Abstract
In this randomized controlled trial we tested the efficacy of an intervention program (CARE: Creating Avenues for Relative Empowerment) for improving outcomes of hospitalized older adults and their family caregivers. Family caregiver-patient dyads (n=407) were randomized into two groups. The CARE group received a two-session empowerment-educational program 1-2 days post-admission and 1-3 days pre-discharge. The attention control group received a generic information program during the same timeframe. Follow-up was at 2 weeks and 2 months post-discharge. There were no statistically significant differences in patient or family caregiver outcomes. However, inconsistent evidence of role outcome differences suggests that CARE may benefit certain family caregiver subgroups instead of being a one-size-fits-all intervention strategy. Closer examination of CARE's mechanisms and effects is needed.
Keywords: RCT, family caregivers, older adult patients, hospital care
National Hospital Discharge Survey data show an increasing rate of hospitalization for persons aged ≥65 years (DeFrancis, Lucas, Buie, & Golosinskiy, 2008). The need for hospital care is attributed to a rising prevalence of chronic conditions in older adult populations and technological advances that offer a wider array of treatment options. Yet, rates of dysfunctional syndromes related to hospitalization (e.g., acute confusion, falls, nutrition problems, functional decline) affect as many as 29-38% of these patients “and are at least 3- to 5-fold higher in older compared with younger patients with similar diagnoses” (Inouye et al., 2008, p. 726). Adverse outcomes of hospitalization pose serious challenges to transitional care planning post-hospital discharge to home or another type of care facility (Popejoy, Moylan, & Galambos, 2009).
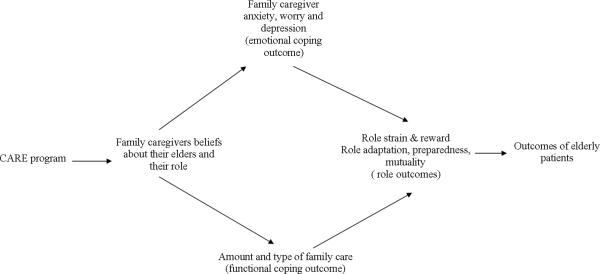
The purpose of this randomized controlled trial, conducted between 2003 and 2006 in a 750-bed academic medical center in Western New York, was to test an intervention program -- Creating Avenues for Relative Empowerment (CARE). The intervention was designed to increase family member participation in the hospital care of elderly relatives in order to prepare them for their anticipated post-hospital caregiver roles. It was hypothesized that (Fig.1):
Family caregivers (FCGs) of hospitalized elders who received the CARE Program versus those who received the attention control comparison program would report: (a) more positive beliefs about their older family members’ responses to hospitalization and their role in the hospital setting, (b) more positive emotional outcomes (less worry, anxiety, and depressive symptoms) during and after hospitalization, (c) more participation in their older family members’ care during hospitalization, and (d) more positive role outcomes (more role rewards, less role strain, more preparedness for family members’ care and a closer relationship with the older family members) both during and after hospitalization.
Hospitalized elders whose FCGs received the CARE Program versus those who received the attention control comparison program would have better outcomes during and after the hospitalization including: (a) fewer incidents of dysfunctional syndromes, (b) shorter hospital stays, (c) fewer readmissions, (d) less depressive symptoms, (e) higher cognitive level, (f) less functional decline, and (g) closer relationships with FCGs.
Figure 1.
Hypothesized Effects of the CARE Program on the Process and Outcomes of Family Caregivers and their Hospitalized Elders.
An additional question was: Does type of relationship (i.e., spouse vs. non-spouse) moderate the effects of the CARE intervention on FCG and patient outcomes?
Methods
Study Design
Using a randomized block design with repeated measures, FCG-patient dyads were randomly assigned to CARE intervention or an attention control group, blocking on FCG type of relationship (spouse or non-spouse). This produced approximately equal numbers of spouses and non-spouses of elders in each of the two study groups. The intent was to assess a main effect of group (CARE or attention control), a main effect of relationship (spouse or non-spouse), and an interaction effect between treatment group and type of relationship, in the event that the CARE intervention proved more effective for either spouses or non-spouses.
Sample
FCGs were included if they: (a) were age 21 years or above; (b) had a relative 65 years or older admitted within the past 24-48 hours; (c) were related to the patient by blood, marriage, adoption, or affinity as a significant other; (d) were the primary FCG (person who performs the most care for the hospitalized older adult); (e) could read and speak English; and (f) lived within a 1-hour drive of the hospital. FCGs were excluded if they were (a) paid care providers or (b) unable to complete questionnaires or provide care due to mental or physical impairment. Patients admitted within the past 24-48 hours were included if they were 65 years or older and had an expected hospital stay of more than 4 days. Patients were excluded if they had been enrolled in the CARE pilot study, were admitted from a long-term care facility, had a diagnosis of dementia prior to admission, or were receiving hospice care.
Intervention Conditions
Two previous investigations informed the CARE program: the Creating Opportunities for Parent Empowerment (COPE) program for parents of hospitalized/critically ill children and low-birth-weight premature infants (Melnyk, Alpert-Gillis, Hensel, Cable-Billing, & Rubenstein, 1997; Melnyk et al., 2001, 2004, & 2006) and the Preparedness, Enrichment, and Predictability (PREP) program for FCGs of frail older adults in home settings (Archbold & Stewart, 2005; Archbold, Stewart, Greenlick, & Valanis, 1993; Archbold et al., 1995). Discussion of CARE origins and theoretical underpinnings can be found in the pilot study report of this research (Li et al., 2003). In brief, CARE intervention strategies are driven by two interrelated theories.
Intervention to empower
Based on role theory (Burr, Leigh, Day, & Constantine, 1979), the mutual agreement component of CARE invites FCGs to choose care activities they wish to perform in association with identified potential problems. The theoretical perspective is that completing the mutually agreed upon family preference contract will help FCGs perceive that they are respected, empowered, and equipped with clear role expectations.
Intervention to educate and inform
Based on self-regulation theory (Johnson, 1999; Johnson & Leventhal, 1974), the educational component of the CARE Program provides FCGs with two 10-minute tapes and written handouts describing common complications of and older patient responses to hospitalization. The theoretical perspective is that clear information about what to expect and how to deal with patient care issues that may arise will strengthen FCG information processing and coping abilities, which will lead to more positive outcomes.
Experimental CARE Intervention Protocol
The CARE Program focus on teaching FCGs how to be more effective, confident caregivers by involving them early in the hospitalization was not the conventional hospital discharge planning protocol followed for all patients and families as time of discharge neared.
Session I
Within 1 to 2 days after hospital admission, CARE Program FCGs were assisted to develop a plan (a Mutual Agreement) for their relatives’ hospital care, based on their abilities and preferences. They also received audio-taped and written materials containing information on (a) common emotional responses, behavioral characteristics, and dysfunctional syndromes of hospitalized older adults and (b) how FCGs can participate in care to prevent or help manage dysfunctional syndromes. Balancing care actions across the three dimensions of providing patient care, working with health care providers, and caring for self was encouraged.
Session II
Session II, initiated 1 to 3 days before discharge, consisted of audio-taped and written materials containing information on how to (a) smooth hospital-to-home transitions, (b) participate in the discharge process, (c) foster a positive caregiver-care receiver relationship, and (d) prepare for follow-up care. Content was designed to increase FCG competence in identifying their relative's care needs (targeting what they should understand) and their self-confidence as caregivers (targeting how they could help with care) during and after the hospital stay.
Attention Control Intervention Protocol
The content of audio-taped and written information provided to FCGs in the attention control group differed from that received by CARE Program participants. It was received in the same amount, at the same time, and in the same manner.
Session I
Session I consisted of materials describing hospital services and policies (e.g., food service, visiting hours, smoking, and parking policies).
Session II
In Session II, FCGs received additional information about the hospital (e.g., specialized facilities, health care team personnel). The content was designed to increase participants’ generic understanding of the hospital's history, organization, and services rather than striving to enhance their understanding of patient needs or how they could help with care.
Procedures
Recruitment and randomization
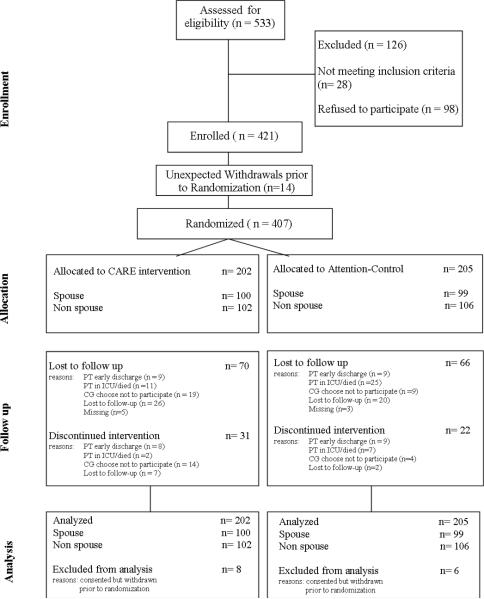
Recruitment and enrollment were conducted by research assistants (RAs). Of 533 FCG-patient dyads assessed for eligibility, 421 were enrolled; 14 of them withdrew before baseline data collection. The remaining 407 (76%) were randomized to either the CARE intervention or attention control group. One hundred and twenty six FCGs refused participation or were ineligible. The most common reasons for refusals were time and energy constraints. Attrition rate over the course of the study was ~30% for both groups. Figure 2 shows the progress of the 407 FCG-patient dyads with approximately equal numbers of spouse and non-spouse FCGs in each study group. The most common reasons for self-withdrawals were patients’ early discharge or death, FCG lack of time to complete the study, travel limitations, or patients’ concerns about FCGs devoting time to the research.
Figure 2.
Flow Chart of Study Participant's Progress in the Study
The majority of participating FCG-patient dyads (n=376: 89%) were recruited from an orthopedic and two general surgery units of an urban-based regional medical center in the northeast United States. These units were chosen because of the number of elderly patients admitted for procedures that placed them at higher risk for developing dysfunctional syndromes during hospitalization (Anderson, Gustafson, & Hallberg, 2001), which, in turn, might place FCGs at higher risk for stress, with associated anxiety and depression (Lenz & Perkins, 2000). A smaller number (n=45: 11%) of participants were recruited from medical units. We excluded patients who were transferred from intensive care after more than a 2-night stay, were hospitalized more than 30 days, died in the hospital or within 2 months post-discharge, or stayed longer than 6 weeks in a nursing home post-hospitalization. Compensating for these planned exclusions led to the inclusion of the medical patients.
Within 48 hours of patients’ hospital admissions, RAs screened potential participants for eligibility, obtained informed consent, and collected baseline data. RAs then obtained the randomization assignment from the project manager. The random allocation sequence was generated by the principal investigator (PI:HL) using a computerized random number system. RAs were responsible for administering either the CARE or the attention control intervention and performing subsequent assessments at three more data collection time points: 1-3 days pre-discharge, and at 2 weeks and 2 months post-discharge. FCGs received $20 for completing questionnaires at each time point. IRB approval was obtained prior to sample recruitment.
Nurses caring for patients participating in the study were blind to and not involved in the administration of the intervention. The relatively short duration of hospital stays was believed to have precluded the likelihood of families having time to become acquainted and exchange information about the manner of their engagement in the study. RAs were blinded to research hypotheses and the intervention assignment of patient-FCG dyads during recruitment, enrollment, and collection of baseline data. They then were unblinded so that they could distribute the appropriate materials to participants by group assignment. Possibility of contamination was reduced by limiting the RA role to a hand-over of materials. Introduction of bias was reduced because data collection was self-report, and delivery of intervention/attention-control information was via written material accompaniments to audio-tapes that FCGs independently listened to before completing paper-and-pencil questionnaires.
A sample size of 280 (70 spouses and 70 non-spouses in each arm) was required to test study hypotheses and analyze the moderating variable of spouse vs. non-spouse relationship between FCGs and elders (Cohen, 1992; Tu et al., 2007). This was based on a power of .8 at the .05 level of significance (a), the small to medium effect sizes found for important outcome variables in the pilot study (Li et al., 2003), the longitudinal statistical procedures to be used in the data analysis, and an estimated attrition rate of 25%.
Research assistant training and intervention fidelity
RAs were registered nurses. They met weekly with the research team to review the study's progress, as documented by flow sheets and checklists. They received one-on-one training from the PI that included observation and simulation. Their activities were routinely monitored by the Project Manager. A Training Manual contained detailed directions regarding all aspects of the study including scripts and tips on how to successfully manage procedural issues and concerns related to enrollment, human subjects, communication, study instruments and timeline for contacts, avoiding missing data, preventing study contamination, and all levels of team member roles and responsibilities. RA interactions with study participants in both groups were narrowly proscribed to maintain intervention integrity. RAs used prepared scripts for screening, enrolling, and collecting baseline and follow-up data from subjects. Unscripted interactions were focused on preventing missing data by verifying that participants did not intentionally leave questionnaire items unanswered. RAs received extensive coaching on their primary responsibilities, which were to provide questionnaires appropriate to participants’ group assignment, in accordance with the designated study timeframes, and to ensure that questionnaires were thoroughly completed.
To assess the fidelity of intervention, study participants in both groups answered eight true-false manipulation check questions after each session as indicators of their processing the appropriate information. FCG perceptions of the program they received were elicited with open-ended questions on whether and how they found the program to be helpful.
Dependent Outcome Measures
Table 1 lists the instruments used to measure dependent variables by times of administration. It includes instrument internal consistency reliabilities from the current study. Results of reliability testing were comparable to reliabilities reported in the original studies. See Li et al. (2003) for more detailed information about instrumentation, including sample items, scoring, and psychometric properties for the measures.
Table 1.
Measures of family caregivers and elders outcomes
| Variable | Measure | Reliability | B | BD | CR | 2wk | 2mo |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||
| Emotional Coping | |||||||
| State Anxiety (SA) | State-Trait Anxiety Inventory (STAI) (Spielberger et al. 1977) | .93-.95 | X | X | X | X | |
| Worry | Family Worry Scale (FWS) (Li, Stewart, & Archbold, 2001c) | .97 | X | X | X | X | |
| Depression | Depression Scale (Beck et al. 1996) | .88-.91 | X | X | Xa | Xa | |
| Functional Coping | |||||||
| Quality of care giving | Family Care Actions Index (FCAI) (Part A and Part B) (Li & Stewart, 2001a) | .93(part A) | X | ||||
| .84(part B) | X | ||||||
| RN observation | Family participation scale (Visual analog scale) | N/A | X | ||||
| Amount of care giving | Reported time spend at bedside | N/A | X | ||||
| Role Outcomes | |||||||
| Preparedness for follow-up care | Family Preparedness Scale (FPS) (Stewart & Archbold, 1994) | .94 | X | ||||
| Role adaptation | Family Role Adaptation Scale (Visual analog scale) | N/A | X | X | X | ||
| Role rewards | Family Role Rewards Scale (Stewart & Archbold, 1994) | .96-.97 | X | X | X | ||
| Role strain | Strain from Lack of Resources | .88-.90 | X | X | X | ||
| Global Strain Scale (Stewart & Archbold, 1994) | .75-.80 | X | X | X | |||
| Mutuality | Mutuality Scale (MS) (Stewart & Archbold, 1994) | .94-.95 | X | X | Xa | Xa | |
| Patient Outcomes | |||||||
| Dysfunctional | Chart review | N/A | X | ||||
| syndromes | Family caregiver report | N/A | X | ||||
| Length of stay | Chart review | N/A | X | ||||
| Re-admission rate | Family caregiver report | N/A | X | X | |||
| Functional status | Functional Status Scale (Adapted from Chyba & Washington, 1990) | .89-.92 | X | X | X | X | |
| Depression | Depression Scale (Adapted from Pearlin et al. 1990) | .88-.90 | X | X | X | X | |
| Cognitive status | Cognitive Status Scale (Adapted from Pearlin et al. 1990) | .86-.90 | X | X | X | X | |
| Mental Status Questionnaire (SPMSQ) (Pfeiffer, 1975) | N/A | X | X | X | |||
| Family caregiver beliefs | Family Caregiver Belief Scale (FBS) (Melnyk & Li, 2001) | .91 | X | ||||
| Clinical variable | Family Preferences Index (FPI) (Li & Stewart, 2001b) | .62 | X | ||||
| Evaluation | Family caregiver report | X |
B=Baseline; BD=Before Discharge; CR=Chart Review; 2wk=2 weeks after patient's discharge; 2mo=2 months after patient's discharge
both patient and Family caregiver were asked to respond separately.
FCG emotional coping outcomes
Anxiety and worry were assessed at all four time points, using the Stait-Trait Anxiety Inventory (STAI; Spielberger, Gorusuch, & Lushene, 1977) and the Family Worry Scale (FWS; Li, Stewart, & Archbold, 2001). FCGs rated each of 35 items on the 5-point FWS scale that ranged from not at all (0) to a great deal (4). Higher scores indicated higher worry levels. Depression was measured at all four time points by the Beck Depression Inventory (BDI-II; Beck, Steer, & Brown, 1996).
FCG functional coping outcomes
The Family Care Actions Index (FCAI; Li & Stewart, 2001a) was used to measure quality/type of care provided by FCGs. Part A, a 70-item checklist, was used to measure what FCGs said they did for relatives during their hospitalization. Part B, an 11-item checklist, was used to measure what FCGs said they did during the discharge process. Higher summed scores indicated greater FCG participation in care. There were two observational measures of FCG level of participation in hospital care. Before discharge each patient's primary nurse was asked to respond to five questions about care the FCG provided, estimating relative amount on 100mm-visual analog scales. Each scale was anchored with the descriptor words not at all (0) and extremely (100). Amount of care giving also was measured by FCG self-reports of the number of hours per week spent at the hospitalized relative's bedside.
FCG role outcomes
Preparedness for follow-up care was measured using the Family Preparedness Scale (FPS; Stewart & Archbold, 1994). FCGs rated each of 9 items on a 5-point scale ranging from not at all prepared (0) to very well prepared (4) with higher summed scores indicating higher preparedness. Role adaptation was measured using 100-mm visual analog scales for FCGs’ estimations of how difficult it was to adapt to the caregiver role. Each scale was anchored with the descriptor words not at all (0) and extremely (100). Role rewards were measured using the Family Role Reward Scale (FRRS; Stewart & Archbold, 1994). FCGs rated items associated with felt rewards of care giving on a 5-point scale ranging from not at all (0) to a great deal (4). Role strain was measured using two subscales of the Caregiver Role Scale (CRS; Stewart & Archbold, 1994). FCGs rated 5 items associated with lack of resources and 4 items related to global strain on a 5-point scale ranging from not at all (0) to a great deal (4). The quality of the relationship between FCGs and patients was measured by the Mutuality Scale (MS; Stewart & Archbold, 1994). FCGs rated each of 15 items on a 5-point scale ranging from not at all (0) to a great deal (4). Higher scores indicated higher mutuality levels.
Patient Outcomes
Rate and duration of dysfunctional syndromes (acute confusion, urinary and fecal incontinence, falls, skin ulcers, and functional decline after discharge) were measured by a post-discharge chart review and FCG report. Length of hospital stay measured in days was collected by chart review. Readmission rates were reported by FCGs. Functional status was measured using a scale from the National Health Interview Survey (NHIS; Chyba & Washington, 1990), adapted by Archbold and Stewart (Archbold, Stewart, Greenlick, & Harvath, 1990; Archbold et al., 1995). FCGs checked no (0) or yes (1) on a 17-item checklist about the need for help with daily activities. Higher scores indicated more impairment in functional status.
Depression level and cognitive status were measured using a scale adapted from Pearlin, Mullan, Semple, and Skaff (1990) by Archbold and Stewart (Archbold et al., 1990, 1995). FCGs rated how often they had to deal with patients’ behaviors on a 4-point scale ranging from 0 days per week (0) to 5-7 days per week (4). Higher scores indicated greater depression. Patient memory and cognitive capacities were rated by FCGs on an 8-item 5-point scale ranging from not at all difficult (0) to can't do it at all (4). Higher scores reflected more cognitive impairment. The Short Portable Mental Status Questionnaire (SPMSQ; Pfeiffer, 1975) also was used to assess cognitive status and was administered by the RAs. This 10-item questionnaire taps orientation, memory, and capacity to perform mathematical tasks. The number of incorrect responses was summed. Higher scores reflected more cognitive impairment. Patients responded separately regarding self-perceived depression and quality of the relationship, measured by the Beck Depression Inventory and the Mutuality Scale, as described above.
FCG Coping Process (FCG Beliefs)
FCG beliefs about their ability to know what to expect and what they could do in caring for their relatives were measured using the 19-item Family Belief Scale: Elders (FBS-E; Melnyk & Li, 2001) adapted from the Parent Belief Scale (PBS; Melnyk et al., 1997). FCGs rated their beliefs on a 5-point scale ranging from strongly disagree (1) to strongly agree (5). Higher scores indicated more positive beliefs.
Demographic variables
FCGs and patients reported on age, gender, race and ethnicity, type of relationship (spouse/non-spouse), education, employment status, marital status, income, and number of children under 18 years old, patients’ medical diagnoses, and number of dysfunctional syndromes. FCGs also completed self-evaluation questionnaires regarding their general feelings and areas of concern; rated the perceived seriousness of their elders’ illness; and indicated preferences for providing care on the Family Preferences Index (FPRI; Li, 2002; Li & Stewart, 2001b; Messecar, Powers, & Nagel, 2008).
Chart review
Length of stay and number of dysfunctional syndromes as measured by number of days were obtained by post-discharge chart review. Interrater reliability was assessed on 10% of the chart reviews and intraclass correlation coefficients (ICC) ranged from .78-.99.
Data Analysis
We used longitudinal statistical methods to examine the hypothesized effects of the CARE intervention on the FCG and patient outcome variables described above. We followed the intent-to-treat (ITT) principle and included all subjects randomized (Polit & Gillespie, 2010).
A key issue arising in the analysis of longitudinal data is missing data. Some of the reasons for missing data are treatment-related while others are not. Random or non-treatment-related missingness is known as missing completely at random (MCAR), while treatment-related missingness is known as missing at random (MAR). As MCAR is a special case of MAR, statistical methods that address MAR naturally account for MCAR.
The two most popular approaches for longitudinal data modeling with strategies for missing data are the weighted generalized estimating equations (WGEE) and the mixed-effects model approach (MM; Kowalski & Tu, 2008). Given our primary interest in treatment effect for this study, both WGEE and MM could be applied. We chose to deploy the MM because WGEE requires a more stringent monotone missing data pattern (MMDP; Kowalski & Tu, 2008), which did not fit our data well because some subjects in our study had intermittent missing data. Although MM provides robust inference under complete data even if normal distribution assumptions for the random-effects and error terms are violated, it generally introduces bias into model estimates under MAR (Lu et al., 2009). To minimize such effects, we first modeled missingness for each primary outcome using logistic regression to identify baseline covariates that were predictive of missing response over time, and then included these covariates in the primary model for the outcome. Although this approach is ad-hoc and may not completely eliminate bias, it is expected to provide better estimates under MAR when applying MM within our context. In this study, the number of months a caregiver was involved in care to other relatives, number of patient co-morbidities, admission type (medical or surgical), and patient illness acuity level significantly predicted dropout. Therefore, we included these four variables as covariates in all the models. Further, to simplify the model for ease of interpretation, we applied MM to post-baseline data by including the baseline value as a covariate in the model. Under this modeling approach, a significant main effect of treatment condition without a significant time by treatment condition interaction implies a constant treatment effect over time; a significant time by treatment condition interaction points to a varying treatment effect across assessment times. In addition, to correct for inflated type I error rates due to multiple comparisons, the false discovery rate (FDR) was used to ensure a correct overall type I error. FDR overcomes the limitation of classic methods such as the Bonferroni correction, which are too conservative for adjusting the type I error for a large number of comparisons as in this study.
Results
There were no statistically significant differences between the CARE and attention control groups at baseline on participants’ demographic or clinical characteristics (Table 2). The study's two hypotheses were tested in accordance with the ITT principle. Table 3 provides estimates of effects and corresponding p-values for each of the variables without any correction for multiple comparisons. We formally assessed the significance of the results using FDR, which provides a better adjustment to ensure an overall type I error at alpha = .01 level (.01 was used because of the large sample size). No reported findings reached this level of statistical significance. In addition, no time by treatment interactions were found nor differences in the main findings by not using an ITT analysis.
Table 2.
Participant Demographics and Clinical Characteristics
| Variables | CARE Group (n=202)* | Comparison Group (n=205)* | p |
|---|---|---|---|
| Family caregivers | |||
| Age Mean (SD) | 60.76 (14.66) | 61.55 (13.40) | .57 |
| Gender | .61 | ||
| Female | 142 (71.4%) | 148 (73.6) | |
| Male | 57 (28.6%) | 53 (26.4) | |
| Race | .75 | ||
| White | 177 (89.4%) | 179 (89.5%) | |
| African American | 15 (7.6%) | 18 (9.0%) | |
| Other | 7 (3.0%) | 3 (1.5%) | |
| Income (yearly) | .89 | ||
| Under $5,000 | 2 (1.2%) | 1 (0.6%) | |
| $5,000 – 19,999 | 18 (10.6%) | 16 (9.5%) | |
| $20,000 – 49,999 | 68 (40.3%) | 72 (42.9%) | |
| $50,000 and over | 81 (47.9%) | 79 (47.0%) | |
| Marital status | .48 | ||
| Married | 149 (74.9%) | 154 (78.2%) | |
| Widowed | 5 (2.5%) | 5 (2.5%) | |
| Divorced | 19 (9.5%) | 14 (7.1%) | |
| Separated | 6 (3.0%) | 4 (2.0%) | |
| Never married | 20 (10.1%) | 20 (10.2%) | |
| Education level | .51 | ||
| Less than high school | 10 (5.0%) | 3 (1.5%) | |
| High school | 40 (20.1%) | 47 (23.6%) | |
| Greater than high | 149 (74.9%) | 149 (74.9%) | |
| Have children < 18 years old | 34 (17.2%) | 36 (18.0%) | .83 |
| Health Mean (SD) | 2.19 (0.94) | 2.28 (1.02) | .35 |
| Employment status | .95 | ||
| Retired | 100 (52.1%) | 100 (50.8%) | |
| Not employed | 6 (3.1%) | 11 (5.6%) | |
| Full time | 57 (29.7%) | 57 (28.9%) | |
| Part time | 29 (15.1%) | 29 (14.7%) | |
| Elderly patients | |||
| Age Mean (SD) | 75.74 (7.14) | 76.56 (7.13) | .71 |
| Gender | .69 | ||
| Female | 104 (52.3%) | 101 (50.2%) | |
| Male | 95 (47.7%) | 100 (49.8%) | |
| Race | .98 | ||
| White | 177 (89.4%) | 180 (89.6%) | |
| African American | 16 (8.1%) | 18 (9.0%) | |
| Other | 5 (2.5%) | 3 (1.5%) | |
| Education | .88 | ||
| Less than high school | 44 (22.2%) | 38 (19.0%) | |
| High School | 50 (25.3%) | 63 (31.5%) | |
| Greater than high | 104 (52.5%) | 99 (49.5%) | |
| Marital status | .89 | ||
| Married | 127 (63.8%) | 126 (63.3%) | |
| Widowed | 57 (28.6%) | 57 (28.6%) | |
| Divorced | 9 (4.5%) | 8 (4.0%) | |
| Separated | 2 (1.0%) | 3 (1.5%) | |
| Never married | 4 (2.0%) | 5 (2.5%) | |
| Illness acuity level (SAPS score) Mean (SD) | 23.95 (8.12) | 24.04 (8.66) | .92 |
N will not always equal to 202 or 205 due to missing data.
SAPS = Simplified Acute Physiology Score
SD = standard deviation
Table 3.
Family Caregivers and Patient Outcomes: Whole Sample
| CARE Group |
Comparison Group |
p | |||||
|---|---|---|---|---|---|---|---|
| M (SE) | Range Actual | Median | M (SE) | Range Actual | Median | ||
| Family Caregivers Emotional outcomes | |||||||
| FCG depression (Time 2) a | 6.13 (.33) | (0-33) | 4 | 5.89 (.30) | (0-34) | 5 | .55 |
| FCG depression (Time 3) | 5.68 (.36) | (0-38) | 3 | 5.33 (.33) | (0-35) | 4 | .46 |
| FCG depression (Time 4) | 5.32 (.41) | (0-30) | 3 | 5.16 (.40) | (0-38) | 3 | .78 |
| FCG anxiety (Time 2) b | 34.24 (.76) | (20-78) | 31 | 33.38 (.72) | (20-72) | 34 | .38 |
| FCG anxiety (Time 3) | 33.04 (.84) | (20-70) | 31 | 32.70 (.78) | (20-69) | 33 | .75 |
| FCG anxiety (Time 4) | 32.03 (.83) | (20-59) | 29 | 32.46 (.79) | (20-67) | 33 | .69 |
| FCG worry (Time 2) c | 36.30 (1.43) | (0-121) | 27 | 35.56 (1.31) | (0-132) | 28 | .67 |
| Functional outcomes | |||||||
| Amount of care giving (hours/week) (Time 2) d | 42.66 (2.51) | (5-168) | 38.5 | 43.15 (2.32) | (4-168) | 38.5 | .87 |
| RN observation (Time 2) | 36.02 (.93) | (0-50) | 38.5 | 36.85 (.85) | (11-50) | 38.5 | .46 |
| Quality of care giving (Time 2) | 40.07 (1.10) | (8-67) | 40 | 38.45 (1.00) | (8-66) | 36 | .23 |
| Quality of care giving (Time 3) | 7.83 (.33) | (0-11) | 8 | 7.38 (.29) | (0-11) | 8 | .24 |
| Role outcomes | |||||||
| Role adaptation (Time 2) (lower score better) e | 1.68 (.23) | (0-10) | 1 | 2.35 (.21) | (0-10) | 1 | .02 |
| Role adaptation (Time 3) | 1.73 (.21) | (0-10) | 1 | 1.98 (.20) | (0-10) | 1 | .34 |
| Role adaptation (Time 4) | 1.84 (.22) | (0-8) | 1 | 2.17 (.20) | (0-10) | 1 | .23 |
| Role rewards (Time 2) e | 63.60 (1.51) | (0-84) | 65 | 61.89 (1.33) | (4-84) | 63 | .34 |
| Role rewards (Time 3) | 67.51 (1.52) | (24-84) | 69 | 63.58 (1.34) | (7-84) | 64 | .03 |
| Role rewards (Time 4) | 67.27 (1.61) | (11-84) | 67 | 64.10 (1.44) | (0-84) | 67 | .10 |
| Role strain: | |||||||
| Lack of resources (Time 2) e | .61 (.08) | (0-4) | .4 | .89 (.07) | (0-3.8) | .6 | .01 |
| Lack of resources (Time 3) | .75 (.08) | (0-3.8) | .4 | .97 (.07) | (0-3.4) | .8 | .03 |
| Lack of resources (Time 4) | .66 (.08) | (0-3.2) | .4 | .77 (.07) | (0-4) | .4 | .24 |
| Global strain (Time 2) e | .88 (.06) | (0-3.2) | .7 | .99 (.06) | (0-3.2) | 1 | .15 |
| Global strain (Time 3) | .88 (.07) | (0-3.2) | .7 | 1.04 (.06) | (0-3.2) | 1 | .04 |
| Global strain (Time 4) | .77 (.07) | (0-2.7) | .7 | .87 (.06) | (0-3.5) | .7 | .26 |
| Preparedness for care (Time 3) e | 28.86 (.70) | (0-36) | 28 | 26.65 (.61) | (8-36) | 28 | .01 |
| Mutuality (Time2)f | 3.41 (.02) | (.6-4.2) | 3.6 | 3.39 (.02) | (1.2-4) | 3.4 | .36 |
| Mutuality (Time 3) | 3.30 (.03) | (.2-4) | 3.4 | 3.30 (.03) | (1-4) | 3.4 | .96 |
| Mutuality (Time 4) | 3.30 (.04) | (.3-4) | 3.4 | 3.26 (.03) | (.6-4) | 3.5 | .34 |
| Positive Beliefs FCG beliefs | 75.25 (.87) | (49-95) | 74 | 73.48 (.79) | (48-95) | 74 | .09 |
| Patient Outcomes | |||||||
| # of dysfunctional syndromes: Chart review | .40 (.06) | (0-3) | 0 | .40 (.05) | (0-3) | 0 | .99 |
| # of dysfunctional syndromes: FGC report g | .41 (.07) | (0-3) | 0 | .37 (.06) | (0-3) | 0 | .63 |
| Length of stay in hospital | 6.62 (.45) | (1-30) | 4 | 6.01 (.40) | (1-30) | 5 | .26 |
| # of Readmissions: 1-15 days after discharge h | .08 (.03) | (0-2) | 0 | .09 (.03) | (0-1) | 0 | .83 |
| # of Readmissions: 16-60 days after discharge | .11 (.03) | (0-2) | 0 | .06 (.03) | (0-1) | 0 | .18 |
| Patient depressive symptoms | |||||||
| Depression (Time 2) i | 1.65 (.20) | (0-11) | 1 | 1.94 (.19) | (0-12) | 1 | .27 |
| Depression (Time 3) | 2.77 (.26) | (0-12) | 2 | 2.33 (.25) | (0-12) | 2 | .20 |
| Depression (Time 4) | 2.06 (.22) | (0-12) | 1 | 2.03 (.21) | (0-12) | 1 | .90 |
| Patient cognitive status | |||||||
| FCG report (Time 2) j | 2.21 (.19) | (0-28) | 1 | 1.87 (.17) | (0-25) | 1 | .15 |
| FCG report (Time 3) | 2.27 (.28) | (0-22) | 0 | 1.79 (.26) | (0-27) | 0 | .19 |
| FCG report (Time 4) | 2.21 (.24) | (0-20) | 0 | 1.68 (.23) | (0-29) | 0 | .10 |
| Patient report (Time 3) | .71 (.11) | (0-6) | 0 | .86 (.10) | (0-9) | 0 | .30 |
| Patient report (Time 4) | .61 (.10) | (0-5) | 0 | .71 (.09) | (0-5) | 0 | .40 |
| Patient functional status | |||||||
| FCG report (Time 2) k | 34.01 (1.00) | (15-63) | 34 | 33.23 (.91) | (0-64) | 32 | .52 |
| FCG report (Time 3) | 25.47 (.88) | (0-68) | 25 | 24.12 (.83) | (0-68) | 22 | .21 |
The following set of control variables were used in all analysis: 1) patient illness acuity level, 2) # of co-morbidities, 3), type of admission, 4) # months caregiver involved in care to other relatives. Additional control variables were used as indicated:
baseline depression scores
baseline anxiety scores
baseline worry scores and discharge to a nursing home
family preferences
family care actions
baseline mutuality and depression scores
patient age
discharge to a nursing home
patient's depression at baseline and family caregiver's depression at baseline and follow-up
baseline cognitive status
baseline functional status and discharge to a nursing home
FCG = Family Care Giver
FCG Coping Outcomes
There were no significant differences between CARE and attention control groups on emotional coping measures for depression, anxiety, and worry or on functional coping measures for amount and quality of care giving (Table 3).
FCG Role Outcomes and Coping Process (Beliefs)
There were some role outcome differences at Time 2 (before discharge) and Time 3, reflecting sub-group differences. CARE FCGs reported less role strain and better preparation to participate in elders’ post-hospital care than attention control FCGs. However, FCG-reported relationship with the patient (mutuality), measured at all time points, and patient-reported mutuality at Times 3 and 4 resulted in no significant differences between CARE and attention control groups at any point in time. There also were no significant differences between CARE and attention control groups in their ability to know what to expect and how to assist in the care of hospitalized older relatives.
Patient (Older Family Member Outcomes)
The clinical trial did not support earlier pilot study findings. There were no significant differences between the study groups on patient outcomes at any time point.
Discussion
We studied the main effects of CARE Program efficacy, following-up an earlier pilot study of its feasibility and preliminary evaluation of its effectiveness (Becker, 2008; Li et al., 2003). We hypothesized that the intervention would increase FCG in-hospital participation in their older relatives’ care in ways that helped them become more confident, effective caregivers, post-hospital discharge. We further hypothesized that the older adult patients would show measurable emotional and functional improvement, both during and after their hospital stay, as a result of improved FCG coping skills. However, there were no statistically significant differences for FCGs or patients. This result, though unexpected, provides us with an opportunity to raise questions and consider possible explanations for why these overall negative findings occurred.
Question: Why did findings of studies using the same theoretical approach differ?
Methodological perspectives
The absence of effects on FCG emotional coping with planned and unplanned hospitalization of older adult relatives is unlike findings from similarly conducted studies of parental coping with children's unplanned hospitalization. In Melnyk and colleagues’ (2001, 2004, 2006) COPE Program investigations, similar interventions (audio-taped and written information) and the same well-established measures (the STAI [Spielberger et al., 1977] and Beck's Depression Inventory [Beck et al., 1996]) were used. Participating mothers of hospitalized premature infants and young critically ill children reported significantly less state anxiety and depressive symptoms. Failure to achieve comparable results from replicating the COPE Program's approach in the CARE study could be due to differences in administration of the intervention. In the trial of COPE for parents of premature infants in neonatal intensive care (Melnyk et al., 2006) the treatment dosage, in terms of timing and content, were different than in the trial of CARE. All participants in the COPE study received three in-hospital intervention sessions and one in-home session at 1 week post-discharge. These sessions included taped and written information as well as specific behavioral activities for parents to complete or perform. All parents received the same activities. Conversely, CARE study participants received 2 in-hospital intervention sessions of solely taped and written information tailored to their initial selection of care activities on which they wished to focus. We do not know if more intense, frequent doses of the intervention would have boosted FCG processing of provided information.
Theoretical Perspectives
Young families may present a different picture than that of intergenerational families as they age across the life span. Consequently, the ability to make generalizations about conjoint efficacy of COPE and CARE intervention programs, based on theoretical and methodological similarities, is constrained by the need to understand research participants’ responses within the broader context of a life-span development framework. For example, results could be affected by the extent to which the illness/hospitalization experience was a first-time versus a recurrent one for FCGs and patients. In contrast to the high anxiety of COPE study parents facing first-time critical hospitalizations of their infants and young children, most CARE study FCGs had previous experience caring for hospitalized and ill elderly persons. Their perceptions of the severity of patients’ illnesses were probably lower because the patients were not critically ill. They likely already had developed cognitive schemas about what to expect during hospitalization; and patients themselves may have evidenced varying degrees of self-efficacy. Therefore, it is possible that CARE study FCG emotional and learning/adjustment curves were not as steep as those of less experienced parents of young children. Our findings are consistent with literature that reports inconclusive results of information-giving to older patients and families (e.g., Smith et al., 2008). Social patterns of intergenerational exchange provide another contrast in situational context between young families providing care to children and late-life families with members who may themselves be in need of care. According to Fingerman and colleagues (2011), a typical pattern is one in which “a majority of middle-aged adults ...engage in a downstream flow of support to each of their progeny, but...may divert from this pattern when elderly parents’ needs are pressing or urgent” (p.96). Thus, given the primacy of parental emotional investment in their offspring, it may be of limited utility to equate concerns about caring for older family members with childrearing concerns of younger birth-cohorts.
Evolutionary Perspectives
Stress and coping theories are widely used in caregiver research. The literature on late-life care giving consistently supports the need to be mindful of caregiver mental health, warning that caregiver anxiety and depression can lead to anger over the burden of care and resentment of the older individual (MacNeil et al., 2010). However, the status of autonomous older adults at the later-life end of the aging spectrum differs from that of younger parents and children at the other end. Berg and Upchurch (2007) described how many couples learn to cope jointly over time, adapting to illness and disability by sharing stressors, pooling resources, and engaging in joint coping efforts. Citing a need to move beyond traditional views of stress and coping, these and other authors recommend the use of different theoretic and analytic approaches to account for the affect that coping by one dyad member has on coping by the other member (e.g., Schindler, Berg, Butler, Fortenberry, & Wiebe, 2010).
Question: Why was the intervention not effective for this particular group of FCGs?
Methodological Perspectives
Despite randomization and blocking on FCG type of relationship (spouse/non-spouse), the end result was a homogeneous FCG sample overall, with high percentages of well educated, older married middle-class White females who rated their health as good-very good (on a scale where 1 equals excellent and 5 equals poor). Results based on spouse/non-spouse comparisons in the intervention and attention control groups were similar in that most caregiver variables did not differ. However, there were interesting exceptions that may be related to positive effects of the intervention for the CARE group non-spouses, who reported more positive beliefs than non-spouses in the attention control group. This suggests that they may be less confident and have more learning needs than spouses with greater longevity and experience in the caregiver role. The moderating influences of spouse versus non-spouse conditions as well as other demographic factors have not been fully analyzed. It may be that the intervention could be more effectively targeted to non-spouse family contexts where levels of stress may be higher and cognitive schemas about what to expect not well formulated.
Theoretical Perspectives
Pinquart and Sörensen's (2011) meta-analytic comparison of available research findings on sociodemographic differences, resources, care giving-related stressors, and psychological distress among spouses, adult children, and children-in-law caregivers of older adults lends credence to the value of more carefully calibrated interventions. They concluded that because these caregivers struggle with such different issues, interventions need to specifically target the unique circumstances of each of these subgroups. In the CARE study, the decision to stratify on spouse/non-spouse relationship variables capitalized on the distinctiveness and documented importance of these categories. Additional stratification in such a large study with so many independent variables would have increased the cost and complexity of sample selection. However, we can learn more about our FCG participants from the dataset to contribute to the literature on caregivers of older adults. Confirmation of the existence of broad-based differences in the care giving experience (by virtue of factors such as relationship, age, sex, race, ethnicity, health, socioeconomic status) substantiates the need for more research and related theories to explain the differential effects of these important variables (e.g., see Haley, Roth, Howard, & Safford, 2010; Mensie & Steffen, 2011; Wang, Shyu, Chen, & Yang, 2010.)
Evolutionary Perspectives
The role of family involvement in managing older relatives’ transitions within and between different care settings is largely unexamined (Levine, Halper, Peist, & Gould, 2010). Jacelon (2006) identified the directive and supportive behaviors family members use to affect and modify older adults’ hospital experience and advocated for more research in this area. Bauer, Fitzgerald, Haesler, and Manfrin (2009) also identified the need for interventions that begin well in advance of older persons’ hospital discharge. However, the body of literature related to families in acute hospital settings is relatively small and dominated by explorations of family needs when their relatives are in critical condition or when they are in the process of being discharged to home or another non-acute setting (Li, 2005). In a review of nursing intervention studies on patients and family members, Mattila, Leino, Paavilainen, and Astedt-Kurki (2009) identified a need to expand the scope and practical application of reported interventions in the development and testing phases to different nursing practice environments.
The design and testing of the CARE intervention were in response to weaknesses in the literature, including a lack of studies that were theoretically driven or that involved FCGs of hospitalized older adults; studies typically conducted at only one transition point (ICU to general unit or hospital-to-home); and the tendency of experimental studies to use usual care as the control condition instead of using a comparison group that controls for time and attention provided to members of the treatment group (Li, Melnyk, & McCann, 2004). The CARE Program is innovative because it involves a theoretically driven intervention, different in focus and content from conventional discharge planning, that combines elements of empowerment (i.e., attention to FCG preferences) with information provided in the early days following admission to the hospital. The success of the COPE Program for parents of young children influenced the decision to package CARE as audio-taped and written information that could be administered at low cost to all FCGs of hospitalized older adults. The intervention's easy reproducibility does not require intensive staff training or interaction. However, this effort at streamlining to facilitate widespread dissemination may have reduced its potential efficacy.
Question: Where do we go from here?
Methodological Perspectives
We think that the timing of the intervention (engaging FCGs soon after a patient's hospital admission) is optimal in terms of preparing FCGs for transitions from hospital to home or another community-based setting. What appear to be weak effects of the intervention for non-spouse participants have not yet been fully explored. This ongoing analysis may identify ways in which the CARE intervention could be strengthened.
Theoretical perspectives
The outcomes of this trial of CARE have helped us to think about refinements of the intervention that could accentuate those aspects of its design dealing with empowerment and education. Aujoulat, d'Hoore, and Deccache (2007) described patient empowerment as “a complex experience of personal change facilitated by care providers” (p.18). This way of thinking, by extension, leads us to believe that including more FCG- hospital staff interaction would strengthen the empowerment aspect of the CARE intervention by allowing introduction of selected nuances to the educational-behavioral portions. Auslander (2011) encouraged health care providers to determine what family members want to do for hospitalized relatives and to identify appropriate tasks that will benefit themselves, patients and hospital staff.
Evolutionary perspectives
On observation of the post-discharge findings, we have directed our thinking beyond CARE as a stand-alone intervention that teaches FCGs of older adults how to be or be better at what they do after leaving the hospital setting and stops there. Families need ongoing community-based support to sustain them in their care giving roles. The urgency of this need is reflected by an expanding literature on the effectiveness of different types of home care nurse interventions (Liebel, Powers, Friedman, Watson, 2012; Monsen, Westra, Oancea, Yu, & Kerr, 2011). The CARE intervention's in-hospital care focus leaves room for thinking about partnerships with programs more specifically targeted to community-based care.
Strengths and Limitations of the Study
The major strength of our study is its use of a rigorous trial design with strong internal validity (randomization, comparability of treatment groups, consistency across treatment arms of methods used to measure outcomes, and similar levels of care/attention in the treatment arms). Design limitations are (a) the reliance on chart reviews, FCG self-reports, and FCG perceptions of patient status as data collection strategies and (b) risks of attrition between the number and spacing of data collection time points. Oversampling, monetary incentives, and home delivery of questionnaires were used to counteract the effects of attrition. Dropouts and missing data were taken into consideration to minimize introduction of bias while using all available data.
Conclusions
The investigation of FCG involvement with hospitalized older relatives and management of their care transitions between settings calls for persistence. The CARE program recognizes the importance of enabling FCGs to become better informed and more confident in their abilities to assume these essential responsibilities. However, it appears that CARE may not work as a one-size-fits-all intervention. It may be more beneficial for subgroups of FCGs, especially those facing stressful new experiences without previously formulated cognitive schemas. While not rising to the level of significance, some small differences in outcomes are intriguing. Thus, the relationship of the intervention to the dynamic diversities of care giving is worthy of continued examination. We believe that progress in understanding the intricate complexities of multidimensional phenomena can spring from negative findings. The work of disentangling what does and does not work in treatment (i.e. laying bare the mechanisms that enable an intervention to produce improvement) is undervalued. In order for science to advance, researchers must be willing to take time to understand both an intervention's efficacy and its mechanisms.
Acknowledgments
This research was supported by NIH, NINR Grant # RO1 NR 008455. The authors thank Ping Sun for contributions as Project Manager, Barbara Stewart for consultation, James McMahon and Craig Sellers for helpful suggestions, and Harriet Kitzman for technical and data management support services of the University of Rochester School of Nursing Center for Research and Evidence-Based Practice. We also are sincerely grateful for the participation of the families, patients and nurses who made this study possible.
Footnotes
The authors declare no conflicts of interest
Bernadette Mazurek Melnyk: Associate VP for Health Promotion, University Chief Wellness Officer & Dean, College of Nursing Robert McCann: Professor of Medicine Christina Koulouglioti: Assistant Professor of Nursing Elizabeth Anson: Research Associate Joyce A. Smith: Doctoral Student Yinglin Xia: Research Assistant Professor of Biostatistics Susan Glose: Doctoral Student Xin Tu: Professor of Biostatistics
References
- Anderson EM, Gustafson L, Hallberg IR. Acute confusional state in elderly orthopedic patients: Factors of importance for detection in nursing care. International Journal of Geriatric Psychiatry. 2001;16:7–17. doi: 10.1002/1099-1166(200101)16:1<7::aid-gps261>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Archbold PG, Stewart BJ. Development of the Family Care Inventory: 20 years of nursing research. Journal of Japan Academy of Nursing Science. 2005;25:104–112. [Google Scholar]
- Archbold PG, Stewart BJ, Greenlick MR, Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Research in Nursing & Health. 1990;13:375–384. doi: 10.1002/nur.4770130605. doi:10.1002/nur.4770130605. [DOI] [PubMed] [Google Scholar]
- Archbold PG, Stewart BJ, Greenlick MR, Valanis B. An evaluation of a caregiving support program. Oregon Health Sciences University; Portland, OR: 1993. Final report to NINR (RO1 NR02088) [Google Scholar]
- Archbold PG, Stewart BJ, Miller LL, Harvath TA, Greenlick MR, VanBuren L, Hagan JM. The PREP system of nursing interventions: A pilot test with families caring for older members. Research in Nursing & Health. 1995;18:3–16. doi: 10.1002/nur.4770180103. doi.10.1002/nur.4770180103. [DOI] [PubMed] [Google Scholar]
- Aujoulat I, d'Hoore W, Deccache A. Patient empowerment in theory and practice: Polysemy or cacophony? Patient Education & Counseling. 2007;66:13–20. doi: 10.1016/j.pec.2006.09.008. doi:10.1016/j.pec.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Auslander GK. Family caregivers of hospitalized adults in Israel: A point-prevalence survey and exploration of tasks and motives. Research in Nursing & Health. 2011;34:204–217. doi: 10.1002/nur.20430. doi:10.1002/nur.20430. [DOI] [PubMed] [Google Scholar]
- Bauer M, Fitzgerald L, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. Journal of Clinical Nursing. 2009;18:2539–2546. doi: 10.1111/j.1365-2702.2008.02685.x. doi:10.1111/j.1365-2702.2008.02685.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory: Second edition manual. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Becker PT. Publishing pilot intervention studies. Research in Nursing & Health. 2008;31:1–3. doi: 10.1002/nur.20268. doi:10.1002/nur.20268. [DOI] [PubMed] [Google Scholar]
- Berg CA, Upchurch R. A developmental-contextual model of couples coping with chronic illness across the adult life span. Psychological Bulletin. 2007;133:920–954. doi: 10.1037/0033-2909.133.6.920. doi: 10.1037/0033-2909.133.6.920. [DOI] [PubMed] [Google Scholar]
- Burr WR, Leigh GK, Day RD, Constantine J. Symbolic interaction and the family. In: Burr WR, Hill R, Nye FI, Reiss IL, editors. Contemporary theories about the family. Vol. 2. The Free Press; New York, NY: 1979. pp. 42–111. [Google Scholar]
- Chyba MM, Washington LR. Questionnaires from the National Health Interview Survey 1980-84. Vital and Health Statistics Series 1: Programs and Collection Procedures: Vital Health Statistics. 1990;N24(1):iii–cvi. 1–203, D1990. [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- DeFrancis CJ, Lucas CA, Buie V, Golosinskiy A. 2006 National Hospital Discharge Survey. National Health Statistics Reports (No.5) 2008 2008 Jul 30; Retrieved from www.cdc.gov/nchs/data/nhsr/nhsr005.pdf. [PubMed]
- Fingerman KL, Pitzer LM, Chan W, Birditt K, Franks MM, Zarit S. Who gets what and why? Help middle-aged adults provide to parents and grown children. Journal of Gerontology: Social Sciences. 2011;66B:87–98. doi: 10.1093/geronb/gbq009. doi:10.1093/geronb/gbq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley WE, Roth DL, Howard G, Safford MM. Caregiving strain and estimated risk for stroke and coronary heart disease among spouse caregivers: Differential effects by race and sex. Stroke. 2010;41:331–336. doi: 10.1161/STROKEAHA.109.568279. doi:10.1161/STROKEAHA.109.568279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye SK, Zhang Y, Jones RN, Shi P, Cupples LA, Calderon HN, Marcantonio ER. Risk factors for hospitalization among community-dwelling primary care older patients: Development and validation of a predictive model. Medical Care. 2008;46:726–731. doi: 10.1097/MLR.0b013e3181649426. doi:10.1097/MLR.0b013e3181649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacelon CS. Directive and supportive behaviors used by families of hospitalized older adults to affect the process of hospitalization. Journal of Family Nursing. 2006;12:234–250. doi: 10.1177/1074840706290264. doi:10.1177/1074840706290264. [DOI] [PubMed] [Google Scholar]
- Johnson JE. Self-regulation theory and coping with physical illness. Research in Nursing & Health. 1999;22:435–448. doi: 10.1002/(sici)1098-240x(199912)22:6<435::aid-nur2>3.0.co;2-q. doi:10.1002/(SICI)1098-240X(199912)22:6,435::AIDNUR2.3.3.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Leventhal H. The effects of accurate expectations and behavioral instructions on reactions during a noxious medical examination. Journal of Personality & Social Psychology. 1974;29:710–718. doi: 10.1037/h0036555. doi:10.1037/h0036555. [DOI] [PubMed] [Google Scholar]
- Kowalski J, Tu XM. Modern applied U statistics. Wiley; New York, NY: 2008. [Google Scholar]
- Lenz ER, Perkins S. Coronary artery bypass graft surgery patients and their family member CGs: Outcomes of a family-focused staged psychoeducational intervention. Applied Nursing Research. 2000;13:142–150. doi: 10.1053/apnr.2000.7655. [DOI] [PubMed] [Google Scholar]
- Levine C, Halper D, Peist A, Gould DA. Bridging troubled waters: Family caregivers, transitions, and long-term care. Health Affairs. 2010;29:116–124. doi: 10.1377/hlthaff.2009.0520. doi: 10.1377/hlthaff.2009.0520. [DOI] [PubMed] [Google Scholar]
- Li H. Family caregivers’ preferences in caring for their hospitalized elderly relatives. Geriatric Nursing. 2002;23:204–207. doi: 10.1067/mgn.2002.126966. doi:10.1067/mgn.2002.126966. [DOI] [PubMed] [Google Scholar]
- Li H. Identifying family care process themes in caring for their hospitalized elders. Applied Nursing Research. 2005;18:97–101. doi: 10.1016/j.apnr.2004.06.015. doi:10.1016/j.apnr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Li H, Melnyk BM, McCann R. Review of intervention studies of families with hospitalized elderly relatives. Journal of Nursing Scholarship. 2004;36:54–59. doi: 10.1111/j.1547-5069.2004.04011.x. doi:10.1111/j.1547-5069.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Li H, Melnyk BM, McCann R, Chatcheydang J, Koulouglioti C, Nichols LW, Ghassemi A. Creating Avenues for Relative Empowerment (CARE): A pilot test of an intervention to improve outcomes of hospitalized elders and family caregivers. Research in Nursing & Health. 2003;26:284–299. doi: 10.1002/nur.10091. doi:10.1002/nur.10091. [DOI] [PubMed] [Google Scholar]
- Li H, Stewart BJ. Family Care Actions Index. 2001a. Unpublished instrument.
- Li H, Stewart BJ. Family Preferences Index. 2001b. Unpublished instrument.
- Li H, Stewart BJ, Archbold Family Worry Scale. 2001. Unpublished instrument.
- Liebel DV, Powers BA, Friedman B, Watson NM. Barriers and facilitators to optimize function and prevent disability worsening: A content analysis of nurse home visits. Journal of Advanced Nursing. 2012;68:80–93. doi: 10.1111/j.1365-2648.2011.05717.x. doi:10.1111/j.1365-2648.2011.05717.x. [DOI] [PubMed] [Google Scholar]
- Lu N, Tang W, He H, Yu Q, Crits-Christoph P, Zhang H, Tu XM. On the impact of parametric assumptions and robust alternatives for longitudinal data analysis. Biometrical Journal. 2009;51:627–643. doi: 10.1002/bimj.200800186. doi:10.1002/bimj.200800186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil G, Kosberg J, Durkin DW, Dooley WK, DeCoster J, Williamson GM. Caregiver mental health and potentially harmful caregiving behavior: The central role of caregiver anger. The Gerontologist. 2010;50:76–86. doi: 10.1093/geront/gnp099. doi:10.1093/geront/gnp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila E, Leino K, Paavilainen E, Astedt-Kurki P. Nursing intervention studies on patients and family members: A systematic literature review. Scandinavian Journal of Caring Sciences. 2009;23:611–622. doi: 10.1111/j.1471-6712.2008.00652.x. doi:10.1111/j.1471-6712.2008.00652.x. [DOI] [PubMed] [Google Scholar]
- Melnyk BM, Alpert-Gillis L, Feinstein NF, Crean H, Johnson J, Fairbanks E, Corbo-Richert B. Creating opportunities for parent empowerment: Program effects on the mental health/coping outcomes of critically ill young children and their mothers. Pediatrics. 2004;113:e597–e607. doi: 10.1542/peds.113.6.e597. doi:10.1542/peds.113.6.e597. [DOI] [PubMed] [Google Scholar]
- Melnyk BM, Alpert-Gillis L, Feinstein NF, Fairbanks E, Schultz-Czarniak J, Hust D, Sinkin RA. Improving cognitive development of low-birth-weight premature infants with the COPE Program: A pilot study of the benefit of early NICU intervention with mothers. Research in Nursing & Health. 2001;24:373–389. doi: 10.1002/nur.1038. [DOI] [PubMed] [Google Scholar]
- Melnyk BM, Alpert-Gillis LJ, Hensel PB, Cable-Billing RC, Rubenstein J. Helping mothers cope with a critically ill child: A pilot test of the COPE intervention. Research in Nursing & Health. 1997;20:3–14. doi: 10.1002/(sici)1098-240x(199702)20:1<3::aid-nur2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Melnyk BM, Feinstein NF, Alpert-Gillis L, Fairbanks E, Crean HF, Sinkin R, Gross SJ. Reducing premature infants length of stay and improving parents’ mental health outcomes with the COPE NICU program: A randomized clinical trial. Pediatrics. 2006;118:1414–1427. doi: 10.1542/peds.2005-2580. doi:10.1542/peds.2005-2580. [DOI] [PubMed] [Google Scholar]
- Melnyk BM, Li H. Family Belief Scale: Elders (FBS-E) 2001. Unpublished instrument.
- Mensie LC, Steffen AM. Predicting in-home respite utilization by family caregivers of older adults: Results of a community study. Home Health Care Management & Practice. 2011;23:109–117. doi:10.1177/1084822310384694. [Google Scholar]
- Messecar D, Powers BA, Nagel CL. How to try this: The Family Preferences Index. American Journal of Nursing. 2008;108:52–60. doi: 10.1097/01.NAJ.0000334527.52341.bd. [DOI] [PubMed] [Google Scholar]
- Monsen KA, Westra BL, Oancea SC, Yu F, Kerr MJ. Linking home care interventions and hospitalization outcomes for frail and non-frail elderly patients. Research in Nursing & Health. 2011;34:160–168. doi: 10.1002/nur.20426. doi:10.1002/nur.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and stress process: An overview of concepts and their measures. The Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sörensen S. Spouses, adult children, and children-in-law as caregivers of older adults: A meta-analytic comparison. Psychology and Aging. 2011;26:1–14. doi: 10.1037/a0021863. doi:10.1037/a0021863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polit DF, Gillespie BM. Intention-to-treat in randomized controlled trials: Recommendations for a total trial strategy. Research in Nursing & Health. 2010;33:355–368. doi: 10.1002/nur.20386. doi:10.1002/nur.20386. [DOI] [PubMed] [Google Scholar]
- Popejoy LL, Moylan K, Galambos C. A review of discharge planning research of older adults 1990-2008. Western Journal of Nursing Research. 2009;31:923–947. doi: 10.1177/0193945909334855. doi:10.1177/0193945909334855. [DOI] [PubMed] [Google Scholar]
- Schindler I, Berg CA, Butler JM, Fortenberry KT, Wiebe DJ. Late-midlife and older couples’ shared possible selves and psychological well-being during times of illness: The role of collaborative problem solving. Journal of Gerontology: Psychological Sciences. 2010;65B:416–424. doi: 10.1093/geronb/gbq030. doi:10.1093/geronb/gbq030. [DOI] [PubMed] [Google Scholar]
- Smith J, Forster A, House A, Knapp P, Wright JJ, Young J. Information provision for stroke patients and their caregivers. Cochrane Database of Systematic Reviews. 2008;(2):CD001919. doi: 10.1002/14651858.CD001919.pub2. Art. No. DOI: 10.1002/14651858.CD001919.pub2. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. The STAI Manual. Consulting Psychologists; Palo Alto, CA: 1977. [Google Scholar]
- Stewart BJ, Archbold PG. Family Caregiving Inventory. 1994. (Available from Patricia G. Archbold, DNSc, RN, FAAN, FGSA, Consultant; Building Academic Geriatric Nursing Capacity; American Academy of Nursing. 802-229-2620).
- Tu XM, Zhang J, Kowalski J, Shults J, Feng C, Sun W, Tan W. Power analyses for longitudinal study designs with missing data. Statistics in Medicine. 2007;26:2958–2981. doi: 10.1002/sim.2773. doi:10.1002/sim.2773. [DOI] [PubMed] [Google Scholar]
- Wang Y-N, Shyu Y-IL, Chen M-C, Yang P-S. Reconciling work and family caregiving among adult-child family caregivers of older people with dementia: effects on role strain and depressive symptoms. Journal of Advanced Nursing. 2010;67:829–840. doi: 10.1111/j.1365-2648.2010.05505.x. doi:10.1111/j.1365-2648.2010.05505.x. [DOI] [PubMed] [Google Scholar]