Abstract
For studies of vertebrate limb regeneration it is often desirable to visualize the regenerated skeleton, which is mostly cartilage, and also section the specimen for histological or immunohistochemical visualization of other tissues. However, the normal skeletal staining techniques are incompatible with immunohistochemistry. Here, we describe a contrast-based micro-computed tomography (microCT) method for direct and nondestructive observation of regenerated cartilage spikes in Xenopus frog limbs. In addition, we show that contrast based microCT imaging is compatible with immunohistochemistry protocols. This approach provides versatile and high contrast images of the cartilage allowing us to measure the regenerated skeletal structure in detail as well as carrying out the other types of analysis. It opens a wide range of potential microCT applications in research on vertebrate limb regeneration.
Keywords: limb regeneration, regenerated skeleton, Xenopus frog, microCT, Hexabrix
INTRODUCTION
Xenopus, an anuran amphibian, has recently emerged as a leading model for regeneration research (Slack et al., 2008; Beck et al., 2009). Complete limb regeneration is possible during the early stages of limb development in the tadpole. This capacity continues until stage 54, but then declines as limb differentiation occurs (Dent, 1962; Muneoka et al., 1986). After metamorphosis, the Xenopus frog can only grow a cartilaginous protrusion, called a “spike,” after limb amputation (Goss and Holt, 1992; Endo, 2000). Because of this stage-dependent regeneration property, Xenopus provides a “gain of function” assay for limb regeneration studies. Potential treatments to increase regeneration can be assessed by applying them to the frog limb then amputating to see if regeneration is, in fact, enhanced.
The extent and pattern of Xenopus limb regeneration is usually evaluated by examining the formation of skeletal structures. The traditional method of analysis is staining with Alcian Blue and Alizarin Red (Inouye, 1976). The principle of using these two dyes is that the Alcian Blue 8GX stains the acid mucopolysaccharides in the matrix of cartilage while the Alizarin Red S binds to the calcium found in bones. Thus, the cartilage is stained a blue color and the calcified bone is stained red. This method is simple, effective and cheap. But the whole process takes 4–7 days depending on the size of the specimen. Moreover, the method is incompatible with other preparation and imaging techniques. For example, in a frog limb regeneration study, one would often need to know the expression and localization of regeneration-related gene products by methods such as immunohistochemistry or in situ hybridization, in addition to observing the skeletal pattern. However, the skeletal staining is destructive and prevents further examination of the same specimen with other histological and immunological approaches. In this report, we have explored some non-destructive ways to directly visualize the internal skeletal structures in the regenerated frog limb.
X-ray imaging has long been applied in medicine because it is safe, convenient, and non-invasive. However, conventional X-rays are mainly used to image calcified bones. For softer tissues such as cartilage and muscle, it has very limited usage. Recently, a contrast-based microcomputed tomography (microCT) technique has been developed to image and quantify the proteoglycans in the cartilage (Palmer et al., 2006). Computed tomography (CT) can generate a three-dimensional image of the inside of an object from a large series of two-dimensional X-ray images taken around a single axis of rotation. So, microCT provides a 3D quantitative morphologic and non-destructive analysis at a micron-level voxel resolution. To broaden the microCT applications to soft tissues, the Levenston group has used an ionic contrast agent to compensate for the poor radiopacity of the cartilage and make it distinguishable in the X-ray image (Palmer et al., 2006). The contrast agent they use is Hexabrix 320 (Guerbet Inc.), which contains ioxaglate, a negatively charged X-ray absorbing hexaiodinated dimer. Before CT scanning, the tissue of interest is equilibrated in the solution containing the Hexabrix, with the aim of increasing the X-ray attenuation of the soft tissues to a detectable level.
We have used Hexabrix in our microCT analysis of frog limb regeneration. We found that with Hexabrix, the regenerated limb cartilage could be visualized in a nondestructive way as a negative image which can be further processed to give high quality sections through the specimen. Furthermore, we demonstrate that Hexabrix based microCT scanning does not destroy the antigens in the structures, so it is compatible with the immunohistochemistry often needed in regeneration research.
METHODS
Hexabrix-Based microCT Scanning
Xenopus were raised past metamorphosis to small frogs, about 3–4 cm in length. For these experiments they were anesthetized in 0.02% MS222 and forelimbs were amputated through the middle ulna-radius. A spike was allowed to regenerate over 6 weeks and then the frogs were imaged. Before the CT scan, the skin of the spikes was removed, they were fixed in 4% formalin fixative (Sigma) for 1 hr, and incubated in 50% Hexabrix/50% Phosphate Buffered Saline (PBS) with rotation at room temperature. Various incubation times were tested. For a spike 5–8 mm long and 2–3 mm wide, 2 hr incubation time was optimal.
For microCT imaging, all scanning was performed in air using a XT H 225 microCT machine that was manufactured by Nikon metrology NV. To visualize the skeletal structure in a hand of a Xenopus frog, a scan setting of 45 kV, 80 μA was used. Because of the presence of the contrast agent, a different microCT setting was required to optimize the images. Hexabrix-based CT scan of regenerated spikes was performed at a setting range of 70–85 kV, 140–160 μA, with a voxel size of 2.7 μm. The image acquisition time was 15–25 min.
3D Reconstruction of the CT Images
Acquired images of spikes were processed and reconstructed using CT Pro 3D software (Nikon Metrology). After reconstruction, volume rendering of the spike was manipulated using VG studio 2.0 software (Volume Graphics GmbH, Germany). No segmentation was performed for the 3D images. Background noise removal and definition of spike tissue type were achieved by manually selecting appropriate grayscale thresholds of different tissue. The sectional image of the spike was created in the sagittal plane using the clipping box tool.
Immunostaining After CT Scanning
Regenerated spikes were fixed in 4% formalin and then incubated in 50% Hexabrix before the microCT scan. After scanning, the samples were washed in PBS to remove the Hexabrix and then processed for cryosectioning. Immunofluorescence staining was performed as described (Chen et al., 2006). Muscle-specific monoclonal antibody 12/101 was used at 1:100 dilution of medium to detect differentiated muscle fibers. The secondary antibody was Alexa Fluor 594 goat anti-mouse IgG at 1:200 dilution (Invitrogen). To detect neuronal tissue in the spike, anti-beta tubulin III antibody (Sigma) was used at 1:200 dilution and followed by a fluorescein anti-rabbit IgG secondary antibody (Vector Laboratories) with 1:200 dilution.
RESULTS AND DISCUSSION
Conventional X-Ray Does Not Detect the Cartilage in Frog Spikes
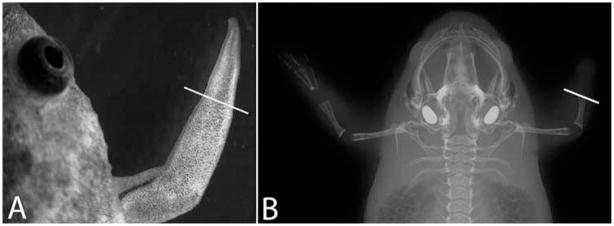
To test how good X-ray imaging is for visualizing the frog limb regeneration, we used a small animal imaging system (In Vivo MS FX-PRO, Carestream Molecular Imaging). It is designed for research animals such as rats and mice but it is difficult to obtain a high-magnification image of the frog limb spike, which is about 2–3 mm × 5–8 mm (Fig. 1A). We tested exposures over the range 1–5 minutes but were not able to visualize the cartilage of the spike (Fig. 1B). We also tested a small dental X-ray machine but this gave a less sharp image and also did not show the cartilage (not shown).
Fig. 1.
X-ray images of Xenopus frogs. A: A bright field image of a regenerated frog spike. B: X-ray image of a frog captured by the In Vivo MS FX PRO imaging system. Limb amputation was performed on the right forelimb only and a spike was formed on the right amputation stump. The white lines indicate the amputation planes.
Detection of Cartilage in Frog Spikes Using microCT with Contrast Agent
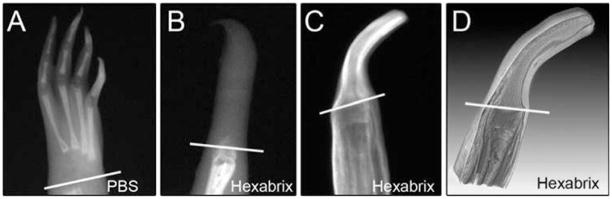
To test the ability of microCT to analyze Xenopus frogs, we first scanned a control frog hand without using any contrast agent. As shown in Fig. 2A, the phalanges of each digit were clearly visible and the resolution of the microradiographs from the microCT machine is much higher than that of the images from the other two X-ray systems. However, the cartilage in the digit joints and the carpals was still invisible. Next, we tested the contrast-based imaging method using regenerated frog spike. We analyzed 10 cases altogether. We tried soaking the spike in the 50% Hexabrix/50%PBS suggested by Guldberg’s group for 1.5 hr and then ran a microCT scan (Xie et al., 2009). But we still could not visualize the cartilage (Fig. 2B). All we saw above the amputation plane was a mass of soft tissue. We noted that the workers in previous studies had removed tissues surrounding the cartilage before the contrast staining (Palmer et al., 2006; Xie et al., 2009), which suggested that penetration of the contrast agent through the skin of the specimen was an issue in the sample preparation.
Fig. 2.
MicroCT images of Xenopus frog forearm and spikes. A: An original microradiograph of an unoperated frog hand obtained by the microCT scanner with a setting of 45 kV, 80 μA. The white line indicates the future limb amputation site. B: An original microradiograph of a frog spike with a setting of 70 kV, 140 μA. The spike with skin was incubated in 50% Hexabrix for 1.5 hr before scanning. C: An original microradiograph of a frog spike with a setting of 85 kV, 160 μA. The spike was skinless and was incubated in 50% Hexabrix for 2 hr before CT scanning. D: A sagittal sectional view from the 3D reconstructed image of the spike in C. Spike tissue was distinguished by distinct gray value thresholds. For better image presentation, the voxels with gray values less than 32,075 were set as full transparent, 32,075–39,700 as bone, 39,700–46,600 as cartilage, and 46,600–59,960 as muscle. The white lines in B–D indicate the limb amputation plane. Image A–C are inverted using photoshop.
Therefore, we removed the skin of the spike and then incubated the skinless spike in 50% Hexabrix/50%PBS for periods of time from 30 min to 3 hr before the microCT scanning. It turned out that a 2-hr incubation is optimal and could generate a negative image that clearly shows the boundary of the cartilage and its surrounding connective tissues (Fig. 2C). Under these conditions, the cartilage protruding from the limb amputation surface is readily distinguishable from the surrounding tissues in the microCT images, but the density of the cartilage is lower than its surrounding tissues. This is because the surrounding tissues had been over stained by the contrast agent so that the cartilage in the spike can be outlined. Figure 2D showed a section through a 3D reconstruction of this specimen. The volume rendering of the reconstruction was performed using the software VGstudio. The thresholds of gray values for different tissues were manually chosen to achieve a very good image of the cartilage. For this sample, using 16 bit images, the gray value ranges were: Bone: 32075–39700; Cartilage: 39700–46600; Muscle: 46600–59960.
The time of incubation in Hexabrix is important: with a shorter or longer period there was no difference in X-ray attenuation between cartilage and muscle. This means that for different sizes and ages of specimen the time should be varied to achieve optimal discrimination. Taken together, these results demonstrate that using Hexabrix as a contrast agent, and with removal of the skin, the microCT technique can be used to visualize small recently regenerated cartilage structures in situ in small animals such as the Xenopus frog.
MicroCT is Compatible with Immunohistochemistry
Since the energy used in microCT scanning was much higher than that in regular X-ray imaging, we were concerned about the preservation of the tissue morphology, and of antigens, both essential if we wanted to combine microCT imaging with other histological analysis. We tested the compatibility of Hexabrix-based microCT with immunohistochemistry on cryosections prepared from specimens following contrast based microCT imaging. After scanning, the tissue was washed in PBS for 2 hr to remove Hexabrix. In this study, we used two antibodies: anti-beta tubulin III for neural tissue detection (Lee et al., 1990), and 12/101 for muscle detection (Kintner and Brockes, 1984). These antibodies worked well on the control spikes that did not receive Hexabrix and X-ray radiation (Fig. 3A, B). In the scanned group, anti-beta tubulin III and 12/101 also worked very well (Fig. 3C, D). So, these results confirm that Hexabrix based microCT and immunohistochemistry can be performed on the same specimen.
Fig. 3.
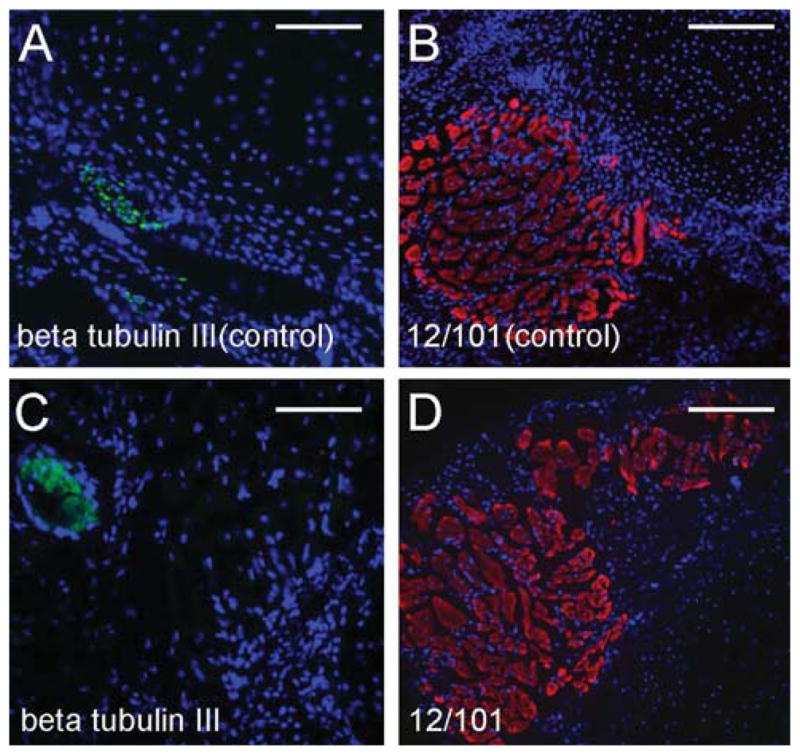
Detection of gene expression by immunofluorescence staining following contrast based microCT imaging and cryosectioning. A and B: Antibody staining was performed using control frog spikes without any treatment or CT scanning. A: Neuron specific beta tubulin III expression (green); B: Muscle specific 12/101 antibody staining (red). C and D: Skinless spikes were fixed in 4% formalin and then incubated in 50% Hexabrix for 2 hr before the microCT scan. The microCT scan was performed at a setting of 85 kV, 160 μA. After scanning, the samples were washed in PBS to remove Hexabrix and then processed for cryosectioning. C: Beta tubulin III expression (green) in a microCT scanned spike; D: 12/101 antibody staining (red) in a microCT scanned spike. Scale bars = 0.1 mm.
It has been reported that contrast-based microCT allows imaging of diverse non-mineralized animal tissues (Metscher, 2009), such as mouse embryos and Xenopus embryos. However, in that study some of the contrast agents used are either toxic or not readily available, and some of the fixatives are not compatible with immunostaining. Thus, their methods do not entirely meet our requirements for regeneration studies. Here, we used the low toxicity and clinically available agent Hexabrix as a contrast medium. This agent can be desorbed by washing with PBS (Xie et al., 2009), and we also have shown that the X-ray radiation did not interfere with the antigen and antibody binding. Thus, this study not only expands our current range of techniques to visualize anatomical structures, but also provides a useful non-destructive assessment tool for regeneration research.
Acknowledgments
Grant sponsor: NIH; Grant number: R01GM088500.
LITERATURE CITED
- Beck CW, Izpisua Belmonte JC, Christen B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn. 2009;238:1226–1248. doi: 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin GF, Slack JMW. Control of muscle regeneration in the Xenopus tadpole tail by Pax7. Development. 2006;133:2303–2313. doi: 10.1242/dev.02397. [DOI] [PubMed] [Google Scholar]
- Dent JN. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J Morphol. 1962;110:61–77. doi: 10.1002/jmor.1051100105. [DOI] [PubMed] [Google Scholar]
- Endo T. Analysis of gene expression during Xenopus forelimb regeneration. Dev Biol. 2000;220:296–306. doi: 10.1006/dbio.2000.9641. [DOI] [PubMed] [Google Scholar]
- Goss RJ, Holt R. Epimorphic vs tissue regeneration in Xenopus forelimbs. J Exp Zool. 1992;261:451–457. doi: 10.1002/jez.1402610412. [DOI] [PubMed] [Google Scholar]
- Inouye M. Differential staining of cartilage and bone in fetal mouse skeleton by Alcian Blue and Alizarin Red S. Congenital Anomalies. 1976:171–173. [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Metscher BD. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009;9:11. doi: 10.1186/1472-6793-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka K, Holler-Dinsmore G, Bryant SV. Intrinsic control of regenerative loss in xenopus-laevis limbs. J Exp Zool. 1986;240:47–54. doi: 10.1002/jez.1402400107. [DOI] [PubMed] [Google Scholar]
- Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc Natl Acad Sci USA. 2006;103:19255–19260. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JMW, Lin G, Chen Y. The Xenopus tadpole—a new model for regeneration research. Cell Mol Life Sci. 2008;65:54–63. doi: 10.1007/s00018-007-7431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Lin AS, Levenston ME, Guldberg RE. Quantitative assessment of articular cartilage morphology via EPIC-microCT. Osteoarthritis Cartilage. 2009;17:313–320. doi: 10.1016/j.joca.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]