Abstract
Bacillus subtilis contains seven extracytoplasmic-function σ factors that activate partially overlapping regulons. We here identify four additional members of the σX regulon, pbpX (penicillin-binding protein), ywnJ, the dlt operon (d-alanylation of teichoic acids), and the pss ybfM psd operon (phosphatidylethanolamine biosynthesis). Modification of teichoic acids by esterification with d-alanine and incorporation of phosphatidylethanolamine into the cell membrane have a common consequence: in both cases positively charged amino groups are introduced into the cell envelope. The resulting reduction in the net negative charge of the cell envelope has been previously implicated as a resistance mechanism specific for cationic antimicrobial peptides. Consistent with this notion, we find that both sigX and dltA mutants are more sensitive to nisin than wild-type cells. We conclude that activation of the σX regulon serves to alter cell surface properties to provide protection against antimicrobial peptides.
Bacillus subtilis encodes seven extracytoplasmic-function (ECF) σ factors. Most studies to date have focused on three: σX, σW, and σM (reviewed in reference 19). sigX and its downstream gene rsiX (encoding the anti-σX factor) were originally observed to be homologous (but not orthologous) to Escherichia coli fecI and fecR, which are involved in expression of ferric citrate transport genes (37). Although expression of sigX in E. coli can complement a fecI mutant (4), the B. subtilis sigX mutant is not affected in any known ferri-siderophore uptake systems (20).
To understand the function of σX, we identified several σX-regulated genes using a consensus promoter search method (22). In these initial studies, we characterized six genes that are preceded by promoters recognized by σX: sigX, abh (an AbrB homolog), csbB (a membrane-bound glucosyl transferase) (2), divIC (a membrane-bound cell-division initiation protein), lytR (a negative regulator of autolysin) (30), and rapD (a response regulator aspartate phosphatase) (43). These results suggested that σX modulates aspects of cell envelope metabolism. Interestingly, most σX-controlled genes are also transcribed by other forms of holoenzyme. For example, csbB has an additional σB-dependent promoter, lytR and rapD both have additional σA-dependent promoters, and sigX itself is preferentially transcribed from an upstream σA-dependent site in addition to the σX-dependent autoregulatory promoter (20, 22). Moreover, in some cases (e.g., abh and divIC) the promoter activated by the EσX holoenzyme can also be recognized by the EσW holoenzyme at least in vitro (21). Similarly, the recently defined bcrC gene (a bacitracin resistance gene) is transcribed from a promoter that is recognized by either σX or σM (7, 38). The unknown function of many σX-controlled genes makes it difficult to predict a phenotype for the sigX mutant. This challenge is exacerbated by the fact that many of the genes are expressed from multiple promoters or by multiple holoenzyme forms activating the same promoter. The latter observation also makes DNA microarray approaches difficult, since many genes that can be activated by σX are also expressed by σX-independent pathways.
In this study we have used both promoter consensus search and in vitro runoff transcription-macroarray analysis (ROMA) (8) to identify four additional σX-dependent operons: dltABCDE, pssA ybfM psd, pbpX, and ywnJ. Both the dlt and the pssA operons encode enzymes that modulate cell surface charge (d-alanylation of teichoic acids and biosynthesis of phosphatidylethanolamine [PE], respectively), and PbpX is a low-molecular-weight penicillin-binding protein of unknown function. These results lead us to propose that one function of σX is to regulate cell surface modification as a defense against cationic antimicrobial peptides.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotides, and growth conditions.
All bacterial strains, plasmids, and oligonucleotides used in this study are listed in Table 1. B. subtilis and E. coli strains were grown at 37°C with vigorous shaking in Luria broth (LB) medium (50) unless otherwise indicated. For E. coli, 100 μg of ampicillin/ml was used to select for Ampr, and 200 μg of spectinomycin/ml was used to select for Spcr. For B. subtilis, antibiotics used for selection were as follows: 100 μg of spectinomycin/ml for Spcr, 10 μg of kanamycin/ml for Kanr, 8 μg of neomycin/ml for Neor, and 1 μg of erythromycin/ml and 25 μg of lincomycin/ml for macrolide-lincomycin-streptogramin B resistance (MLSr).
TABLE 1.
Strains, plasmids, and oligonucleotides used in this work.
| Strain, plasmid, or oligonucleotide | Genotype, characteristic, or sequence | Reference |
|---|---|---|
| E. coli strain | ||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Lab stock |
| B. subtilis strains | ||
| CU1065 | W168 attSPβ trpC2 | Lab stock |
| ZB307A | W168 SPβc2Δ2::Tn917::pBSK10Δ6 | 60 |
| HB7007 | CU1065, but sigX::spc | 20 |
| HB7013 | CU1065, but rsiX::pVA29 (MLSr) | 20 |
| HB0042 | CU1065, sigW::kan | 6 |
| HB0010 | CU1065, rsiW::kan | 6 |
| HB0030 | CU1065, sigX::spc, sigW::MLS | 8 |
| HB4035 | CU1065, sigD::kan | Lab stock |
| HB4229 | HB1000, flgM::mini-Tn10 (Spcr) | 15 |
| HB0059 | CU1065, flgM::mini-Tn10 (Spcr) | This work |
| SDB01 | Marburg 160, but psd1::neo | 33 |
| SDB02 | Marburg 160, but pssA10::spc | 33 |
| HB0036 | ZB307A transduced with SPβ(PdltA1-cat-lacZ) | This work |
| HB0037 | ZB307A transduced with SPβ(PdltA2-cat-lacZ) | This work |
| HB0038 | CU1065, but dltA::pMUTIN | This work |
| HB0048 | CU1065, dltA::spc | This work |
| HB0094 | CU1065, but dltA::pMUTIN and pssA::spc | This work |
| HB0095 | CU1065, but dltA::spc and psd::neo | This work |
| HB0089 | ZB307A transduced with SPβ(PywnJ-cat-lacZ) | This work |
| HB4514 | ZB307A transduced with SPβ(PpbpX-cat-lacZ) | This work |
| HB4509 | ZB307A transduced with SPβ(PpssA2-cat-lacZ) | This work |
| HB4533 | ZB307A transduced with SPβ(PpssA1-cat-lacZ) | This work |
| HB4519 | CU1065, but psd::neo | This work |
| HB4520 | CU1065, but pssA::spc | This work |
| Plasmids | ||
| pJM122 | Vector for integration of reporter fusions into SPβ | 51 |
| pGEM-cat-3Zf(+) | Cloning vector for gene knockout | 59 |
| pJM114 | Kanamycin resistance cassette vector | 42 |
| pMUTIN4 | Cloning vector for integration, allows lacZ fusion at locus | 56 |
| pKF59 | Spectinomycin resistance cassette vector | 5 |
| pMC54 | PdltA1-cat-lacZ in pJPM122 | This work |
| pMC55 | PdltA2-cat-lacZ in pJPM122 | This work |
| pMC57 | dltA cloned in SacI-PstI of pGEM-cat-3Zf(+) | This work |
| pMC58 | spc cassette cloned in pMC57 (dltA::spc) | This work |
| pMC59 | an internal fragment of dltA cloned in EcoRI-BamHI of pMUTIN4 | This work |
| pMC94 | PywnJ-cat-lacZ in pJPM122 | This work |
| pJQ20 | PpbpX-cat-lacZ in pJPM122 | This work |
| pJQ22 | PpssA2-cat-lacZ in pJPM122 | This work |
| pJQ23 | PpssA1-cat-lacZ in pJPM122 | This work |
| Oligonucleotides | ||
| 368 dltA-r123 | 5′-TCGCGGATCCATAATTCCTGATACGTGA-3′ | This work |
| 371 dltA-in-f | 5′-CCCGGAATTCCGTCCGAACGGATTG-3′ | This work |
| 372 dltA-in-r | 5′-CCCGGATCCATCAGGCACATTTGC-3′ | This work |
| 373 dltA-f1 | 5′-GCCCAAGCTTATTTGTTTGGTTCATCTTCC-3′ | This work |
| 374 dltA-f3 | 5′-GCCCAAGCTTTATTATGAATCAGCTCGAAAC-3′ | This work |
| 427 dltA-f | 5′-AAGCGAGCTCCTTGCAGGTATAAAGATT-3′ | This work |
| 428 dltA-r | 5′-AAAACTGCAGCCAAGCAGTATAAAGAAT-3′ | This work |
| 413 pbpX-f | 5′-AATGATAAGCTTGGCTGAGTGAAAAACTCAGC-3′ | This work |
| 414 pbpX-r | 5′-CAGGGATCCTCTTTTATTTAGTTTTCTCCG-3′ | This work |
| 422 pssA-f1 | 5′-TTTGGGAAGCTTATCTCTGGATCAGCCAG-3′ | This work |
| 407 pssA-f2 | 5′-TTTGGGAAGCTTCTATGTTATCATGCTTATTGG-3′ | This work |
| 408 pssA-r | 5′-TGAGGATCCAGCAATCCGCAAATGAAG-3′ | This work |
| 537 psd-in-f | 5′-GGATTTTGCGAGTCGAAA-3′ | This work |
| 538 psd-in-r | 5′-CTCCAATTCAGTACGGGT-3′ | This work |
| 571 ywnJ-f | 5′-CGCAAGCTTACCCAAGAAACAGAAGAA-3′ | This work |
| 572 ywnJ-r | 5′-CCCGGATCCACAGACAGAAAGCAGGAT-3′ | This work |
Construction of mutants.
CU1065 chromosomal DNA was amplified with primers #427 and #428. The PCR fragment was digested with SacI and PstI and ligated into pGEM-cat-3Zf(+) (59) to generate plasmid pMC57. pMC57 was digested with HincII and SnaBI and ligated with a Spcr cassette (PCR amplified from pKF59 [5] using T3 and T7 primers) to generate pMC58. B. subtilis CU1065 was transformed with pMC58 (linearized with ScaI) with selection for Spcr to generate HB0048 (dltA::spc). Thus, a ∼630-bp internal fragment of dltA was replaced with a Spcr cassette.
Primers #371 and #372 were used to amplify an internal fragment of dltA (∼490 bp). The PCR fragment was digested with EcoRI and BamHI and cloned into pMUTIN4 (56), generating plasmid pMC59. This plasmid was inserted into CU1065 by Campbell integration and selection for MLSr to generate strain HB0038 (dltA::pMUTIN).
CU1065 was transformed with chromosomal DNA from SDB01 (psd::neo) (33) and SDB02 (pssA::spc) (33) to generate the psd::neo (HB4519) and pssA::spc (HB4520) mutants, respectively. The dltA pssA (HB0094) and dltA psd (HB0095) double mutants were generated by using chromosomal DNA from SDB02 and SDB01 to transform HB0038 (dltA::pMUTIN) and HB0048(dltA::spc), with selection for (MLSr plus Spcr) and (Spcr plus Neor), respectively.
Construction of promoter-cat-lacZ fusions.
The putative promoter regions were amplified and cloned into pJPM122 (51). The sequence of the promoter region in each plasmid was verified by DNA sequencing (Cornell DNA sequencing facility). The promoter fusions were introduced into the SPβ prophage by double-crossover recombination, in which each pJPM122 derivative was linearized by digestion with ScaI and used to transform B. subtilis ZB307A (60) with selection for Neor. SPβ lysates were prepared by heat induction and used to transduce various recipient strains, and β-galactosidase activity was measured on each sample in early stationary phase (when σX activity is at a maximum) (21) as described by Miller (34).
Purification of RNAP and σ factors.
Preparation of B. subtilis core RNA polymerase (RNAP) and σA, σD, σX, σW and δ proteins was previously described (10, 20, 21, 25, 31).
ROMA.
The ROMA experiment was performed as described previously (8). A typical transcription reaction (50 μl) contains 1.3 pmol of core RNAP, 16 pmol of σX, 15 pmol of δ, and 1 μg of digested genomic DNA mixed in transcription buffer (20 mM Tris-HCl [pH 8.0], 10 mM MgCl2, 50 mM KCl, 0.5 mM dithiothreitol, 0.1-mg/ml bovine serum albumin, 5% [vol/vol] glycerol, and RNasin from Promega [10 U/reaction]) and NTP mixture (40 nmol of ATP, GTP, CTP, and 8 nmol of [α-33P]UTP [3,000 Ci/mmol] from NEN). The Panorama B. subtilis gene arrays (catalog no. PRBS0002) were purchased from Sigma-Genosys Biotechnologies, Inc.
In vitro runoff transcription assays for candidate genes.
A typical runoff reaction mixture (20 μl) contains 0.36 pmol of core RNAP, 4.5 pmol of σX, 4.2 pmol of δ, 0.04 pmol of PCR-amplified template DNA (normally the same fragments used for generating promoter fusions) and NTP mixture (10 nmol of ATP, GTP, CTP, 1 nmol of UTP, and 0.6 pmol of [α-32P]UTP [3,000Ci/mmol]). Reactions were incubated and processed as described for the ROMA experiments (8).
Primer extension assays.
RNA was either purified from in vitro runoff transcription reaction mixtures or extracted from late-log-phase B. subtilis cells using phenol-chloroform extraction as described previously (22). A PCR fragment containing the promoter region studied was sequenced using the same primer to index the transcription start site.
Nisin MIC assays.
Nisin was obtained from Sigma Chemical Co. and dissolved in 20 mM HCl. Overnight cultures were diluted 1:100 into fresh LB medium in the presence of nisin at the indicated concentration. After incubation for 6 h with shaking, the optical density at 600 nm (OD600) was measured.
Autolysis test.
CU1065 (wild type), sigX::spc, dltA::spc, and dltA::pMUTIN strains were grown in LB or minimal medium (9). Cells were harvested at exponential growth phase (OD600, ∼0.7), washed twice with cold Tris-HCl buffer (pH 7.1), and resuspended in 50 mM Tris-HCl buffer (pH 7.1) containing 0.05% Triton X-100. Incubation was at 37°C, and autolysis was monitored by measuring the decrease of OD600 at 30-min intervals.
Northern blot analysis.
Primers #537 and #538 were used to amplify an internal fragment (∼570 bp) of psd from CU1065 chromosomal DNA. After HindIII digestion, the fragment was labeled with [α-32P]dATP by the 3′ fill-in method using a Klenow fragment of DNA polymerase (Exo−; New England BioLabs). The probe was hybridized with membranes containing total RNA from wild-type, sigX, and rsiX strains (same RNA sample used for primer extension; see above). The NorthernMax formaldehyde-based system (Ambion, Inc.) was used to perform the Northern analysis. Ten micrograms of total RNA was denatured and loaded on 1% formaldehyde agarose gel. Hybridization was performed at 42°C overnight. The second day, the blot was washed twice with low-stringency buffer (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) at room temperature followed by two washes with high-stringency buffer (0.1× SSC) at 42°C. The blot was wrapped in plastic wrap and exposed to a Phosphor screen (Molecular Dynamics).
RESULTS
Defining the σX regulon using promoter consensus search.
Previously, saturation mutagenesis of the sigX autoregulatory promoter was used to identify those bases important for σX-dependent promoter recognition. The resulting consensus was used to search the partially sequenced B. subtilis genome (63% complete at the time the analysis was done [22]) to identify candidate promoters. While this approach identified several σX target genes, others were subsequently found to be primarily controlled by σW or σM, which recognize promoters with closely related sequences (22, 35).
The availability of the complete genome sequence (28), together with a better understanding of the rules for promoter recognition by σX and its paralog σW (21, 48), encouraged us to repeat the consensus search procedure. Although we explored the use of several different search patterns to identify likely candidates, one of the most successful searches used the degenerate consensus tgtaACtttt n12-13 CG(A,T)C to screen the SubtiList database (36) for those sites within 250 bp of an annotated start codon. This search pattern is based on the observation that many identified σX-dependent promoters contain a T-rich region in the downstream portion of the −35 element and the AC base pairs are highly conserved. We allowed up to three mismatches in this extended −35 element (in the positions in lowercase) and none in the −10 element. By including those promoters with −10 elements of either CGTC or CGAC, we expected to identify some sites already defined as largely dependent on σW. The resulting list of candidate promoters includes one site with no mismatches (the sigX autoregulatory site), three with one mismatch (preceding lytR, ywnJ, and dltA), and four with two mismatches (divIC, ydjA, abh, and yrhH; underlined sites are known to be at least partially σX dependent in vivo or in vitro) (21, 22). Among the 15 sites with three mismatches in the −35 element, we focused our attention on those preceding pssA and pbpX, since these genes are of known function (Table 2).
TABLE 2.
Genes preceded by promoters recognized by σX
| Genea | Promoter region (σX dependent)b | 5′UTRc | ROMAd | Overlapping recognition by other ECF σ factor(s)e | Reporter fusionf | Reference(s) |
|---|---|---|---|---|---|---|
| −35 −10 . . | ||||||
| sigX | tgtaaTGTAACTTTTcaagctattcataCGACaaaaaag | 17 | + | + | 20 | |
| csbB | aaaatTGTAACaaaaaacag-gtttaaaCGACtttaaaa | 80 | +++* | + | 22 | |
| lytR | aacaaTGAAACTTTTtttta-taaaaaaCGACtatttta | 84 | + | + | 22 | |
| rapD | taaaaTGTAACcaactgtcaatgagagcCGTCaaaagtt | 45 | − | + | 22 | |
| divlC | atgttTGAAACTTcTtcctgtgaaaatgCGTCtaacttt | 113 | + | σW (RO), σM (RF) | + | 22, 35 |
| abh | aagcggGAAACTTTTtcaaagtttcattCGTCtacgata | 62 | − | σW (RO) | + | 22 |
| bcrC | ttattTGAAACTTTTcatgagtaagattaGTCtactaaa | 24 | ++ | σM (RF, PE) | + | 7 |
| ywnJ | ttttcTGTtACTTTTtgctttgttttacCGTCtatgtag | 38 | +++ | σW (RO) | − | This work |
| pbpX | tttttgacAACTTTTttagggctttattCGTCtaacaaa | 40 | − | σW (RF) | + | This work |
| dltA | aaaaaTGAAACTTTTtgagc-atctgatCGTCaaataat | 204 | + | + | This work | |
| pssA | tttccTGTAACgcTattcga-tcactatCGTCaaataat | 34 | ++* | + | This work |
The first gene in each known or putative σX-dependent operon and its (σX-dependent) promoter region are listed.
Note that the promoter consensus defined here includes an extended −35 region that includes the downstream T-rich segment and two additional bases in the −10 region that appear to be important for promoter discrimination by σX (double underline) or σW (single underline) (see the text).
The known or estimated distance from the transcription start site to the start codon of the gene is indicated in nucleotides.
The strength of the signal detected in the ROMA (EσX) experiment (Fig. 1) is indicated. An asterisk indicates that the downstream genes were also identified by ROMA.
This promoter is recognized by other ECF σ factors, as confirmed by runoff transcription (RO), reporter fusion (RF), or primer extension (PE).
+, the indicated promoter region gives σX-dependent β-galactosidase activity with a reporter fusion. −, no activity was detected for the reporter fusion.
Defining the σX regulon using ROMA.
As a complementary mechanism to identify candidate σX target genes, we performed in vitro ROMA analysis (8). The ROMA approach generates 33P-labeled runoff transcripts using σX-containing holoenzyme to transcribe total genomic DNA that has been restricted with either EcoRI or HindIII. The resulting runoff transcripts are then used to probe a DNA macroarray (Sigma/GenoSys) containing 4,107 B. subtilis open reading frames. Candidates (genes whose signal became stronger in the presence of σX) were chosen for further analysis if they had a particularly strong signal or if they were associated with a plausible promoter site as identified by the consensus search approach described above. In addition to signals corresponding to promoters known to be recognized by σX (e.g., sigX, csbB, lytR, divIC, and bcrC) (Fig. 1), the ROMA experiment revealed strong signals for the pssA and ywnJ genes and a weaker signal for dltA. Note that in many cases, these same genes appeared in reactions using σW holoenzyme instead of σX (Fig. 1), consistent with the known overlap between the sets of promoters recognized by these two σ factors (22).
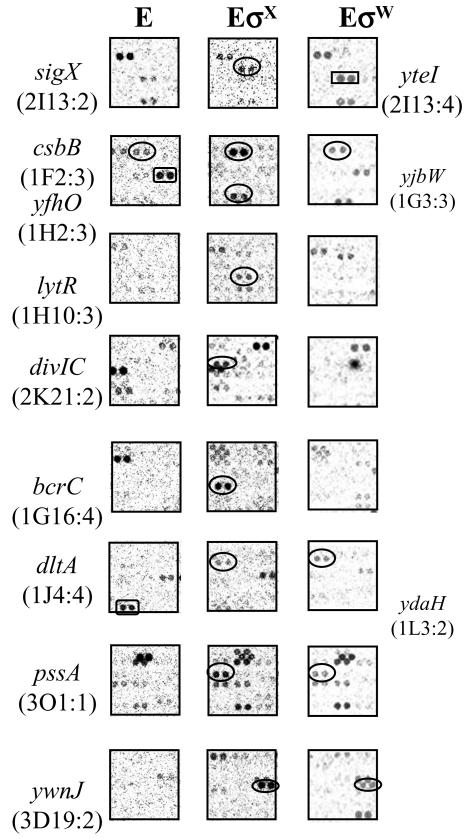
FIG.1.
Identification of σX regulon genes by ROMA. Total B. subtilis chromosomal DNA was digested with EcoRI and transcribed in vitro with core alone (E [left column]) or core with an excess of σX (EσX [central column]). For comparison, the same regions from previous ROMA experiment with EσW are placed in the right column. The σX-regulated genes are apparent in experiments with EσX (ovals). yteI (rectangle) is a σW-dependent gene. Since the core is contaminated with trace amounts of other σ factors, several nonspecific spots appeared on the membrane even in the core-alone experiment. Some spots disappeared or were greatly decreased in abundance upon supplementation with a large molar excess of σX or σW (e.g., yjbW and ydaH, rectangles in left column). Other genes, such as sigX and csbB, which have multiple promoters are found in the RNA population transcribed by core as well as the σX- or σW-supplemented reactions. Three genes identified in this study (dltA, pssA, and ywnJ) are found in both EσX and EσW reactions, but only ywnJ can be transcribed by both σX and σW as confirmed by runoff transcription assays. The location of each gene on the Sigma/GenoSys macroarray is indicated in parentheses.
Confirmation of promoters for pbpX and ywnJ.
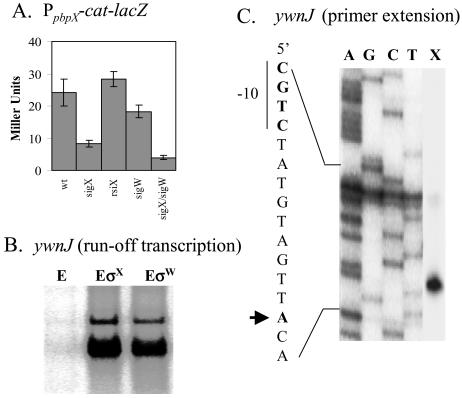
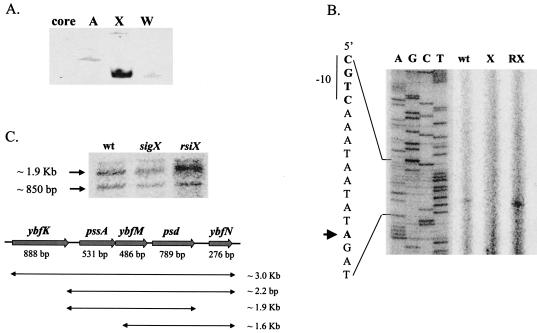
We used a reporter fusion to demonstrate that pbpX is dependent on sigX in vivo, with a further reduction in expression in the sigX sigW double mutant (Fig. 2A). DNA microarray analyses reveal that the expression of pbpX decreased 2.7-fold in the sigX mutant (data not shown) but not in the sigW mutant (8). The reporter fusion for the putative ywnJ promoter had very low activity, so in vitro transcription was used to demonstrate that this site could be recognized by both the σX and σW holoenzymes (Fig. 2B). Transcription initiates at the expected site, as measured by primer extension mapping of the resulting in vitro transcripts (Fig. 2C).
FIG. 2.
Confirmation of the pbpX and ywnJ targets. (A) Expression of PpbpX-cat-lacZ in various genetic backgrounds. Each result is the average for three individual β-galactosidase measurements. (B) In vitro recognition of the putative ywnJ promoter by both the B. subtilis σX (EσX) and the σW (EσW) holoenzymes. The RNAP core enzyme (E) was used as a negative control. (C) RNA generated by runoff transcription using EσX as shown in panel B was used as a template for primer extension mapping of the ywnJ transcription start site (lane X). The same primer was used to sequence this region to index the start site (lanes A, G, C, and T).
The dltABCDE operon is largely dependent on σX.
The B. subtilis dltABCDE operon is responsible for d-alanine esterification of both lipoteichoic acids (LTA) and wall teichoic acids (WTA) (44). Transcription of the dltABCDE operon was originally proposed to be largely (∼70%) σD dependent, with the residual activity perhaps due to two putative σA-dependent promoters (Fig. 3A, P1 and P4) (44).
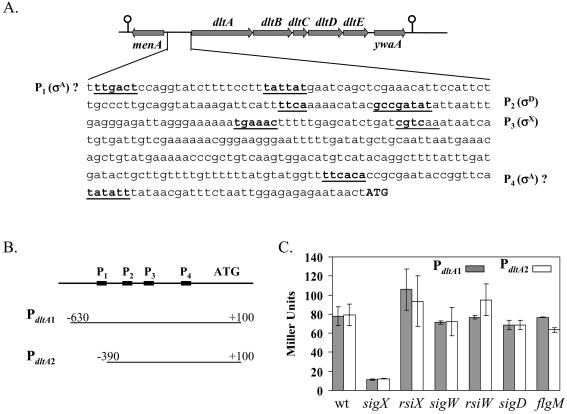
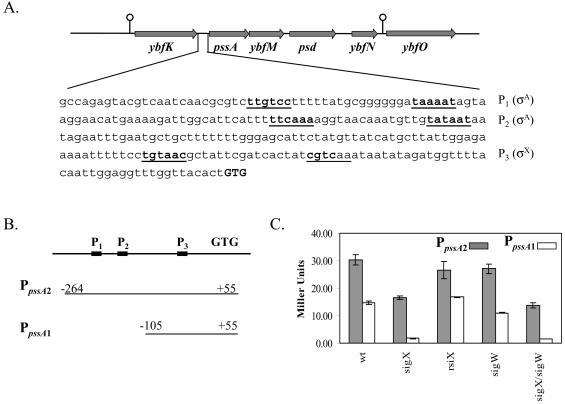
FIG. 3.
Regulation of the dlt operon by σX. (A) The location of the dltABCDE operon on the B. subtilis chromosome and the dltA promoter region. The σX-dependent promoter (P3), the σD-dependent promoter (P2), and two putative σA-dependent promoters (P1 and P4) are underlined. The translation start codon (ATG) is shown in bold capital letters. (B and C) Graphic presentation of the two PdltA promoter fusions (B) and their activities in various genetic backgrounds (C) (each result is the average and standard deviation from three individual measurements).
To examine the regulation of the dlt operon, we integrated two lacZ transcriptional fusions ectopically at the SPβ locus. The PdltA1-cat-lacZ fusion consists of a ∼730-bp fragment (from −630 bp to +100 bp relative to the start codon) and includes all four putative promoters (P1 to P4). The PdltA2-cat-lacZ fusion consists of a shorter fragment (from −390 bp to +100 bp) and includes P2 through P4 (Fig. 3B). Results with both promoter fusions indicate that expression is reduced by about 85% in the sigX mutant and slightly increased in the rsiX mutant (defective in the anti-σ factor that targets σX [20]), confirming the existence of a σX-dependent promoter in this region (Fig. 3C). Expression was unaffected in the sigW and rsiW mutant strains, despite the presence of a CGTC motif is the predicted −10 region (see Discussion). Under our growth conditions, σD does not seem to play a role in dlt transcription, since the activities from both fusions neither decreased in a sigD mutant nor increased in a flgM (anti-σD) mutant. Moreover, the first putative σA-dependent promoter (P1) apparently did not contribute to the dlt transcription, since expression from PdltA1 and PdltA2 was similar. The residual activity (∼11 Miller units) in the sigX mutant might be due to recognition of the σX-dependent promoter by another ECF σ factor or might be due to another promoter (maybe P4).
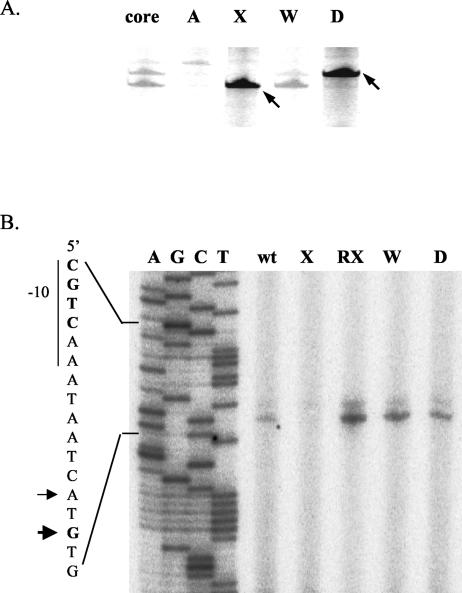
We extended these in vivo results using in vitro runoff transcription and primer extension assays. When the long PCR fragment (PdltA1) was incubated with RNAP core enzyme in the presence of σA, σX, σW or σD, appropriately sized transcripts were generated by both the σX and σD holoenzymes (Fig. 4A). Although σW could weakly recognize the σX-dependent site in vitro (Fig. 4A), it did not appear to play a major role in vivo (Fig. 3C). While EσD could initiate transcription from the σD-dependent promoter in vitro, in vivo transcripts were detected only for the σX-dependent promoter (Fig. 4B). Primer extension reactions indicate that transcription of dltA initiates primarily from a G residue 11 bases downstream of the −10 CGTC motif and secondarily from an A residue 2 bases upstream. Both signals became stronger in the rsiX mutant and were greatly reduced in the sigX mutant. No other strong start sites were visible in this region. We conclude that dlt expression is largely dependent on σX in vivo.
FIG. 4.
Identification of the σX-dependent promoter for the dlt operon. (A) Runoff transcription from the dltA promoter region in the presence of B. subtilis RNAP core enzyme and the indicated σ factor: σA (A), σX (X), σW (W), or σD (D). In the first lane (core), no σ factor was added in the reaction. Major transcripts are indicated by arrows. (B) Primer extension mapping of the in vivo dltA transcription start site. RNA samples were prepared from wild-type (wt), sigX (X), rsiX (RX), sigW (W), or sigD (D) mutant strains. Equal amounts (100 μg) of total RNA were annealed with radiolabeled oligonucleotide #368 for reverse transcription. The transcription start sites corresponding to the σX-dependent promoter are indicated by arrows.
The pssA ybfM psd operon is partially controlled by σX.
Our identification of a candidate σX-dependent promoter upstream of the pssA gene suggests a possible role for σX in regulating the phospholipid content of the membrane. The pssA gene is part of a predicted operon including ybfM and psd. Together, the PssA and Psd proteins catalyze the synthesis of PE. Okada et al. (39) proposed two putative σA-dependent promoters (P1 and P2) upstream of pssA, and our results suggest a third σX-dependent promoter (P3) (TGTAAC-N16-CGTCaa) (Fig. 5A).
FIG. 5.
Regulation of the pssA ybfM psd operon by σX. (A) Locations of the pssA, ybfM, and psd genes on the B. subtilis chromosome and DNA sequence of the pssA promoter region. The σX-dependent promoter (P3) and two putative σA-dependent promoters (P1 and P2) are underlined. The translation start codon (GTG) is shown in bold capital letters. (B and C) Graphic presentation of the construction of two PpssA promoter fusions (B) and their activities in various genetic backgrounds (C) (each result is the average of three individual measurements).
To test the contribution of each promoter to pssA expression, we constructed two lacZ fusions: one contains the complete promoter region (P1, P2, and P3), the other contains only P3 (Fig. 5B). β-Galactosidase assays demonstrate that about one-half of the expression derives from the σX-dependent promoter (P3), with the other half from the region containing P1 and P2 (Fig. 5C).
Recognition of P3 by σX holoenzyme was confirmed in vitro by runoff transcription assays (Fig. 6A). A faint, larger band was observed in reactions containing EσA, probably resulting from one of the σA-dependent promoters. We used primer extension assays to localize the transcription start site for the σX holoenzyme to an A residue 10 bp downstream from CGTC (Fig. 6B). A weak transcript was detected in the wild type (CU1065) but not in the sigX mutant strain. The amount of transcript increased in the rsiX mutant, as expected for a σX-dependent promoter.
FIG. 6.
Identification of the σX-dependent promoter for the pssA ybfM psd operon. (A) Runoff transcription from the pssA promoter region in the presence of B. subtilis RNAP core enzyme and the indicated σ factor: σA (A), σX (X), or σW (W). In the first lane (core), no σ factor was added in the reaction. (B) Primer extension mapping of the pssA transcription start site. RNA samples were prepared from the wild type (wt) or from sigX (X) or rsiX (RX) mutant strains. Equal amounts (100 μg) of total RNA were annealed with radiolabeled oligonucleotide #408 for reverse transcription. The transcription start site is indicated by the arrow. (C) Northern blot analysis demonstrates that pssA, ybfM, and psd are cotranscribed. The combinations and sizes of possible transcripts are listed. Two bands were observed: the top band is about 1.9 kb, representing the transcript from pssA to psd, while the lower band (∼850 bp) can only be assigned to the psd mRNA, probably due to RNA processing.
To test whether pssA and psd are in one operon, we conducted Northern blot analysis using a 32P-labeled internal fragment of the psd gene as a probe. A large transcript (∼1.9 kb) was detected, consistent with an mRNA extending from pssA through psd. A smaller transcript (∼850 bp) likely corresponds to the psd gene and may have been produced by RNA processing, since it varies in intensity with the full-length transcript (Fig. 6C). The density of both bands decreased about 50% in the sigX mutant and increased in the rsiX mutant, consistent with the previous conclusion that σX contributes ∼50% of the expression of PpssA.
sigX mutants are altered in autolysis and sensitivity to cationic antimicrobial peptides.
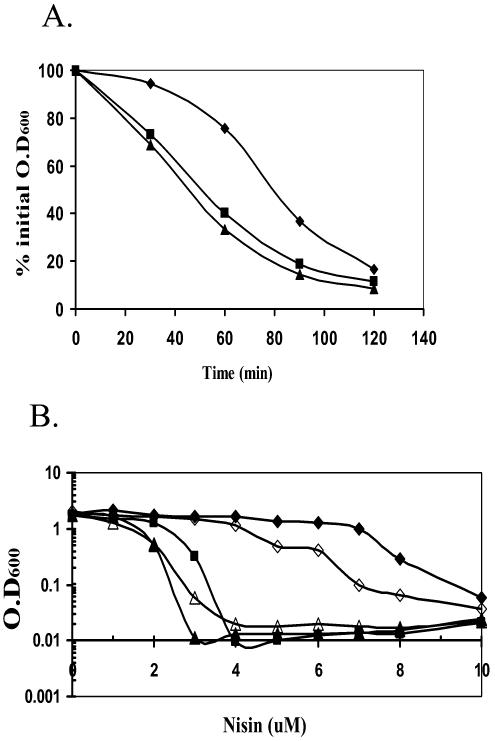
Since σX regulates both d-alanylation of teichoic acids and PE biosynthesis, we tested the effects of a sigX mutation on two phenotypes previously shown to be affected by cell surface charge: autolysis and resistance to cationic antimicrobial peptides. We first compared the Triton X-100-induced autolysis rates (58) of the wild type and the sigX and dltA::spc mutants. Autolysins are a group of positively charged cell wall hydrolytic enzymes that bind more avidly to the cell wall of dlt mutant strains (58). As expected, both the sigX and dltA mutants have a twofold increase in the rate of autolysis compared to the wild type (Fig. 7A). Similar results were observed with cells grown in LB or minimal medium.
FIG. 7.
Effects of sigX, dlt, and psd on autolysis and nisin sensitivity. (A) Autolysis rates. B. subtilis CU1065 (wild type; diamonds) and the sigX::spc (squares) and dltA::spc (triangles) mutants were grown to exponential growth phase (OD600, ∼0.7). The cell pellets were washed twice with cold Tris buffer (pH 7.1) and resuspended in 50 mM Tris-HCl buffer (pH 7.1) containing 0.05% Triton X-100. Incubation was at 37°C, and autolysis was monitored by measuring the decrease of OD600 at 30-min intervals. (B) MIC of nisin for the growth of B. subtilis wild-type (closed diamonds), sigX::spc (closed squares), dltA::spc (closed triangles), psd::neo (open diamonds), and dltA psd (open triangle) strains. All strains were grown for 6 h after dilution into LB medium containing the indicated concentration of nisin. This experiment was repeated three times, and representative results are shown.
Cationic antimicrobial peptides (CAMPs) are a broadly distributed family of peptides that kill bacteria. Many are thought to act by accumulating within the cytoplasmic membrane to a critical concentration that allows the assembly of structures that permeabilize the cell (16-18). To test whether σX plays a role in resistance to CAMPs, we measured the MICs of nisin for the wild type and the sigX, dltA, pssA, and psd mutants: a positively charged (+3) peptide produced by Lactococcus lactis (24). As expected, the sigX and dltA mutants were more sensitive to nisin than the wild type (Fig. 7B). The psd mutant had only slightly increased sensitivity, while the pssA mutant was unaffected. A psd dltA double mutant behaved much like the dltA single mutant. In addition to nisin, the sigX and dltA mutants were more sensitive to several other tested CAMPs (S. Farmer and R. Hancock, personal communication) but not to gramicidin, a neutral peptide. In contrast, the mutants were unaltered in their sensitivity to vancomycin, tunicamycin, or lysozyme (data not shown), although dlt mutants have been previously reported to display an increased susceptibility to methicillin (57).
DISCUSSION
Using a promoter consensus search and ROMA approaches, we have defined four additional σX-dependent operons. Together with the results of our previous analyses (22), we conclude that most members of the σX regulon control processes related to the composition or metabolism of the cell envelope. For example, LytR is a negative regulator of autolysin activity (30), CsbB is a membrane-bound glucosyl transferase likely involved in cell wall biosynthesis (2), PbpX is a penicillin-binding protein, DltA, DltB, DltC and DltD are responsible for D alanylation of the WTA and LTA (44), and PssA and Psd are enzymes for PE biosynthesis (33). We note that most of these operons are expressed from complex promoter regions: lytR is controlled by both σA and σX, csbB is also regulated by σB, pbpX is partially regulated by σW, and σX and σA each contribute to pssA ybfM psd expression. Perhaps due to this overlapping regulation, the sigX mutant strain does not display dramatic growth defects under most tested conditions, although some increased sensitivity to oxidative stress and heat stress has been noted (20). Here, we have extended the phenotypes of the sigX mutant to include increased rates of autolysis and increased sensitivity to cationic antimicrobial peptides.
Promoter recognition by σX.
As noted previously, σX recognizes −10 elements with sequence CGaC, σW recognizes CGTa, and both can recognize CGTC (lowercase reflects a noncritical base for recognition) (21, 22, 48). In Table 2 we compile the 11 promoters that are the best candidates for regulation by σX in vivo. Note that some of these sites can also be recognized by either σW or σM. For example, both bcrC (7, 38) and pbpX (Fig. 2A) seem to be under dual control in vivo, and σW recognizes several other sites in vitro (Table 2). In addition, a number of other promoters previously studied (22) can be recognized by σX in vitro, but an in vivo role for σX has not been documented, and it seems likely that they may be primarily dependent on σW or σM for in vivo expression (22). Indeed, in B. subtilis W23, expression of the divIC gene is partially σM dependent (35).
Inspection of Table 2 allows a refinement of our previous models for promoter discrimination among ECF σ factors. Specifically, we note that most of the newly characterized promoters identified in this study contain a CGTC −10 motif, previously shown to be also recognized by σW holoenzyme. However, all four promoters (abh, divIC, pbpX, and ywnJ) that are also recognized by σW share a common extended −10 region of CGTCta. In contrast, the other three (rapD, dltA, and pssA) that are recognized only by σX have a −10 region of “CGTCaa (Table 2). This is consistent with the observation that the highly specific autoregulatory sites for sigX and sigW contain −10 elements of “CGACaa” and “CGTAta,” respectively. Furthermore, in a previous promoter mutagenesis study we found that changing the sigX promoter (−10) region CGACaa to CGTCaa resulted in a site that retained high selectivity for σX. In contrast, when the sigW (−10) region CGTAta was changed to CGTCta, both σX and σW could recognize this promoter (48). We therefore conclude that (i) the preferred −10 consensus sequences for σX (CGaCaa) and σW (CGTata) differ in two positions (italics) rather than one position and (ii) there is considerable overlap between these two regulons.
Biological role of σX and the σX regulon.
Distinctive aspects of the gram-positive bacterial cell envelope include the presence of a thick cell wall containing peptidoglycan, WTA, and LTA (14). In B. subtilis 168 strains, the negatively charged teichoic acids contain an alternating glycerol phosphate copolymer, whereas in B. subtilis W23 strains ribitol replaces glycerol. Recent results indicate that ribitol-based teichoic acid synthesis in W23 strains is regulated by both σX and σM (29, 35).
In general, the WTA and LTA polymers are highly modified by esterification on the sugar residues with sugar, amino sugar, or amino acid substituents. For B. subtilis, LTA chains contain between 24 and 33 glycerol phosphate monomers and carry, on average, 0.35 to 0.55 d-alanine constituents and 0.2 to 0.4 glycosyl substituents per monomer (14). The d-Ala residues on LTA are subject to rapid turnover, both by spontaneous hydrolysis and by transesterification to WTA (14, 26). d-Alanylation of WTA and LTA is catalyzed by the products of the dlt operon. The dltA and dltC genes encode the d-alanine-d-alanyl carrier protein ligase (Dcl) and the d-alanyl carrier protein (Dcp), respectively. DltB and DltD may function in transport and the actual esterification reaction (45).
The modification with d-Ala introduces free amino groups (NH3+) into the cell envelope and thereby reduces the net negative charge of the surface (44). Genetic studies with several microorganisms indicate that dlt mutants are pleiotropic, with phenotypes including altered patterns of autolysis, increased sensitivity to CAMPs (1, 45-47), altered colonization properties (11), altered carbohydrate metabolism (52), enhanced UV sensitivity, and loss of acid tolerance (3). In addition, d-alanylation affects protein folding and secretion (23, 54). Our results suggest that conditions leading to activation of the σX regulon will lead to enhanced expression of the dlt operon and thereby result in a decrease in the net negative charge of the cell wall. The factors that activate expression of the σX regulon are not well defined, but they are likely to act through the RsiX anti-σ, shown previously to inhibit σX activity (20). It has also been shown that transposon insertions in the yitG multidrug efflux system, the manA gene encoding mannose-6-phosphate isomerase, the srfAB surfactin biosynthesis gene, the ytxJ general stress protein, the ywpH single-stranded DNA-binding protein, and the yogA alcohol dehydrogenase locus also lead to enhanced expression from a σX-dependent promoter (55), although the significance of these observations is not yet clear.
The bacterial cell membrane also contains a net negative charge due to the abundance of anionic phospholipids. However, PE, a neutral (zwitterionic) lipid, makes up as much as 50% of the B. subtilis membrane (33). The biosynthesis of PE in B. subtilis is carried out by two membrane-localized enzymes: CDP-diacylglycerol-dependent phosphatidylserine (PS) synthase (PssA) and phosphatidylserine decarboxylase (Psd). The genes (pssA and psd) encoding the enzymes are separated by another gene, ybfM, on the chromosome. All three genes are cotranscribed (Fig. 6C). The psd mutant contains no PE and accumulates PS, while the pssA mutant contains no PE or PS. The absence of PE in B. subtilis cells does not have any adverse effects on cell growth, probably due to compensation from increased glucosyldiacylglycerol content in the membrane (33). Interestingly, the E. coli psd gene has recently been shown to have a σE-dependent promoter (49), suggesting that this system may also be controlled, at least in part, by an ECF σ factor in this organism.
In B. subtilis, σX may serve to regulate the net charge in the cell envelope by affecting the expression of both the dlt and pssA operons (Fig. 8), and this, in turn, may affect sensitivity to CAMPs. CAMPs share several common features, including broad-spectrum antimicrobial activity and cationic charge at physiological pH (16-18). CAMPs act by an initial electrostatic binding to the anionic moieties on the microbial membrane, followed by membrane disruption (16-18). In eukaryotes, CAMPs (including defensins) are the major form of defense against bacterial infection and are induced by bacteria or lipopolysaccharides (12, 17).
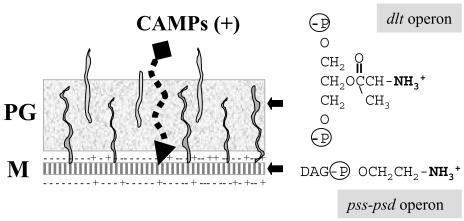
FIG. 8.
Roles of the B. subtilis σX protein in resistance to CAMPs. The B. subtilis cell envelope includes both a cytoplasmic membrane (M) and a thick peptidoglycan layer (PG). Two of the operons controlled by σX are involved in modulating the net charge of the cell envelope. The dlt operon encodes proteins involved in the d-alanylation of both LTA and WTA by esterification of the glycerol moieties with d-alanine. Since both LTA and WTA are glycerol-phosphate copolymers, the introduction of d-alanine esters reduces the net negative charge of the cell wall. Similarly, the cytoplasmic membrane contains an abundance of anionic phospholipids (indicated by −), and the net charge of the membrane can be modulated by the incorporation of neutral constituents, such as glycolipids and the zwitterionic PE. The synthesis of PE requires the products of the pssA ybfM psd operon, which is partially under σX control. The ability of CAMPs to penetrate the cell wall and permeabilize the membrane is reduced by the incorporation of these positively charged groups into the cell envelope.
Bacteria can acquire resistance to CAMPs by modification of their surface properties, although in general the underlying regulatory mechanisms have not been described. For example, a nisin-resistant Listeria monocytogenes strain contains elevated levels of zwitterionic PE and a reduction in phosphatidylglycerol and cardiolipin (13). Similarly, a nisin-resistant strain of the rumen bacterium Streptococcus bovis has decreased negative surface charge (32). A recent study found that Staphylococcus aureus achieves resistance to defensins and CAMPs by modifying anionic membrane lipids with l-lysine (27). In gram-negative bacteria, resistance often involves modification of lipopolysaccharides. For example, CAMP resistance in Salmonella enterica involves addition of palmitate or 4-aminoarabinose to lipid A, a process regulated by the PmrA-PmrB two-component regulatory system (53).
In this study, we demonstrate that sensitivity of B. subtilis to CAMPs is affected by an ECF σ factor that contributes to the expression of two operons that modulate surface charge (Fig. 8). Other σX regulon proteins (e.g., LytR, PbpX, and CsbB) may also participate in this adaptive response. In other bacteria, related cell wall homeostasis functions may be controlled by two-component regulatory systems instead of, or in addition to, ECF σ factors. For example, the Streptomyces coelicolor CseC-CseB two-component system activates expression of σE, in response to unknown signals, which then functions to modify cell wall structure (40, 41). In Streptococcus agalactiae, up-regulation of the dlt operon when d-alanine incorporation into LTA is deficient is controlled by the DltS-DltR two-component system (46). Our studies provide evidence linking ECF σ factors to the biosynthesis and modification of the cell envelope and suggest that these regulatory proteins may participate in an inducible defense response providing resistance to CAMPs.
Acknowledgments
We would like to thank previous lab members who purified proteins used in this study: Y. L. Juang (RNAP and σA), Y. F. Chen (σD), X. J. Huang (σX and σW), and F. J. Lopez de Saro (δ). Thanks also go to Y. Chai for construction of the PpssA-cat-lacZ fusions, J. Qiu for construction of the PpbpX-cat-lacZ fusion, S. Farmer and R. Hancock for tests of CAMP sensitivity, and K. Matsumoto for providing the original pssA and psd mutant strains.
This work was supported by NIH grant GM-47446 (to J.D.H.).
REFERENCES
- 1.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 2.Akbar, S., and C. W. Price. 1996. Isolation and characterization of csbB, a gene controlled by Bacillus subtilis general stress transcription factor σB. Gene 177:123-128. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, D. A., D. G. Cvitkovitch, A. S. Bleiweis, M. Y. Kiriukhin, D. V. Debabov, F. C. Neuhaus, and I. R. Hamilton. 2000. Defects in D-alanyl-lipoteichoic acid synthesis in Streptococcus mutans results in acid sensitivity. J. Bacteriol. 182:6055-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brutsche, S., and V. Braun. 1997. SigX of Bacillus subtilis replaces the ECF sigma factor FecI of Escherichia coli and is inhibited by RsiX. Mol. Gen. Genet. 256:416-425. [DOI] [PubMed] [Google Scholar]
- 5.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 6.Cao, M., B. A. Bernat, Z. Wang, R. N. Armstrong, and J. D. Helmann. 2001. FosB, a cysteine-dependent fosfomycin resistance protein under the control of σW, an extracytoplasmic-function sigma factor in Bacillus subtilis. J. Bacteriol. 183:2380-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao, M., and J. D. Helmann. 2002. Regulation of the Bacillus subtilis bcrC bacitracin resistance gene by two extracytoplasmic function sigma factors. J. Bacteriol. 184:6123-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, M., P. A. Kobel, M. M. Morshedi, M. F. Wu, C. Paddon, and J. D. Helmann. 2002. Defining the Bacillus subtilis sigma(W) regulon: a comparative analysis of promoter consensus search, run-off transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J. Mol. Biol. 316:443-457. [DOI] [PubMed] [Google Scholar]
- 9.Chen, L., L. P. James, and J. D. Helmann. 1993. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially regulated by metal ions. J. Bacteriol. 175:5428-5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y.-F., and J. D. Helmann. 1995. The Bacillus subtilis flagellar regulatory protein σD: overproduction, domain analysis, and DNA-binding. J. Mol. Biol. 249:743-753. [DOI] [PubMed] [Google Scholar]
- 11.Clemans, D. L., P. E. Kolenbrander, D. V. Debabov, Q. Zhang, R. D. Lunsford, H. Sakone, C. J. Whittaker, M. P. Heaton, and F. C. Neuhaus. 1999. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregations. Infect. Immun. 67:2464-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, A. M., and T. Ganz. 2000. Human antimicrobial peptides: analysis and application. BioTechniques 29:822-826, 828, 830-831. [DOI] [PubMed] [Google Scholar]
- 13.Crandall, A. D., and T. J. Montville. 1998. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl. Environ. Microbiol. 64:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, W. 1988. Physiology of lipoteichoic acids in bacteria. Adv. Microb. Physiol. 29:233-302. [DOI] [PubMed] [Google Scholar]
- 15.Fredrick, K., and J. D. Helmann. 1996. FlgM is a primary regulator of σD activity, and its absence restores motility to a sinR mutant. J. Bacteriol. 178:7010-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, R. E., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helmann, J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47-110. [DOI] [PubMed] [Google Scholar]
- 20.Huang, X., A. Decatur, A. Sorokin, and J. D. Helmann. 1997. The Bacillus subtilis σX protein is an extracytoplasmic function sigma factor contributing to survival at high temperature. J. Bacteriol. 179:2915-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, X., K. L. Fredrick, and J. D. Helmann. 1998. Promoter recognition by Bacillus subtilis σW: autoregulation and partial overlap with the σX regulon. J. Bacteriol. 180:3765-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, X., and J. D. Helmann. 1998. Identification of target promoters for the Bacillus subtilis σX factor using a consensus-directed search. J. Mol. Biol. 279:165-173. [DOI] [PubMed] [Google Scholar]
- 23.Hyyrylainen, H. L., M. Vitikainen, J. Thwaite, H. Wu, M. Sarvas, C. R. Harwood, V. P. Kontinen, and K. Stephenson. 2000. D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J. Biol. Chem. 275:26696-26703. [DOI] [PubMed] [Google Scholar]
- 24.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of Gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juang, Y. L., and J. D. Helmann. 1994. The delta subunit of Bacillus subtilis RNA polymerase. An allosteric effector of the initiation and core-recycling phases of transcription. J. Mol. Biol. 239:1-14. [DOI] [PubMed] [Google Scholar]
- 26.Koch, H. U., R. Doker, and W. Fischer. 1985. Maintenance of d-alanine ester substitution of lipoteichoic acid by reesterification in Staphylococcus aureus. J. Bacteriol. 164:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristian, S. A., M. Durr, J. A. Van Strijp, B. Neumeister, and A. Peschel. 2003. MprF-mediated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect. Immun. 71:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 29.Lazarevic, V., F. X. Abellan, S. B. Moller, D. Karamata, and C. Mauel. 2002. Comparison of ribitol and glycerol teichoic acid genes in Bacillus subtilis W23 and 168: identical function, similar divergent organization, but different regulation. Microbiology 148:815-824. [DOI] [PubMed] [Google Scholar]
- 30.Lazarevic, V., P. Margot, B. Soldo, and D. Karamata. 1992. Sequencing and analysis of the Bacillus subtilis lytRABC divergon: a regulatory unit encompassing the structural genes of the N-acetylmuramoyl-L-alanine amidase and its modifier. J. Gen. Microbiol. 138:1949-1961. [DOI] [PubMed] [Google Scholar]
- 31.Lopez de Saro, F. J., A. Y. Woody, and J. D. Helmann. 1995. Structural analysis of the Bacillus subtilis delta factor: a protein polyanion which displaces RNA from RNA polymerase. J. Mol. Biol. 252:189-202. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani, H. C., and J. B. Russell. 2001. Nisin resistance of Streptococcus bovis. Appl. Environ. Microbiol. 67:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto, K., M. Okada, Y. Horikoshi, H. Matsuzaki, T. Kishi, M. Itaya, and I. Shibuya. 1998. Cloning, sequencing, and disruption of the Bacillus subtilis psd gene coding for phosphatidylserine decarboxylase. J. Bacteriol. 180:100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 35.Minnig, K., J. L. Barblan, S. Kehl, S. B. Moller, and C. Mauel. 2003. In Bacillus subtilis W23, the duet σXσM, two sigma factors of the extracytoplasmic function subfamily, are required for septum and wall synthesis under batch culture conditions. Mol. Microbiol. 49:1435-1447. [DOI] [PubMed] [Google Scholar]
- 36.Moszer, I., P. Glaser, and A. Danchin. 1995. SubtiList: a relational database for the Bacillus subtilis genome. Microbiology 141:261-268. [DOI] [PubMed] [Google Scholar]
- 37.Ochs, M., S. Veitinger, I. Kim, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: fecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 38.Ohki, R., K. Tateno, Y. Okada, H. Okajima, K. Asai, Y. Sadaie, M. Murata, and T. Aiso. 2003. A bacitracin-resistant Bacillus subtilis gene encodes a homologue of the membrane-spanning subunit of the Bacillus licheniformis ABC transporter. J. Bacteriol. 185:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada, M., H. Matsuzaki, I. Shibuya, and K. Matsumoto. 1994. Cloning, sequencing, and expression in Escherichia coli of the Bacillus subtilis gene for phosphatidylserine synthase. J. Bacteriol. 176:7456-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paget, M. S., E. Leibovitz, and M. J. Buttner. 1999. A putative two-component signal transduction system regulates sigmaE, a sigma factor required for normal cell wall integrity in Streptomyces coelicolor A3(2). Mol. Microbiol. 33:97-107. [DOI] [PubMed] [Google Scholar]
- 41.Paget, M. S. B., L. Chamberlin, A. Atrih, S. J. Foster, and M. J. Buttner. 1999. Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 181:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perego, M. 1993. Integrational vectors for genetic manipulation in Bacillus subtilis, p. 615-624. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 43.Perego, M., P. Glaser, and J. A. Hoch. 1996. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol. Microbiol. 19:1151-1157. [DOI] [PubMed] [Google Scholar]
- 44.Perego, M., P. Glaser, A. Minutello, M. A. Strauch, K. Leopold, and W. Fischer. 1995. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 270:15598-15606. [DOI] [PubMed] [Google Scholar]
- 45.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 46.Poyart, C., M. C. Lamy, C. Boumaila, F. Fiedler, and P. Trieu-Cuot. 2001. Regulation of D-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J. Bacteriol. 183:6324-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in D-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 49:1615-1625. [DOI] [PubMed] [Google Scholar]
- 48.Qiu, J., and J. D. Helmann. 2001. The −10 region is a key promoter specificity determinant for the Bacillus subtilis extracytoplasmic-function sigma factors σX and σW. J. Bacteriol. 183:1921-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezuchova, B., H. Miticka, D. Homerova, M. Roberts, and J. Kormanec. 2003. New members of the Escherichia coli sigmaE regulon identified by a two-plasmid system. FEMS Microbiol. Lett. 225:1-7. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1990. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 51.Slack, F. J., J. P. Mueller, and A. L. Sonenshein. 1993. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J. Bacteriol. 175:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spatafora, G. A., M. Sheets, R. June, D. Luyimbazi, K. Howard, R. Hulbert, D. Barnard, M. el Janne, and M. C. Hudson. 1999. Regulated expression of the Streptococcus mutans dlt genes correlates with intracellular polysaccharide accumulation. J. Bacteriol. 181:2363-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamayo, R., S. S. Ryan, A. J. McCoy, and J. S. Gunn. 2002. Identification and genetic characterization of PmrA-regulated genes and genes involved in polymyxin B resistance in Salmonella enterica serovar Typhimurium. Infect. Immun. 70:6770-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thwaite, J. E., L. W. Baillie, N. M. Carter, K. Stephenson, M. Rees, C. R. Harwood, and P. T. Emmerson. 2002. Optimization of the cell wall microenvironment allows increased production of recombinant Bacillus anthracis protective antigen from B. subtilis. Appl. Environ. Microbiol. 68:227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner, M. S., and J. D. Helmann. 2000. Mutations in multidrug efflux homologs, sugar isomerases, and antimicrobial biosynthesis genes differentially elevate activity of the σX and σW factors in Bacillus subtilis. J. Bacteriol. 182:5202-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144(Part 11):3097-3104. [DOI] [PubMed] [Google Scholar]
- 57.Wecke, J., M. Kazimierz, and W. Fischer. 1997. The absence of D-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology 143:2953-2960. [DOI] [PubMed] [Google Scholar]
- 58.Wecke, J., M. Perego, and W. Fischer. 1996. D-alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb. Drug Resist. 2:123-129. [DOI] [PubMed] [Google Scholar]
- 59.Youngman, P. 1990. Use of transposons and integrational vectors for mutagenesis and construction of gene fusions in Bacillus species, p. 221-266. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for bacillus. John Wiley and Sons, Ltd., Chichester, United Kingdom.
- 60.Zuber, P., and R. Losick. 1987. Role of AbrB and SpoOA- and SpoOB-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]