Abstract
Recent findings implicate group II metabotropic glutamate receptors (mGluR2/3) in the reinforcing effects of psychostimulants and have identified these receptors as potential treatment targets for drug addiction. Here, we investigated the effects of mGluR2/3 stimulation on cue- and drug-primed reinstatement in rats with different histories of methamphetamine (METH) self-administration training, under two conditions: 16 daily sessions of short access (90 min/day, ShA), or 8 daily sessions of short access followed by 8 sessions of long access (6 hr/day, LgA). Following self-administration and subsequent extinction training, rats were pretreated with the selective mGluR2/3 agonist LY379268 (variable dose, 0 – 3 mg/kg), exposed to METH-paired cues or a priming injection of METH (1 mg/kg), and tested for reinstatement of METH-seeking behavior. LgA rats self-administered greater amounts of METH during the second half of training, but when pretreated with vehicle, ShA and LgA rats showed cue- and drug-primed reinstatement at equivalent response rates. However, LgA rats demonstrated greater sensitivity to mGluR2/3 stimulation with attenuated responding during cue-induced reinstatement after 0.3 mg/kg and higher doses of LY379268, whereas ShA rats decreased cue-induced reinstatement behavior following 1.0 mg/kg and 3.0 mg/kg LY379268. Additionally, both LgA and ShA rats exhibited decreased METH-primed reinstatement behavior following 0.3 mg/kg and higher doses of LY379268. A separate group of control rats was trained to self-administer sucrose pellets, and demonstrated attenuated cue-induced sucrose-seeking behavior following 1.0 and 3.0 mg/kg LY379268. Together, the results indicate that LY379268 has differential attenuating effects on cue-induced reinstatement behavior in rats with different histories of METH intake.
Keywords: escalation, methamphetamine, mGluR, reinstatement
1. Introduction
Methamphetamine (METH) abuse is characterized by repeated episodes of craving and relapse, even when the medical and social consequences of drug use are recognized (Hartz et al., 2001; McLellan et al., 2000; Rutkowski and Maxwell, 2009). The development of a persistent and irrepressible desire to procure and self-administer METH is associated with adaptations of multiple neurotransmitter systems, particularly catecholaminergic systems, induced over time by intoxication and withdrawal (Barr et al., 2006; Hanson and Fleckenstein, 2009). Unfortunately, there are no existing medications that are approved for preventing relapse in abstinent METH abusers (Rose and Grant, 2008). However, there is burgeoning evidence that characterization of the contributions of glutamatergic neurotransmission to METH taking and relapse behaviors may lead to additional treatment possibilities (Gass et al., 2009; Reichel and See, 2010). This interest in glutamatergic mechanisms has rapidly grown in studies of addiction to other drugs of abuse as well (Gass and Olive, 2008; Kalivas et al., 2009; Tzschentke and Schmidt, 2003).
Development of animal self-administration models that incorporate extended periods of daily training have shown that rats subjected to this regimen rapidly escalate intake of psychostimulants (Ahmed and Koob, 1998; Kitamura et al., 2006; Wee et al., 2007a). This method arguably provides a closer approximation of the compulsive drug intake experienced by severely addicted individuals (American Psychiatric Association, 2000). Escalated daily rates of drug intake in rats are also strongly associated with elevations of intracranial self-stimulation thresholds (Ahmed et al., 2002) as well as excessive time spent on defensive burying following an aversive stimulus (Aujla et al., 2008), providing compelling evidence of physiological consequences of severe drug intake manifesting as dysphoria and anxiety-like behaviors. These behaviors in animals (Koob and Le Moal, 1997) in turn validate the modeling of negative affect observed in human addicts (Sinha et al., 2000). Prolonged exposure to addictive drugs has been linked with the destabilization of glutamate homeostasis, leading to hyperreactivity to drug-related stimuli and an enhanced sensitivity to stressful events (see for review Kalivas, 2009). The presence of these neuroadaptations suggests that glutamatergic contributions to METH craving and relapse can be beneficially investigated in animals subjected to prolonged drug exposure by extended access to drug self-administration.
Glutamate is the primary excitatory neurotransmitter in the central nervous system and is responsible for up to 70% of neuronal communication, and ligands acting on ionotropic glutamate receptors have been shown to interfere with the development of addictive behaviors but also have a plethora of undesirable side effects (Uys and LaLumiere, 2008). Research interest has consequently turned toward developing compounds that target the metabotropic glutamate receptors (mGluRs), which modulate extracellular glutamate release and mediate experience-dependent alterations in the glutamatergic system (Olive, 2009). Recent experiments utilizing animal models of drug consumption and drug-motivated behavior have shown a therapeutic potential of agonists of group II metabotropic glutamate (mGluR2/3) receptors, a subfamily of mGluRs that are mainly localized to presynaptic and perisynaptic elements, and exert a dampening effect on synaptic glutamate levels (Benarroch, 2008; Schoepp, 2001). Stimulating mGluR2/3 receptors with the specific agonist LY379268 has been shown to attenuate heroin-, cocaine- and alcohol-seeking behavior in rats previously trained to self-administer these drugs (Backstrom and Hyytia, 2005; Baptista et al., 2004; Bossert et al., 2005; Martin-Fardon and Weiss, 2011; Peters and Kalivas, 2006). Self-administration of amphetamine was reduced in rats with a history of amphetamine exposure, when the stimulant was co-administered with LY379268 (Kim et al., 2005). While this compound has been associated with interference of responding for non-drug rewards as well as reduction of general locomotor behavior (Backstrom and Hyytia, 2005; Bossert et al., 2006; Peters and Kalivas, 2006), drug-specific effects of LY379268 have been observed in rats with histories of prolonged drug exposure (Hao et al., 2010; Kufahl et al., 2011; Sidhpura et al., 2010).
Multiple studies have identified glutamate as an important substrate of relapse-like behaviors following METH self-administration (Kufahl and Olive, 2011). Treatment with modafinil, a wakefulness-promoting drug with dopaminergic and glutamatergic actions (Ballon and Feifel, 2006), reduced reinstatement of METH seeking following re-exposure to a drug-paired environment (Reichel and See, 2010). Also, blocking the function of postsynaptic mGluR5 receptors has been shown to dampen reinstatement of METH seeking as well as responding for the drug under a progressive ratio schedule of reinforcement (Gass et al., 2009). However, there is reason to believe that the effectiveness of glutamatergic ligands to change in animals with histories of extensive METH intake: rats exposed to prolonged sessions of cocaine self-administration have been found to be more sensitive to the effects of LY379268 but somewhat less sensitive to mGluR5 antagonist treatment (Hao et al., 2010). Recent studies of rats exposed to repeated daily prolonged (6 hr or more) sessions have reported increased drug seeking triggered by METH priming (Rogers et al., 2008) and augmented behavioral sensitivity to treatment with a dopamine D2 receptor agonist (Wee et al., 2007b). However, the role of mGluRs in METH-motivated behaviors in these “post-escalated” animals has so far been unexplored.
This study examined the role of mGluR2/3 receptors in METH-seeking behavior of rats with histories of restricted and extended access to METH self-administration. Following self-administration and extinction training, rats were pretreated with LY379268 and tested for reinstatement of METH-seeking behavior triggered by exposure to METH-paired sensory cues or a non-contingent METH priming injection. To balance the effects of cue conditioning between the groups with different self-administration histories, rats with extended drug access were given the same amount of METH-paired cues as the restricted-access rats, and subjected to additional daily training where METH reinforcement was delivered without presentation of the cues. To test for the effects of LY379268 on behavior motivated by non-drug rewards, we also trained a separate group rats to self-administer sucrose pellets and tested for cue-elicited reinstatement of sucrose-seeking behavior.
2. Materials and Methods
2.1. Animals
A total of 74 male Sprague-Dawley rats (Harlan Laboratories, Livermore, CA; 250–275 g upon arrival) were single-housed and maintained on a 12 h/12 h reversed light/dark cycle, and all training and testing was conducted without food or water restriction during the dark phase of the cycle. All experimental procedures were conducted in accordance to the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Care and Use Committee of Arizona State University.
2.2. Experimental Design
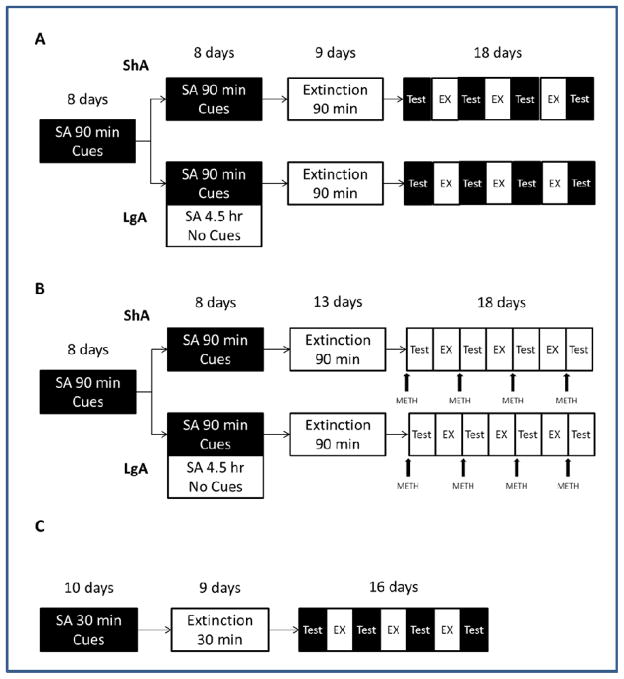
In Experiments 1 (initial n=28), 2A (n=34) and 2B (n=12), rats were trained to self-administer (SA) METH over a period of 16 days by pressing a lever, subjected to extinction training for at least 11 days and then given four tests for reinstatement of METH-seeking behavior following pretreatment with different doses of LY379268 (Fig. 1). The reinstatement tests were separated by a series of additional 4–7 extinction sessions to reestablish baseline extinction responding. In Experiment 1, reinstatement was triggered by exposure to METH-paired sensory cues. In Experiments 2A and 2B, reinstatement was elicited by a 1 mg/kg METH priming injection prior to testing. Rats in these experiments were divided into short access (ShA) and long access (LgA) groups: ShA rats were given 90-min sessions for the entire span of SA training, and LgA were given 90-min sessions for the first 8 days and 6-hr sessions for the last 8 days of SA training. Experiment 2B was included to duplicate the procedure of Experiment 2A, utilizing a different range of LY379268 doses (0, 0.1 and 1 mg/kg) prior to drug-primed reinstatement testing.
Figure 1.
Diagram illustrating the experimental procedure for Experiment 1 (A), Experiments 2A and 2B (B) and Experiment 3 (C). Dark boxes denote sessions where operant responding results in the presentation of reinforcer-paired cues, and white boxes indicate no presentation of cues.
In Experiment 3 (n=12), rats were trained to SA sucrose pellets by pressing a lever during 10 daily 30-min sessions. This was followed by 9 days of extinction training, and subsequently four tests for the reinstatement of sucrose-seeking behavior following pretreatment with different doses of LY379268. Like the other experiments, the reinstatement tests were separated by a series of 3–4 extinction sessions to reestablish baseline extinction responding.
2.3. Surgical Procedures
Prior to arrival at the animal facility, 74 of the rats were surgically pre-implanted with Silastic rounded-tip jugular indwelling catheters at Harlan Laboratories, and the catheters were filled with HepLock solution to prevent loss of patency during shipment. 24 hr after arrival, rats were anesthetized with isoflurane (2% v/v, Butler Animal Health Supply, Dublin, OH) vaporized in oxygen at a flow rate of 2 L/min. The rats were also given pre-incision injections of buprenorphine (0.05 mg/kg, s.c., Reckitt Benckiser, Richmond, VA) and meloxicam (1 mg/kg, s.c., Boehringer Ingelheim, St. Joseph, MO). The skin area where the catheter exited between the scapulae was cleaned with 1% iodine (Purdue Products, Stamford, CT). A 2-cm incision was then made to connect the catheter to a back-mounted threaded vascular access port (Plastics One, Roanoke, VA). The catheter was anchored to the port with SNAP dental resin (Parkwell, Edgewood, NY) and secured to the surrounding tissue with a polyethylene mesh collar (Plastics One). The wound was then treated with 0.2 ml bupivacaine hydrochloride (0.25% v/v, Hospira, Lake Forest, IL), closed with nylon sutures (Ethicon, San Lorenzo, Puerto Rico) and topically treated with lidocaine (Hi-Tech Pharmacol, Amityville, NY) and a triple antibiotic gel (G&W Laboratories, South Plainfield, NJ). The HepLock solution was evacuated from the catheter, which was then flushed with 0.2 ml heparin (100 U/ml, Sagent Pharmaceuticals, Schaumberg, IL) and 0.3 ml cefazolin (West-Ward Pharmaceuticals, Estontown, NJ). The access port was then sealed with a plastic obturator and a threaded protective cap (Plastics One). Rats were given two injections of 0.9% saline (5 ml each, s.c.) and small portions of “Froot Loops” cereal to facilitate postsurgical rehabilitation. Following surgical procedures, rats were allowed to recover for 5 days. Throughout the experiment, the rats received daily intravenous infusions of 0.1 ml cefazolin and 0.2 ml heparin to minimize infections and maintain catheter patency.
2.4. Drugs
Methamphetamine hydrochloride (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% sterile saline for intravenous (i.v.) self-administration and intraperitoneal (i.p.) injection. LY379268 ((1R, 4R, 5S, 6R)-4-Amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarbolic acid; Tocris, Ellisville, MO) was dissolved in sterile water and delivered subcutaneously (s.c.) in a volume of 1 ml/kg, 30 min before behavioral testing.
2.5. Behavioral Training and Testing
2.5.1. Operant Methamphetamine Self-administration
Starting the day after surgery, rats in Experiments 1, 2A and 2B received 3–5 sucrose pellets (45 mg, TestDiet, Richmond, IN) daily in their home cages. After five days of recovery, rats were placed into the operant chambers for two daily 90-min pre-training sessions. Sessions were initiated by extension of an active lever, and thereafter responses on the active lever were reinforced with delivery of a sucrose pellet on a continuous reinforcement schedule. Responses on the inactive lever were registered but resulted in no schedules consequences for the entirety of the experiment. Following pre-training, rats were placed into the operant chambers for daily 90-min self-administration sessions. Each response on the active lever resulted in activation of an infusion pump delivering for 2 sec, delivering 0.05 mg/kg METH in 0.06 ml saline. METH reinforcement was accompanied by simultaneous activation of a cue light and tone for 5 sec, and a 20 sec timeout period during which responses on the active lever had no consequences. After completion of eight 90-min SA sessions, rats were divided in ShA and LgA groups, counterbalanced for total METH exposure. ShA rats received eight additional 90-min training sessions with light and tone cues. LgA rats also received eight 90-min sessions with cues, but each session was immediately followed by another 4.5 hr session where active lever responses were reinforced with 0.05 mg/kg METH infusions without presentation of cues to maintain an equal number of drug-cue exposures between ShA and LgA groups.
Rats in Experiment 3, which were not subjected to surgery but were given sucrose pellets (45 mg, TestDiets, Richmond, IN) in their home cages for five days, were also trained to respond on the active lever for sucrose pellet reinforcement. These training sessions were 30 min and paired pellet delivery with activation of light and tone cues for 5 sec and a 20 sec timeout period. Sucrose SA training continued for ten consecutive sessions.
2.5.2. Extinction Training
Following self-administration training, rats in all experiments underwent daily sessions under extinction conditions until active lever responding in the last three sessions reached the criterion of < 20% of baseline (average of last three sessions) SA responding. These sessions were 90 min in Experiments 1 and 2, and 30 min in Experiment 3. During this time, levers were presented but responses on either lever resulted in no programmed consequences.
2.5.3. Pharmacological Testing during Cue-Induced Reinstatement
Reinstatement tests began one day after the final extinction session. In Experiment 1, these tests lasted 90 min under conditions where responses on the active lever resulted in the presentation of METH-paired cues but not METH reinforcement. LY379268 (0.3, 1, or 3 mg/kg) or vehicle was administered 30 min prior to the test sessions. Every dose of LY379268 was tested in all rats, in a randomized Latin Square design. Between reinstatement tests, rats underwent extinction training until baseline criterion was reached.
2.5.4. Pharmacological Testing during Drug-primed Reinstatement
In Experiment 2, reinstatement tests lasted 90 min under conditions identical to extinction training, where responses on either lever resulted in no programmed consequences. Starting 30 min prior to each test session, rats were injected with LY379268 (0.3, 1, or 3 mg/kg in Experiment 2A; 0.1 or 1 mg/kg in Experiment 2B) or vehicle, and 10 min later were injected with a priming dose of METH (1 mg/kg, i.p.). In each experiment, all LY379268 doses were tested in each rat, in a randomized Latin Square design. Between reinstatement tests, rats underwent extinction training until baseline criterion was reached.
2.5.5. Pharmacological Testing during Cue-Induced Reinstatement to Sucrose Seeking
In Experiment 3, reinstatement tests lasted 30 min under conditions where responses on the active lever resulted in presentation of sucrose-paired cues but not pellet delivery. LY379268 (0.3, 1, or 3 mg/kg) or vehicle was administered 30 min prior to the test sessions. Each dose of LY379268 was tested in all rats, in a randomized Latin Square design. Between reinstatement tests, rats underwent additional extinction training until baseline extinction criterion was again reached.
2.6. Statistical Analysis
Statistical analyses of data from the three experiments were analyzed separately. Daily METH-reinforced responses were analyzed by 2 × 16 mixed factorial analysis of variance (ANOVA) with drug history (ShA or LgA) as a between-subjects factor and training day as a within-subjects factor. Average METH-reinforced responses were also analyzed by 2 × 2 mixed factorial ANOVA, with drug history as a between-subjects factor and training phase (first 8 sessions or second 8 sessions) as a within-subjects factor. Training phase averages of active lever responses during the entire session, during the first 90 min of each session and inactive lever responses were similarly analyzed. Extinction responses were analyzed by 2-way mixed factorial ANOVA, using drug history as a between-subjects factor and extinction day as a within-subjects factor. Responses on the active and inactive lever were analyzed separately. The effects of LY379268 on reinstatement of METH seeking in ShA and LgA rats were analyzed by 2 × 4 (2 × 3 in Experiment 2B) repeated measures ANOVAs with test condition (extinction or cues) and LY379268 dose as within-subjects factors. To better illustrate the apparent different differences in LY379268 potency across the METH history conditions, active lever responses recorded during the reinstatement tests were converted to fractional inhibition scores by normalizing to reinstatement responding following vehicle treatment. Fractional inhibition scores associated with corresponding LY379268 doses were then compared between ShA and LgA rats.
For Experiment 3, daily responding for sucrose pellets was analyzed by one-way ANOVA with training day a within-subjects factor. The effects of LY379268 on reinstatement of sucrose seeking were analyzed by one-way ANOVA with LY379268 dose (0, 0.3, 1 or 3 mg/kg) as a within-subjects factor. Significant main effects or interactions were followed by reduced ANOVA models, Fisher’s LSD tests or simple effects ANOVAs. All data are presented as mean ± SEM.
3. Results
Of the 62 rats that were implanted with jugular vein catheters, 14 rats were removed from the study prior to reinstatement testing because of infections and/or catheter patency issues. All 12 rats in Experiment 3 completed the entire study.
3.1. Experiment 1
3.1.1. METH Self-administration and Extinction
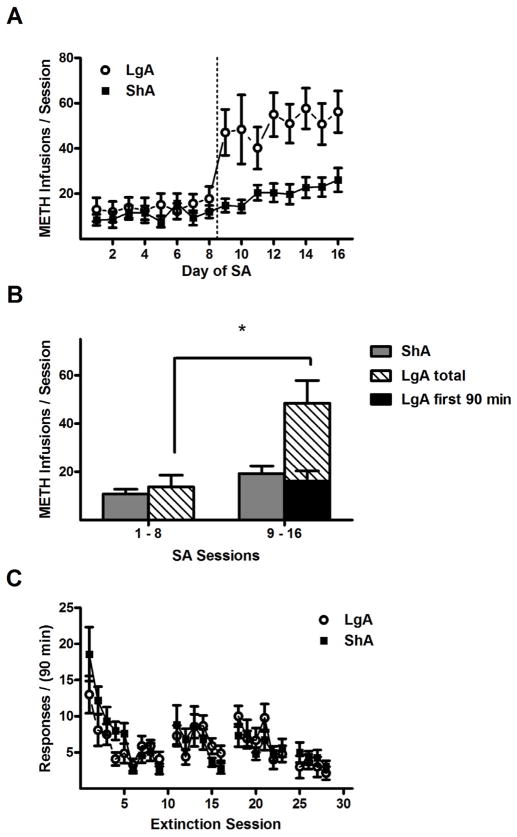
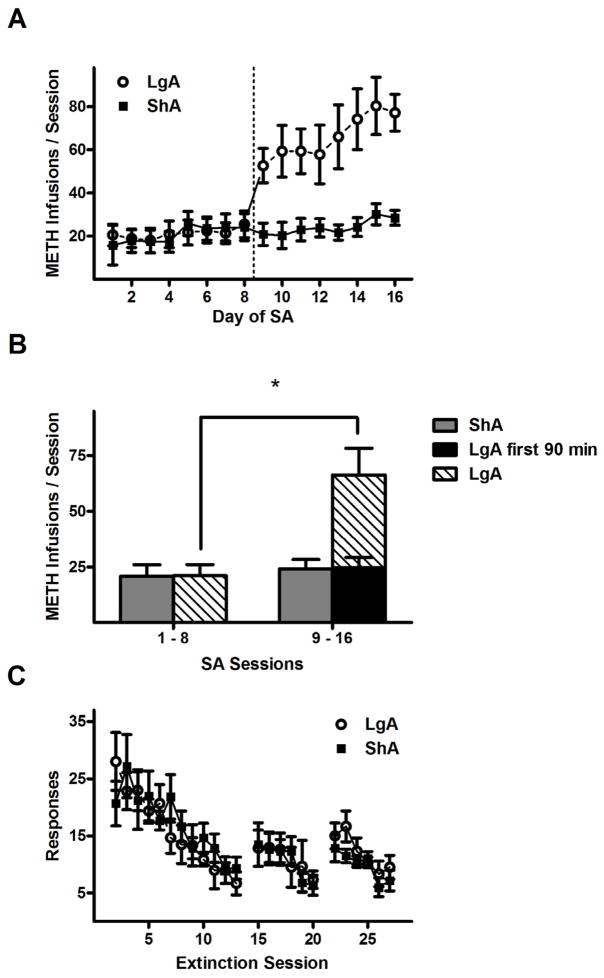
In Experiment 1, the length of the conditioning sessions during the second half of SA training exerted a dramatic influence on the amount of METH consumed (Fig. 2A). ANOVA of the daily METH infusions revealed a significant drug history × training day interaction (F15,285 = 7.4, p < 0.005) as well as main effects of drug history (F1,285 = 7.7, p < 0.05) and training day (F15,285 = 21.2, p < 0.005). ANOVA of the average METH infusions also revealed a significant drug history × training phase interaction (F1,19 = 18.3, p < 0.005). Subsequent post hoc comparisons demonstrated that, on average, LgA rats received more infusions in the second half of SA training than the first half (50.5 ± 8.6 vs. 14.7 ± 4.4, Fisher’s LSD test, p < 0.05), and during the second half they received more infusions than ShA rats (50.5 ± 8.6 vs. 18.0 ± 3.1, Fisher’s LSD test, p < 0.05). These infusion totals corresponded to average daily METH intake estimates of 0.69 (ShA) and 0.73 mg/kg (LgA) for the first half and 0.90 (ShA) and 2.52 mg/kg (LgA) for the second half. Similar results were found in the active lever pressing data (drug history × training phase: F1,19 = 13.6, p < 0.005; LgA second half responses > LgA first half responses and ShA second half responses, Fisher’s LSD tests, p < 0.05). In contrast, no significant effects were found between responses or infusions recorded in the first 90 min of the LgA sessions during the second half of SA training, and the corresponding totals from the LgA sessions during the first half and ShA sessions during the second half of SA training (Fig. 2B). These results indicate that the LgA rats exhibited more responses and received more METH during the second half of SA training, but the escalated activity occurred within the extended sessions in the absence of METH-paired cues. No significant effects were found in analysis of responding on the inactive lever.
Figure 2.
METH self-administration and extinction responding in the ShA (short access) and LgA (long access) conditions in Experiment 1. (A) Mean (±SEM) number of METH reinforcements per training session, which is 90 min in sessions 1–8 and 9–16 for ShA rats, and 4.5 hr in sessions 9–16 for LgA rats. Dotted line indicates division between the two phases of the self-administration procedure. (B) Mean (± SEM) number of METH reinforcements per 90 min session, averaged across all sessions of each training phase. (C) Mean (± SEM) number of active lever responses per 90 min extinction session. Gaps in the time series correspond to days where reinstatement tests were performed. Sample sizes (N): ShA = 11 and LgA = 10. * P < 0.05, difference between first and second phase of self-administration training.
During extinction training, responding on the active lever progressively decreased (Fig. 2C) as confirmed by a main effect of extinction day (F8,152 = 15.8, p < 0.005), but no other main effects or interactions. Responding decreased from the first day average of 13.0 ± 2.6 to a final day mean of 4.1 ± 1.0 in LgA rats, and from the first day mean of 18.6 ± 3.4 to a final day mean of 2.7 ± 0.7 in ShA rats.
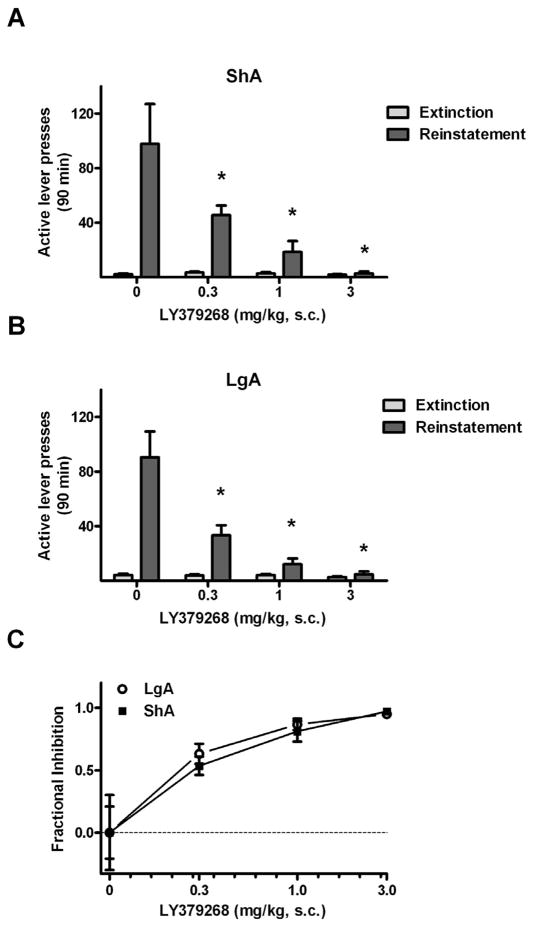
3.1.2. Effects of LY379268 on Cue-Elicited Reinstatement of METH-Seeking Behavior
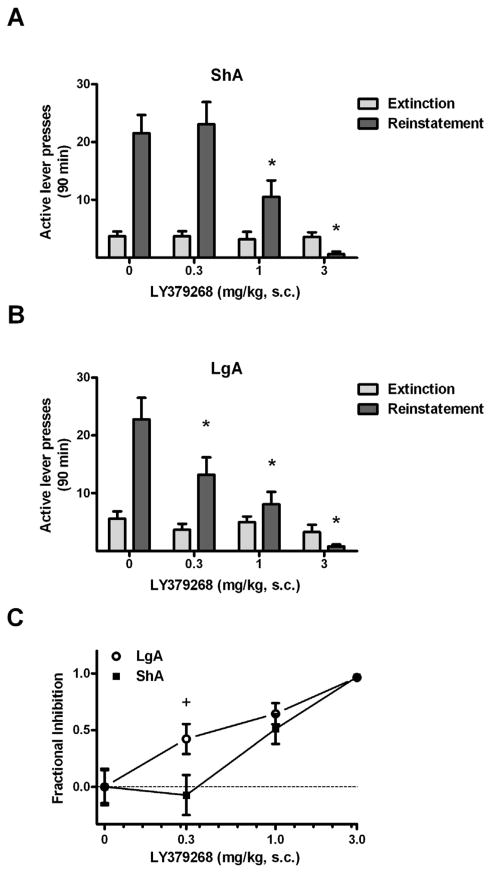
LY379268 dose-dependently attenuated reinstatement behavior in both drug history conditions, as confirmed by the presence of significant test condition × LY379268 dose interactions in the reinstatement data of both ShA (Fig. 3A, F3,60 = 13.8, p < 0.0005) and LgA rats (Fig. 3B, F3,54 = 16.7, p < 0.0005). Specifically, no significant difference from vehicle responding (21.5 ± 3.1) with the 0.3 mg/kg LY379268 dose (23.1 ± 3.8) in the ShA rats, whereas this dose significantly reduced reinstatement in the LgA group (13.2 ± 3.0 vs. 22.8 ± 3.7, Fisher’s LSD, p < 0.05). Moreover, there was a trend toward a significant difference between the responding with this dose between the ShA and LgA rats (unpaired t-test, P = 0.056). The responding was significantly higher during the cue test sessions than the corresponding extinction baselines in the ShA group at 0 (Fisher’s LSD, p < 0.005), 0.3 (p < 0.005) and 1.0 mg/kg LY379268 (p < 0.05), and in the LgA group at 0 (p < 0.005) and 0.3 mg/kg LY379268 (p < 0.005), indicating that responding in the presence of METH-paired cues decreased to extinction baseline levels at smaller doses (1.0 and 3.0 mg/kg) in the LgA rats than in the ShA rats (3.0 mg/kg only). However, the overall effect of LY379268 on reinstatement in both groups was confirmed by significant main effects of LY379268 dose (ShA: F3,60 = 14.3, p < 0.005, LgA: F3,54 = 17.2, p < 0.005). The overall triggering effect of METH-paired cues in both groups was shown by the presence of significant main effects of test condition (ShA: F1,60 = 33.8, p < 0.005, LgA: F1,54 = 12.6, p < 0.05).
Figure 3.
Effects of the mGluR2/3 agonist LY379268 on cue-elicited reinstatement in rats of the ShA and LgA conditions in Experiment 1. (A) Mean (± SEM) number of active lever presses exhibited by ShA rats during the last two days of extinction training and during reinstatement test sessions, where they were pretreated with 0, 0.3, 1.0 or 3.0 mg/kg LY379268 30 min prior to testing. N = 11. (B) Mean (± SEM) number of active lever presses exhibited by LgA rats during the last two days of extinction training and during reinstatement testing. N = 10. (C) Active lever responses expressed as the fractional inhibition of responding relative to reinstatement responding following vehicle treatment. Sample sizes (N): ShA = 11 and LgA = 10. * P < 0.05, vs. vehicle treatment. + P < 0.05 difference between ShA and LgA rats.
Comparisons of fractional inhibition scores at each data point confirmed that the dose-response for the effects of LY379268 on reinstatement differed across METH history conditions (Fig. 3C), with a significant difference between LgA and ShA rats at 0.3 mg/kg (LgA: 0.42 ± 0.13, ShA: −0.07 ± 0.18; Fisher’s LSD test, p < 0.05), but not at any other doses.
3.2. Experiment 2A
3.2.1. METH Self-administration and Extinction
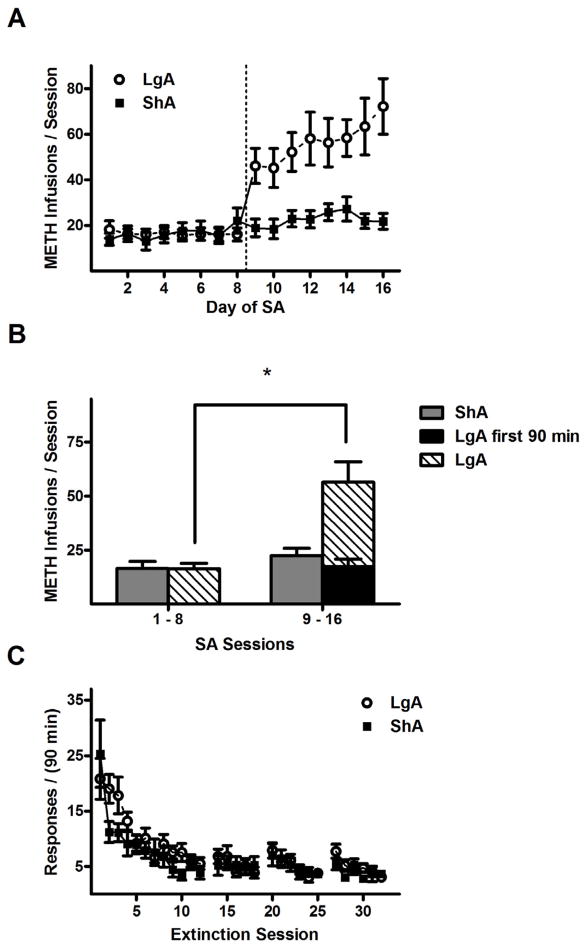
In Experiment 2A, the length of the conditioning sessions during the second half of SA training also had a significant influence on the amount of METH consumed (Fig. 4A). ANOVA of the daily METH infusions revealed a significant drug history × training day interaction (F15,285 = 10.0, p < 0.005) as well as main effects of drug history (F1,285 = 6.8, p < 0.05) and training day (F15,285 = 17.8, p < 0.005). ANOVA of the average METH infusions (Fig. 4B) also revealed a significant drug history × training phase interaction (F1,19 = 14.6, p < 0.005). Subsequent post hoc comparisons demonstrated that, on average, LgA rats received more infusions in the second half of SA training than the first half (56.5 ± 9.4 vs. 16.4 ± 2.4, Fisher’s LSD test, p < 0.005), and during the second half they received more infusions than ShA rats (56.5 ± 9.4 vs. 22.5 ± 3.4, Fisher’s LSD test, p < 0.05). These infusion totals corresponded to average daily METH intake estimates of 0.83 (ShA) and 0.82 mg/kg (LgA) for the first half and 1.12 (ShA) and 2.83 mg/kg (LgA) for the second half. Similar results were found in the active lever pressing data (main effects of drug history: F1,18 = 15.1, p < 0.005, and training phase: F1,18 = 4.5, p < 0.05; LgA second half responses > LgA first half responses and ShA second half responses, Fisher’s LSD tests, p < 0.05). In contrast, no significant effects were found between responses or infusions recorded in the first 90 min of the LgA sessions during the second half of SA training, and the corresponding totals from the LgA sessions during the first half and ShA sessions during the second half of SA training. Like in Experiment 1, the LgA rats exhibited more responses and received more METH during the second half of SA training, but the escalated activity occurred within the extended sessions in the absence of METH-paired cues. No significant effects were found in analysis of responding on the inactive lever.
Figure 4.
METH self-administration and extinction responding in ShA and LgA conditions in Experiment 2A. (A) Mean (±SEM) number of METH reinforcements per training session, which is 90 min in sessions 1–8 and 9–16 for ShA rats, and 4.5 hr in sessions 9–16 for LgA rats. Dotted line indicates division between the two phases of the self-administration procedure. (B) Mean (± SEM) number of METH reinforcements per 90 min session, averaged across all sessions of each training phase. (C) Mean (± SEM) number of active lever responses per 90 min extinction session. Gaps in the time series correspond to days where reinstatement tests were performed. Sample sizes (N): ShA = 10 and LgA = 11. * P < 0.05, difference between first and second phase of self-administration training.
During extinction training, responding on the active lever progressively decreased (Fig. 4C) as confirmed by a main effect of extinction day (F11,198 = 14.5, p < 0.005), but no other main effects or interactions. Responding decreased from the first day average of 20.8 ± 3.7 to a final day mean of 5.5 ± 1.0 in LgA rats, and from the first day mean of 25.3 ± 6.1 to a final day mean of 3.9 ± 1.2 in ShA rats.
3.2.2. Effects of LY379268 on Drug-Primed Reinstatement of METH-Seeking Behavior
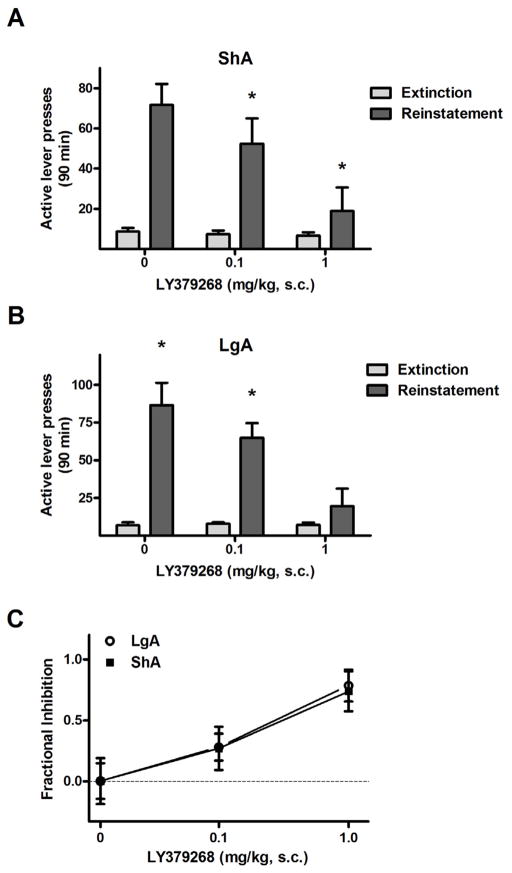
LY379268 dose-dependently attenuated reinstatement behavior in both drug history conditions, as confirmed by the presence of significant test condition × LY379268 dose interactions in the reinstatement data of both ShA (Fig. 5A, F3,48 = 8.2, p < 0.0005) and LgA rats (Fig. 5B, F3,60 = 15.1, p < 0.0005). Unlike the reinstatement data in Experiment 1, the effects of LY379268 in ShA rats mirrored the effects of LY379268 in LgA rats: both groups exhibited attenuated reinstatement of responding after pretreatment by the 1.0 (ShA: 16.8 ± 7.4, LgA: 11.1 ± 3.9) and 3.0 mg/kg (ShA: 3.0 ± 1.7, LgA: 4.3 ± 2.0), relative to vehicle responding (ShA: 88.6 ± 27.8, LgA: 82.9 ± 18.8; Fisher’s LSD tests, p < 0.05). The only exception to this pattern was that the difference between 0.3 mg/kg and vehicle treatment was significant in LgA rats (30.6 ± 7.2, Fisher’s LSD, p < 0.05) but not ShA rats (40.9 ± 7.9).
Figure 5.
Effects of the mGluR2/3 agonist LY379268 on METH-primed reinstatement in ShA and LgA rats in Experiment 2A. (A) Mean (± SEM) number of active lever presses exhibited by ShA rats during the last two days of extinction training and during reinstatement test sessions, where they were pretreated with 0, 0.3, 1.0 or 3.0 mg/kg LY379268 30 min prior to testing,. N = 11. (B) Mean (± SEM) number of active lever presses exhibited by LgA rats during the last two days of extinction training and during reinstatement testing. N = 10. (C) Active lever responses expressed as the fractional inhibition of responding relative to reinstatement responding following vehicle treatment. Sample sizes (N): ShA = 11 and LgA = 10. * P < 0.05, vs. vehicle treatment.
Responding was significantly higher during the drug priming test sessions compared to the corresponding extinction baselines in both ShA and LgA groups at 0 (Fisher’s LSD tests, p < 0.005) and 0.3 mg/kg LY379268 (p < 0.005), but not at 1.0 or 3.0 mg/kg, indicating that drug-induced METH seeking in LgA and ShA rats were similarly affected by mGluR2/3 pretreatment. Additionally, the overall effect of LY379268 on reinstatement in both groups was confirmed by significant main effects of LY379268 dose (ShA: F3,48 = 8.2, p < 0.005, LgA: F3,60 = 15.8, p < 0.005). The overall reinstatement effect of the METH priming injection in both groups was confirmed by the presence of significant main effects of test condition (ShA: F1,48 = 17.2, p < 0.005, LgA: F1,60 = 27.9, p < 0.0005).
Comparisons of fractional inhibition scores at each data point demonstrated that the dose-response for the effects of LY379268 on reinstatement was similar for the two METH history conditions (Fig. 5C), with no significant differences between LgA and ShA rats at any doses (Fisher’s LSD tests).
3.3. Experiment 2B
3.3.1. METH Self-administration and Extinction
In Experiment 2B, the length of the conditioning sessions during the second half of SA training also had a significant influence on the amount of METH consumed (Fig. 6A). ANOVA of the daily METH infusions revealed a significant drug history × training day interaction (F15,150 = 9.9, p < 0.005) as well as main effects of drug history (F1,150 = 5.8, p < 0.05) and training day (F15,150 = 14.4, p < 0.005). ANOVA of the average METH infusions (Fig. 6B) also revealed a significant drug history × training phase interaction (F1,10 = 16.3, p < 0.005). Subsequent post hoc comparisons demonstrated that, on average, LgA rats received more infusions in the second half of SA training than the first half (66.4 ± 11.9 vs. 21.1 ± 4.9, Fisher’s LSD test, p < 0.01), and during the second half they received more infusions than ShA rats (66.4 ± 11.9 vs. 24.1 ± 4.3, Fisher’s LSD test, p < 0.01). These infusion totals corresponded to average daily METH intake estimates of 1.04 (ShA) and 1.05 mg/kg (LgA) for the first half and 1.21 (ShA) and 3.32 mg/kg (LgA) for the second half. A drug history × training phase interaction was also found in the active lever pressing data (F1,10 = 16.4, p < 0.005), accompanied by similar post hoc results (LgA second half responses > LgA first half responses and ShA second half responses, Fisher’s LSD tests, p < 0.05). In contrast, no significant effects were found between responses or infusions recorded in the first 90 min of the LgA sessions during the second half of SA training, and the corresponding totals from the LgA sessions during the first half and ShA sessions during the second half of SA training. Like in Experiment 2A, the LgA rats exhibited more responses and received more METH during the second half of SA training, but the escalated activity occurred within the extended sessions in the absence of METH-paired cues. No significant effects were found in analysis of responding on the inactive lever.
Figure 6.
METH self-administration and extinction responding ShA and LgA rats in Experiment 2B. (A) Mean (±SEM) number of METH reinforcements per training session, which is 90 min in sessions 1–8 and 9–16 for ShA rats, and 4.5 hr in sessions 9–16 for LgA rats. Dotted line indicates division between the two phases of the self-administration procedure. (B) Mean (± SEM) number of METH reinforcements per 90 min session, averaged across all sessions of each training phase. (C) Mean (± SEM) number of active lever responses per 90 min extinction session. Gaps in the time series correspond to days where reinstatement tests were performed. Sample sizes (N): ShA = 6 and LgA = 6. * P < 0.05, difference between first and second phase of self-administration training.
During extinction training, responding on the active lever progressively decreased (Fig. 6C) as confirmed by a main effect of extinction day (F12,120 = 18.8, p < 0.005), but no other main effects or interactions. Responding decreased from the first day average of 49.0 ± 9.8 to a final day mean of 6.7 ± 2.0 in LgA rats, and from the first day mean of 54.2 ± 9.3 to a final day mean of 9.3 ± 1.9 in ShA rats.
3.3.2. Effects of LY379268 on Drug-Primed Reinstatement of METH-Seeking Behavior
LY379268 dose-dependently attenuated reinstatement behavior in both drug history conditions, as confirmed by the presence of significant test condition × LY379268 dose interactions in the reinstatement data of both ShA (Fig. 7A, F2,20 = 16.9, p < 0.005) and LgA rats (Fig. 7B, F2, 20= 10.7, p < 0.001). Like the reinstatement data in Experiment 2A, the effects of LY379268 in ShA rats matched the effects of LY379268 in LgA rats: both groups exhibited attenuated reinstatement of responding after pretreatment by the 1.0 (ShA: 18.8 ± 11.8, LgA: 19.5 ± 11.7) but not the 0.1 mg/kg dose (ShA: 52.3 ± 12.6, LgA: 64.8 ± 9.8), relative to vehicle responding (ShA: 71.7 ± 10.5, LgA: 86.5 ± 15.0; Fisher’s LSD tests, p < 0.05).
Figure 7.
Effects of the mGluR2/3 agonist LY379268 on METH-primed reinstatement in ShA and LgA rats in Experiment 2B. (A) Mean (± SEM) number of active lever presses exhibited by ShA rats during the last two days of extinction training and during reinstatement testing, where they were pretreated with 0, 0.1 or 1.0 mg/kg LY379268 30 min prior to testing. N = 6. (B) Mean (± SEM) number of active lever presses exhibited by LgA rats during last two days of extinction training and during reinstatement testing. N = 6. (C) Active lever responses expressed as the fractional inhibition of responding relative to reinstatement responding following vehicle treatment. Sample sizes (N): ShA = 6 and LgA = 6. * P < 0.05, vs. vehicle treatment.
Responding was significantly higher during the priming test sessions compared to the corresponding extinction baselines in both ShA and LgA groups at 0 (Fisher’s LSD tests, p < 0.005) and 0.1 mg/kg LY379268 (p < 0.05), but not at 1.0 mg/kg, largely duplicating the observations of Experiment 2A. The overall effect of LY379268 on reinstatement in both groups was confirmed by significant main effects of LY379268 dose (ShA: F2,20 = 19.5, p < 0.0005, LgA: F2,20 = 10.7, p < 0.001). The overall triggering effect of the METH prime in both groups was confirmed by the presence of significant main effects of test condition (ShA: F1,20 = 19.5, p < 0.0005, LgA: F1,20 = 29.7, p < 0.0005).
As with the data of Experiment 2A, the fractional inhibition of the nonzero doses of LY379268 used in Experiment 2B (Fig. 7C) contained no significant differences between LgA and ShA rats (Fisher’s LSD tests).
3.4. Experiment 3
3.4.1. Sucrose Self-administration and Extinction
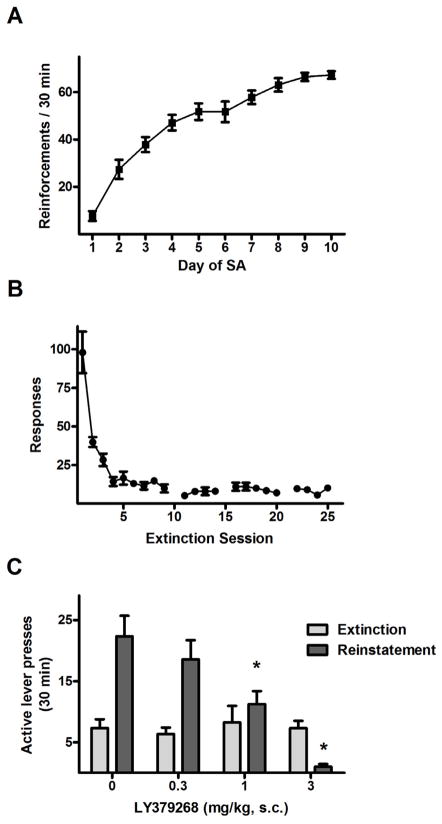
Rats trained to self-administer sucrose rapidly acquired the behavior and reached a stable baseline over the final three sessions (Fig. 8A). Sucrose reinforcements increased from the first session (7.7 ± 2.0) to the final session (67.3 ± 1.6), as confirmed by a significant effect of training day (F9,99 = 72.9, p < 0.0005). During extinction training (Fig. 8B), responding on the active lever progressively decreased as confirmed by a main effect of extinction day (F8,88 = 2.7, p < 0.005). Responding decreased from the first day average of 98.0 ± 13.5 to a final day mean of 9.8 ± 2.5.
Figure 8.
Sucrose self-administration and extinction responding and the effects of the mGluR2/3 agonist LY379268 on cue-elicited reinstatement of sucrose-seeking in Experiment 3. (A) Mean (±SEM) number of sucrose reinforcements per 30 min session. (B) Mean (± SEM) number of active lever responses per 30 min extinction session. Gaps in the time series correspond to days where reinstatement tests were performed. (C) Mean (± SEM) number of active lever presses exhibited during the last two days of extinction training and during reinstatement testing, where they were pretreated with 0, 0.3, 1.0 or 3.0 mg/kg LY379268 30 min prior to testing. N = 12. * P < 0.05, vs. vehicle treatment.
3.4.2. Effects of LY379268 on Cue-Elicited Reinstatement of Sucrose-Seeking Behavior
LY379268 dose-dependently attenuated reinstatement behavior in the sucrose-trained rats, as confirmed by the presence of significant test condition × LY379268 dose interaction in the reinstatement data (Fig. 8C, F3,22 = 9.8, p < 0.0005). Like the reinstatement data in Experiment 2 (but not in Experiment 1), rats exhibited attenuated reinstatement of responding after pretreatment by the 1.0 (11.3 ± 2.1) and 3.0 mg/kg (1.0 ± 0.5), relative to vehicle responding (22.3 ± 3.4, Fisher’s LSD tests, p < 0.05). In contrast, the difference in responding between 0.3 mg/kg (18.6 ± 3.1) and vehicle treatment was not significant.
The responding was significantly higher during the cue test sessions than the corresponding extinction baselines at 0 (Fisher’s LSD tests, p < 0.005) and 0.3 mg/kg LY379268 (p < 0.005), but not at 1.0 and 3.0 mg/kg; moreover, at 3.0 mg/kg responding to sucrose-paired cues was significantly less than the corresponding baseline levels (Fisher’s LSD test, p < 0.05). Additionally, the overall effect of LY379268 on reinstatement in both groups was confirmed by significant main effects of LY379268 dose (F3,22 = 8.8, p < 0.0005). The overall triggering effect of sucrose-paired cues in both groups was confirmed by the presence of significant main effects of test condition (F1,22 = 15.5, p < 0.005).
4. Discussion
Activation of mGluR2/3 receptors by the selective agonist LY379268 dose-dependently reduced cue-induced and drug-primed reinstatement of METH seeking. In the case of cue-triggered reinstatement, this attenuating effect was marked by increased potency in rats with a history of extended access to METH reinforcement during SA training, compared to rats with a history of restricted METH access. However, the potency of LY379268 was unchanged between METH history groups when used to attenuate drug-primed reinstatement of METH seeking. The low dose of LY379268 (0.3 mg/kg) that reduced responding in LgA but not ShA rats during cue reinstatement (Experiment 1) was equally effective in reducing responding in both groups during drug reinstatement (Experiment 2A). Additionally, a smaller dose of LY379268 (0.1 mg/kg) produced a small attenuating effect on drug-primed reinstatement behavior with no differences between LgA and ShA groups (Experiment 2B). These findings are largely in agreement with previous reports of greater potency of LY379268 in attenuating behavior motivated by other drugs of abuse, when administered to rats with histories of escalated intake or drug dependence (Hao et al., 2010; Kufahl et al., 2011; Sidhpura et al., 2010). Accumulating evidence therefore points to an important role for mGluR2/3 receptors in drug-conditioned behaviors demonstrated by rat models that incorporate experiences of extensive drug exposure.
4.1. Behavioral changes as a result of prolonged METH self-administration sessions
In each experiment, LgA rats subjected to extended (6 hr) sessions during the second half of SA training demonstrated an elevated daily intake of METH, compared to the ShA rats which were subjected to restricted (90 min) sessions throughout the entire SA training regimen. This result was consistent with previous reports (Kitamura et al., 2006; Rogers et al., 2008; Schwendt et al., 2009; Wee et al., 2007b), but was not accompanied by higher METH intake during the first 90 min of the extended SA sessions by LgA rats, compared to intake exhibited by the ShA rats or the LgA rats during the first half of SA training. Higher daily METH intake during the extended SA sessions has been associated with an increased motivation to consume METH in a manner analogous to previous studies of escalated cocaine intake. However, no statistical differences were found in extinction responding between ShA and LgA rats, an observation consistent with published findings in escalated cocaine (Kippin et al., 2006; Knackstedt and Kalivas, 2007) and METH (Rogers et al., 2008). While this does not necessarily indicate equivalent levels of motivation for METH (Rogers et al., 2008), it argues against the possibility that the difference between ShA and LgA groups in sensitivity to mGluR2/3 receptor ligands in subsequent testing is a consequence of differences in learning. A recent study investigating memory loss induced by escalated METH intake concluded that learning deficits were associated with changes in expression of mGluR5 but not mGluR2/3 receptor protein (Reichel et al., 2011). Furthermore, the extended access sessions experienced by the LgA rats were divided into 90 min of cue-paired METH reinforcements and 4.5 hr of METH reinforcement without cues, and the number of reinforcements attained by the LgA rats with the cues did not exceed the amount obtained by the ShA group. This effectively eliminated any systematic differences in METH-cue pairings that could later manifest in reinstatement testing. The discussion of the reinstatement behavior that follows can therefore examine the differences in mGluR2/3 receptor sensitivity independent of learning factors that would have altered baseline levels of reinstatement.
In addition, it has recently been demonstrated that compulsive cocaine use in rats (as measured by resistance to extinction during punished responding) is more dependent on the number of drug infusions than the number of drug-cue pairings under extended access conditions (Jonkman et al., 2012). Although these investigators did not measure cue-induced reinstatement, their findings suggest that habitual drug-seeking may be more driven by neural adaptations to drug reward rather than drug-cue associations. While it remains to be determined empirically, it is possible that methamphetamine self-administration under extended access conditions may also be driven to a higher degree by drug reinforcement than by drug-associated cues.
METH-trained rats reinstated to roughly equivalent levels of active lever responding after the vehicle pretreatment, irrespective of METH exposure history (in Experiment 1, ShA: 21.5 ± 3.1, LgA: 22.8 ± 3.7; in Experiment 2A, ShA: 88.6 ± 27.8, LgA: 82.9 ± 18.8; in Experiment 2B, ShA: 71.7 ± 10.5, LgA: 86.5 ± 15.0). That this occurred during METH-primed reinstatement testing is at variance with previous studies, where LgA rats exhibited the same reinstatement levels as ShA rats when triggered by cues, but reinstated to markedly higher levels of responding after a METH priming injection (Rogers et al., 2008; Schwendt et al., 2009). The explanation for this difference is not immediately clear: the METH priming doses were the same (1 mg/kg, i.p.) in both studies, and the times between priming injection and start of the test session were similar (20 min vs. 30 min). The discrepancy may be a result of one or more differences in experimental design. Rather than performing both types of reinstatement testing in the same animals (Rogers et al., 2008; Schwendt et al., 2009), the present study executed them in separate experiments. Also notable were the shorter sessions during the initial phase of SA training (90 min vs. 2 hr) and the smaller number of sessions in both the initial (8 days vs. 10 days) and extended access (8 days vs. 14 days) phases in the present study. The most likely explanation may be that extended access to METH SA beyond eight 6-hr sessions engenders additional behavioral and/or neurochemical alterations that manifest in augmented drug-primed but not cue-elicited METH seeking. However, this idea would be most appropriately asserted after comparing these escalation procedures in animals submitted to testing by a single reinstatement modality.
Although the restriction of cue-paired METH reinforcement to the first 90 min of the extended self-administration sessions of the LgA rats resulted in a total number of cue exposures equivalent to that experienced by the ShA rats, a concern arises from the large proportion of METH reinforcement not associated with cues resulting in an altered level of stimulus control. During the first half of training (as well as during the initial 90 min of each extended session), the discrete light/tone cue and operant chamber contextual elements form a cue combination that acquires Pavlovian associative strength. During the cue-free part of each LgA training session, METH reinforcement is paired with the context alone, possibly resulting in backward blocking of the discrete cue. However, while backward blocking is regularly observed in studies of human learning (Beckers et al., 2005), this phenomenon is reportedly very difficult to attain in the rat model (Urushihara and Miller, 2010), making this effect an unlikely influence of reinstatement performance in LgA rats. However, repeated lever-contingent delivery of METH in the absence of the discrete cue may act to degrade the correlative relationship between the cue and reinforcement. Although the present study does not feature any instances where the cue is presented without METH reinforcement prior to cue reinstatement testing, the predictive value of cues have been found to be critical to the behavioral consequences of certain learning regimens, including fear conditioning (Rescorla, 1968). Partial degradation of the predictive qualities of the cue is theoretically possible but not found in the extinction data (no significant interaction, or trend of such an interaction, in any of the METH experiments, data not shown) or cue reinstatement behavior following the administration of vehicle. Further investigation is required to conclusively determine whether alteration of contiguity (i.e., how many times the cue is paired with reinforcement, which is equivalent between LgA and ShA rats in the present study) or correlation between cue and METH presentations is more important in driving group differences in subsequent extinction and reinstatement behavior.
4.2. Attenuation of reinstatement of METH seeking by LY379268
The effects of LY379268 on cue-elicited reinstatement in ShA rats were significant at the 1.0 and 3.0 mg/kg doses, which replicates earlier findings associated with ShA cocaine-trained rats (Baptista et al., 2004) as well as nondependent alcohol-trained rats (Kufahl et al., 2011; Zhao et al., 2006). However, LgA rats were notably more sensitive to LY379268 treatment with significant attenuation of reinstatement at the lowest (0.3 mg/kg) dose, suggesting a leftward shift in the dose-response function. This finding extends prior observations that LY379268 is more effective in reducing the self-administration breakpoint of cocaine in rats with a history of extended access (Hao et al., 2010). It also is in agreement with a recent study that found enhanced sensitivity to the anti-reinstatement actions of LY379268 in rats with a history of ethanol dependence versus nondependent controls (Kufahl et al., 2011). These papers describe evidence of increased mGluR2/3 receptor function in post-dependent rats in the form of increased [35S]GTPγS binding in brain areas associated with drug reinforcement and mediation of stress responses (Hao et al., 2010; Kufahl et al., 2011). Additionally, stress-induced reinstatement to ethanol responding was attenuated by LY379268 with greater potency in rats with a history of ethanol intoxication and withdrawal (Sidhpura et al., 2010).
The very high comorbidity between drug addiction and clinical anxiety has contributed to growing interest in mGluR2/3 receptor ligands, which are known to have anxiolytic effects (Monn et al., 1997; Olive, 2009; Palucha and Pilc, 2007). Rats with a history of escalated cocaine intake have shown increased sensitivity to the anxiolytic actions of LY379268 (Aujla et al., 2008), suggesting that at least part the motivation to procure psychostimulants in escalated rats could be anxiety-related. Feelings of anxiety and dysphoria are reported symptoms of withdrawal experienced by abstinent METH addicts (McGregor et al., 2005), as well as abstinent cocaine addicts (Kampman et al., 1998). In animals, psychostimulant withdrawal has been shown to produce effects in rats which partially generalized to the anxiogenic compound pentylenetetrazole (Emmett-Oglesby et al., 1990; Wood and Lal, 1987). METH reinstatement to cues has been linked to stress mechanisms in a study using central microinfusions of a corticotrophin-releasing factor receptor agonist in rats with a limited amount of self-administration training (Moffett and Goeders, 2007). Reinstatement of METH seeking has also been successfully triggered by intermittent footshock and pretreatment by the α-2 adrenoreceptor antagonist yohimbine (Shepard et al., 2004). Further investigation of the anxiogenic properties of withdrawal in rats with a history of escalated METH intake could therefore provide greater insight into the motivational attributes of METH-paired cues.
LY379268 pretreatment also had pronounced effects on METH-primed reinstatement, with the lowest dose (0.3 mg/kg) significantly attenuating responding in both groups of rats. This is in agreement with an earlier report that found significant attenuation of drug-primed reinstatement for cocaine (Peters and Kalivas, 2006). Interestingly, escalated METH intake did not enhance the effects of LY379268 treatment on drug-primed reinstatement. This is notable since current models of glutamate neuroplasticity associated with the development of psychostimulant addiction are built around experimental observations of reinstatement to cocaine seeking and identify mGluR2/3 receptors as potential treatment targets (Kalivas and McFarland, 2003; Moussawi and Kalivas, 2010). Briefly, mGluR2/3 receptor function may be compromised by chronic drug abuse via neuroadaptations such as a withdrawal-induced increase in the regulation protein Activator of G protein signaling 3 (AGS3) (Bowers et al., 2004; Xi et al., 2002), decrease in cystine-glutamate exchanger function (Baker et al., 2003) and reduced basal extracellular glutamate levels (Kalivas et al., 2003). Persistent changes in surface expression of mGluR2/3 in the medial prefrontal cortex have been recently reported in rats subjected to a regimen prolonged METH self-administration sessions, but analogous mGluR2/3 changes found in the striatum were reversed by post-METH extinction training experiences (Schwendt et al., 2012). Further understanding of how these mechanisms exist in models of METH exposure could rely on resolving the differences between reinstatement behaviors by priming and cues.
4.3. Effects of LY379268 on reinstatement of sucrose seeking
While previous studies have utilized systemic injections of LY379268 to successfully identify mGluR2/3 receptors as a promising target for drug abuse therapy, effects on generic locomotor activity and behavior motivated by non-drug reinforcement have been reported (Imre, 2007). The highest dose investigated in this paper (3.0 mg/kg) has produced reductions in pellet-primed reinstatement of food seeking (Peters and Kalivas, 2006) and cue-elicited reinstatement for sweetened condensed milk (Baptista et al., 2004). Locomotor effects were also discovered at 3.0 mg/kg (Kufahl et al., 2011) and higher doses (Backstrom and Hyytia, 2005) of LY379268. However, administration of 1.0 mg/kg and smaller doses of LY379268 have resulted in the attenuation of drug-motivated behaviors in most (Baptista et al., 2004; Kufahl et al., 2011; Peters and Kalivas, 2006) but not all (Backstrom and Hyytia, 2005) of these studies. The LY379268 dose-responses of effects on cocaine- and alcohol-related activity appeared to be shifted leftward, and hence farther from the range of nonspecific effects, in rats that were exposed to greater amounts of these drugs (Hao et al., 2010; Kufahl et al., 2011; Sidhpura et al., 2010). Furthermore, studies have shown that repeated exposure to LY379268 resulted in the development of tolerance to locomotor effects while the potentially therapeutic actions were retained (Cartmell et al., 2000; Imre et al., 2006). While LY379268 treatment has been found in most cases to exert drug-specific actions on conditioned or cue-elicited reinstatement of lever responding, novel effects of this drug should be interpreted with an examination of non-specific effects (Imre, 2007; Olive, 2009).
To test whether the effects of LY379268 found on reinstatement of METH seeking generalize to non-drug rewards, this study included an assessment of the effects of this drug on reinstatement to sucrose seeking. Sucrose reinstatement was markedly attenuated after 1.0 mg/kg LY379268 treatment, as opposed to the 3.0 mg/kg LY379268 required to significantly reduce cue-elicited sucrose reinstatement in a prior report (Bossert et al., 2006). This discrepancy may be explained by the fact that all animals in the present study had free access to food throughout the experiment, whereas the Long-Evans rats in the other study were restricted to 20 g of food per day. Without the added motivation of food restriction, the dose-dependent effects of LY379268 on sucrose reinstatement overlap with its effects on METH reinstatement, except when METH seeking is triggered in LgA rats by METH-paired cues. A similar difference was revealed in the dose-response of this drug on lever responding reinforced by the delivery of food pellets: 3.0 mg/kg LY379268 did not interfere with responding in nutritionally deprived rats (Zhao et al., 2006), whereas it significantly attenuated responding in freely-feeding animals (Liechti et al., 2007). Nonetheless, the findings of Experiment 3 appear to place some limitations on the therapeutic potential of mGluR2/3 receptor ligands, thereby motivating further study with more specific compounds such as the mGluR2 agonists BINA (Jin et al., 2010) and BINA-14 (Dhanya et al., 2011), which have both been found to be effective in attenuating cocaine-motivated behaviors. However, the results also demonstrate that incorporating elements of METH dependence into the reinstatement model can serve to identify effects that are drug-specific and dependent on drug history.
5. Conclusion
Systemic pretreatment with the mGluR2/3 receptor agonist LY379268 attenuated reinstatement of METH-seeking behavior triggered by conditioned cues or by a METH prime. In the case of cue-triggered reinstatement, rats with a history of prolonged METH exposure exhibited an enhanced sensitivity to LY379268, experiencing significant treatment effects at a dose smaller than that shown by rats with a history of limited METH self-administration and by rats trained with sucrose reinforcement. The relatively narrow window between the doses effective against METH seeking and sucrose seeking suggests that while group II mGluRs comprise an important target for the treatment of drug relapse in severely addicted individuals, more specific compounds may be required to develop a truly effective therapeutic strategy.
Highlights.
We tested the effects of the specific mGluR2/3 agonist LY379268 on drug seeking.
Stimulation of mGluR2/3 attenuated reinstatement of methamphetamine seeking.
A regimen of greater drug exposure elevated sensitivity to mGluR2/3 treatment.
The specificity of LY379268 effects was also shown using sucrose-trained rats.
Acknowledgments
The authors would like to acknowledge Piroska Barabas, Elisabeth Moore, Corina Lucero, Kaveish Sewalia, Joanne Alonso and Evan Armstrong of Arizona State University for their technical contributions, and to Dr. Rémi Martin-Fardon of The Scripps Research Institute for insightful conversations regarding the experimental design. This work was supported by PHS grant DA025606 to MFO. The authors declare that they have no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Literature Cited
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–566. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Beckers T, De Houwer J, Pineno O, Miller RR. Outcome additivity and outcome maximality influence cue competition in human causal learning. J Exp Psychol Learn Mem Cogn. 2005;31:238–249. doi: 10.1037/0278-7393.31.2.238. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Metabotropic glutamate receptors: synaptic modulators and therapeutic targets for neurologic disease. Neurology. 2008;70:964–968. doi: 10.1212/01.wnl.0000306315.03021.2a. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. Tolerance to the motor impairment, but not to the reversal of PCP-induced motor activities by oral administration of the mGlu2/3 receptor agonist, LY379268. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:39–46. doi: 10.1007/s002109900151. [DOI] [PubMed] [Google Scholar]
- Dhanya RP, Sidique S, Sheffler DJ, Nickols HH, Herath A, Yang L, Dahl R, Ardecky R, Semenova S, Markou A, Conn PJ, Cosford ND. Design and synthesis of an orally active metabotropic glutamate receptor subtype-2 (mGluR2) positive allosteric modulator (PAM) that decreases cocaine self-administration in rats. J Med Chem. 2011;54:342–353. doi: 10.1021/jm1012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Mathis DA, Moon RT, Lal H. Animal models of drug withdrawal symptoms. Psychopharmacology (Berl) 1990;101:292–309. doi: 10.1007/BF02244046. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Fleckenstein AE. Basic neuropharmacological mechanisms of methamphetamine. In: Roll JM, Rawson RA, Ling W, Shoptaw S, editors. Methamphetamine addiction. Guilford Press; New York: 2009. pp. 30–60. [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imre G, Salomons A, Jongsma M, Fokkema DS, Den Boer JA, Ter Horst GJ. Effects of the mGluR2/3 agonist LY379268 on ketamine-evoked behaviours and neurochemical changes in the dentate gyrus of the rat. Pharmacol Biochem Behav. 2006;84:392–399. doi: 10.1016/j.pbb.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Pelloux Y, Everitt BJ. Drug Intake is Sufficient, but Conditioning is not Necessary for the Emergence of Compulsive Cocaine Seeking After Extended Self-Administration. Neuropsychopharmacology. 2012;37:1612–1619. doi: 10.1038/npp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci. 2003;1003:169–175. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D’Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21:295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther. 2007;322:1103–1109. doi: 10.1124/jpet.107.122861. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR 2/3 agonist LY379268 and increased functional activity of mGluR 2/3 in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36:2762–2773. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Olive MF. Investigating Methamphetamine Craving Using the Extinction-Reinstatement Model in the Rat. J Addict Res Ther. 2011;S1 doi: 10.4172/2155-6105.s1-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. (−)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine (MTEP) similarly attenuate stress-induced reinstatement of cocaine seeking. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue- and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115:116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berl) 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rogers JL, De Santis S, See RE. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose ME, Grant JE. Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry. 2008;20:145–155. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- Rutkowski BA, Maxwell JC. Epidemiology of methamphetamine use. In: Roll JM, Rawson RA, Ling W, editors. Methamphetamine addiction: from basic science to treatment. Guilford Press; New York: 2009. pp. 6–29. [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schwendt M, Reichel CM, See RE. Extinction-Dependent Alterations in Corticostriatal mGluR2/3 and mGluR7 Receptors following Chronic Methamphetamine Self-Administration in Rats. PLoS One. 2012;7:e34299. doi: 10.1371/journal.pone.0034299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Urushihara K, Miller RR. Backward blocking in first-order conditioning. J Exp Psychol Anim Behav Process. 2010;36:281–295. doi: 10.1037/a0016773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS Neurol Disord Drug Targets. 2008;7:482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007a;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007b;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Lal H. Anxiogenic properties of cocaine withdrawal. Life Sci. 1987;41:1431–1436. doi: 10.1016/0024-3205(87)90619-9. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]