Summary
DiGeorge syndrome, caused by a 22q11 microdeletion or mutation of the TBX1 gene, varies in severity greatly, even among monozygotic twins. Epigenetic phenomena have been invoked to explain phenotypic differences in individuals of identical genetic composition, although specific chromatin modifications relevant to DiGeorge syndrome are elusive. Here we show that lack of the histone acetyltransferase MOZ (MYST3/KAT6A) phenocopies DiGeorge syndrome, and the MOZ complex occupies the Tbx1 locus, promoting its expression and histone 3 lysine 9 acetylation. Importantly, DiGeorge syndrome-like anomalies are present in mice with homozygous mutation of Moz and in heterozygous Moz mutants when combined with Tbx1 haploinsufficiency or oversupply of retinoic acid. Conversely, a Tbx1 transgene rescues the heart phenotype in Moz mutants. Our data reveal a molecular mechanism for a specific chromatin modification of the Tbx1 locus intersecting with an environmental determinant, modeling variability in DiGeorge syndrome.
Highlights
► Homozygous mutation of Moz causes DiGeorge syndrome-like anomalies ► MOZ is required for palate, thymus, aortic arch, and cardiac septal development ► MOZ acts through H3K9 acetylation and promotion of transcription at the Tbx1 locus ► Mutation of Moz sensitizes embryonic development to environmental insults
Voss et al. show that loss of the histone acetyltransferase Moz disrupts histone H3K9 acetylation and transcription of Tbx1. A Tbx1 transgene partially rescues DiGeorge syndrome-like phenotypes in Moz mutant mice. Moz mutants offer opportunities to study how environmental insults such as retinoids influence genetic models of disease.
Introduction
DiGeorge/velo-cardio-facial/22q11 deletion syndrome (DGS, VCFS, 22q11 delS) is the most common deletion syndrome in humans (Singh et al., 2002) and is characterized by severe cardiac and aortic arch defects, thymus dysplasia, cleft palate, and craniofacial abnormalities (Lindsay, 2001). Despite its characteristic genetic lesion, the syndrome shows a remarkable variability in phenotypic severity. Newborn infants who lack the same 22q11 deletion interval can present with severe aortic arch and heart defects requiring surgery or, alternatively, have no detectable cardiovascular defects. Even monozygotic twins can exhibit this level of discordance, suggesting that the phenotypic variability cannot be explained on the basis of genetic differences alone (Goodship et al., 1995). As a consequence, chromatin modifiers are of great interest because they have the potential to integrate environmental effects and directly alter expression levels of key regulators of aortic arch and heart development.
Among the genes in the 1.5–3 Mb hemizygous deletion interval on chromosome 22q11 causing DGS, the TBX1 gene is a major contributor to the characteristic clinical features seen in patients. The TBX1 gene encodes a T box DNA-binding transcription factor, and mutations in one allele of the TBX1 gene can lead to the same syndrome without 22q11 deletion (Paylor et al., 2006; Stoller and Epstein, 2005; Torres-Juan et al., 2007; Yagi et al., 2003; Zweier et al., 2007). Furthermore, heterozygous mutations of the Tbx1 gene in mice model DGS (Jerome and Papaioannou, 2001; Lindsay et al., 2001; Merscher et al., 2001). We show here that a chromatin modifier, the monocytic leukemia zinc finger protein (MOZ/MYST3/KAT6A), is a critical regulator of the Tbx1 gene in vivo.
MOZ, a member of the MYST family of histone acetyltransferases (Reifsnyder et al., 1996; Thomas and Voss, 2007; Voss and Thomas, 2009), was first identified as a target of recurrent translocations causing a particularly aggressive form of acute myeloid leukemia (Borrow et al., 1996). The Moz gene is expressed widely at moderate levels (Thomas et al., 2006; Voss et al., 2009), but mutation of the Moz gene has surprisingly specific effects. MOZ is required for the development of hematopoietic stem cells (Katsumoto et al., 2006; Thomas et al., 2006), thymus development (Thomas et al., 2006), histone 3 lysine 9 (H3K9) acetylation, and transcriptional activity at Hox gene loci and body segment identity specification (Voss et al., 2009).
Through the use of Moz and Tbx1 mutant mice, expression analysis, chromatin immunoprecipitation (ChIP), and pharmacological treatment, we report here a role of MOZ in cardiac, pharyngeal apparatus, and facial development. We show that MOZ is required for H3K9 acetylation (H3K9ac) and transcription at the Tbx1 locus in vivo, and not essential for a range of other loci regulating cardiac development. Significantly, we demonstrate that Moz mutation leads to the spectrum of defects seen in DGS, that a Tbx1 transgene rescues the heart defects in Moz mutant mice, that Moz exhibits synergistic genetic interaction with Tbx1, and that Moz haploinsufficiency synergizes with oversupply of retinoic acid (RA).
Results
Moz Deficiency Leads to Anomalies Characteristic of DGS and Tbx1 Deficiency
Two Moz mutant alleles were used in this study: MozΔ and Moz−. The carboxy-terminal deletion allele, MozΔ, does not produce detectable protein, as examined by immunoblotting (Thomas et al., 2006). MozΔ/Δ homozygous mice are born at a normal Mendelian ratio and die within the first hour after birth. In the Moz− allele exons 3–7 were deleted (Voss et al., 2009). Moz−/− embryos die between embryonic day 14.5 (E14.5) and birth, depending on the genetic background. Homozygosity of either allele causes the absence of transplantable hematopoietic stem cells (Thomas et al., 2006; unpublished data), anterior homeotic transformation (Voss et al., 2009), and the defects reported here. Based on the differences in time of death and in the severity of the defects between the two alleles, the Moz− allele is the more severe loss-of-function allele, and it is inferred that the MozΔ allele must produce some residual, albeit not detectable and truncated, MOZ protein. MozΔ/Δ homozygotes were used to study the full spectrum of anatomical defects at birth, and Moz−/− homozygotes were used for biochemical studies.
Newborn MozΔ/Δ mice (33 of 33) were of normal size. The morphology and histology of most organs were normal. However, all MozΔ/Δ mutant newborn pups had micrognathia (Figures 1A and 1B) and cleft palate (Figures 1C–1F; see Figure S1 available online; Table 1). In addition, we have previously reported the absence or hypoplasia of the thymus in 100% of MozΔ/Δ mutant newborn pups (Thomas et al., 2006).
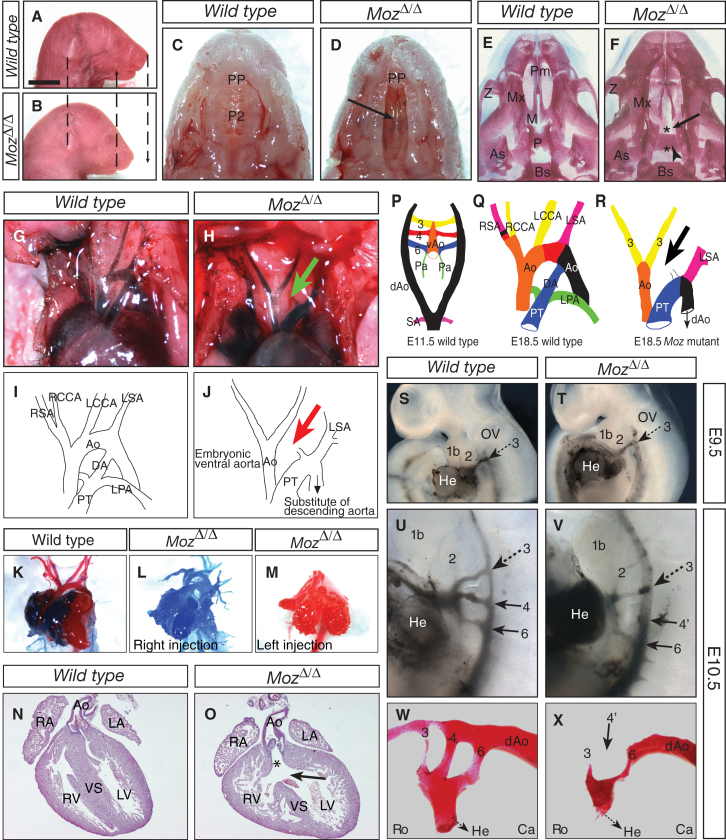
Figure 1.
Craniofacial, Palate, Aortic Arch, and Heart Abnormalities in MozΔ/Δ Mutant Pups
Moz+/+ (A, C, E, G, I, K, N, S, U, and W) and MozΔ/Δ (B, D, F, H, J, L, M, O, T, V, and X) newborn mouse pups (A–F), E18.5 pups (G–O), E9.5 (S and T), and E10.5 (U–X) embryos. Lateral view of the head (A and B), ventral view of the palate (C and D), and of the bone structure of the palate (E and F). Note the shortened face and mandible of the mutant (A and B), the lack of midline fusion of the secondary palate (arrow in D and F). Asterisks in (F) indicate location where the maxillary shelf and the palatine shelf should have fused. Aortic arch and other great vessels, ventral view, ink injected (G and H), and traced (I and J). Note the absence of the aortic arch between the LCCA and the LSA, i.e., an IAA-B (arrow in H and J) and the connection of the pulmonary trunk with the descending aorta (H and J). Resin cast of heart and large blood vessels (K–M) injected with red (left ventricle) and blue (right ventricle), H&E sections of hearts, ventral view (N and O). Note the complete filling of both sides of the MozΔ/Δ hearts by injection from either the right (L) or left (M) ventricle, the ventricular septum defect (arrow in O), and overriding aorta (asterisk in O). Schematic drawing of aortic arch development from E11.5 to E18.5 (P and Q) and abnormal vessels in E18.5 MozΔ/Δ mutants (R). Ink-injected aorta-pharyngeal complex at E9.5 (S and T) and E10.5 (U and V). Resin cast of the aorta-pharyngeal complex at E10.5 (W and X). The connection between the ascending and descending aspects of the aorta is derived from the left fourth pharyngeal artery (red in P and Q), missing in MozΔ/Δ (arrow, R). The left fourth pharyngeal artery is not yet developed at E9.5 in either Moz+/+ or MozΔ/Δ (S and T). Note, however, the presence of a fourth PAA in Moz+/+ E10.5 embryos (U and W), which is absent in MozΔ/Δ E10.5 embryos (4′ in V and X). 1b, mandibular portion of the first pharyngeal arch; 2, second pharyngeal arch; 3, 4, 6, third, fourth, and sixth PAA; arrowhead in (F), presphenoid bone, only visible through cleft palate; Ao, aorta; As, alisphenoid; Bs, basisphenoid; dAo, dorsal aorta; Ca, caudal; DA, ductus arteriosus; He, heart; LA, left atrium; LCCA, left common carotid artery; LSA, left subclavian artery; LPA, left pulmonary artery; LV, left ventricle; M, maxillary shelf; Mx, maxilla; OV, otic vesicle; P, palatine shelf; P2, secondary palate; Pa, pulmonary artery; Pm, premaxilla; PP, primary palate; PT, pulmonary trunk; RA, right atrium; RCCA, right common carotid artery; Ro, rostral; RSA, right subclavian artery; RV, right ventricle; SA, subclavian artery; vAo, ventral aorta; VS, ventricular septum. Z, zygomatic process. Scale bar, 5 mm (A and B), 2 mm (C and D), 1.5 mm (E and F), 940 μm (G and H), 2.3 mm (K), 1.5 mm (L), 1.2 mm (M), 550 μm (N and O), 1150 μm (S and T), 290 μm (U and V), and 230 μm (W and X). n = 33 MozΔ/Δ, 45 MozΔ/+, and 35 Moz+/+ newborn pups (A–F), n = 19 MozΔ/Δ,13 MozΔ/+, and 15 Moz+/+ newborn pups (G–J), n = 19 MozΔ/Δ, 16 MozΔ/+, and 17 Moz+/+ newborn pups (K–O). n = 12 MozΔ/Δ, 14 MozΔ/+, and 9 Moz+/+ E9.5 embryos (S and T), n = 4 MozΔ/Δ, 20 MozΔ/+, and 13 Moz+/+ control E10.5 embryos (U–X). See also Figure S1.
Table 1.
Incidence of Specific Anomalies Observed in MozΔ/Δ Mutant Neonatal Mice Compared to Incidence Reported in Patients with DGS and Tbx1 Mouse Mutants
| Finding |
MozΔ/Δ Mouse Neonatesa |
Patients with DGS (%)b |
Mouse Neonates within a Series of Tbx1 Mutant Alleles (%)c |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number/Total | % | Botto et al. (2003) | Kobrynski and Sullivan (2007) | −/− | Neo/Neo | −/Neo2 | Neo2/Neo | Neo2/Neo2 | +/− | +/Neo | +/Neo2 | |
| Any major finding | 33/33 | 100 | 100 | – | 100 | 100 | 100 | 100 | 100 | – | – | – |
| Palatal anomalies | 33/33 | 100 | 16 | 69–100 | 100 | – | – | – | – | – | 0 | – |
| Cleft bony palate | 33/33 | 100 | – | 11 | 100 | – | – | – | – | – | 0 | – |
| Absent or hypoplastic thymus | 33/33 | 100 | 28 | – | 100 | √ | √ | 100 | 100 | 41 | √ | 29 |
| Skeletal | 33/33 | 100 | 9 | 17–19 | 100 | – | – | – | – | – | – | – |
| Vertebrae anomalies | 33/33 | 100 | 7 | 19 | 100 | – | – | – | – | – | – | – |
| Cardiovascular defects | 19/19 | 100 | 81 | 49–83 | 100 | – | – | – | – | – | – | – |
| Cardiac defects | 18/19 | 95 | – | – | 100 | 100 | 100 | 85 | – | – | – | – |
| Ventricular septum defect | 18/19 | 95 | 69 | 30–36 | 100 | 100 | 100 | 85 | – | – | – | – |
| Atrioventricular septum defect | 3/12 | 25 | 3 | – | – | – | – | – | – | – | – | – |
| Aortic arch defects | 14/19 | 74 | 63 | – | 100 | 100 | 90 | √ | 100 | 38 | 42 | 11 |
| Interrupted aortic arch | 14/19 | 74 | 23 | 14–15 | 87 | – | – | – | 20 | 3 | – | 0 |
| Right descending aorta | 5/19 | 26 | 43 | – | 13 | – | – | – | – | – | – | – |
Data are derived from this study.
Data are derived from Botto et al. (2003), who examined 35 patients with DGS (43 with respect to some findings), and Kobrynski and Sullivan (2007), who summarized data from multiple sources. Note the high degree of variation among patients with DGS.
Data are derived from Jerome and Papaioannou (2001), Vitelli et al. (2002), Xu et al. (2004), and Zhang and Baldini (2008). Tbx1 mRNA ranges from 0% in the Tbx1−/− to 70% of wild type in the Tbx1+/Neo2. √ indicates that defect is present but penetrance not stated. – indicates t hat no data are available.
Macroscopic examination, ink injection, and resin casting showed that MozΔ/Δ mutants most commonly exhibited an interrupted aortic arch between the left common carotid artery and the left subclavian artery, i.e., interrupted aortic arch type B (IAA-B) (74%; Figures 1G–1J; Table 1). In this condition, the ascending aorta is not connected to the descending aorta (Figures 1H and 1J). In 26% of MozΔ/Δ mutants, the pulmonary trunk was curved to the right side, forming an abnormal right aortic arch and a right descending aorta (Figure S1). Other abnormalities observed included retropharyngeal/retrotracheal right subclavian artery (RSA), abnormal origin of the RSA, and incompletely remodeled great vessel walls, which appear dilated in diameter and thin walled (Figure S1). The aortic arch defects in MozΔ/Δ newborn pups closely resembled anomalies observed in patients with DGS and reported in mice in an allelic series of Tbx1 mutations (Table 1).
Ink injection into the inferior vena cava and resin casts of the heart cavities (Figures 1K–1M) showed that 95% of MozΔ/Δ mutant newborns lacked separation of the left and right side of the heart at the level of the ventricles (n = 19 MozΔ/Δ, 16 MozΔ/+, and 17 Moz+/+ newborn pups). Some MozΔ/Δ mutant newborns showed atrial septal defects in addition (Table 1). Serial histological sections showed overriding aorta and ventricular septal defects (VSDs) of varying severity affecting either large parts of the septum (Figures 1N and 1O) or the superior, membranous part of the ventricular septum (Figure S1). Varying degrees of cardiac defects are also observed in approximately 80% of patients suffering from DGS (Table 1).
Overall, MozΔ/Δ mutant newborn mice exhibited the full spectrum of facial, palatal, thymic, aortic arch, and cardiac septal defects described in DGS and encompass defects observed in mice in a series of Tbx1 mutant alleles (Table 1; Botto et al., 2003; Jerome and Papaioannou, 2001; Kobrynski and Sullivan, 2007; Lindsay et al., 2001; Merscher et al., 2001; Singh et al., 2002; Vitelli et al., 2002; Xu et al., 2004; Yagi et al., 2003; Zhang and Baldini, 2008).
Embryonic Pathogenesis of the Moz Mutant Aortic Arch Defects
Examination of the developmental origin of the aortic arch defects uncovered a failure in fourth pharyngeal arch artery (PAA) development. Between E9.5 and E10.5 (4–5 weeks in human), the fourth PAAs develop. Between E10.5 and E12.5 (5–8 weeks in human), asymmetric remodeling of the PAAs begins and results in the formation of the aortic arch from the fourth PAA on the left side of the body, which then connects the ascending aorta to the descending aorta (Figures 1P–1R). Although the aorta/PAA system was normal in MozΔ/Δ embryos at E9.5 (Figures 1S and 1T; overview in Figure S1), the fourth PAA had failed to develop on the left side in E10.5 MozΔ/Δ embryos (Figures 1U–1X), resulting in the failure to connect the ascending and descending aorta (interrupted aortic arch; Figures 1J and 1R). The fourth PAA on the right body side is reduced in diameter in MozΔ/Δ embryos (Figure S1).
We conclude that the aortic arch anomalies observed in MozΔ/Δ mutant newborn arise from a failure of fourth PAA development between E9.5 and E10.5. Hypoplasia or aplasia of the fourth PAA at E10.5 has been described as the “hallmark of Tbx1 haploinsufficiency” (Randall et al., 2009).
T box Gene Expression Is Reduced in Moz-Deficient Embryos
Three findings suggested that the pathogenic mechanism of the Moz mutant phenotype involved the Tbx1 gene: (1) the Moz loss-of-function defects were similar to the anomalies observed in patients with DGS; (2) the Moz loss-of-function defects encompassed those reported in mice within an allelic series of Tbx1 mutation; and (3) based on our previous study of the role of MOZ in the regulation of Hox gene expression, we postulated that the expression of putative MOZ target gene(s) responsible for the velo-cardio-facial anomalies may only be reduced by approximately one-half. Because haploid mutation of the TBX1 gene in humans can cause DGS (Paylor et al., 2006; Stoller and Epstein, 2005; Torres-Juan et al., 2007; Yagi et al., 2003; Zweier et al., 2007), we determined mRNA expression levels of the Tbx1 gene in Moz mutant and control embryos. In addition, we determined expression of the immediate genomic neighbors of Tbx1 (Figure 2A; Gnb1l, Gp1bb, Sept5) and another gene deleted in the larger 22q11 deletion interval, Crkl, because homozygous mutations in Crkl in mice cause DGS-like defects (Guris et al., 2001). Furthermore, we examined genes encoding other transcription factors involved in heart development (Figure 2B; Nkx2.5, Gata4, Tbx2, Tbx5, Tbx20, Pitx2, Foxa2, Foxc1, Foxc2), along with other genes encoding T-box proteins for comparison (Tbx3, Tbx4) and three housekeeping genes (Pgk1, Hsp90ab1, Gapdh).
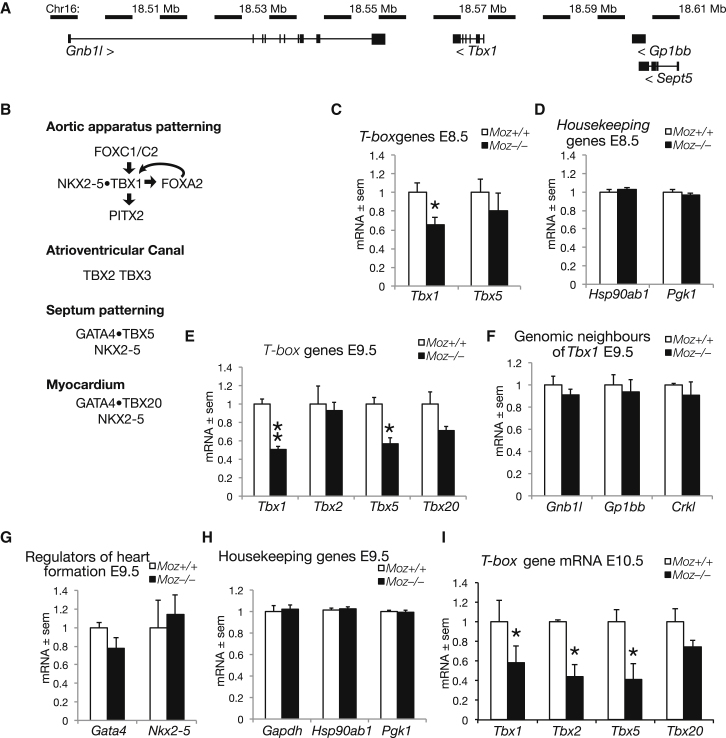
Figure 2.
mRNA Expression Levels of Genes Regulating Heart Development and Genes within the 22q11 Deletion Interval
(A) Schematic drawing of the genomic context of the Tbx1 gene and genes flanking the locus on chromosome 16 in the mouse, a region syntenic with the 22q11 deletion interval in human.
(B) Schematic representation of major regulators of transcription during heart development.
(C–I) mRNA expression levels as assessed by RT-qPCR and normalized to housekeeping genes in Moz+/+ and Moz−/− embryos at E8.5 (C and D), E9.5 (E–H), and E10.5 (I); mRNAs encoded by genes as indicated in the graphs. ∗p < 0.05 and ∗∗p < 0.01. Exact p values are given below. Significantly lower in Moz−/− embryos were mRNAs encoded by Tbx1 at E8.5 (C, p = 0.029), at E9.5 (E, p = 0.001), and E10.5 (I, p = 0.039); Tbx5 at E9.5 (E, p = 0.011) and E10.5 (I, p = 0.006) and Tbx2 at E10.5 (I, p = 0.014). Not significantly changed were the mRNA expression levels of housekeeping genes (D and H), other genes in the 22q11 deletion interval (F), and major regulators of heart development, Gata4 and Nkx2-5 (G). Data are presented and were analyzed as described in the Experimental Procedures. Data were derived from 36 embryos, n = 6 Moz+/+ and 6 Moz−/− per gene at each developmental stage.
Tbx1 was the only one of seven genes examined at E8.5 that showed significantly reduced mRNA levels in Moz−/− mutant embryos as compared to wild-type littermate controls (Figures 2C and 2D), which corresponds to the timing requirement of Tbx1 for fourth PAA formation (Xu et al., 2005). One day later at E9.5, mRNA levels of Tbx1 and Tbx5 were reduced in Moz−/− mutant embryos (Figure 2E). mRNA levels of other genes, including Nkx2-5, Gata4, and Crkl, were normal (Figures 2E–2H). Expression levels of Tbx1 in E9.5 Moz−/− mutant embryos were approximately 50% of wild-type levels. By E10.5, mRNA levels of Tbx1, Tbx2, and Tbx5 were reduced in Moz−/− mutant embryos (Figure 2I). Differences in Tbx1 mRNA levels were also detectable at E11.5 as assessed by northern blot and densitometry (data not shown). Of interest, given the high frequency of PAA remodeling defects in Moz mutants, reduction of Tbx1 to just 70% of the wild-type expression level is sufficient to result in fourth PAA patterning defects and hypoplasia (Zhang and Baldini, 2008).
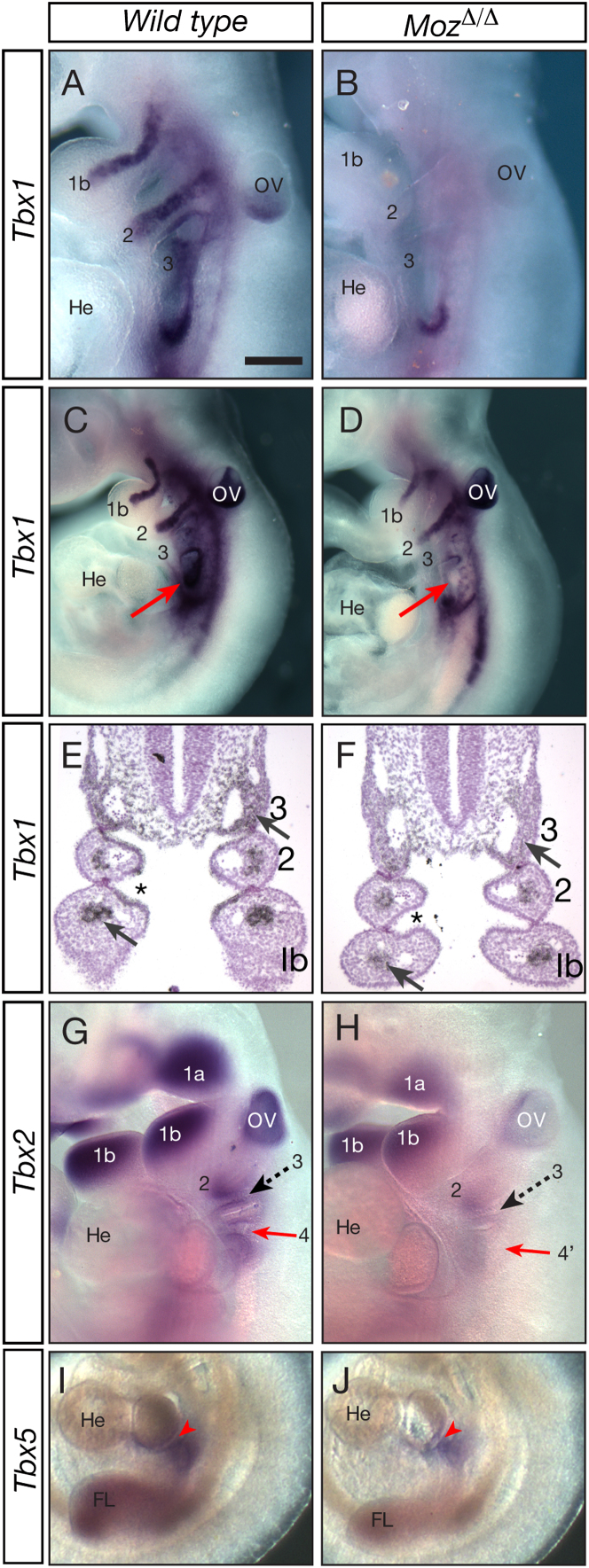
RNA/RNA in situ hybridization of E9.5 sections and whole-mount E10.5 embryos revealed that, whereas wild-type embryos exhibited the previously published expression patterns (Davenport et al., 2003; Naiche and Papaioannou, 2003), expression of Tbx1 was almost absent in some MozΔ/Δ mutant embryos (Figures 3A and 3B) or was reduced (Figures 3C–3F). Similarly, expression levels of Tbx2 (Figures 3G and 3H) and Tbx5 (Figures 3I and 3J), as well as Tbx3 (Figure S2), were reduced. Overall, the expression analysis suggests that of the cardiac development genes examined here, the first affected by the absence of MOZ is Tbx1, whereas effects on Tbx5 and Tbx2 are delayed by 24 and 48 hr, respectively.
Figure 3.
Reduced Expression of Tbx Genes in MozΔ/Δ Embryos Detected by In Situ Hybridization
Moz+/+ (A, C, E, G, and I) and MozΔ/Δ (B, D, F, H, and J) embryos, whole mount at E10.5 (A–D and G–J), and sections at E9.5 (E and F). Expression of Tbx1 (A–F), Tbx2 (G and H), and Tbx5 (I and J). Note the substantial reduction (B) or more moderate reduction (D and F) in Tbx1 mRNA signal in the MozΔ/Δ embryos (dark purple in whole mount), reduced Tbx1 mRNA signal in the mesodermal core of the pharyngeal arches (black-silver grains, arrows E and F), and in the pharyngeal endoderm (asterisks, E and F) in the MozΔ/Δ embryos on sections. The region of the fourth pharyngeal arch is indicated (arrows, C and D). Note the reduction in Tbx2 (G and H) and Tbx5 mRNA signals (I and J, arrowhead indicates Tbx5 expression maintained in the inflow tract at E10.5). 1a and 1b, maxillary and mandibular, respectively, aspect of the first pharyngeal arch; 2, 3, 4, second, third, and fourth pharyngeal arch; 4′, region where the fourth pharyngeal arch should be present in the MozΔ/Δ; FL, forelimb bud; He; Heart; OV, otic vesicle. Scale bar, 190 μm (A and B), 390 μm (C and D), 142 μm (E and F), 350 μm (G and H), and 530 μm (I and J). Data were derived from 42 embryos, n = 6 Moz+/+ and 6 MozΔ/Δ per gene at E10.5 and 3 Moz+/+ and 3 MozΔ/Δ at E9.5. See also Figure S2.
Moz Mutant Embryos Lack H3K9ac at T box Gene Loci
We have previously shown that MOZ is required for H3K9ac at Hox gene loci in E10.5 embryos (Voss et al., 2009). High levels of H3K9ac are associated with the transcriptionally active state of gene loci (Strahl and Allis, 2000). In contrast, H3K9 trimethylation (H3K9me3) is associated with transcriptional repression, recruitment of silencing factors (Bannister et al., 2001; Lachner et al., 2001), and DNA methylation (Estève et al., 2006).
We examined histone modification levels in vivo at Tbx gene loci (Figure 4A) in E10.5 Moz−/− mutant and control embryos by ChIP-quantitative PCR (qPCR) (Figure 4). Histone modifications were unaffected by the Moz mutation at a total of six reference genes (B2m, Pgk1, Rpl13a, Hsp90ab1, Alb, Hbb; data not shown). We observed that the levels of H3K9ac were reduced in Moz−/− mutant embryos at the Tbx1 and Tbx5 loci near the start site of transcription (Figure 4B), 0.7–1 kb upstream of the start site of the Tbx1, Tbx2, and Tbx5 gene (Figure 4C) and in the Tbx1 coding region (Figure 4D). In contrast to H3K9ac, presence or absence of MOZ had no effect on H3K14ac (Figures 4E–4G). The loss of H3K9ac was accompanied by a corresponding increase in the repressive H3K9 modification mark, H3K9me3 (Figures 4H and 4I). Our data reveal a requirement for MOZ for acetylation of H3K9 and transcriptional activity of the Tbx1, Tbx2, and Tbx5 loci in vivo.
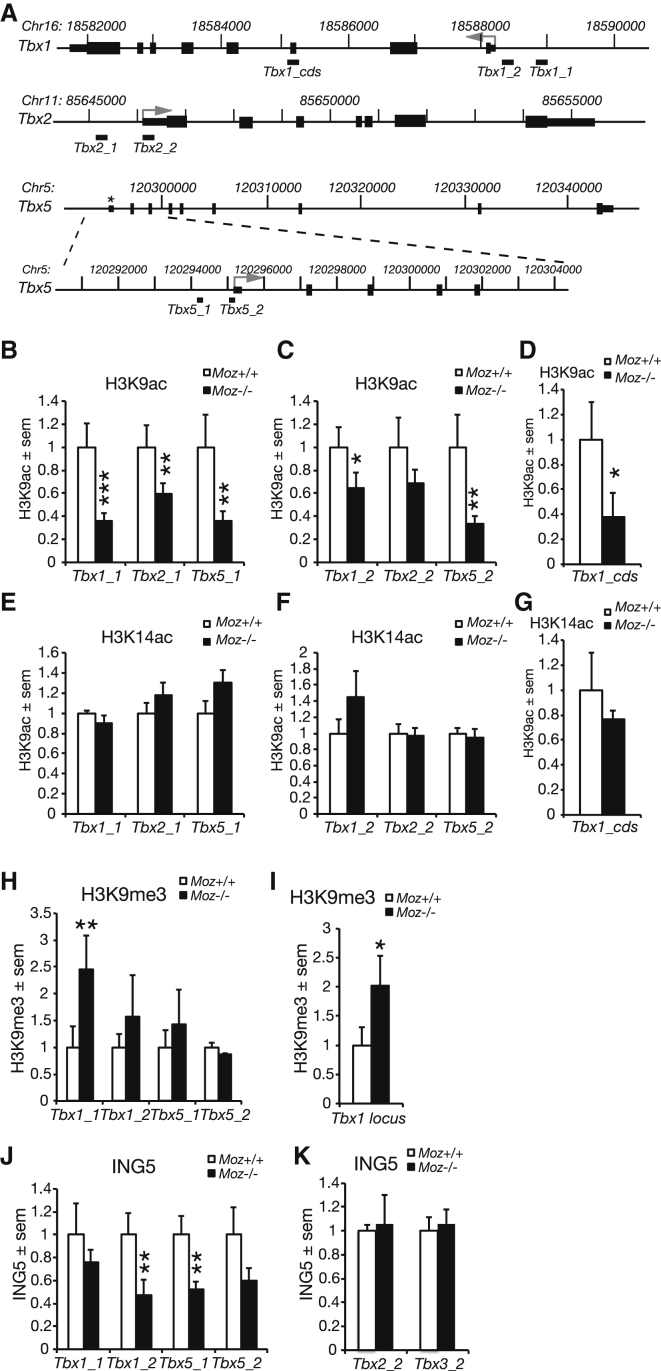
Figure 4.
H3K9ac and MOZ Complex Protein ING5 Occupancy Is Reduced at the Tbx1 and Tbx5 Gene Loci in the Absence of MOZ
(A) Schematic drawing of the genomic loci assayed for histone modifications and ING5 occupancy by ChIP. “Tbx1_1, Tbx1_2, Tbx2_1 etc.” denote sequences amplified in (B)–(K). H3K9ac (B–D), H3K14ac (E–G), H3K9me3 (H and I), and ING5 (J and K) were assessed by ChIP-qPCR of Moz−/− and Moz+/+ E10.5 embryos for sequences −0.7 to −1.0 kb upstream of the TSS (B, E, H, and J; tiles_1), in proximity of the transcription start site (TSS; C, F, H, J, and K; tiles_2) and in the coding region (D and G). Note the reduction in H3K9ac in Moz−/− mutants −0.7 to −1 kb upstream of the TSS of Tbx1, Tbx2, and Tbx5 (B; p < 0.0005, p = 0.003, and p = 0.003, respectively), near the TSS of Tbx1 and Tbx5 (C; p = 0.032 and p = 0.002, respectively), and in the Tbx1 coding region (D; p = 0.0151), but no reduction in H3K14ac (E–G) and an increase in H3K9me3 (H and I; p = 0.0071). Notice the reduction in binding of the MOZ complex protein ING5 to Tbx1 and Tbx5 in Moz−/− mutants (J; p = 0.001 and p < 0.0005, respectively), but not Tbx2 and Tbx3 (K). For the effects of Moz mutation, p values <0.05, <0.01, and <0.001 are indicated by one, two, and three asterisks, respectively. Data are presented and were analyzed as described in the Experimental Procedures. Data are derived from a total of 39 embryos, n = 6 Moz+/+ and 6 Moz−/− per ChIP antibody (B–G), 3 Moz+/+ and 3 Moz−/− (H, I, and K), and 4 Moz+/+ and 5 Moz−/− (J).
In HeLa cells, MOZ associates with ING5 and acetylated histone 3 as part of the MOZ-ING5 complex (Doyon et al., 2006). Because ChIP-quality antibodies to MOZ are not available, we examined binding of the MOZ complex protein ING5 to Tbx loci in lieu of MOZ (Figures 4J and 4K). ING5 associated with Tbx1 and Tbx5 in wild-type embryos showing that components of the MOZ complex occupy these loci. In contrast, ING5 occupancy of the Tbx1 and the Tbx5 locus was significantly reduced in embryos homozygous for the Moz null allele, Moz−/− (Figure 4J), whereas ING5 occupancy at the Tbx2 and Tbx3 loci was not affected by MOZ (Figure 4K).
If the effects of MOZ on histone modification and expression of the Tbx1 and Tbx5 loci were indirect, one would expect to see all histone modification marks associated with gene activation, including H3K14ac, reduced in the Moz mutants. Our observation that Moz mutation reduces the binding of the MOZ complex protein ING5 to the Tbx1 and Tbx5 loci and H3K9ac, but not H3K14ac, suggests that MOZ acts directly on the Tbx1 and Tbx5 loci to acetylate H3K9 and regulates their expression.
Effects of MOZ on Regulators and Targets of TBX1
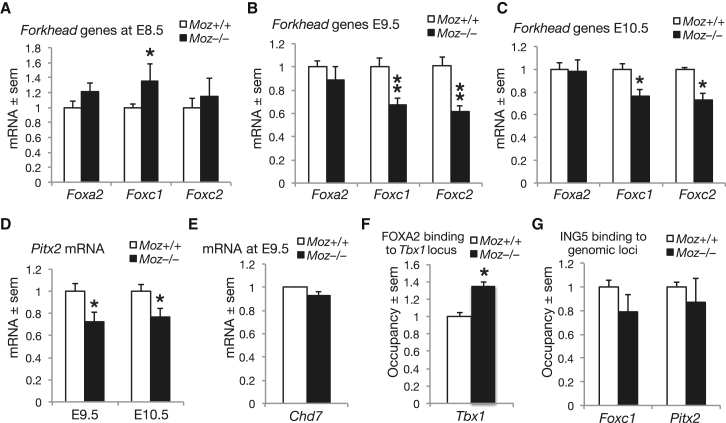
To examine the regulatory network of transcription factors that cooperate with TBX1 in heart development, we determined the effects of loss of Moz on a number of genes encoding transcription factors that were reported to induce Tbx1 gene transcription, i.e., FOXA2, FOXC1, and FOXC2 (Figure 2B; Yamagishi et al., 2003) and a known target gene of TBX1, Pitx2 (Figure 2B; Nowotschin et al., 2006). At E8.5, none of the forkhead genes, Foxa2, Foxc1, or Foxc2, exhibited a reduction in mRNA levels in the Moz−/− mutant embryos. In fact, Foxc1 mRNA levels were elevated in the Moz−/− mutant embryos as compared to controls (Figure 5A). At E9.5 and E10.5, Foxa2 mRNA levels were not different between Moz−/− mutant embryos and controls, whereas Foxc1 and Foxc2 mRNA levels were now reduced in the Moz−/− mutant embryos (Figures 5B and 5C). Expression of Pitx2 requires TBX1 as a transcriptional activator (Nowotschin et al., 2006). As expected in a TBX1-deficient state, Pitx2 mRNA levels were reduced in E9.5 and E10.5 Moz−/− mutant embryos as compared to control (Figure 5D). The Chd7 gene encodes, like Moz, a chromatin modifier, namely the chromodomain helicase DNA-binding protein CHD7 and interacts genetically with the Tbx1 locus (Randall et al., 2009). In contrast to the TBX1 target gene Pitx2, Chd7 gene expression was unaffected by the mutation of Moz (Figure 5E). Of the three forkhead proteins, ChIP-quality antibodies could only be obtained for FOXA2. The Tbx1 locus is regulated by forkhead transcription factors through binding to a number of mutually redundant transcription factor binding sites (Zhang and Baldini, 2010). We examined a consensus forkhead binding site, which is more than 14 kb upstream of exon 1 of the Tbx1 locus and highly conserved between humans and mice (Yamagishi et al., 2003) (for details see Supplemental Experimental Procedures). The site was bound by FOXA2 protein, and occupancy was increased in the Moz−/− mutant embryos, as compared to wild-type controls (Figure 5F), suggesting that the reduced Tbx1 mRNA (or protein) levels induced recruitment of the transcriptional activator to the Tbx1 locus via a feedback mechanism. However, despite this increased recruitment of FOXA2 to the Tbx1 locus, Tbx1 gene transcription was approximately half of normal in the absence of MOZ (Figure 2), indicating that MOZ is specifically required for normal Tbx1 transcription, and the forkhead transcription factors are unable to drive normal levels of transcription without MOZ. The reduction in Foxc1 and Foxc2 mRNA levels at E9.5 and E10.5 conflicted with unchanged or increased mRNA levels at E8.5, suggesting that these were indirect consequences of loss of MOZ. Nevertheless, we examined if MOZ affected the occupancy of the transcription start site of the Foxc1 and Foxc2 gene by ING5, the member of the MOZ complex. ING5 occupancy of the Foxc1 locus was low and not significantly different in Moz−/− mutant and control embryos (Figure 5G), and ING5 did not bind to the Foxc2 locus (data not shown), indicating that the Foxc1 and Foxc2 genes are not direct targets of MOZ. Occupancy of the Pitx2 locus by ING5 was not affected by the absence or presence of MOZ (Figure 5G), suggesting that the reduction in Pitx2 transcriptional activity was an indirect effect of MOZ, presumably via reduction of TBX1 levels.
Figure 5.
Expression of Genes Encoding Regulators of the Tbx1 Gene, Genes Regulated by TBX1 Protein, and Genes Showing Genetic Interaction with the Tbx1 Locus
mRNA levels of genes as indicated assessed by RT-qPCR (A–E) and occupancy of gene loci as indicated by FOXA2 (F) and by the MOZ complex protein ING5 (G). Expression of the genes encoding the forkhead protein FOXA2 (A–C) reported to upregulate Tbx1 gene expression, as well as the Chd7 gene (E), reported to interact genetically with the Tbx1 locus, is unaffected by Moz mutation at E8.5 (A), E9.5 (B and E), and E10.5 (C). Tbx1 locus occupancy assessed by ChIP-qPCR by FOXA2 is increased in E9.5 Moz−/− mutants (F; p = 0.015), albeit without being able to induce normal Tbx1 expression levels in the absence of MOZ (Figure 2). Expression of Foxc1 is increased at E8.5 (A, p = 0.04), and expression of Foxc1 and Foxc2 is decreased at E9.5 (B, p = 0.008 and p = 0.001, respectively) and E10.5 (C, p = 0.034 and p = 0.011, respectively) in Moz−/− mutants without changes in occupancy of Foxc1 locus by ING5 (G; p = 0.251) and without occupancy of the Foxc2 locus by ING5 (data not shown). The expression of the Pitx2 gene, a target gene of TBX1, is decreased at E9.5 and E10.5 in Moz−/− mutants (D, p = 0.023) without changes in ING5 occupancy of the Pitx2 locus (G). For the effects of Moz mutation, p values <0.05 and <0.01 are indicated by one and two asterisks, respectively. Data are presented and were analyzed as described in the Experimental Procedures. Data were derived from 48 embryos, n = 6 Moz+/+ and 6 MozΔ/Δ per developmental stage (A–E) and 3 Moz+/+ and 3 Moz−/− (F and G).
Genetic Interaction between the Moz and the Tbx1 Locus
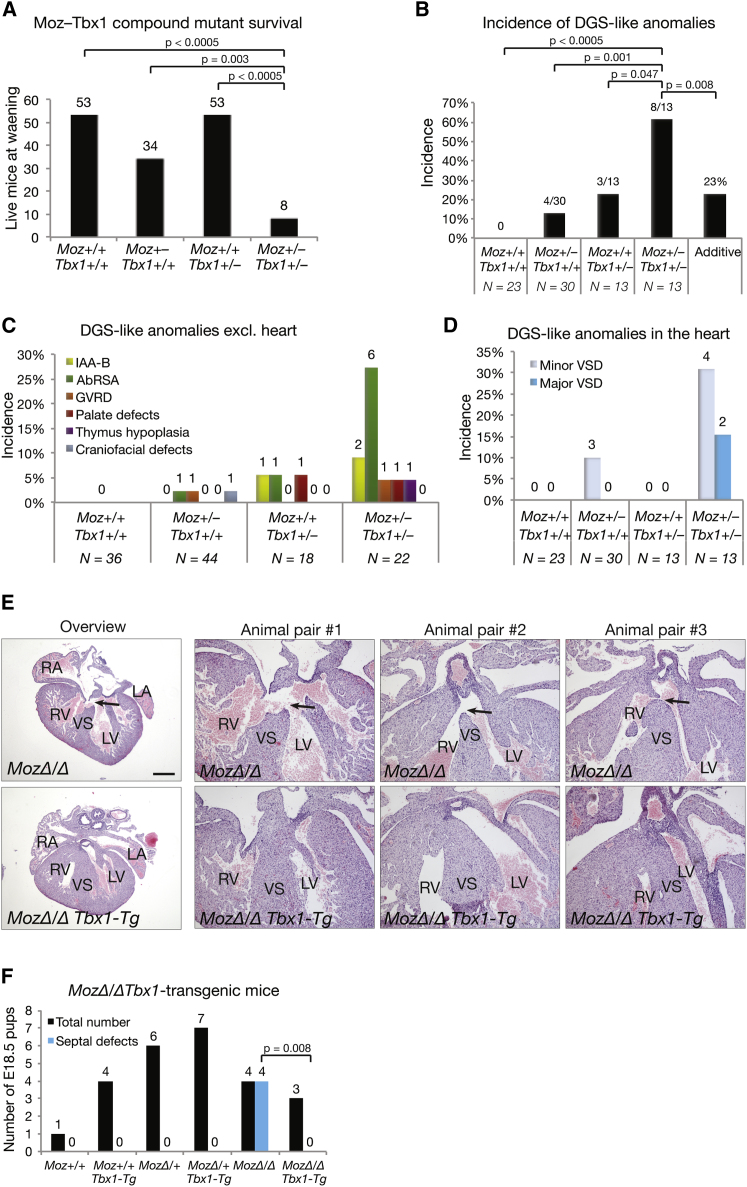
If deficiencies in MOZ contributed to the phenotypic variability of velo-cardio-facial defects via a failure of activation of Tbx1 gene expression, a genetic interaction between the Moz and the Tbx1 gene would be expected. Therefore, we examined wild-type, Tbx1lacZ/+, and Moz+/− single- and double-heterozygous mice for viability and anomalies. Among 148 offspring of Tbx1lacZ/+ by Moz+/− matings were only 8 Tbx1lacZ/+Moz+/− double heterozygous at weaning (Figure 6A), indicating that 80% of Tbx1lacZ/+Moz+/− double heterozygotes had died. At E17.5–E18.5 a large proportion of Tbx1lacZ/+Moz+/− double heterozygous exhibited DGS-like anomalies, significantly larger than the frequency calculated for mere additive effects of Moz and Tbx1 heterozygosity (Figure 6B). A total of 120 E17.5–E18.5 fetuses were examined for defects by macroscopic dissection (Figure 6C) and 79 by serial sectioning and examination of the heart (Figure 6D). Wild-type littermate controls showed no anomalies. Among Moz+/− single heterozygotes we observed RSA, great vessel wall remodeling, and craniofacial defects, as well as minor VSDs (Figures 6B–6D). Among Tbx1lacZ/+ single heterozygotes we saw aortic arch, RSA, and palate defects (Figures 6B–6D). DGS-like anomalies were more frequent and more severe in the Tbx1lacZ/+Moz+/− double heterozygotes (Figure 6B) and included cleft palate, thymus hypoplasia, IAA-B, and abnormal RSAs (Figure 6C) and large VSDs (Figure 6D). The more than additive rise in incidence of DGS-like anomalies and the increase in severity in the double heterozygotes demonstrate synergistic epistasis between Moz and Tbx1 mutation.
Figure 6.
Genetic Synergy between Moz and Tbx1 Haploinsufficiency and Rescue of Moz Mutant Heart Defects by a Tbx1 Transgene
(A) Survival of wild-type, Moz+/− and Tbx1+/− single-heterozygous, and Moz+/−Tbx1+/− double-heterozygous mice to weaning. Note that 80% of Moz+/−Tbx1+/− double-heterozygous mice are not viable.
(B) Incidence of DGS-like anomalies observed in Moz+/−Tbx1+/− double- and single-heterozygous mice at E17.5–E18.5.
(C and D) Details of DGS-like anomalies in locations other than the heart (C) and in the heart (D) observed in the Moz+/−Tbx1+/− double and single heterozygotes.
(E and F) E18.5 offspring in three litters of MozΔ/+Tbx1-Tg by MozΔ/+ matings. (E) Overview and high-resolution images of VSDs in three MozΔ/Δ animals (arrows, as in Figure 1 and Table 1) and the rescue of the septal defects by one copy of the Tbx1-containing BAC RP23-35B9 in three MozΔ/ΔTbx1-Tg animals (serial images in Figure S3). (F) Summary of all 25 offspring. The septal defects observed in the four MozΔ/Δ were three VSDs and one aortopulmonary septal defect. Data are presented and analyzed as described in the Experimental Procedures. AbRSA, abnormal right subclavian artery including retrotracheal and abnormal origin; GVRD, great vessel wall remodeling defects, i.e., dilated in diameter and thin walled; LA, left atrium; LV, left ventricle; IAA-B, interrupted aortic arch type B; RA, right atrium; RV, right ventricle; VS, ventricular septum; VSD, ventricular septal defect. Scale bar, 445 μm in the left two panels of (E) and 185 μm in all other panels of (E).
Rescue of the MozΔ/Δ Heart Phenotype by a Tbx1 Transgene
Because we hypothesized that the reduction in Tbx1 transcriptional activity in the Moz mutants is a mechanistic contributor to the DGS-like defects in the Moz mutant mice, we expected that Tbx1 overexpression might rescue the DGS-like defects of Moz mutant mice at least partially. We generated Tbx1 BAC transgenic mice with one exogenous copy of the Tbx1 gene, resulting in an elevation of Tbx1 mRNA levels to 128% ± 7% (SEM) of wild-type on the Moz wild-type background and a rescue of Tbx1 mRNA levels to 88% ± 8% of wild-type on the MozΔ/Δ background (n = 10 E9.5 embryos per genotype). Although one additional copy of Tbx1 was unable to rescue the aortic arch and palate defects, one copy of the Tbx1-containing BAC rescued the cardiac septal defects, in all three MozΔ/Δ Tbx1-transgenic animals examined (Figures 6E, 6F, and S3). Examination of serial sections of MozΔ/Δ mutant hearts revealed cardiac septation defects in all four MozΔ/Δ hearts in this experiment (three with VSDs and one with an aortopulmonary septal defect), as expected based on the septal defects observed in 18 of 19 MozΔ/Δ mutant hearts examined in experiments described above (95% penetrance, Figure 1; Table 1). In contrast, all three MozΔ/Δ Tbx1-transgenic hearts had intact cardiac and aortopulmonary septa. The rescue indicates that the deficiency in Tbx1 gene expression underlies the cardiac septal defects observed in the Moz homozygous mutant mice.
RA Increases Penetrance of DGS-like Defects in Moz+/− Mice
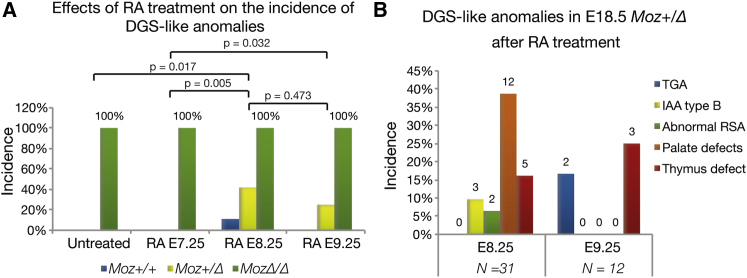
We hypothesized that Moz haploinsufficiency might render an individual sensitive to environmental influence. To test this hypothesis, we investigated if dietary supplementation with RA affected the incidence of DGS-like anomalies in Moz heterozygotes because oversupply with vitamin A or RA has been reported to cause DGS-like anomalies (Lammer et al., 1985). Of 30 mice heterozygous for the Moz− null allele, three exhibited small VSDs (Figure 6D). In contrast, none of the mice heterozygous for the carboxy-terminal deletion allele MozΔ had DGS-like anomalies (Figure 7A, Untreated). We used the MozΔ allele because it appeared to provide just enough MOZ activity in MozΔ/+ heterozygotes to prevent DGS-like defects to occur spontaneously. We examined the effects of RA during a critical phase of PAA and outflow tract development, i.e., from E7.25 to E9.25. Dietary RA at E7.25 had no effects on aortic arch morphology in E17.5–E18.5 fetuses. However, dietary RA at E8.25 and E9.5 caused DGS-like anomalies in 42% and 25% of MozΔ/+ heterozygotes, respectively (Figure 7A). In contrast, only 11% of the RA-treated wild-type controls at E8.5 and none of the controls at E9.5 showed DGS-like symptoms. The anomalies observed in the RA-treated MozΔ/+ heterozygotes included cleft secondary palate, hypoplasia of the thymus, abnormal RSA, transposition of the great arteries, and IAA-B (Figure 7B).
Figure 7.
Sensitization to RA by Loss of One Copy of Moz
(A) Effects of RA treatment at E7.25–E9.25 on Moz+/+, MozΔ/+, and MozΔ/Δ E17.5–E18.5 pups on the incidence of DGS-like anomalies. Note the induction of DGS-like anomalies in heterozygotes treated with RA at E8.25 and E9.25.
(B) Details of anomalies observed in the RA-treated MozΔ/+ heterozygous animals. Data are presented and analyzed as described in the Experimental Procedures. Abnormal RSA, abnormal right subclavian artery including retrotracheal and abnormal origin; IAA type B, interrupted aortic arch type B; TGA, transposition of the great arteries.
Collectively, our findings show that homozygous mutation of the Moz gene or Moz heterozygous mutation combined with either Tbx1 haploinsufficiency or oversupply of RA during pregnancy causes anomalies that resemble DGS with high penetrance (40%–60% in haploid state and 100% in the homozygous state) and include severe defects, i.e., IAA-B and VSD. Importantly, one copy of a Tbx1 transgene rescued the cardiac septal defects in Moz homozygous mutants.
Discussion
We have shown that
-
(1)
MOZ has highly specific functions in the development of the palate, facial structures, thymus, and the cardiovascular system, and lack of MOZ produces defects closely resembling DGS.
-
(2)
MOZ is required for normal levels of Tbx1 mRNA and
-
(3)
normal levels of H3K9ac at the Tbx1 locus.
-
(4)
The MOZ complex protein ING5 binds to the Tbx1 locus in the presence of MOZ.
-
(5)
Homozygous mutation of Moz alone or heterozygous mutation of Moz in combination with either
-
(6)
Tbx1 haploinsufficiency or
-
(7)
oversupply of RA in the diet causes DGS-like anomalies, including IAA-B, thymus aplasia, or hypoplasia, cleft palate, and VSD.
-
(8)
One copy of a Tbx1 transgene rescues the cardiac septal defects seen in Moz mutant mice.
The structures affected in the Moz mutant embryos are derived from Tbx1-positive aspects of the pharyngeal arches (aortic arch, thymus, palate) and the Tbx1 and Pitx2-positive derivatives of the second heart field. One-third of newborn Tbx1 heterozygous display minor great vessel remodeling defects, but overt IAA-B occurs at a low frequency (Lindsay and Baldini, 2001). However, the combination of Moz and Tbx1 haploinsufficiency generates the severe defects (IAA-B and large VSDs) as well as other DGS-like defects at a higher frequency than would be expected from additive effects of the two haploinsufficiencies, showing a synergistic effect of haploid loss of Moz and Tbx1. Unlike other genes reported to exhibit genetic interaction with the Tbx1 locus in causing DGS-like defects (Fgf8, Crkl, Gbx2, Pitx2, Chrd), Moz and another chromatin modifier, Chd7, showed DGS-like defects in the single-heterozygote state (data reported here and Randall et al., 2009 for Chd7).
Our study does not exclude the possible involvement of other MOZ target genes. Indeed, we show here that apart from Tbx1, the Tbx5 locus is also regulated by MOZ, albeit later than Tbx1. In addition, we have previously shown that MOZ is required for H3K9ac and expression of Hox loci and is critical for body segment identity specification (Voss et al., 2009). RA treatment of Moz mutant embryos rescued Hox gene expression, H3K9ac, and segment identity. In contrast, RA treatment enhanced the DGS-like defects showing that the upregulation of Hox gene expression does not ameliorate the DGS-like defects, rendering it unlikely that the DGS-like defects were mediated by reduced Hox gene expression in the MOZ-deficient state. The fact that one additional copy of Tbx1 rescues the cardiac septal defects observed in Moz mutant mice demonstrates that MOZ acts via the Tbx1 gene in cardiac septal development. The observation that one additional copy of Tbx1 does not rescue the aortic arch and palate defects in Moz mutant mice suggests that one additional copy of Tbx1 may not be sufficient but may also indicate that other genes are involved in the development of these structures in addition to Tbx1.
Discordance of anomalies is observed between monozygotic twins with DGS (Goodship et al., 1995) and C57BL/6 inbred Tbx1lacZ/+ mice (our own observation), showing that genetic factors alone are an inadequate explanation for phenotypic variation. The effects of environmental factors mediated by chromatin modifications must therefore play an important role in determining the phenotypic outcome in DGS. Although other chromatin modifiers are known to be required for heart development (Chang et al., 2004; Gottlieb et al., 2002; Lickert et al., 2004; Montgomery et al., 2007; Nimura et al., 2009; Randall et al., 2009; Shirai et al., 2002; Stankunas et al., 2008; Wang et al., 2004), the effects of loss of these on chromatin at the Tbx1 locus were either not examined, or no effect was observed (Gottlieb et al., 2002; Lickert et al., 2004; Stankunas et al., 2008). In contrast, our results show effects of a chromatin modifier, MOZ, on the transcriptional activity through discrete changes of the chromatin at the Tbx1 locus.
Environmental factors known to affect aortic arch and heart development include, among others, vitamin A oversupply and pharmacological overexposure to RA, which can mimic the palate, VSD, thymus, and aortic arch defects seen in DGS (Lammer et al., 1985). RA is known to suppress Tbx1 gene expression (Roberts et al., 2005), and the phenotypic outcome in models of DGS appears to be exquisitely sensitive to gene dosage and time course of Tbx1 gene expression (Liao et al., 2004; Xu et al., 2005; Zhang and Baldini, 2008). Indeed, mice heterozygous for a hypomorphic Moz allele, which do not display DGS-like defects, responded sensitively to oversupply of RA and develop defects in aortic arch, thymus, and palate development, in the time frame consistent with sensitivity to Tbx1 gene expression levels.
In conclusion, we postulate that MOZ, via H3K9ac, drives transcriptional activity of the Tbx1 locus and is essential for aortic arch, heart, thymus, and palate development. The robust development of these structures depends on the presence of two copies of the Moz gene. Importantly, this system is susceptible to perturbations both at the genetic and chromatin level and exquisitely sensitive to environmental insults at key developmental stages.
Experimental Procedures
Mice
All animal experiments were approved by the Walter and Eliza Hall Institute Animal Ethics Committee and conducted in accordance with the Australian code of practice for the care and use of animals for scientific purposes. Mice heterozygous for a carboxy-terminal deletion of MOZ, MozΔ/+ (Thomas et al., 2006), or carrying a Moz null allele, Moz+/− (Voss et al., 2009), on an FVB/BalbC hybrid background were used as indicated in the text and figure legends. Tbx1 BAC transgenic mice were generated by pronuclear microinjection of BAC RP23-35B9, which contains the Tbx1 gene at its center. Tbx1 BAC transgenic line T1-33, which contained one copy of the Tbx1 transgene, was used. Apart from the Tbx1 gene, the BAC contains Sept5, which is not expressed at the relevant developmental stage, as well as Gp1bb and part of Gnbl1. The expression of neither of these two genes is affected by the presence or absence of Moz (Figure 2F). Although the DGS-like defects were highly penetrant in Moz−/− homozygous mice regardless of the genetic background, Tbx1lacZ/+(Lindsay et al., 2001) and Moz+/− mice, both on a C57BL/6 background, were used for the genetic interaction studies because the penetrance of defects in heterozygotes of both strains was largely reduced on an outbred background. Noon following the morning observation of a vaginal mating plug was termed E0.5. Embryos and pups were collected at the developmental stages indicated in the text and figure legends. Palate, thymus, and aortic arch defects were scored during dissection prior to genotyping. Extraembryonic membranes or tails were used for genotyping by three-way PCR as described previously (Lindsay et al., 2001; Thomas et al., 2006; Voss et al., 2009). Oral supplementation with all-trans RA (Sigma-Aldrich; R2625) was performed as described previously (Voss et al., 2009) at the developmental stages indicated in the figures.
Expression Analysis
Whole-mount and radioactive in situ hybridization, total RNA isolation, northern blot analysis, and densitometry were conducted using standard techniques as previously described (Thomas et al., 2007; Voss et al., 2000). cRNA and cDNA probes are listed in the Supplemental Experimental Procedures. qPCR was performed using a Roche LightCycler 480 and SYBR Green based detection. Details on primers are given in the Supplemental Experimental Procedures.
ChIP
ChIP followed by qPCR was carried out as described previously (Voss et al., 2009, 2012) using primers described in Supplemental Experimental Procedures. Antibodies used for ChIP, immunoblotting, and immunofluorescence were directed against ING5 (Abnova; H00084289-B01), FOXA2 (HNF-3b [M-20]: sc-6554; Santa Cruz Biotechnology), H3K9ac (Millipore; Upstate 07-352), H3K9me3 (Abcam; ab8898), and H3K14ac (Millipore; Upstate 07-353). The histone antibodies detect the modified forms preferentially over the other forms. Anti-IgG was used as a control in ChIP.
Statistics
Data were analyzed using Intercooled Stata 10 software. RT-qPCR and ChIP-qPCR data are presented as standardized mean ± SEM of cDNA and gDNA concentration normalized for the housekeeping genes Hsp90ab1, Pgk1, and Gapdh for RT-qPCR and for either β-2-microglobulin and Hsp90b for transcriptionally active loci or albumin and hemoglobin β for transcriptionally inactive loci in the ChIP-qPCR experiments. Data were analyzed by one or two-factorial ANOVA with a number of intact Moz alleles with or without genomic locus as independent factors, followed by Bonferroni's post hoc test. Frequencies of genotypes at weaning and frequencies of DGS-like anomalies at E17.5–E18.5 in RA treatment experiments, genetic interaction, and rescue studies were examined by Pearson's chi-square test.
Acknowledgments
We thank N. Downer, C. Gatt, and L. Sampurno for excellent technical assistance. We gratefully acknowledge A. Baldini for helpful advice on the manuscript and provision of the Tbx1 mutant mice. We thank V.E. Papaiaonnou for supplying T box cDNA probes. This work was supported by the Australian National Health and Medical Research Council (project grants, senior research fellowships to A.K.V. and T.T.; scholarship to B.N.S.), the Australian Stem Cell Centre (program module to A.K.V. and T.T.; scholarship to B.N.S.), the British Heart Foundation (PG/10/032/28333 RG/10/13/28570 to P.S.), and Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
Published online: August 23, 2012
Footnotes
Supplemental Information includes three figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2012.07.010.
Contributor Information
Anne K. Voss, Email: avoss@wehi.edu.au.
Tim Thomas, Email: tthomas@wehi.edu.au.
Supplemental Information
References
- Bannister A.J., Zegerman P., Partridge J.F., Miska E.A., Thomas J.O., Allshire R.C., Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Borrow J., Stanton V.P., Jr., Andresen J.M., Becher R., Behm F.G., Chaganti R.S., Civin C.I., Disteche C., Dubé I., Frischauf A.M. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat. Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- Botto L.D., May K., Fernhoff P.M., Correa A., Coleman K., Rasmussen S.A., Merritt R.K., O'Leary L.A., Wong L.Y., Elixson E.M. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Chang S., McKinsey T.A., Zhang C.L., Richardson J.A., Hill J.A., Olson E.N. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol. Cell. Biol. 2004;24:8467–8476. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport T.G., Jerome-Majewska L.A., Papaioannou V.E. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- Doyon Y., Cayrou C., Ullah M., Landry A.J., Côté V., Selleck W., Lane W.S., Tan S., Yang X.J., Côté J. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell. 2006;21:51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Estève P.O., Chin H.G., Smallwood A., Feehery G.R., Gangisetty O., Karpf A.R., Carey M.F., Pradhan S. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev. 2006;20:3089–3103. doi: 10.1101/gad.1463706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodship J., Cross I., Scambler P., Burn J. Monozygotic twins with chromosome 22q11 deletion and discordant phenotype. J. Med. Genet. 1995;32:746–748. doi: 10.1136/jmg.32.9.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P.D., Pierce S.A., Sims R.J., Yamagishi H., Weihe E.K., Harriss J.V., Maika S.D., Kuziel W.A., King H.L., Olson E.N. Bop encodes a muscle-restricted protein containing MYND and SET domains and is essential for cardiac differentiation and morphogenesis. Nat. Genet. 2002;31:25–32. doi: 10.1038/ng866. [DOI] [PubMed] [Google Scholar]
- Guris D.L., Fantes J., Tara D., Druker B.J., Imamoto A. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nat. Genet. 2001;27:293–298. doi: 10.1038/85855. [DOI] [PubMed] [Google Scholar]
- Jerome L.A., Papaioannou V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Katsumoto T., Aikawa Y., Iwama A., Ueda S., Ichikawa H., Ochiya T., Kitabayashi I. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 2006;20:1321–1330. doi: 10.1101/gad.1393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrynski L.J., Sullivan K.E. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370:1443–1452. doi: 10.1016/S0140-6736(07)61601-8. [DOI] [PubMed] [Google Scholar]
- Lachner M., O'Carroll D., Rea S., Mechtler K., Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- Lammer E.J., Chen D.T., Hoar R.M., Agnish N.D., Benke P.J., Braun J.T., Curry C.J., Fernhoff P.M., Grix A.W., Jr., Lott I.T. Retinoic acid embryopathy. N. Engl. J. Med. 1985;313:837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Liao J., Kochilas L., Nowotschin S., Arnold J.S., Aggarwal V.S., Epstein J.A., Brown M.C., Adams J., Morrow B.E. Full spectrum of malformations in velo-cardio-facial syndrome/DiGeorge syndrome mouse models by altering Tbx1 dosage. Hum. Mol. Genet. 2004;13:1577–1585. doi: 10.1093/hmg/ddh176. [DOI] [PubMed] [Google Scholar]
- Lickert H., Takeuchi J.K., Von Both I., Walls J.R., McAuliffe F., Adamson S.L., Henkelman R.M., Wrana J.L., Rossant J., Bruneau B.G. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432:107–112. doi: 10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- Lindsay E.A. Chromosomal microdeletions: dissecting del22q11 syndrome. Nat. Rev. Genet. 2001;2:858–868. doi: 10.1038/35098574. [DOI] [PubMed] [Google Scholar]
- Lindsay E.A., Baldini A. Recovery from arterial growth delay reduces penetrance of cardiovascular defects in mice deleted for the DiGeorge syndrome region. Hum. Mol. Genet. 2001;10:997–1002. doi: 10.1093/hmg/10.9.997. [DOI] [PubMed] [Google Scholar]
- Lindsay E.A., Vitelli F., Su H., Morishima M., Huynh T., Pramparo T., Jurecic V., Ogunrinu G., Sutherland H.F., Scambler P.J. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Merscher S., Funke B., Epstein J.A., Heyer J., Puech A., Lu M.M., Xavier R.J., Demay M.B., Russell R.G., Factor S. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Montgomery R.L., Davis C.A., Potthoff M.J., Haberland M., Fielitz J., Qi X., Hill J.A., Richardson J.A., Olson E.N. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–1802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche L.A., Papaioannou V.E. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–2693. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- Nimura K., Ura K., Shiratori H., Ikawa M., Okabe M., Schwartz R.J., Kaneda Y. A histone H3 lysine 36 trimethyltransferase links Nkx2-5 to Wolf-Hirschhorn syndrome. Nature. 2009;460:287–291. doi: 10.1038/nature08086. [DOI] [PubMed] [Google Scholar]
- Nowotschin S., Liao J., Gage P.J., Epstein J.A., Campione M., Morrow B.E. Tbx1 affects asymmetric cardiac morphogenesis by regulating Pitx2 in the secondary heart field. Development. 2006;133:1565–1573. doi: 10.1242/dev.02309. [DOI] [PubMed] [Google Scholar]
- Paylor R., Glaser B., Mupo A., Ataliotis P., Spencer C., Sobotka A., Sparks C., Choi C.H., Oghalai J., Curran S. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall V., McCue K., Roberts C., Kyriakopoulou V., Beddow S., Barrett A.N., Vitelli F., Prescott K., Shaw-Smith C., Devriendt K. Great vessel development requires biallelic expression of Chd7 and Tbx1 in pharyngeal ectoderm in mice. J. Clin. Invest. 2009;119:3301–3310. doi: 10.1172/JCI37561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifsnyder C., Lowell J., Clarke A., Pillus L. Yeast SAS silencing genes and human genes associated with AML and HIV-1 Tat interactions are homologous with acetyltransferases. Nat. Genet. 1996;14:42–49. doi: 10.1038/ng0996-42. [DOI] [PubMed] [Google Scholar]
- Roberts C., Ivins S.M., James C.T., Scambler P.J. Retinoic acid down-regulates Tbx1 expression in vivo and in vitro. Dev. Dyn. 2005;232:928–938. doi: 10.1002/dvdy.20268. [DOI] [PubMed] [Google Scholar]
- Shirai M., Osugi T., Koga H., Kaji Y., Takimoto E., Komuro I., Hara J., Miwa T., Yamauchi-Takihara K., Takihara Y. The Polycomb-group gene Rae28 sustains Nkx2.5/Csx expression and is essential for cardiac morphogenesis. J. Clin. Invest. 2002;110:177–184. doi: 10.1172/JCI14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.M., Murphy B., O'Reilly R. Monozygotic twins with chromosome 22q11 deletion and discordant phenotypes: updates with an epigenetic hypothesis. J. Med. Genet. 2002;39:e71. doi: 10.1136/jmg.39.11.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankunas K., Hang C.T., Tsun Z.Y., Chen H., Lee N.V., Wu J.I., Shang C., Bayle J.H., Shou W., Iruela-Arispe M.L., Chang C.P. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev. Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller J.Z., Epstein J.A. Identification of a novel nuclear localization signal in Tbx1 that is deleted in DiGeorge syndrome patients harboring the 1223delC mutation. Hum. Mol. Genet. 2005;14:885–892. doi: 10.1093/hmg/ddi081. [DOI] [PubMed] [Google Scholar]
- Strahl B.D., Allis C.D. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Thomas T., Voss A.K. The diverse biological roles of MYST histone acetyltransferase family proteins. Cell Cycle. 2007;6:696–704. doi: 10.4161/cc.6.6.4013. [DOI] [PubMed] [Google Scholar]
- Thomas T., Corcoran L.M., Gugasyan R., Dixon M.P., Brodnicki T., Nutt S.L., Metcalf D., Voss A.K. Monocytic leukemia zinc finger protein is essential for the development of long-term reconstituting hematopoietic stem cells. Genes Dev. 2006;20:1175–1186. doi: 10.1101/gad.1382606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Loveland K.L., Voss A.K. The genes coding for the MYST family histone acetyltransferases, Tip60 and Mof, are expressed at high levels during sperm development. Gene Expr. Patterns. 2007;7:657–665. doi: 10.1016/j.modgep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Torres-Juan L., Rosell J., Morla M., Vidal-Pou C., García-Algas F., de la Fuente M.A., Juan M., Tubau A., Bachiller D., Bernues M. Mutations in TBX1 genocopy the 22q11.2 deletion and duplication syndromes: a new susceptibility factor for mental retardation. Eur. J. Hum. Genet. 2007;15:658–663. doi: 10.1038/sj.ejhg.5201819. [DOI] [PubMed] [Google Scholar]
- Vitelli F., Morishima M., Taddei I., Lindsay E.A., Baldini A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum. Mol. Genet. 2002;11:915–922. doi: 10.1093/hmg/11.8.915. [DOI] [PubMed] [Google Scholar]
- Voss A.K., Thomas T. MYST family histone acetyltransferases take center stage in stem cells and development. Bioessays. 2009;31:1050–1061. doi: 10.1002/bies.200900051. [DOI] [PubMed] [Google Scholar]
- Voss A.K., Thomas T., Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127:1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- Voss A.K., Collin C., Dixon M.P., Thomas T. Moz and retinoic acid coordinately regulate H3K9 acetylation, Hox gene expression, and segment identity. Dev. Cell. 2009;17:674–686. doi: 10.1016/j.devcel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Voss A.K., Dixon M.P., McLennan T., Kueh A.J., Thomas T. Chromatin immunoprecipitation of mouse embryos. Methods Mol. Biol. 2012;809:335–352. doi: 10.1007/978-1-61779-376-9_23. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhai W., Richardson J.A., Olson E.N., Meneses J.J., Firpo M.T., Kang C., Skarnes W.C., Tjian R. Polybromo protein BAF180 functions in mammalian cardiac chamber maturation. Genes Dev. 2004;18:3106–3116. doi: 10.1101/gad.1238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Morishima M., Wylie J.N., Schwartz R.J., Bruneau B.G., Lindsay E.A., Baldini A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- Xu H., Cerrato F., Baldini A. Timed mutation and cell-fate mapping reveal reiterated roles of Tbx1 during embryogenesis, and a crucial function during segmentation of the pharyngeal system via regulation of endoderm expansion. Development. 2005;132:4387–4395. doi: 10.1242/dev.02018. [DOI] [PubMed] [Google Scholar]
- Yagi H., Furutani Y., Hamada H., Sasaki T., Asakawa S., Minoshima S., Ichida F., Joo K., Kimura M., Imamura S. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- Yamagishi H., Maeda J., Hu T., McAnally J., Conway S.J., Kume T., Meyers E.N., Yamagishi C., Srivastava D. Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev. 2003;17:269–281. doi: 10.1101/gad.1048903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Baldini A. In vivo response to high-resolution variation of Tbx1 mRNA dosage. Hum. Mol. Genet. 2008;17:150–157. doi: 10.1093/hmg/ddm291. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Baldini A. Manipulation of endogenous regulatory elements and transgenic analyses of the Tbx1 gene. Mamm. Genome. 2010;21:556–564. doi: 10.1007/s00335-010-9304-4. [DOI] [PubMed] [Google Scholar]
- Zweier C., Sticht H., Aydin-Yaylagül I., Campbell C.E., Rauch A. Human TBX1 missense mutations cause gain of function resulting in the same phenotype as 22q11.2 deletions. Am. J. Hum. Genet. 2007;80:510–517. doi: 10.1086/511993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.