |
Moncton Sewerage Commission (27) |
| Eastern Coastal Shelf | 7,943 (4.7%) | 3.97 (1.8%) | See footnoteb | Yarmouth Sewage Treatment Plant (9) |
| Northern Coastal Shelf | 14,760 (8.6%) | 7.38 (3.4%) | Penobscot (12%) Kennebec + Androscoggin (13%) Saco (3%) Merrimack (6%) |
WWTPs: Kennebec Sanitation District (9); Greater Lawrence (27); Lynn (23); Haverhill (9) |
| Southern Coastal Shelf | 8,244 (4.8%) | 4.12 (1.9%) | See footnoteb | WWTPs: Deer Island (105); South Essex (41); Manchester (14) |
| Offshore Regions | ||||
| Browns Bank | 2,953 (1.7%) | 2.21 (1.0%) | n/a | n/a |
| Central Gulf of Maine | 59,040 (34.6%) | 88.6 (40.8%) | n/a | n/a |
| Georges Bank | 41,930 (24.6%) | 42.4 (19.5%) | n/a | n/a |
| Great South Channel | 5,198 (3.0%) | 3.90 (1.8%) | n/a | n/a |
| Georges Basin | 4,110 (2.4%) | 15.2 (7.0%) | n/a | n/a |
| Jordan Basin | 6,695 (2.4%) | 20.8 (9.6%) | n/a | n/a |
| Wilkinson Basin | 7,078 (4.1%) | 19.1 (8.8%) | n/a | n/a |
From NOAA (NOAA, 1994);
No rivers with flow > 3% total freshwater discharge.
Total discharge from river flows into the Gulf of Maine (McAdie, 1995).
The most productive finfish fisheries and biological foraging grounds of the Gulf of Maine are found in offshore environments (Thompson, 2010), although the lucrative lobster fisheries are predominantly inshore in Maine and Nova Scotia. Both surface and deeper water in the offshore regions are heavily influenced by surface inflows from the Scotian Shelf and deeper, nutrient rich flows through the Northeast Channel (Sutcliffe et al., 1976; Townsend et al., 2010; Xue et al., 2000). The Scotian Shelf and Northeast Channel inflows are, in turn, influenced by a combination of relatively fresh Labrador Sea Water and freshwater discharges from the St. Lawrence River Basin (Sutcliffe et al., 1976; Townsend et al., 2010). Primary productivity is elevated in the offshore Georges Bank region due to upwelling of nutrient rich waters reaching 470 g C m−2 year−1 compared to an average of 290 g C m−2 year−1 for the rest of the region (Hameedi et al., 2002; Smith et al., 1984).
The nearshore coastal regions of the Gulf of Maine include the Bay of Fundy and the Eastern, Northern, and Southern Coastal Shelves (Figure 1). Water circulation patterns in these regions are highly influenced by freshwater discharges from Gulf of Maine watershed (Geyer et al., 2004; Xue et al., 2000). For example, large freshwater discharges into the Bay of Fundy from the St. John River travel south in a coastal current in the eastern Gulf of Maine, which is eventually deflected offshore near the entrance to Penobscot Bay (Geyer et al., 2004). The dominant coastal current (Western Maine Coastal Current) flows southwestward around the perimeter of the Gulf of Maine (Geyer et al., 2004).
In the nearshore coastal environment, Hg is input directly through sewage and industrial effluent outfalls along the coast as well as from point watershed sources along rivers and streams. These terrestrial sources impact chemical dynamics of the inshore more than the offshore areas. A large gradient in human population density across these coastal areas also influences the extent of anthropogenic Hg inputs. Most urban development is concentrated around the city of Boston in the Southern Coastal Shelf region (Thompson, 2010).
Hg dynamics are also influenced by large-scale patterns in sediment transport and deposition. Fader et al. (1977) estimated that significant sediment accumulation occurs in less than 30% of the surface area of the Gulf of Maine. Deposition regions with elevated clay-silt content and higher levels of organic matter tend to have higher Hg concentrations (Loring, 1979; 1982). Surface sediments of offshore regions are most commonly defined as gravel, sand and mud (Kelley and Belknap, 1991). Concentrations of Hg in sandy sediments of these dynamic erosional areas of the Bay of Fundy tend to be near background concentrations in geological materials (~15–20 ppb) (Loring, 1979; 1982).
3.0 Hg Inputs to the Gulf of Maine
The following sections summarize best-available information on Hg inputs to the Gulf of Maine. Tables 1 and 2 summarize inputs to the different physioregions of the Gulf of Maine estimated by scaling the measurements described below to the water surface areas (atmospheric deposition) or water flow rates (rivers, wastewater, oceanographic exchange).
Table 2.
Hg inputs to the Gulf of Maine. All values are for total Hg in kg/year unless noted. Ranges are based on standard deviations of reported Hg concentrations where available or upper and lower bounds for inputs based on different data sources that are described in the main text. n/a = insufficient data available to characterize mass flows. NDI = no direct inputs.
| Region | Direct Atmospheric |
Rivers | Point Sources | Seawater Inflow |
|---|---|---|---|---|
| Gulf of Maine: Total Hg | 1964 (1708–2049) | 385 (94–1289) | 586 (8–586) | 4178 (2873–5483) |
| Gulf of Maine: MeHga | 17 (5–29) | 19 (9–31) | 29 (0.4–29) | 1022 (635–1410) |
| Coastal Regions: | ||||
| Bay of Fundy | 147 | >122 | 109 | 1046 |
| Eastern Coastal Shelf | 91 | n/a | 9 | n/a |
| Northern Coastal Shelf | 170 | >119 | 153 | n/a |
| Southern Coastal Shelf | 95 | n/a | 214 | n/a |
| Offshore Regions: | ||||
| Browns Bank | 34 | NDI | NDI | n/a |
| Central Gulf of Maine | 679 | NDI | NDI | n/a |
| Georges Bank | 482 | NDI | NDI | n/a |
| Great South Channel | 60 | NDI | NDI | n/a |
| Georges Channel | 47 | NDI | NDI | n/a |
| Jordan Basin | 77 | NDI | NDI | n/a |
| Wilkinson Basin | 81 | NDI | NDI | n/a |
Insufficient data were available to characterize the magnitude of MeHg exchange for individual physioregions of the Gulf of Maine.
3.1 Atmospheric Deposition
Most modern atmospheric Hg emissions sources release predominantly elemental Hg (Hg0) (Streets et al., 2011). The long atmospheric lifetime of Hg0 means that atmospheric Hg deposition reflects the combined influences of local, regional, and global anthropogenic sources, in addition to recycled and natural Hg (Corbitt et al., 2011). Waste incinerators, coal-fired electricity generation, and metal smelters are the main atmospheric Hg emission sources in Eastern Canada and the Northeastern U.S. (NEG-ECP, 2003; Sunderland et al., 2008). Coal-fired power plants are the largest anthropogenic Hg sources in the U.S., Canada and globally and accordingly account for largest component of anthropogenic Hg deposition (Corbitt et al., 2011; Pacyna et al., 2010; Streets et al., 2011). Sunderland et al. (2008) showed that anthropogenic Hg emissions in the U.S. and Canada resulted in between 28–33% of total deposition to the Gulf of Maine in the early 2000s, with the rest from global anthropogenic (41–53%) and natural (14–32%) sources.
Atmospheric Hg enters ecosystems directly through wet and dry deposition processes. Measurement techniques for dry deposition are currently under development (Lai et al., 2011) and thus monitoring programs have typically focused on collecting wet deposition measurements. Data on wet deposition for the Gulf of Maine region have been available from the National Atmospheric Deposition Program’s Mercury Deposition Network (MDN) since 1996. Two MDN sites are still active (Kejimkujik, Nova Scotia, Canada, NS-01 and Hancock County, Maine, USA, ME-98), while the site in St. Andrews, New Brunswick, Canada (NB-02) ceased operation in 2003. In 2003, reported wet deposition at these stations ranged from 6.0 to 7.2 ug m−2 year−1. In 2009, only the stations in Maine and Nova Scotia were still operating and the measured wet deposition rates were 7.0 and 7.4 ug m−2 year−1, respectively (MDN, 2011). Ritchie et al. (2006) measured Hg deposited in fog in the Bay of Fundy and found that although this deposition pathway was not a major contributor to near coastal areas (0.4–7.5% of wet deposition), the contribution was much greater for Grand Manan Island (31–74% of wet deposition).
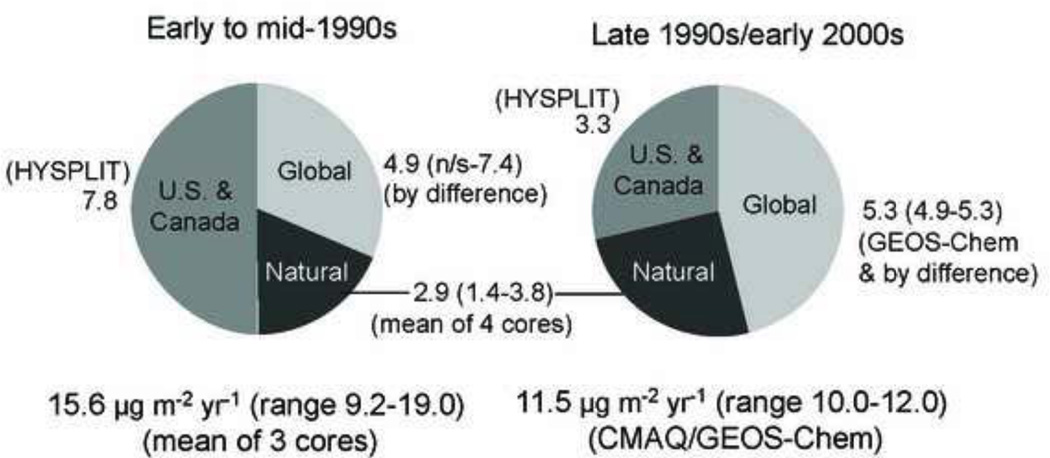
To estimate total (wet + dry) atmospheric Hg deposition in the Gulf of Maine region, Sunderland et al. (2008) combined the MDN measurements with modeling data (spanning the years between 1990 and 2003) and sediment core data on past atmospheric deposition (Figure 2). Dry deposition was modeled with a standard resistance-in-series scheme based on local surface type and turbulence (Wesely, 1989). The GEOS-Chem modeling data for the Gulf of Maine reported in Sunderland et al. (2008) also included uptake of Hg0 by vegetation, as described in Selin et al. (2008).
Figure 2.
Changes in atmospheric deposition rates in the Bay of Fundy/Gulf of Maine regions inferred from a combination of modeling and sediment core data. From: Sunderland et al. (2008).
Across all archives and modeling results, contemporary deposition rates ranged between 10.0 and 12.0 ug m−2 year−1 (Sunderland et al., 2008). Sunderland et al. (2010) estimated MeHg deposition rates of 0.10±0.07 ug m−2 year−1 for the Gulf of Maine region using Environment Canada data on MeHg in precipitation at several sites in Maritime Canada (Dalziel et al., 2010). Total atmospheric inputs to the Gulf of Maine based on these deposition rates are 1964 kg Hg/year (range 1708–2049) and 17 kg MeHg/year (range 5–29).
3.2 Rivers
Export of fine-grained sediments from rivers to estuaries and continental shelf areas of the Gulf of Maine may be an important vector for Hg transport, extending the influence of riverine Hg on this system. Due to the relatively small area of the Gulf’s estuaries compared to the rivers draining into these estuaries, most fine-grained sediment is directly transported from the rivers into major shelf basins (Stumpf and Goldschmidt, 1992). This is especially noteworthy during high flow events. For example, Stumpf and Goldschmidt (1992) showed a sediment plume from the Kennebec-Androscoggin river system deposited 105 metric tons of fine-grained sediment 30 km from the coast onto the Gulf’s continental shelf after a storm in 1987. Bothner et al. (1998) reported that 50% to 90% of the fine-grained suspended sediments that are transported across Boston Harbor are exported to Massachusetts Bay. They proposed that sediment resuspension due to storms or benthic organisms leads to transport outside of Boston Harbor into Massachusetts Bay even in areas where net sediment accumulation is expected.
Data on total Hg and MeHg concentrations in major tributaries flowing into the Gulf of Maine are limited other than the work by Dalziel et al. (2010) for the Bay of Fundy. Strong seasonal dynamics in river flows means a large number of samples are needed to accurately estimate Hg loads (Quemerais et al., 1999). Annually averaged concentrations of total and methyl Hg in unfiltered river water (weighted by concentrations sampled seasonally and associated discharges) were 4.37 ng Hg/L (range 1.07–14.62) and 0.22 ng MeHg/L (range 0.10–0.35) between 1998–2002 (Dalzeil et al., 1998; 2010). Concentrations of suspended particulate matter in these rivers averaged 3.43±1.03 mg/L over the same time period. Sunderland et al. (2010) used these measured solids concentrations and partition coefficients for inorganic and MeHg to calculate the dissolved fraction of Hg and MeHg river load entering the estuarine region to be 51% for total Hg (annual average 2.23 ng Hg/L) and >90% for MeHg (annual average 0.20 ng MeHg/L). Penobscot river filtered total Hg (0.75–2.6 ng L−1) and MeHg (0.02–0.31 ng L−1) concentrations measured between 2006–2007 are in the same range as the Bay of Fundy data, and typical of an unimpacted site despite the presence of legacy contamination from a chlor-alkali facility (Bodaly et al., 2008).
To estimate inputs of Hg and MeHg from rivers, we multiplied the total freshwater inputs flowing into Gulf of Maine from major tributaries (Table 1) by the average concentrations for total Hg and MeHg (unfiltered) reported by Dalziel et al. (2010). Results suggest total inputs of Hg and MeHg are 385 kg/year (range 94–1289) and 19 kg/year (9–31), respectively.
3.3 Wastewater Treatment and Industrial Point Sources
Greater than 10 million people live in the Gulf of Maine watershed region and the major urban effluent point sources for Hg are the >350 wastewater treatment plants (NOAA, 1994; Table 1). Most of this population is concentrated in the Southern Coastal Shelf watershed region in the city of Boston and surrounding areas (Thompson, 2010). A variety of industrial point sources are located in the watershed of the Northern Coastal shelf region, including several pulp and paper mills and historically a large chlor-alkali facility on the Penobscot River (NOAA, 1994). Similarly, several industries that discharged Hg were historically located along the St. Croix and St. John Rivers in the Bay of Fundy watershed, although these sources have been eliminated or presently release only minor amounts of Hg (Doiron et al., 1998; Sunderland and Chmura, 2000b).
Only two estimates of Hg discharges into the Gulf of Maine from wastewater treatment facilities were available in the literature. NOAA (1994) characterized Hg inputs from major wastewater treatment facilities and industrial point sources for 1991 and reported total inputs of Hg at 586 kg/year. All of the top ten major sources were wastewater and sewage treatment plants (Table 1). The largest point source, the Deer Island wastewater treatment facility in Boston, MA, discharges ~5×108 m3/year of wastewater into Massachusetts Bay each year (Wu, 2011). NOAA (1994) reported an associated Hg input of 105 kg/year from this facility. More recent measurements of total Hg in wastewater from the Deer Island treatment facility (Wu, 2011) were much lower (6.91 ng Hg/L). Multiplying this concentration by the total wastewater discharge from Deer Island results in a source of only 3.4 kg Hg/year from Deer Island and a total Hg input of 7.8 kg/year from all wastewater effluent sources in the Gulf of Maine, assuming comparable Hg concentrations. The difference between these inputs as compared to the early 1990s is likely attributable to secondary sewage treatment in 1997 and associated declines in Hg inputs to Boston Harbor (Benoit et al., 2006). Uncertainty in the magnitude of Hg discharges to the Gulf of Maine from these sources is high but is likely bounded by these two ranges.
Dean and Mason (2007) measured the fraction of MeHg in wastewater effluents from a number of sources and found concentrations ranged between 1–10% of total Hg. Assuming 5% of the total Hg discharges into the Gulf of Maine are MeHg results in an input of between 0.4 to 29 kg/year depending on the total Hg inputs assumed.
3.4 Oceanographic Inputs
Despite low concentrations of Hg in marine waters, oceanographic currents transport large volumes of seawater and therefore large amounts of Hg and MeHg in and out of the Gulf of Maine and are thus a major determinant of ambient concentrations in seawater. The major seawater inflows are from the Scotian Shelf (9.4 × 1012 m3/year) and surface (4.5 × 1012 m3/year) and deep water (3.8 × 1012 m3/year) flows through the Northeast Channel (Smith et al., 2001). No studies have directly measured concentrations in inflowing seawater. Dalziel (1992) measured vertical profiles of reactive Hg on the Scotian Shelf in 1985 and reported concentrations between 441 and 461 pg/L. More recently, Dalziel et al. (2010) measured concentrations at the mouth of the Bay of Fundy during several cruises between 2000 and 2002 in regions corresponding to inflowing waters from the Scotian Shelf and Northeast Channel. Average concentrations of total Hg and MeHg were 237±74 pg/L and 58±22 pg/L, respectively. Sample sizes and analytical precision were insufficient to distinguish concentration differences among major water masses in the sampling region (Dalziel et al., 2010).
Based on seawater concentrations measured by Dalziel et al. (2010), estimated total inflows from oceanographic circulation are 4178 kg Hg/year (range 2873–5483) and 1022 kg MeHg/year (range 635–1410). If the concentration data from Dalziel (1992) are considered, this estimate for total Hg increases to >5600 kg/year. Resolving these high levels of uncertainty regarding seawater Hg concentrations in the Gulf of Maine and major inflows should clearly be a priority for future study since they are a dominant determinant of the Hg concentrations in much of the Gulf of Maine region, particularly the productive offshore regions.
3.5 Spatial Patterns in Hg Inputs
The relative importance of Hg inputs to the different Gulf of Maine basins varies by physioregion (Table 2). Based on estimates presented in sections 3.1–3.4, oceanographic circulation is the dominant source of total Hg inputs to the entire Gulf of Maine region (59%), followed by atmospheric deposition (28%), wastewater and industry (8%), and rivers (5%) (see Table 2 for upper and lower bounds for these estimates). Gulf-wide budgets for total Hg obscure the importance of Hg from riverine and wastewater/industry sources in nearshore coastal areas (Table 2). For example, in the Bay of Fundy, inputs of total Hg from atmospheric deposition, rivers, and wastewater/industry are comparable in magnitude (Table 2). Oceanographic inflows are particularly large in the Bay of Fundy (Gregory et al., 1993) but are less pronounced in the various embayments of the Gulf of Maine Northern and Southern Coastal Shelf regions (Hetland and Signell, 2005). Data were not readily available to characterize oceanographic exchanges in most coastal areas but the importance of riverine sources of total Hg to the Northern Coastal Shelf and wastewater treatment/industrial point sources in the Southern Coastal Shelf is apparent in Table 2. These results are comparable to those of Harris et al. (this issue) for the Gulf of Mexico showing that oceanographic inflows dominate the mass budgets for Hg on a system-wide basis but individual regions are much more affected by inflow from rivers and atmospheric sources.
The corresponding inputs of MeHg from atmospheric deposition, wastewater/industry, rivers, and oceanographic inflows also vary across physioregions (Table 2). Of the sources quantified in Table 2, oceanic inflows accounted for 94% (>1000 kg/year) of MeHg inputs with only relatively small amounts from atmospheric deposition (2%), rivers (2%) and wastewater/industry (3%) (Table 2). A major uncertainty in these calculations is therefore characterizing the spatial and temporal variability in inflowing MeHg concentrations in seawater. Presently, we have little understanding of the source of the MeHg to the overlying waters. Since the estimates presented here are based on measurements from the mouth of the Bay of Fundy, an in situ production source is also possible. Several studies have shown accumulation and production of MeHg in open ocean waters (Cossa et al., 2009; 2011; Heimburger et al., 2010; Lehnherr et al., 2011; Sunderland et al., 2009). Such a phenomenon has not been investigated in offshore waters of productive coastal ecosystems like the Gulf of Maine and Gulf of Mexico (Harris et al., this issue; Hetland and Signell, 2005).
The majority of inorganic Hg in coastal systems originates from external sources such as atmospheric deposition and the coastal watershed. In contrast, MeHg is actively produced in coastal sediments, including the Bay of Fundy (Heyes et al., 2006; Sunderland et al., 2004), and only trace concentrations are present in precipitation, as discussed above. Thus, coastal sediments may act as an important source to the water column if sufficient transport takes place through sediment-to-water diffusion and resuspension that is not balanced by MeHg inputs to the sediments from settling particulate matter. To quantify these fluxes we used data from Benoit et al. (2009) from Boston Harbor and Sunderland et al. (2010) from Passamaquoddy Bay at the mouth of the Bay of Fundy (Table 3). Estimates of MeHg diffusion and resuspension from Boston Harbor and Passamaquoddy Bay likely provide effective bounds for these fluxes in other regions of the Gulf of Maine since Hg concentrations in sediments from these systems span the range of MeHg concentrations observed in the Gulf of Maine, with the exception of the point source impacted Penobscot Bay (Table 4). Diffusive MeHg fluxes shown in Table 3 are in the same range as those reported for the continental shelves in the mid-Atlantic (Hollweg et al., 2010) and southern New England (Hammerschmidt and Fitzgerald, 2006) from 0.22 to 25 ng m−2 d−1, respectively.
Table 3.
Estimated fluxes of MeHg between benthic sediments and overlying waters of the Gulf of Maine from coastal shelf regions (Bay of Fundy, Eastern, Northern, Southern Coastal Shelf regions).
| Process | Reported MeHg Flux (ng m−2 day−1) |
aGulf of Maine Mass Flow (kg/year) |
|---|---|---|
| Sediment to water diffusive flux | ||
| Bay of Fundy Boston Harbor |
0.3–2.1 0.4–4.8 |
5–61 |
| Sediment to water resuspension flux | ||
| Bay of Fundy Boston Harbor |
0.2 34.5 |
4–551 |
| Settling flux | ||
| Bay of Fundy | 5.3±1.7 | 85 |
Based on the coastal shelf surface area of the Gulf of Maine (Table 1) of 4.38×1010 m2. Bay of Fundy data from Sunderland et al. (2010); Boston Harbor (Southern Coastal Shelf) data from Benoit et al. (2009). No data were available for the Eastern and Northern Coastal shelf areas.
Table 4.
Mercury concentrations in sediments (ng g−1 dry weight) of the major physioregions of the Gulf of Maine.
| Description | Total Hg | MeHg | Year(s) | Reference |
|---|---|---|---|---|
| Bay of Fundy | ||||
| Passamaquoddy Bay Bay of Fundy mouth Intertidal marshes Bay of Fundy wide |
42±13 16±6.2 7–79 30 (20–90) |
0.30±0.09 0.10±0.02 |
2000–2002 1997–2002 1977 |
Sunderland et al. (2004; 2006) (Hung and Chmura, 2006) Loring (1982) |
| Eastern Coastal Shelf | No data | No data | - | - |
| Northern Coastal Shelf | ||||
| Penobscot Rivera Penobscot River estuary Upper estuary Lower estuary Mount Desert Island, ME Wells, ME Casco Bay, ME Great Bay estuary, NH: Mudflats Coastal salt marsh |
200–86,200 200–1,450 20–140 8.5–9.0 8–83 n/d – 490 40–1420 43–440 |
0.1–74.2 7.5–20 0.2–1.7 0.37 0.15–0.59 0.08–3.88 0.04–2.20 |
1998–2000 2006–2007 2006–2007 2006–2007 2000–2001 2008–2010 |
(Camp Dresser & McKee Inc., 2001) Bodaly et al. (2008) Chen et al. unpublished data Wade et al. (2008) Amirbahman et al. unpublished data |
| Southern Coastal Shelf | ||||
| Boston Harbor | 501–2200 250–1000 |
1.0–9.0 | 2003 1993 |
Benoit et al. (2006; 2009) Bothner et al. (1998) |
| Offshore Regionsb | n/d-20 | No data | 1990s | (Buchholtz ten Brink et al., 2002) |
n/d = below detection limits.
The Penobscot River received large amounts of industrial Hg contamination from several pulp and paper mills and a chlor-alkali facility that closed in 2000.
Includes Brown’s Bank, Central Gulf of Maine, Georges Bank, Great South Channel, Georges Channel, Jordan Basin, and Wilkinson Basin.
Table 3 shows estimated maximal net MeHg fluxes of 527 kg/year from sediments to overlying waters in the Gulf of Maine based on fluxes reported by Benoit et al (2009) and a net sink for MeHg to sediments based on fluxes reported in Sunderland et al. (2010). Note that both estimates rely on settling flux estimates from the Bay of Fundy, which is likely an underestimate for the Boston Harbor data. Benoit et al. (2009) observed an extremely high resuspension flux in Boston Harbor that the authors attributed to bioturbation and high benthic infaunal activity. Sunderland et al. (2004) also showed methylation rates and Hg concentrations in benthic biota in the Bay of Fundy were enhanced in regions with significant tidal mixing. Thus, better characterizing benthic sediment resuspension fluxes of MeHg should be viewed as a priority for understanding the magnitude of the sediment source to the water column in the Gulf of Maine.
If the MeHg flux from benthic sediments is significantly enhanced by resuspension, one potential management concern is the effect of benthic trawling activities throughout the Gulf of Maine. Pilskaln et al. (1998) reported that in 1993 between 35–45% of the surface area of Wilkinson Basin and Georges Basin were disturbed by monthly bottom trawling activity and many inland coastal regions are trawled several times over in a month. They estimated that bottom trawling mobilizes 9.08 kg m−2 of sediment and 6.14×109 m3 of sediment pore water annually in the Gulf of Maine. We expect that these physical mixing processes influence sediment Hg accumulation, and MeHg production (Sunderland et al., 2004) and flux in the Gulf of Maine through changes in sediment biogeochemistry as well as disturbance of benthic infauna and epifauna that bioturbate sediments and MeHg.
Several tidal power installations are presently in operation or are planned along the Gulf of Maine, especially in the Bay of Fundy due to its extreme tidal ranges (e.g., Karsten et al., 2008). These installations reduce tidal energy flow and tidal amplitude, and as a result, alter sediment accretion zones (van Proosdij and Townsend, 2005). Modeling of tidal sediment transport processes show that particulates settle behind the tidal barrage due to a lack of turbulence (Neill et al., 2009). Such a process would potentially extend the area of sediment-associated Hg accumulation in salt marshes, tidal flats and estuaries of the upper Bay of Fundy. Salt marshes are known to be areas of active Hg methylation and MeHg export (Bergamaschi et al., 2011; Mitchell and Gilmour, 2008), including in the Bay of Fundy (O’Driscoll et al., 2011). Thus, increasing the spatial extent of these regions and/or Hg load through tidal power development may exacerbate accumulation in biota.
4.0 Hg Concentrations in the Gulf of Maine
4.1 Water and Sediments
Tables 4 and 5 show a compilation of sediment and seawater data on total and methyl Hg concentration in the major physioregions of the Gulf of Maine. Data on Hg concentrations in seawater are only available for the Bay of Fundy and the Northern Coastal Region and appear to be comparable at these two locations. MeHg concentrations in seawater from the Northern Coastal Zone appear to be lower than the Bay of Fundy, although it difficult to interpret spatial trends given the lack of data.
Table 5.
Hg concentrations in water (filtered, pg L−1) of the major physioregions of the Gulf of Maine.
| Description | Total Hg | MeHg | Year(s) | Reference |
|---|---|---|---|---|
|
Bay of Fundy Passamaquoddy Bay and outer Bay of Fundy |
210±66 | 57±21 | 2000–2002 | Dalziel et al. (2010); Sunderland et al. (2010) |
| Eastern Coastal Shelf | No data | No data | - | - |
| Northern Coastal Shelf | ||||
| Mount Desert Island, ME Wells, ME Great Bay, NH Portsmouth Harbor, NH |
80–160 70–243 150–550 705 |
2 1–9 7 5 |
2003–2008 | Chen et al., unpublished data (all Northern Coastal Shelf data) |
| Southern Coastal Shelf | No data | No data | - | - |
| Offshore Regionsa | No data | No data | - | - |
Includes Brown’s Bank, Central Gulf of Maine, Georges Bank, Great South Channel, Georges Channel, Jordan Basin, and Wilkinson Basin.
The National Coastal Assessment program (USEPA Environmental Monitoring and Assessment Program, http://www.epa.gov/emap/nca/index.html) provides fairly comprehensive data on total Hg concentrations in nearshore sediments (Figure 3) but almost no information is available for offshore sediments (Table 4). Moreover, it is not possible to examine the relationship between water column and bottom sediment concentrations of total Hg and MeHg, given the lack of information on the water column (Table 5).
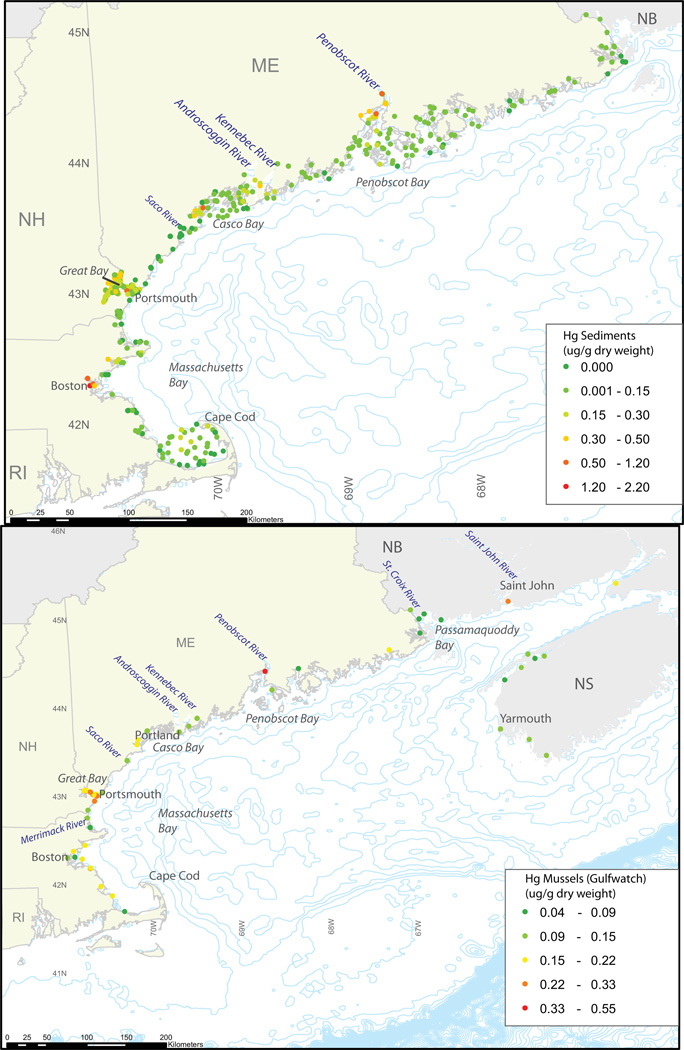
Figure 3.
Reported concentrations of total Hg for sediments (2000–2006) and mussels (Gulfwatch 2003–2008) in nearshore areas of the Gulf of Maine from the National Coastal Assessment Program (NCA) (U.S. EPA Environmental Monitoring and Assessment Program, http://www.epa.gov/emap/nca/index.html).
Among the nearshore regions, highest sediment Hg levels are found in Boston Harbor, the Penobscot River estuary, and Great Bay estuary (Table 4). Sediment total Hg concentrations along the Penobscot River are highly variable largely due to the presence of a chlor-alkali plant in Orrington, Maine that discharged high concentrations of Hg into the river from 1967 to 2000. Recently, Bodaly et al. (2008) conducted an extensive survey to assess the extent of Hg contamination in sediment, water and biota of the Penobscot River and estuary. They found that sediment Hg was the highest in the upper estuary close to the chlor-alkali plant and decreased in the downstream direction. The extent of total Hg and MeHg contamination, especially close to the chlor-alkali plant, is comparable to other highly contaminated sites close to industrial sources of Hg contamination (Covelli et al., 2001; Tomiyasu et al., 2006). Boston Harbor and Great Bay estuaries have sediment total Hg and MeHg concentrations comparable to several other estuaries located close to major urban areas, such as San Francisco Bay (Heim et al., 2007), Chesapeake Bay (Heyes et al., 2006), Hudson Bay, NY (Heyes et al., 2004) and Baltimore Harbor (Mason and Lawrence, 1999).
High sediment total Hg concentrations are also associated with urban areas (Figure 3). Across the nearshore U.S. coastal region (Figure 3), 61% have Hg concentrations higher than the NOAA’s Effects Range-Low (ERL) threshold of 150 ng g−1, and 27% have Hg concentrations higher than the Effects Range Medium (ERM) threshold of 710 ng g−1 d.w. The ERL threshold represents the concentration level below which toxicity rarely is observed, and the ERM represents the median concentration of the compiled samples above which effects frequently occur (NOAA, 2004). Most of the sediments above the ERL and ERM are located close to urban population centers (Figure 3).
Spatial patterns in sediment Hg concentrations throughout the Gulf of Maine appear to be influenced mainly by the presence of past and present industry and wastewater discharges associated with urban centers. Organic carbon content of sediments, which has also been shown to exert a strong control on Hg distribution in other regions, is highly correlated with grain size and sediment composition in this region (Loring, 1982). Organic carbon inputs are also affected by other anthropogenic pollutants such as nutrient loading, which is often spatially correlated with Hg because a dominant source is wastewater treatment facilities (Driscoll et al., this issue). Strong correlations between total organic carbon content of sediments, total Hg and methyl Hg have been documented in Boston Harbor (Benoit et al., 2006; 2009), across the entire Bay of Fundy (Loring, 1982), along the Northeast Coastal Shelf region of the Gulf of Maine (Chen et al., 2009) and in Passamaquoddy Bay and the outer Bay of Fundy (Sunderland et al., 2006).
4.2 Biota
Jones et al. (2009) examined the connections between the spatial distribution of Hg in mussel tissue and sediment, and found elevated Hg levels in both blue mussel tissue and sediments in Great Bay (NH), Casco Bay (ME), Penobscot Bay (ME), Boston Harbor (MA), Passamaquoddy Bay (ME & NB) and St. John Harbour (NB). Figure 3 shows the co-occurrence of urbanized areas and high Hg concentrations in sediments and blue mussels along the coastal regions of the Gulf of Maine from the National Coastal Assessment (NCA) dataset and the Gulfwatch dataset (GOMC). The spatial correspondence between high Hg levels in sediment and blue mussels suggests that sediment concentrations are driving exposures of benthic communities. No other comparable biological data sets are available for the Gulf of Maine region so it is not possible to interpret spatial trends for other species.
Concentrations of Hg in sediments and biota (lower trophic level fish and invertebrates) are co-located across four sites in the Northeast Coastal Shelf Region (Chen et al., 2009) at Mount Desert Island ME, Wells ME, and two sites in Great Bay NH. However, sediment Hg concentrations alone were a poor predictor of concentrations in organisms (partial r2 < 0.3) and the 2-to-4-fold range in organism Hg and MeHg concentrations for each taxa across sites did not reflect the 100 fold range of sediment Hg concentrations. Organic carbon (%TOC) increased (10-fold) with increased Hg in sediments and played a role in reducing the bioavailable pool of Hg in sediments (Chen et al. 2009).
Most of the other biological data for the Gulf of Maine have been collected in the Bay of Fundy/Quoddy region (Table 6). These data span a large range of species and sampling years, thus it is no possible to make inferences about spatial and temporal trends in Hg concentrations. For the intertidal regions of the Northern Coastal Shelf, Chen et al. (2009) measured Hg concentrations in green crab (Carcinus maenus), killifish (Fundulus heteroclitus), blue mussels (Mytilus edulis), and periwinkle (Littorina littorea). These organisms varied in trophic level measured as delta 15N (Fundulus and Carcinus>periwinkle>mussel) and in their source of carbon measured as delta 13C (Carcinus>Littorina>Fundulus>mussel). Results showed that MeHg concentrations did not relate to trophic level but the percent of total as MeHg increased with trophic level. Moreover, MeHg concentrations were higher in more pelagic-feeding than benthic feeding fauna, reinforcing the importance of better understanding factors controlling water column MeHg distribution.
Table 6.
Concentrations of total Hg (ng g−1 wet weight) in muscle tissue of various species from the Bay of Fundy region.
| Species | Hg (ng g−1 wet) | N | Year |
|---|---|---|---|
| Phytoplankton (25–63 um)a | 2.8 (1.9–4.3) | 9 | 2000–02 |
| Copepod (Calanus finmarchicus)b | 4.3±2.2 | 26 | 1981 |
| Herring Brit (Clupea harengus)c | 4.0±0.5 | 12 | 1978–84 |
| Harbour Pollock (Pollachius virens)c | 5.0±0.9 | 17 | 1978–84 |
| Euphausiids (T. inermis)c | 3.0±0.6 | 2 | 1980–83 |
| Euphausiids (M. norvegica)c | 6.0±1.7 | 9 | 1980–83 |
| Amphipod (Gammarus sp.)d | 24±3.1 | 5 | 2001 |
| Amphipod (Gammarus sp.)a | 2 | 1 | 1983 |
| Polychaete (Nephtys sp.)e | 11±3 | 13 | 2001 |
| Polychaete (Nephtys sp.) b | 9±6.7 | 2 | 1983 |
| Blue mussel (Mytilus edulis)f | 26.7±11.5 | 3 | 1998 |
| Oyster (Crassostrea virginica)f | 16.0±5.5 | 5 | 1998 |
| Periwinkle (Littorina littorea)f | 30.0±17.3 | 3 | 1996–98 |
| Lobster (Homarus americanus)f | 98.3±21.4 | 6 | 1990–96 |
| Lobster (Homarus americanus)g | 143.0 | 2 | 1970 |
| Soft shelled clam (Mya areanaria)f | 50 | 1 | 1998 |
| Sea urchin (Strongylocentrus droebachiensis)f | 26.7±15.3 | 3 | 1996–97 |
| Sea scallop (Placopecten magellanicus)g | 111 | 1 | 1970 |
| Witch flounder (Glyptocephalus cynoglossus)a | 33–440 | 4 | 1970 |
| Longhorn sculpin (Myoxocephalus octodecemspinosus)a | 55–132 | 5 | 1970 |
| Atlantic herring (Clupea harengus)h | 11.1±2.3 | 22 | 1981 |
| Alewife (Alosa pseudoharengus)g | 55 | 1 | 1970 |
| Atlantic salmon (Salmo salar)f | 69.2±21.4 | 13 | 1994–97 |
| Spiny dogfish/shark (Squalus acanthias)f | 270±64 | 145 | 1993 |
| Common tern (Sterna hirundo)h | 166 | 30 | 1978–84 |
| Arctic tern (Sterna paradisaea)h | 89 | 36 | 1978–84 |
| Red phalaropes (Phalaropus fulicarius)h | 46.0 | 13 | 1978–84 |
| Bonapart's gull (Larus philadephia)h | 75.0 | 145 | 1978–84 |
| Kittiwake (Rissa tridactyla)h | 37 | 20 | 1978–84 |
| Herring gull (Larus argentatus)h | 101 | 4 | 1978–84 |
| Black guillemot (Cepphus grille)h | 113 | 4 | 1978–84 |
| Common eider (Somateria mollissima)h | 153 | 11 | 1978–84 |
| Double-crested cormorant (Phalacrocorax auritus)h | 606 | 3 | 1978–84 |
| Harbour seals (Phocoena vitulina)i | 590(160–1540) | 11 | 1971 |
Harding et al.., unpublished data.
Health Canada, unpublished data.
Goodale et al. (2008) reviewed Hg levels in the eggs and blood of 17 marine bird species nesting on 35 islands across the Gulf of Maine. Hg levels were generally higher in piscivorous birds than in invertivores, and the three species with the highest Hg levels were Leach’s storm-petrel (Oceanodroma leucorhoa), razorbill (Alca torda) and black guillemot (Cepphus grille). There were no differences in egg Hg levels among breeding colonies for common terns (Sterna hirundo) and double-crested cormorants (Phalacrocorax auritus), but spatial differences were found among five colonies of black guillemots in Maine. Goodale et al. (2008) suggested collecting eggs from common eider (Somateria molissima), Leach’s storm-petrel, double-crested cormorant and black guillemot as the most effective biomonitoring program for Hg in the Gulf of Maine. There are currently no coordinated monitoring programs for Hg levels in predatory fish, marine mammals or seabirds across the entire Gulf of Maine/Bay of Fundy.
5.0 Temporal Trends in Hg Concentrations
5.1 Temporal Trends in Inputs
Sunderland et al. (2008) showed declining atmospheric deposition rates from 15.6 ug m−2 year−1 in the early to mid-1990s (range 9.2–19.0) to 11.5 ug m−2 year−1 in latter part of the decade and early 2000s (range 10.0–12.0) (Figure 2). Regulations controlling Hg releases from waste incinerators in the mid-1990s resulted in lower deposition from U.S. and Canadian sources in recent years (Sunderland et al., 2008). However, the dominant portion of atmospheric deposition (71%) around the year 2000 originated from global and natural sources (Sunderland et al., 2008). Similarly, Han et al. (2008) found that deposition from local sources in the region had declined between 1996 and 2002. Butler et al. (2008) analyzed trends in the MDN data from the Northeast, including sites surrounding the Gulf of Maine, between 1998–2005 and also noted a statistically significant decline over this period.
Prior to the mid-1970s, large quantities of Hg were released as both effluents and atmospheric emissions from chlor-alkali facilities and pulp and paper mills in the Gulf of Maine watershed (NOAA, 1994; Sunderland and Chmura, 2000a; 2000b). Several studies of sediment archives have been used to infer long-term (1850-present) trends in atmospheric deposition of Hg in this area (Kamman and Engstrom, 2002; Lamborg et al., 2002; Sunderland et al., 2008). These studies all documented Hg deposition increases of two to six fold since 1850 with an increase in deposition between 1850 that peaked between 1950 and 2000. Kamman et al. (2002) also noted that watershed retention of atmospheric Hg appears to have declined in New Hampshire over time, resulting in additional atmospherically deposited Hg leaching through the watershed into lakes. Declining watershed retention of Hg would also have resulted in additional inputs into the Gulf of Maine over time, although no temporal monitoring data are available to verify this.
5.2 Temporal Trends in Water and Sediments
There is no information available on Hg levels in seawater for temporal trends analysis. Temporal trends in sediment total Hg concentrations are reported for Boston Harbor (Bothner et al., 1998) and Casco Bay (Wade et al., 2008) estuaries. Bothner et al. (1998) found that concentrations of several metals, including Hg, decreased from 1977 to 1993 in the surficial sediment of Boston Harbor. Historical data show a 53% decrease in Hg concentrations (n = 140, r2 = 0.19, p ≤ 0.0001) in surface sediments of Boston Inner Harbor between 1977 and 1993, likely corresponding to increased retention of Hg by sewage treatment plants (Bothner et al., 1998).
Wade et al. (2008) studied the decadal trends of several organic and trace metal contaminants in the sediment of Casco Bay, ME. They found that even though some sediment organic contaminants and trace metal concentrations decreased from 1991 to 2001, Hg concentrations remained relatively unchanged during this period. Sunderland et al. (2004) found peak subsurface concentrations of total Hg in sediment cores taken from the mouth of the St. Croix River in Passamaquoddy Bay that reflects historical enrichment from an up-river pulp and paper facility that operated in the 1970s. This enhanced Hg deposition signal in the sediments was not present in cores from the central areas of Passamaquoddy Bay, likely due to a combination of source attenuation and physical mixing.
Sunderland et al. (2010) showed with model simulations that there is a long-lag time required by sediments to reach steady state with current Hg inputs due to the deep active sediment layer in parts of the Bay of Fundy. As a result, MeHg concentrations in the well-mixed Bay of Fundy sediments will continue to increase if Hg emission levels do not change because concentrations observed in sediments do not reflect present input levels to the system. The same study showed concentrations of Hg in the water column respond much more rapidly (on the order of months) to changes in loading.
5.3 Temporal Trends in Biota
The Gulf of Maine Council’s Gulfwatch monitoring program measures concentrations of many organic contaminants and metals (including Hg) in blue mussels (Mytilus edulis) at 58 sampling stations spread along the Gulf of Maine in the USA and Canada (Jones et al., 2009). Although Gulfwatch began in 1993, Hg data collected prior to 2003 do not meet the program’s quality-control criteria.
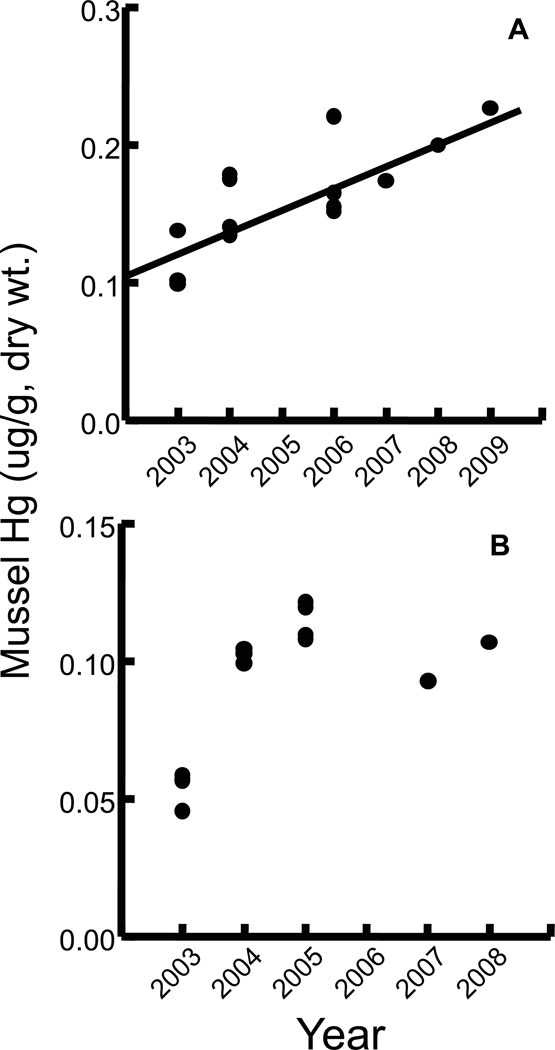
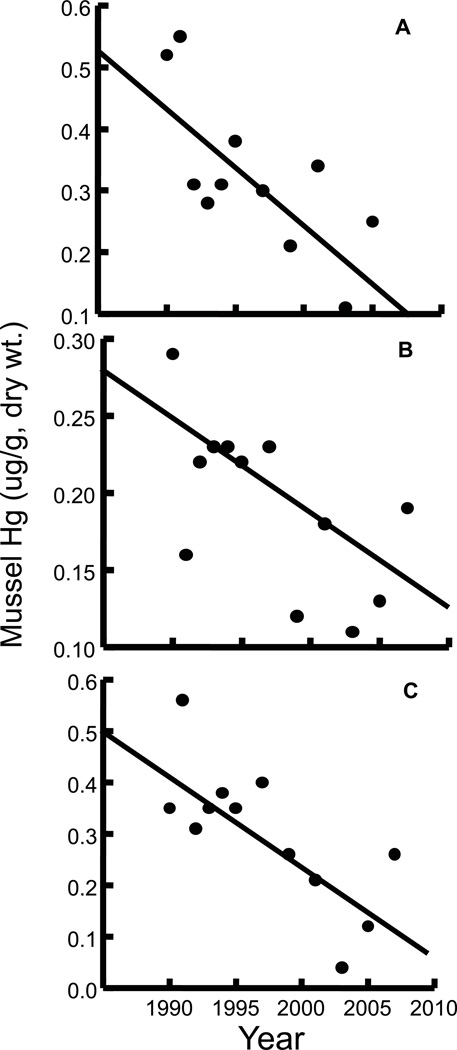
For the six stations with more than five years of Hg data since 2003, we assessed temporal trends using rank regression analysis (SYSTAT 2009). Only one station had a significant trend: Hg levels increased in blue mussels at the Kennebec River, Maine (Figure 4A). An apparently increasing trend in Hg observed at Sandwich, Massachusetts also approached significance (p = 0.06; Figure 4B). Blue mussels at the remaining four sampling stations in Maine, New Hampshire and Nova Scotia showed no significant temporal trends in Hg concentrations. Hg trends have also been measured in blue mussels by the NOAA Mussel Watch program within the Gulf of Maine (Kimbrough et al., 2008). Monitoring began in 1990 and includes 15 sampling stations in coastal Massachusetts, New Hampshire and Maine. Rank regression was used to assess temporal trends and significant decreasing Hg trends were observed at Brewster Island, Mass., Gap Head, Mass., and Sears Island, Maine (Figure 5). No significant temporal trends in Hg were found at the other 12 stations (Kimbrough et al., 2008).
Figure 4.
Mean total Hg concentrations (ug/g, dry wt.) in blue mussels from the Gulf of Maine – Gulfwatch Program: A. Kennebec River, Maine, and B. Sandwich, Mass. Each point represents a composite sample of blue mussels. Significant rank regression: A. Hg = 0.015 Year − 30.623.
Figure 5.
Mean total Hg concentrations (ug/g, dry wt.) in blue mussels from the Gulf of Maine - NOAA Mussel Watch Program: A. Brewster Island, Mass., B. Gap Head, Mass., and C. Sears Island, Maine. Each point represents a composite sample of blue mussels. Significant rank regression: A. Hg = −0.018 Year + 36.246, B. Hg = −0.006 Year + 12.686, C. Hg = −0.018 Year + 35.235.
Variability in observed trends in Hg concentrations in mussel tissue mainly reflects differences in impacts from wastewater discharges and industry, and associated management actions. Increasing concentrations at the Sandwich, MA site, and decreasing concentrations at the other two Massachusetts Bay sites reflect the re-location of the treated Boston municipal wastewater outfall closer to Sandwich, MA in 2000. The closing of the chlor-alkali plant upstream on the Penobscot River, Maine in 2000 may have resulted in lowering levels downstream at the Sears Island site, but the reason for increasing Hg at the Kennebec River site is not presently known.
Historical data are available for Hg concentrations in liver of harbor porpoise from the Gulf of Maine from 1969–77 (mean ± standard deviation: 12.4±17.4 ug/g), 1991 (1.4±1.0 ug/g) and 2001–2003 (8.1±14.6 ug/g) (Gaskin et al., 1979; Harding et al., 2006; Tilbury et al., 1997). Qualitatively there appears to be a declining trend in liver Hg concentrations over time, but this can not be verified statistically. One potential confounding factor is the mean age of the porpoise samples, since Hg concentrations increase with age in harbor porpoise liver (Gaskin et al., 1979). The mean ages of these porpoise samples were 3.8 years in 1969–77 (n=81), 2.0 years in 1991 (n=3), and unknown in 2001–2003 (n=10).
Tracking contaminant concentrations in seabird eggs provides an indication of toxic chemical trends in broader marine ecosystems (Braune, 2007; Furness, 1993; Gilbertson et al., 1987). By monitoring several seabird species that feed in different parts of the ocean, Hg trends can be tracked in different marine food webs (Monteiro and Furness, 1995; Walsh, 1990). Since seabirds are often top predators, they can face relatively high exposures to Hg, which is biomagnified as it passes up marine food chains.
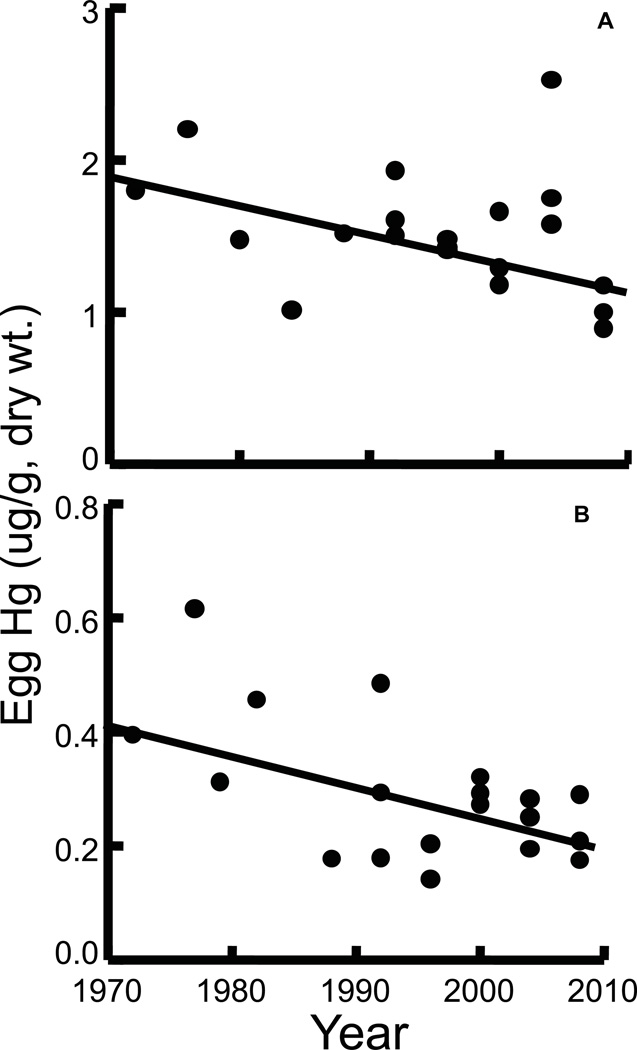
Total Hg concentrations have been monitored in seabird eggs from the Bay of Fundy since 1972 by Environment Canada (Pearce et al., 1979; Burgess et al., 2012). Eggs were collected from island colonies of Atlantic puffins, double-crested cormorants, herring gulls, and Leach’s storm-petrels every four years, up to 2008. Archived composite egg samples were analyzed for total Hg between 1997 and 2008. Hg concentrations were highest in all seabird species and locations between 1972 and 1980. Since the data were not normally distributed, rank regressions were used to assess trends in the Hg levels over time (SYSTAT 2009). Significant declines in Hg levels over time were found in eggs of double-crested cormorants (one outlier removed) and herring gulls at Manawagonish Island (Figure 6). However, after the Hg data were adjusted for seabird dietary shifts using stable nitrogen isotope ratios, the Hg decline in herring gull eggs from Manawagonish Island was no longer significant (Burgess et al., 2012). We found no significant Hg trends in eggs from Atlantic puffins at Machias Seal Island, and herring gulls and Leach’s storm-petrels at Kent Island. The Hg declines observed in cormorants and herring gulls (which are year-round inshore feeders) at Manawagonish Island probably reflect reductions in local Hg releases from heavy industry in the Saint John, New Brunswick area (Sunderland and Chmura, 2000b). The Hg declines are similar to those observed in herring gull eggs from the Great Lakes (Koster et al., 1996), but contrast with increasing Hg levels found in eggs of several seabirds in the Canadian Arctic (Braune, 2007). Machias Seal and Kent Islands are more remote from local industrial sources, and seabirds there probably reflect trends in longer range sources of Hg. Moreover, puffins and storm-petrels feed far offshore for most of the year, and their Hg levels likely reflect long-range atmospheric transport more than local land-based sources. In summary, Hg levels declined or remained stable in eggs from four seabird species collected in the Bay of Fundy since 1972.
Figure 6.
Total Hg concentrations (ug/g, dry wt.) in seabird eggs from Manawagonish Island, New Brunswick: A. double-crested cormorant, and B. herring gull. Each point is a composite sample of five eggs. Significant rank regressions: A. Hg = −0.017 Year + 36.26 (p=0.02) and B. Hg = −0.0052 Year + 10.67 (p<0.01). One outlier (open circle) omitted from regression in A. Data from Burgess et al. (2012) and N. Burgess, unpublished data.
In conclusion, monitoring of Hg trends over time in marine biota in the Gulf of Maine revealed declining or stable Hg levels at most locations, with a couple of exceptions showing increasing trends. These monitoring programs provide a valuable means to assess the success of regulatory efforts to reduce Hg pollution in the Gulf of Maine at local, regional and national scales. The largest immediate challenge for many of these environmental monitoring programs is sustainable funding. Ideally, monitoring for temporal Hg trends should be done in a cooperative manner across the entire Gulf of Maine/Bay of Fundy and should include at a minimum marine sediment, estuarine and coastal invertebrates, insectivorous and piscivorous birds (e.g. common eider, black guillemot), commercial fish species commonly harvested in the Gulf of Maine (e.g. striped bass, winter flounder, bluefish), and marine mammals such as the harbor seal (Evers et al 2007).
6.0 Human and Ecological Exposure to Hg in the Gulf of Maine Region
Marine fish and shellfish are the main source of human exposure to MeHg in the U.S. (Sunderland, 2007). Commercially harvested species in the Gulf of Maine are critical for the economy of this region supporting ~20,000 fishers (Gulf of Maine Council on the Marine Environment, 1994). Major catches in the region include American lobster (42% landings revenue), sea scallops, haddock, Atlantic mackerel and Atlantic herring (Thompson, 2010). While Gulfwatch data show higher Hg concentrations in mussels located near industrialized areas along the coast, spatial patterns of fish Hg concentrations are poorly known because of either lack of information from the offshore areas or inconsistencies in the species of fish sampled in nearshore areas, as in the U.S. National Coastal Assessment program. Moreover, there are little Hg data for fish caught on Georges Bank, one of the most important fishing areas in the Gulf of Maine. This lack of spatially explicit fish data makes it presently impossible to determine if there are areas of enhanced MeHg bioaccumulation in the Gulf of Maine basin.
Based on the National Health and Nutrition Examination Survey (NHANES) data for 1999–2004, Mahaffey et al. (2009) reported that Atlantic coastal residents had the highest blood Hg levels in the U.S. and greatest frequency of fish meals. We conducted a literature review of fish consumption patterns in the Gulf of Maine region to better understand Hg exposures from fisheries and risks to human health. For the literature review, we defined the bounds of the Gulf of Maine watershed to include studies conducted in New England (New Hampshire, Vermont, Maine, Massachusetts, and Connecticut) and southeastern Canada. In populations surveyed in those studies, over 80% of participants had consumed fish and/or shellfish in the past year, including high-risk groups like women of childbearing age and children (Table S1, Electronic supplement). Commonly consumed fish species include salmon, trout and canned tuna (Wessells et al., 1994), similar to the results of the NHANES survey that reported tuna, salmon and shellfish as the most important seafood categories (Mahaffey et al, 2009).
To understand the link between Hg levels in the Gulf of Maine and exposure of local residents, data on the geographic origin of fish consumed are needed. Unfortunately, such information is not readily available at this time. Based on the types of fish consumed by Gulf of Maine watershed residents, we can infer the possibility of a local origin (i.e., fish species that are commonly harvested in the Gulf of Maine are also consumed by residents), but a quantitative relationship cannot be established without additional data collection. Concentrations of Hg in commercially caught fish species in the Gulf of Maine need to be quantified and linked to specific basins within the Gulf. People in the region consume significant amounts of marine fish and shellfish but the source of this seafood is not known and therefore cannot be linked to Gulf of Maine fisheries.
Mercury monitoring data are not available for most marine mammals. Thirty two species of marine mammals are present in the Gulf of Maine including baleen whales (North Atlantic right whale, humpback whale, fin whale, sei whale, and minke whale), toothed whales (harbor porpoises and numerous dolphin species) and two species of seal (Mac et al., 1998). Concentrations of Hg in sediments near urbanized areas exceed NOAA’s Probable Effects Level (PEL: 0.696 µg/g DW) suggesting that there may be adverse impacts on benthic communities (NOAA, 2004). In particular, Hg contamination in the Penobscot River and Estuary likely from the former chlor-alkali plant in Orrington ME has resulted in concentrations in sediments well above the NOAA PEL. Concentrations in songbirds in the area indicate possible toxic effects (Bodaly et al., 2008). Moreover, lobster samples showed levels of MeHg that exceeded the Maine DEP and U.S. EPA criteria for protection of human health (Bodaly et al., 2008). In general, there is a need to better assess ecotoxicological risks across the Gulf of Maine in the more heavily contaminated areas due to both Hg and other contaminants (Pesch and Wells, 2004). Chemical stressors associated with aquaculture, petroleum exploration, mining, coastal development, and industry can have cumulative or interactive effects with Hg and with one another. Moreover, these cumulative chemical impacts occur against a backdrop of alterations to the physical, chemical and biological environment associated with climate change.
7.0 Sustaining Hg Monitoring and Management Programs
Since the 1990s, monitoring programs around the Gulf of Maine have provided local data on Hg trends in atmospheric deposition, sediments, bivalves, finfish, marine mammals, and bird concentrations over various time periods (Table 7). The ever-changing landscape for Hg monitoring in the Gulf of Maine is a challenge to scientists and managers who need consistent data to determine trends for Hg levels in the marine ecosystem. Various environmental agencies and organizations around the Gulf of Maine track Hg levels for their own purposes. States use the data for 305(b) reporting, which is part of the Clean Water Act. EPA National Estuaries Programs in Massachusetts (Massachusetts Bay), New Hampshire (Piscataqua Region), and Maine (Casco Bay) use Hg as a key indicator of contamination for monitoring purposes. The analyses used vary from the simple to more in-depth time trend analysis, yet much of the existing data remains under-analyzed and poorly integrated across environmental matrices.
Table 7.
Summary of Hg monitoring programs in the Gulf of Maine.
| EPA- NCCAa |
GoM Council Gulfwatchb |
NOAA Mussel Watchc |
MDNd | MWRAd | BRIe | MERIf | Environment Canada |
|
|---|---|---|---|---|---|---|---|---|
| Gulf of Maine area | US | US & Canada | US | US & Canada | Massachusetts Bay & Boston Harbor | US | US & Canada | Canada |
| Sites in Gulf of Maine | 422 | 58 | 14 | 3 | various | various | various | 3 |
| Time period | 2000–06, 2010 | 1993–2010 | 1986–2010 | 1996–2010 | 1991–2010 | 1995–2010 | 1991–2010 | 1972–2008 |
| Sediments | YES | no | no | no | YES | no | no | no |
| Finfish species | YES | no | no | no | YES | no | YES | no |
| Bivalve species | no | YES | YES | no | no | no | no | no |
| Atmospheric deposition | no | no | no | YES | no | no | no | no |
| Avian species | no | no | no | no | no | YES | no | YES |
| Pinnipeds | no | no | no | no | no | no | YES | no |
EPA-NCCA is the U.S. Environmental Protection Agency’s National Coastal Condition Assessment.
GoM Council Gulfwatch is chemical-contaminants monitoring program organized and administered by the Gulf of Maine Council on the Marine Environment (http://www.gulfofmaine.org/library/gulfwatch/).
NOAA Musselwatch is the longest running continuous contaminant monitoring program in U.S. coastal and Great Lakes waters administered by the U.S. National Oceanic and Atmospheric Adminstration.
MWRA = U.S. state of Massachusetts Water Resources Authority.
BRI = Biodiversity Resources Institute in Gorham, ME.
MERI = Marine Environmental Research Institute.
Benefits of controlling environmental Hg releases have already been realized in the Gulf of Maine in several industrialized areas with large historical inputs that now show significant declines (e.g., Figure 5). The New England Governors and Eastern Canadian Premiers established a Mercury Action Plan in 1998 with the goal of reducing regional emissions by 75% in 2010 (NEG-ECP, 1998). By 2003, a 54% decline in emissions had been achieved, however, the most recent assessment is not yet available (NEG-ECP, 2003). Other successes of this program include various educational and outreach activities on Hg and the exchange of information with the United Nations Environment Program (UNEP) that will be useful in assessing successes and challenges in an international context. A combination of ongoing monitoring, process studies and modeling are needed to monitor the effectiveness of local emissions reductions at reducing ecological and human exposures to Hg in the Gulf of Maine.
Over the past two decades, monitoring programs around the Gulf of Maine have provided data on Hg concentrations in biotic [finfish, seals (pinnipeds), mussels (bivalve), and bird (avian) species] and abiotic compartments including sediments and atmospheric deposition (Table 7). Most of these monitoring programs are ongoing in terms of geographical coverage and longevity, though most in a diminished capacity. The National Coastal Condition Assessment (NCCA) and the Massachusetts Water Resources Authority (MWRA) programs have been scaled back as monitoring questions and program goals have evolved. The National Oceanic and Atmospheric Administration (NOAA) Mussel Watch Program had no operating budget in 2011, yet still recruited local groups to collect mussel samples and the samples are being archived with the potential for analysis at a later date. This latest development follows a continuous decrease in funding that forced the program to cut back on the frequency of sampling and the collection and analysis of replicate samples. The Gulf of Maine Council’s Gulfwatch Program was only partially funded for the 2010 field season, with funding to support only trace metal analyses while archiving mussel samples for organic analysis.
8.0 Conclusion
The physical, biological and chemical characteristics of the diverse physioregions in the Gulf of Maine greatly influence sources, fate and bioaccumulation of Hg. While this region is known for its productive offshore fishing grounds, there are extremely limited data on Hg concentrations and cycling in offshore regions. Estimated Gulf-wide Hg inputs are dominated by oceanographic inflow but coastal margins are highly influenced by Hg inputs from rivers. Atmospheric Hg deposition from local and regional emissions sources has decreased in response to emissions reductions but increasing contributions to deposition from global sources has offset some of these changes. Accordingly, there is little indication of declines in mussels and birds in the region as a whole, outside of remediated regions that have been heavily impacted by historic Hg sources. In addition to proximity to wastewater discharges and industrial point sources of Hg pollution, factors related to higher sediment concentrations of Hg in the Gulf of Maine appear to be total organic carbon and grain size. Bottom trawling of the depositional basins in the Gulf of Maine may result in enhanced transfer of MeHg to the water column and pelagic fisheries but no research has been conducted on this possible interaction. Although the connection between Gulf of Maine fisheries and exposure of human populations in the region is likely, there is no information on MeHg in commercially caught species in the Gulf of Maine or tracking of where those fish are sold and consumed. Thus, future studies are needed to better understand processes controlling MeHg formation and biological uptake in offshore areas of the Gulf of Maine and also the pathways from food webs and fisheries to human consumption.
Supplementary Material
Highlights.
High levels of mercury are present in fish and marine mammals
Highest sediment and mussel Hg concentrations co-located with urban areas and industry
Trawling of shelf regions may enhance MeHg transfer from sediments
Atmospheric Hg deposition has declined in recent years
Most biological concentrations are stable or declining
Acknowledgements
We acknowledge support for this study from the National Institute of Environmental Health Sciences, NIH Grant Number P42 ES007373. EMS acknowledges new investigator support from the HSPH-NIEHS Center for Environmental Health (P30 ES00002). MRK and EK were additionally supported by grants from NIEHS (P20 ES018175R01) and the US Environmental Protection Agency (RD-83459901). We thank James Shine (Harvard) for helpful discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benoit J, Shull D, Harvey R, Beal S. Effect of bioirrigation on sediment-water exchange of methylmercury in Boston Harbour, Massachusetts. Environmental Science & Technology. 2009;43:3669–3674. doi: 10.1021/es803552q. [DOI] [PubMed] [Google Scholar]
- Benoit J, Shull D, Robinson P, Ucran L. Infaunal burrow densities and sediment monoethyl mercury distributions in Boston Harbour, Massachusetts. Marine Chemistry. 2006;102:124–133. [Google Scholar]
- Bergamaschi BA, Fleck JA, Downing BD, Boss E, Pellerin B, Ganju NK, Schoellhamer DH, Byington AA, Heim WA, Stephenson M, Fujii R. Methyl mercury dynamics in a tidal wetland quantified using in situ optical measurements. 2011;56(4):1355–1371. [Google Scholar]
- Bodaly RA, Rudd JWM, Fisher NS, Whipple CG Penobscot River Mercury Study. Report to: Judge Gene Carter. Portland, ME: U.S. District Court (District of Maine); [24 January, 2008]. Phase I of the Study: 2006–2007. [Google Scholar]
- Bothner MH, Buchholtz ten Brink M, Manheim FT. Metal concentrations in surface sediments of Boston Harbor changes with time. Marine Environmental Research. 1998;45:127–155. [Google Scholar]
- Braune BM. Mercury accumulation in relation to size and age of Atlantic herring Clupea clupea harengus from the southwestern Bay of Fundy, Canada. Archives of Environmental Contamination and Toxicology. 1987;16:311–320. doi: 10.1007/BF01054948. [DOI] [PubMed] [Google Scholar]
- Braune BM. Temporal trends of organochlorines and mercury in seabird eggs from the Canadian Arctic, 1975–2003. Environ. Pollut. 2007;148:599–613. doi: 10.1016/j.envpol.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Braune BM, Gaskin DE. A mercury budget for Bonaparte's gull during autumn molt. Ornis Scandivica. 1987a;18:244–250. [Google Scholar]
- Braune BM, Gaskin DE. Mercury levels in Bonaparte's Gulls (Larus philadelphia) during autumn molt in the Quoddy region, New Brunswick, Canada. Archives of Environmental Contamination and Toxicology. 1987b;16:539–549. [Google Scholar]
- Buchholtz ten Brink MR, et al. Contaminated sediments database for the Gulf of Maine. U.S. Geological Survey Open-file Report No. 02-403, 2002. 2002 Online at http://pubs.usgs.gov/of/2002/of02-403/.
- Butler TJ, Cohen MD, Vermeylen FM, Likens GE, Schmeltz D, Artz RA. Regional precipitation mercury trends in eastern USA, 1998–2005: Declines in the Northeast and Midwest, no trends in the Southeast. Atmospheric Environment. 2008;42:1582–1592. [Google Scholar]
- Burgess NM, Bond AL, Hebert CE, Neugebauer E, Champoux L. Mercury in herring gull (Larus argentatus) eggs from eastern Canada, 1972–2008: temporal change, or dietary shift? Environmental Pollution. 2012 doi: 10.1016/j.envpol.2012.09.001. accepted. [DOI] [PubMed] [Google Scholar]
- Burgess NM, Bond AL, Hebert CE, Neugebauer E, Champoux L. Temporal trends of mercury in seabird eggs from Atlantic Canada, 1968–2008. In review. 2012b [Google Scholar]
- Camp Dresser & McKee Inc. Document prepared in the matter of Maine Peoples’ Alliance and Natural Resources Defense Council v. HoltraChem Manufacturing Company, L.L.C. and Mallinckrodt, Inc.; 2001. Evaluation of Ecological Health of the Lower Penobscot River. Case No. 00-69B. [Google Scholar]
- Chen CY, Dionne M, Mayes B, Ward D, Sturup S, Jackson B. Mercury bioavailability and bioaccumulation in the estuarine food webs of the Gulf of Maine. Environmental Science & Technology. 2009;43:1804–1810. doi: 10.1021/es8017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Critical Reviews in Toxicology. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Corbitt E, Jacob D, Holmes C, Streets D, Sunderland E. Global source-receptor relationships for mercury deposition under present-day and 2050 emissions scenarios. Environmental Science and Technology. 2011;45:10477–10484. doi: 10.1021/es202496y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossa D, Averty B, Pirrone N. The origin of methylmercury in open Mediterranean waters. Limnology and Oceanography. 2009;54:837–844. [Google Scholar]
- Cossa D, Heimburger L-E, Lannuzel D, Rintoul SR, Butler ECV, Bowie AR, Averty B, Watson RJ, Remenyi T. Mercury in the Southern Ocean. Geochimica et Cosmochimica Acta. 2011;75:4037–4052. [Google Scholar]
- Covelli S, Faganeli J, Horvat M, Brambati A. Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic sea) Applied Geochemistry. 2001;16:541–558. [Google Scholar]
- Dalzeil JA. Reactive mercury on the Scotian Schelf and in the adjacent Northwest Atlantic Ocean. Marine Chemistry. 1992;37:171–178. [Google Scholar]
- Dalzeil JA, Yeats PA, Amirault BP. Inorganic chemical analysis of major rivers flowing into the Bay of Fundy, Scotian Schelf, and Bras d'Or Lakes. Canadian Technical Report of Fisheries and Aquatic Sciences. 1998;2226:140. [Google Scholar]
- Dalziel J, Harding G, Sunderland E, Vass P. Mercury in the Bay of Fundy, Gulf of Maine. Threats to the Health of the Bay of Fundy: Potential Problems Posed by Pollutants. In: Burt MDB, Wells PG, editors. Proceedings of a workshop organized under the auspices of BoFEP's Working Group on Stress and Cumulative Effects; Fairmont Algonquin Hotel; 30 April 2010; St. Andrews, New Brunswick. p. 2010. BoFEP Technical Report No. 5. December 2010. [Google Scholar]
- Dean JD, Mason RP. Estimation of Mercury Bioaccumulation Potential from Wastewater Treatment Plants in Receiving Waters: Phase I Report. Draft Final. Water Environment Resources Federation (WERF) 2007:222. [Google Scholar]
- Doiron CC, Roberts GC, Rutherford LA. Inventory of Anthropogenic Sources of Mercury in Atlantic Canada. Dartmouth, Nova Scotia: Environment Canada; 1998. [Google Scholar]
- Driscoll C, Chen CY, Hammerschmidt CR, Mason RP, Gilmour CC, Sunderland EM, Greenfield B, Buckman K, Lamborg C. Nutrient supply and mercury dynamics in marine ecosystems: A conceptual model. Environmental Research. 2012 doi: 10.1016/j.envres.2012.05.002. Accepted, this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers DC, Mason RP, Kamman NC, Chen CY, Bogomolni AL, Taylor DL, Hammerschmidt CR, Jones SH, Burgess NM, Munney K, Parson KC. An integrated mercury monitoring program for temperate estuarine and marine ecosystems on the North American Atlantic Coast. Ecohealth. 2008;5:426–441. doi: 10.1007/s10393-008-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader G, King L, Maclean B. Surficiial geology of the eastern Gulf of Maine and Bay of Fundy. Canadian Hydrographic Service, Marine Science Paper No. 19,22 p. Geological Survey of Canada Paper, Paper 76-17. 1977 [Google Scholar]
- Furness RW. Birds as monitors of pollutants. In: Furness RW, Greenwood JJD, editors. Birds as Monitors of Environmental Change. London: Chapman & Hall; 1993. pp. 86–143. [Google Scholar]
- Gaskin DE, Ishida K, Frank R. Mercury in harbour porpoises (Phocoena phocoena) in the Bay of Fundy. Journal Fisheries Research Board of Canada. 1972;29:1. [Google Scholar]
- Gaskin DE, Stonefield KI, Suda P. Changes in mercury levels in harbour porpoises from the Bay of Fundy, Canada and adjacent waters. Environmental Contamination and Toxicology. 1979;8:733–762. doi: 10.1007/BF01054874. [DOI] [PubMed] [Google Scholar]
- Gaskin GE, Frank R, Holdrinet M, Ishida K, Walton CJ, Smith M. Mercury, DDT, and PCB in harbour seals (Phoca vitulina) from the Bay of Fundy and Gulf of Maine. Journal of Fisheries Research Board of Canada. 1973;30:471–475. [Google Scholar]
- Geyer WR, Signell RP, Fong DA, Wang J, Anderson DM, Keafer BA. The freshwater transport and dynamics of the western Maine coastal current. Continental Shelf Research. 2004;24:1339–1357. [Google Scholar]
- Gilbertson M, Elliott JE, Peakall DB. Seabirds as indicators of marine pollution. In: Diamond AW, Filion FL, editors. The Value of Birds. International Council for Bird Preservation, Cambridge, Tech. Pub. No. 6. Cambridge: 1987. pp. 231–248. [Google Scholar]
- Goodale MW, Evers DC, Mierzykowski SE, Bond AL, Burgess NM, Otorowski CI, Welch LJ, Hall CS, Ellis JC, Allen RB, Diamond AW, Kress SW, Taylor RJ. Marine foraging birds as bioindicators of mercury in the Gulf of Maine. EcoHealth. 2008;5:409–425. doi: 10.1007/s10393-009-0211-7. [DOI] [PubMed] [Google Scholar]
- Gregory D, Petrie B, Jordan F, Langille P. Oceanographic, geographic and hydrological parameters of Scotia-Fundy and southern Gulf of St. Lawrence inlets. Canadian Technical Report of Hydrographic Ocean Sciences. 1993:248. [Google Scholar]
- Gulf of Maine Council on the Marine Environment, Marine Environmental Quality in the Gulf of Maine: Fact Sheet 94-1. Boston, MA: Gulf of Maine Council on the Marine Environment; 1994. p. 16. [Google Scholar]
- Hameedi MJ, Pait AS, Warner RA. Environmental Contaminant Monitoring in the Gulf of Maine. Durham, NH: Northeast Coastal Monitoring Summit; 2002. p. 8. [Google Scholar]
- Hammerschmidt C, Fitzgerald W. Methylmercury cycling in sediments on the continental shelf of southern New England. Geochimica et Cosmochimica Acta. 2006;70:918–930. [Google Scholar]
- Han Y-J, Holsen TM, Evers DC, Driscoll CT. Reduced mercury deposition in New Hampshire from 1996 to 2002 due to changes in local sources. Environmental Pollution. 2008;156:1348–1356. doi: 10.1016/j.envpol.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Harding G, Dalziel J, Sunderland E. Prevalence and bioaccumulation of methylmercury in the food web of the Bay of Fundy, Gulf of Maine. 8th International Conference on Mercury as a Global Pollutant; Madison, WI. 2006. [Google Scholar]
- Harris R, et al. Mercury in the Gulf of Mexico: Sources to Receptors. Environmental Research. 2012 doi: 10.1016/j.envres.2012.08.001. Accepted, this issue. [DOI] [PubMed] [Google Scholar]
- Harris R, et al. Whole ecosystem study shows rapid fish-mercury response to changes in mercury deposition. Proceedings of the National Academy of Science; USA. 2007. pp. 16586–16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim WA, Coale KH, Stephenson M, Choe KY, Gill GA, Foe C. Spatial and habitat-based variations in total and methyl mercury concentrations in surficial sediments in the San Francisco Bay-Delta. Environmental Science & Technology. 2007;41:3501. doi: 10.1021/es0626483. [DOI] [PubMed] [Google Scholar]
- Heimburger LE, Cossa D, Marty JC, Migon C, Averty B, Dufour A, Ras J. Methyl mercury distributions in relation to the presence of nano- and picophytoplankton in an oceanic water column (Ligurian Sea, North-western Mediterranean) Geochimica et Cosmochimica Acta. 2010;74:5549–5559. [Google Scholar]
- Hetland RD, Signell RP. Modeling coastal current transport in the Gulf of Maine. Deep-Sea Research II. 2005;52:2430–2449. [Google Scholar]
- Heyes A, Mason R, Kim E, Sunderland E. Mercury methylation in estuaries: Insights from measuring rates using stable mercury isotopes. Marine Chemistry. 2006;102:134–147. [Google Scholar]
- Heyes A, Miller C, Mason RP. Mercury and methylmercury in the Hudson River sediment: impact of resuspension on partitioning and methylation. Marine Chemistry. 2004;90:75–89. [Google Scholar]
- Hildebrand L, Pebbles V, Ross HS. Cooperative Ecosystem Management: Approaches and Experiences of Programs in the Gulf of Maine, Great Lakes, and Puget Sound/Georgia Basin, Report tabled for discussion at Coastal Zone 97. Boston, Massachusetts: 1997. Jul 23, p. 5. 1997. [Google Scholar]
- Hollweg T, Gilmour C, Mason R. Mercury and methylmercury cycling in sediments of the mod-Atlantic continental shelf and slope. Limnology and Oceanography. 2010;55:2703–2722. [Google Scholar]
- Hung GA, Chmura G. Mercury accumulation in surface sediments of salt marshes in the Bay of Fundy. Environmental Pollution. 2006;142:418–431. doi: 10.1016/j.envpol.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Jones S, Christian K, Harding G. Distrbution of mercury and trace metals in shellfish and sediments in the Gulf of Maine. ICMSS09. Nantes, France: 2009. Jun, p. 9. 2009. [Google Scholar]
- Kamman NC, Engstrom DR. Historical and present fluxes of mercury to Vermont and New Hampshire lakes inferred from Pb-210 dated sediment cores. Atmospheric Environment. 2002;36:1599–1609. [Google Scholar]
- Karsten RH, McMillan JM, Lickley MJ, Haynes RD. Assessment of tidal current energy in the Minas Passage, Bay of Fundy. Proceedings of the Institution of Mechanical Engineers, Part A-Journal of Power and Energy. 2008;222:493–507. [Google Scholar]
- Kelley J, Belknap D. Physiography, surficial sediments and quaternary stratigraphy of the inner continental shelf and nearshore region of the Gulf of Maine. Continental Shelf Research. 1991;11:1265–1283. [Google Scholar]
- Kimbrough KL, Johnson WE, Lauenstein GG, Christensen JD, Apeti DA. An Assessment of Two Decades of Contaminant Monitoring in the Nation's Coastal Zone. Silver Spring, MD: NOAA Technical Memorandum NOS NCCOS 74; 2008. p. 105. [Google Scholar]
- Knightes C, Sunderland E, Barber M, Johnston J, Ambrose RJ. Application of ecosystem scale fate and bioaccumulation models to predict fish mercury response times to changes in atmospheric deposition. Environmental Toxicology and Chemistry. 2009;28:881–893. doi: 10.1897/08-242R.1. [DOI] [PubMed] [Google Scholar]
- Koster MD, Ryckman DP, Weseloh DVC, Struger J. Mercury levels in Great Lakes herring gull (Larus argentatus) eggs. Environmental Pollution. 1996;93:261–270. doi: 10.1016/s0269-7491(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Lai S-O, Huang J, Hopke P, Holsen T. An evaluation of direct measurement techniques for mercury dry deposition. Science of the Total Environment. 2011;409:1320–1327. doi: 10.1016/j.scitotenv.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Lamborg CH, Fitzgerald WF, Damman AWH, Benoit JM, Balcom PH, Engstrom DR. Modern and historic atmospheric mercury fluxes in both hemispheres: Global and regional mercury cycling implications. Global Biogeochemical Cycles. 2002;16:51, 1–11. [Google Scholar]
- Lehnherr I, St. Louis V, Hintelmann H, Kirk JL. Methylation of inorganic mercury in polar marine waters. Nature Geoscience. 2011;4:298–302. [Google Scholar]
- Loring DH. Baseline levels of transition and heavy metals in the bottom sediments of the Bay of Fundy. Proceedings of the Nova Scotia Institute of Science. 1979;29:335–351. [Google Scholar]
- Loring DH. Geochemical factors controlling the accumulation and dispersal of heavy metals in the Bay of Fundy region. Canadian Journal of Earth Sciences. 1982;19:930–944. [Google Scholar]
- Mac MJ, Opler PA, Haecker CEP, Doran PD. Status and Trends of the Nation's Biological Resources. Vol. 2. Reston, VA: U.S. Department of the Interior, U.S. Geological Survey; 1998. pp. 437–964. Reston, VA. [Google Scholar]
- Mahaffey K, Clickner R, Jeffries R. Adult women's blood mercury concentrations vary regionally in USA: Association with patterns of fish consumption (NHANES 1999–2004) Environmental Health Perspectives. 2009;117:47–53. doi: 10.1289/ehp.11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey K, Sunderland EM, Chan HM, Choi AL, Grandjean P, Marien K, Oken E, Sakamoto M, Schoeny R, Weihe P, Yan C-H, Yasutake A. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutrition Reviews. 2011;69(9):493–508. doi: 10.1111/j.1753-4887.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Lawrence AL. Concentration, distribution, and bioavailability of mercury and methylmercury in sediments of Baltimore Harbor and Chesapeake Bay, Maryland, USA. Environmental Toxicology and Chemistry. 1999;18:2438–2447. [Google Scholar]
- McAdie HG. Atmospheric Deposition to the Gulf of Maine. Toronto: International Joint Comission; 1995. p. 73. [Google Scholar]
- National Atmospheric Deposition Program; 2011. [Accessed: 15 August, 2011]. MDN, National Atmospheric Deposition Program Mercury Deposition Network. [Available: http://nadp.sws.uiuc.edu/sites/mdnmap.asp]. [Google Scholar]
- Mitchell CPJ, Gilmour CC. Methylmercury production in a Chesapeake Bay salt marsh. Journal of Geophysical Research. 2008;113 G00C04. [Google Scholar]
- Monteiro LR, Furness RW. Mercury as a global pollutant. Kluwer Academic Publishers; Kluwer Academic Publishers; 1995. Seabirds as monitors of mercury in the marine environment. (Originally published in Water, Air, and Soil Pollution 80: 851–870, 1995) pp. 851–870. [Google Scholar]
- NEG-ECP, Mercury Action Plan; Conference of New England Governors and Eastern Canadian Premiers; Halifax, Nova Scotia. 1998. [Google Scholar]
- NEG-ECP, Report to the New England Governors and Eastern Canadian Provincea on Mercury Projects; Conference on New England Governors and Eastern Canadian Premiers; August, 2003; 2003. [accessed 29 August 2011]. Available: http://www.cap-cpma.ca/images/pdf/eng/2003reportmercury.pdf. [Google Scholar]
- Neill SP, Litt EJ, Couch SJ, Davies AG. The impact of tidal stream turbines on large-scale sediment dynamics. Renewable Energy. 2009;34:2803–2812. [Google Scholar]
- Silver Spring, MD: Pollution Characterization Branch, Strategic Environmental Assessments Division, National Oceanic and Atmospheric Administration; 1994. NOAA, Gulf of Maine Point Source Inventory: A Summary by Watershed for 1991; p. 164. [Google Scholar]
- NOAA, Screening quick reference tables. Hazmat Report 99-1. 2004:12. updated February 2004.
- O’Driscoll NJ, Canario J, Crowell N, Webster T. Mercury speciation and distribution in coastal wetlands and tidal mudflats: Relationship with sulphur speciation and organic carbon. Water, Air and Soil Pollution. 2011;220(1–4):313–326. [Google Scholar]
- Pacyna E, Pacyna J, Sundseth K, Munthe J, Kindbom K, Wilson S, Steenhuisen F, Maxson P. Global emission of mercury to the atmosphere from anthropogenic sources in 2005 and projections to 2020. Atmospheric Environment. 2010;44:2487–2499. [Google Scholar]
- Pearce PA, Peakall DB, Reynolds LM. Shell thinning and residues of organochlorides and mercury in seabird eggs, Eastern Canada, 1970–76. Pesticides Monitoring Journal. 1979;13:61–68. [PubMed] [Google Scholar]
- Pesch GG, Wells PGE. Tides of Change Across the Gulf. An Environmental Report on the Gulf of Maine and Bay of Fundy. Prepared for the the Gulf of Maine Summit: Committing to Change, Fairmon Algonquin Hotel, St. Andres, New Brunswick, Canada, October 26–29th, 2004. Gulf of Maine Council on the Marine Environment and the Global Programme of Action Coalition for the Gulf of Maine. 2004:81. www.gulfofmaine.org. [Google Scholar]
- Pilskaln CH, Churchill JH, M ML. Resuspension of sediment by bottom trawling in the Gulf of Maine and potential geochemical consequences. Conservation Biology. 1998;12:1223–1229. [Google Scholar]
- Quemerais B, Cossa D, Rondeau B, Pham TT, Gagnon P, Fortin B. Sources and fluxes of mercury to the St. Lawrence River. Environmental Science and Technology. 1999;33:840–849. [Google Scholar]
- Ritchie CD, Richards W, Arp PA. Mercury in fog in the Bay of Fundy (Canada) Atmospheric Environment. 2006;40:6321–6328. [Google Scholar]
- Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. Ambio. 2007;36(1):12–19. doi: 10.1579/0044-7447(2007)36[12:eoemot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Selin NE, Jacob DJ, Yantosca RM, Strode S, Jaegle J, Sunderland EM. Global 3-D land-ocean-atmosphere model for mercury: Present-day versus preindustrial cycles and anthropogenic enrichment factors for deposition. Global Biogeochemical Cycles. 2008;22 GB2011. [Google Scholar]
- Sinclair M, Wilson S, Subba Roa DV. Overview of the Biological Oceanography of the Gulf of Maine in Proceedings of the Gulf of Maine Scientific Workshop, January 8–10, 1991. In: Wiggins J, Mooers C, editors. Urban Harbors Institute, University of Massachusetts; 1991. [Google Scholar]
- Smith GJD, Jovellanos CL, Gaskin DE. Canadian Technical Report of Fisheries and Aquatic Sciences. St. Andrews: N.B.; 1984. Near-Surface Bio-Oceanographic Phenomena in the Quoddy Region, Bay of Fundy; p. 9. [Google Scholar]
- Smith P, Houghton R, Fairbanks R, Mountain D. Interannual variability of boundary fluxes and water mass properties in the Gulf of Maine and on Georges bank: 1993–1997. Deep Sea Research II. 2001;48:37–70. [Google Scholar]
- Streets DG, Devane MK, Lu Z, Bond TC, Sunderland EM, Jacob DJ. All-time releases of mercury to the atmosphere from human activities. Environmental Science and Technology. 2011;45:10485–10491. doi: 10.1021/es202765m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf RP, Goldschmidt PM. Remote sensing of suspended sediment discharge into the western Gulf of Maine during the April 1987 100-year flood. Journal of Coastal Research. 1992;8:218–225. [Google Scholar]
- Sunderland EM. Development of a Marine Mercury Cycling Model for Passamaquoddy Bay, New Brunswick. School of Resource and Environmental Management. Burnaby, British Columbia: Simon Fraser University; 2003. p. 264. [Google Scholar]
- Sunderland EM. Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environmental Health Perspectives. 2007;115:235–242. doi: 10.1289/ehp.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Chmura GL. The history of mercury emissions from fuel combustion in Maritime Canada. Environmental Pollution. 2000a;110:1–10. doi: 10.1016/s0269-7491(99)00301-2. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Chmura GL. An inventory of historical mercury pollution in Maritime Canada: Implications for present and future contamination. The Science of the Total Environment. 2000b;256:39–57. doi: 10.1016/s0048-9697(00)00468-x. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Cohen M, Selin N, Chmura G. Reconciling models and measurements to assess trends in atmospheric mercury deposition. Environmental Pollution. 2008;156:526–535. doi: 10.1016/j.envpol.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Gobas FAPC, Branfireun BA, Heyes A. Environmental controls on the speciation and distribution of mercury in coastal sediments. Marine Chemistry. 2006;102:111–123. [Google Scholar]
- Sunderland EM, Gobas FAPC, Heyes A, Branfireun BA, Bayer AK, Cranston RE, Parsons MB. Speciation and bioavailability of mercury in well-mixed estuarine sediments. Marine Chemistry. 2004;90:91–105. [Google Scholar]
- Sunderland EM, Krabbenhoft DP, Moreau JW, Strode SA, Landing WM. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Global Biogeochemical Cycles. 2009;23:14. [Google Scholar]
- Sunderland EM, Dalziel J, Heyes A, Branfireun BA, Krabbenhoft DP, Gobas FAPC. Response of a macrotidal estuary to changes in anthropogenic mercury loading between 1850 and 2000. Environmental Science and Technology. 2010;44:1698–1704. doi: 10.1021/es9032524. [DOI] [PubMed] [Google Scholar]
- Sutcliffe WHJ, Loucks PH, Drinkwater KF. Coastal circulation and physical oceanography of the Scotian Shelf and Gulf of Maine. Journal of Fisheries Research Board of Canada. 1976;33:98–115. [Google Scholar]
- Thompson C. The Gulf of Maine in Context: State of the Gulf of Maine Report. Dartmouth, NS: Gulf of Maine Council on the Marine Environment; 2010. [Google Scholar]
- Tilbury KL, Stein JE, Meador JP, Krone CA, Chan S-L. Chemical contaminants in harbour porpoise (Phocoena phocoena) from the North Atlantic coast: tissue concentrations and intra- and inter-organ distribution. Chemosphere. 34:2159–2181. doi: 10.1016/s0045-6535(97)00076-3. [DOI] [PubMed] [Google Scholar]
- Tomiyasu T, Matsuyama A, Eguchi T, Fuchigami Y, Oki K, Horvat M, Rajar R, Akagi H. Spatial variations of mercury in sediment of Minamata Bay, Japan. Science of the Total Environment. 2006;368:283–290. doi: 10.1016/j.scitotenv.2005.09.090. [DOI] [PubMed] [Google Scholar]
- Townsend DW, Rebuck ND, Thomas MA, Karp-Boss L, Gettings RM. A changing nutrient regime in the Gulf of Maine. Continental Shelf Research. 2010;30:820–832. [Google Scholar]
- U.S. EPA, Regulatory Impact Analysis of the the Clean Air Mercury Rule, Final Report. Vol. EPA-452/R-05-003. Office of Air Quality Planning and Standards. Research Triangle Park, NC: U.S. Environmental Protection Agency; 2005. [Google Scholar]
- van Proosdij D, Townsend SM. Sedimentation and mechanisms of salt marsh colonization on the Windsor mudflats. In: Percy JA, Evans AJ, Wells PG, Rolston SJ, editors. 6th Bay of Fundy Workshop. Cornwallis, NS: Environment Canada; 2005. [Google Scholar]
- Wade TL, Sweet ST, Klein AG. Assessment of sediment contamination in Casco Bay, Maine, USA. Environmental Pollution. 2008;152:505–521. doi: 10.1016/j.envpol.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Walsh PM. The use of birds as monitors of heavy metals in the marine environment. In: Furness RW, Rainbow PS, editors. Heavy Metals in the Marine Environment. Boca Raton, FL: CRC Press; 1990. pp. 183–204. [Google Scholar]
- Wesely ML. Parameterization of surface resistances to gaseous dry deposition in regional-scale numerical models. Atmospheric Environment. 1989;23(6):1293–1304. [Google Scholar]
- Wessells C, Morse S, Manalo A, Gempesaw C. Consumer Preferences for Northeastern Aquaculture Products: Report on the Results of a Survey of Northeastern and Mid-Atlantic Consumers. Rhode Island Experiment Station Publication No. 3100. 1994 [Google Scholar]
- Wu D. Massachusetts Water Resources Authority, Environmental Quality Department. Report ENQUAD 2011-04. Boston, Massachusetts: 2011. NPDES compliance summary report, fiscal year 2009; p. 135. [Google Scholar]
- Xue H, Chai F, Pettigrew NR. A model study of the seasonal circulation of the Gulf of Maine. Journal of Physical Oceanography. 2000;30:1111–1135. [Google Scholar]
- Zitko V, Finlayson B, Wildish D, Anderson J, Kohler A. Methylmercury in freshwater and marine fishes in New Brunswick, in the Bay of Fundy, and on the Nova Scotia Banks. Journal of the Fisheries Research Board of Canada. 1971;28:1285–1291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.