Abstract
Incenp is an essential mitotic protein that, together with Aurora B, Survivin, and Borealin, forms the core of the chromosomal passenger protein complex (CPC). The CPC regulates various mitotic processes and functions to maintain genomic stability. The proper subcellular localization of the CPC and its full catalytic activity require the presence of each core subunit in the complex. We have investigated the mitotic tasks of the CPC using a function blocking antibody against Incenp microinjected into cells at different mitotic phases. This method allowed temporal analysis of CPC functions without perturbation of complex assembly or activity prior to injection. We have also studied the dynamic properties of Incenp and Aurora B using fusion protein photobleaching. We found that in early mitotic cells, Incenp and Aurora B exhibit dynamic turnover at centromeres, which is prevented by the anti-Incenp antibody. In these cells, the loss of centromeric CPC turnover is accompanied by forced mitotic exit without the execution of cytokinesis. Introduction of anti-Incenp antibody into early anaphase cells causes abnormalities in sister chromatid separation through defects in anaphase spindle functions. In summary, our data uncovers new mitotic roles for the CPC in anaphase and proposes that CPC turnover at centromeres modulates spindle assembly checkpoint signaling.
Introduction
Mitotic errors such as precocious chromosome segregation and lagging anaphase chromatids are a potential source of genomic instability that is characteristic of human cancer cells (Yuen et al. 2005). To prevent these errors, cell division is safeguarded by the spindle assembly checkpoint (SAC), which generates cell cycle inhibitory signals in response to incorrect microtubule-chromosome attachments and reduced inter-kinetochore tension (Gorbsky 2001; Musacchio and Salmon 2007). Many proteins that are involved in SAC signaling associate transiently with kinetochore–centromere complexes during early phases of mitosis (Howell et al. 2004; Kallio et al. 2002a; Shah et al. 2004), a phenomenon that is believed to be important for the rapid distribution of cell cycle inhibitory signals in response to attachment errors.
Aurora B, the catalytic subunit of the chromosomal passenger protein complex (CPC), regulates SAC activity (Ruchaud et al. 2007). The kinase has roles in kinetochore recruitment of SAC proteins (Ditchfield et al. 2003) and in the correction of erroneous microtubule–kinetochore attachments, thereby contributing to proper SAC signaling (Cimini et al. 2006; Pinsky et al. 2006). Although the mechanism is not completely understood, inhibition of Aurora B by small molecules or function neutralizing antibodies causes premature escape from microtubule drug-induced mitotic arrest (Gadea and Ruderman 2005; Hauf et al. 2003; Kallio et al. 2002b). The escape is due to precocious SAC inactivation and possibly involves changes in the centromeric turnover of CPC and other regulatory proteins. For example, Survivin undergoes constant exchange between centromeres and the cytoplasm at a rapid rate (Beardmore et al. 2004; Delacour-Larose et al. 2004), but it remains unclear if alterations in CPC subunit turnover can directly modulate SAC signaling.
Aurora B kinase activity depends on its binding to Incenp, which becomes phosphorylated by the kinase in a self-stimulatory loop (Adams et al. 2000; Bishop and Schumacher 2002; Honda et al. 2003). Depletion of Aurora B or Incenp by RNAi, or disruption of normal CPC function with dominant negative Incenp mutants, have been reported to cause severe mitotic defects such as errors in chromosome alignment, improper segregation of chromosomes, and failure of cytokinesis (Adams et al. 2001; Honda et al. 2003; Mackay et al. 1998). Due to the strong spatial and functional interdependencies of the CPC subunits (Jeyaprakash et al. 2007; Klein et al. 2006), genetic perturbation of any core subunit leads to improper assembly and mislocalization of the complex in early mitosis (Adams et al. 2001; Honda et al. 2003). This has hampered detailed analysis of CPC tasks during late mitotic phases. It has also remained unclear whether the mitotic defects that follow Incenp and Aurora B inhibition are due to direct effects on the two proteins or caused by mistakes in the assembly and subcellular localization of the CPC in early mitosis.
Here, we addressed two key questions: what are the consequences of functional perturbation of the CPC at different stages of mitosis, and will changes in the turnover of CPC subunits modulate SAC signaling? To answer these questions, we injected a function blocking antibody against Incenp (Incenp−ab) into Xenopus tissue culture cells (Xeno S3) at different mitotic stages from prophase to late anaphase. We analyzed the turnover of Incenp and Aurora B at inner centromeres during normal mitosis and after introducing Incenp−ab by measuring fluorescence recovery after photo-bleaching (FRAP). Our results indicate that in early anaphase cells CPC activity is needed for the normal poleward motility of sister chromatids. Introduction of Incenp−ab into mitotic cells generates notable anomalies in the function of spindle microtubules throughout cell division. Furthermore, we demonstrate that both Incenp and Aurora B undergo dynamic exchange at inner centromeres, and that this is blocked by the Incenp−ab, an event that coincides with override of SAC.
Materials and methods
Reagents and cell culture
Cell culture media, supplements, and chemicals were purchased from Sigma unless stated otherwise. HeLa and LLC-PK cells were grown at 37°C in a humidified incubator with 5% CO2. Cells were grown in DMEM supplemented with 10% fetal bovine serum, 20 mM HEPES, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, and 0.1 mg/ml penicillin or streptomycin. Xeno S3 cells were grown at RT in Leibovitz’s L-15 medium supplemented with 15% fetal bovine serum, 2 mM L-glutamine, 0.1 mg/ml penicillin/streptomycin, and 15% H2O. Nocodazole, Taxol (Paclitaxel, Molecular Probes), ZM447439 (a generous gift from AstraZeneca), and MG132 were used in the experiments at 3, 0.6, 20, and 20 µM concentrations, respectively.
Antibody injection and imaging
Incenp−ab has been characterized previously (Bolton et al. 2002). For microinjections and live cell analysis Xeno S3, HeLa and LLC-PK cells were grown on 35-mm chambers with class bottom (MatTek, Corp.), and for fixed cell analysis, cells were grown on coverslips. Incenp−ab microinjections were performed using a Narishige MN-151 micromanipulator and Narishige PN-30 needle puller (World Precision Instruments, Inc.) at 5.0-mg/ml needle concentration (microinjection buffer: 0.1 M KCl, 1.7 mM NaCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4). The average injected volume was 0.1 pL equaling to a mean intracellular concentration of 0.125 mg/ml of Incenp−ab in a Xenopus cell with volume of 4 pL. Control cells were injected with unspecific rabbit IgG (Jackson Immunoresearch) at 5 mg/ml or microinjection buffer. In experiments where nocodazole, taxol, or MG132 were used, cells were preincubated with the drugs for 20–60 min before microinjection. Xeno S3 cells were imaged at RT using phase-contrast and/or fluorescence illumination. Images were captured with a Zeiss Axiovert microscope equipped with 63× (N.A. 1.4) and 100× (N.A. 1.4) objectives, Hamamatsu Orca 2 camera (Hamamatsu Photonics), and Metamorph imaging software (Universal Imaging Corp). Immunofluorescence images acquired from fixed samples were deconvoluted using Metamorph software.
Plasmid construction and transfection
GFP-hIncenp (human sequence) was a kind gift from Erich Nigg and was described previously (Klein et al. 2006). GFP-xIncenp (Xenopus sequence) was constructed from pCS2-YFP-xIncenp by amplification of xIncenp using primers that create restriction sites for Xho1 and BamH1 and cloning into pEGFP-C1. pCS2-YFP-xIncenp plasmid was generated from Pet28B-xINCENP (Bolton et al. 2002) by subcloning with Sal1 and Xba1 sites into PCS2 + YFP, which was a kind gift from David Wotton (Kagey et al. 2003). For construction of xAurora B-YFP the xAurora B (xAIRK2) coding sequence was amplified from cDNA from Xeno S3 cells using primers that introduce specific restriction sites at the 5′ and 3′-ends (HindIII and BamHI) and cloned into pEYFP-N1 (BD Biosciences). pEGFP-tubulin (BD Biosciences) was used to create Xeno S3 GFP-tubulin cell line. HeLa and LLC-PK cells were transfected with GFP-hIncenp using Effectene (Qiagen) and used for FRAP analyses 24–30 h after transfection. Xeno S3 cells were electroporated with GFP-xIncenp or xAurora B-YFP using the ECM 830 electroporator (BTX, Holliston, MA, USA) and used for microinjections and FRAP analyses 48 h later.
In vitro kinase assay
To study the in vitro effect of INCENP antibody on Aurora-B kinase activity, 23 pmol recombinant Aurora-B/IN-Box (Rosasco-Nitcher et al. 2008) was treated with the indicated concentration of antibody for 10 min at room temperature. Subsequently, kinase activity assays were started upon addition of substrate (GST-Histone H3 (1–40)) and γ-32P-ATP. Assay time points were quenched in sodium dodecyl sulfate (SDS) sample buffer, analyzed by SDS-polyacrylamide gel electrophoresis, and PO−4 incorporation on histone H3 was quantified on a phosphoimager.
Fluorescence recovery after photobleaching
Confocal images were captured using Zeiss LSM510 META confocal microscope with 63× (N.A. 1.4) objective using LSM5 3.2 software with Physiology option (Carl Zeiss Corporation, Jena, Germany). Experiments with HeLa and LLC-PK cells were performed at 37°C and 5% CO2 in a humidified chamber, and experiments with Xeno S3 cells at RT. Three images were captured prior to photobleaching, after which a small region of interest covering one or two centromeres was bleached with 488-nm laser irradiation. Fluorescence recovery was followed by scanning the sample with 488 nm low intensity laser irradiation at regular intervals. The frame capture interval was 5–20 s and total time-lapse duration 5–10 min. Acquired data was corrected for background and fitted to f(t) = A(1-exp(kt)) using FRAPCalc® (Rolf Sara, Turku Centre for Biotechnology) to calculate protein turnover half-time (t1/2) and total recovery of fluorescence (recf). Graphs presenting FRAP curves in Fig. 3 were plotted with Prism 3.0 software (GraphPad Software, Inc.).
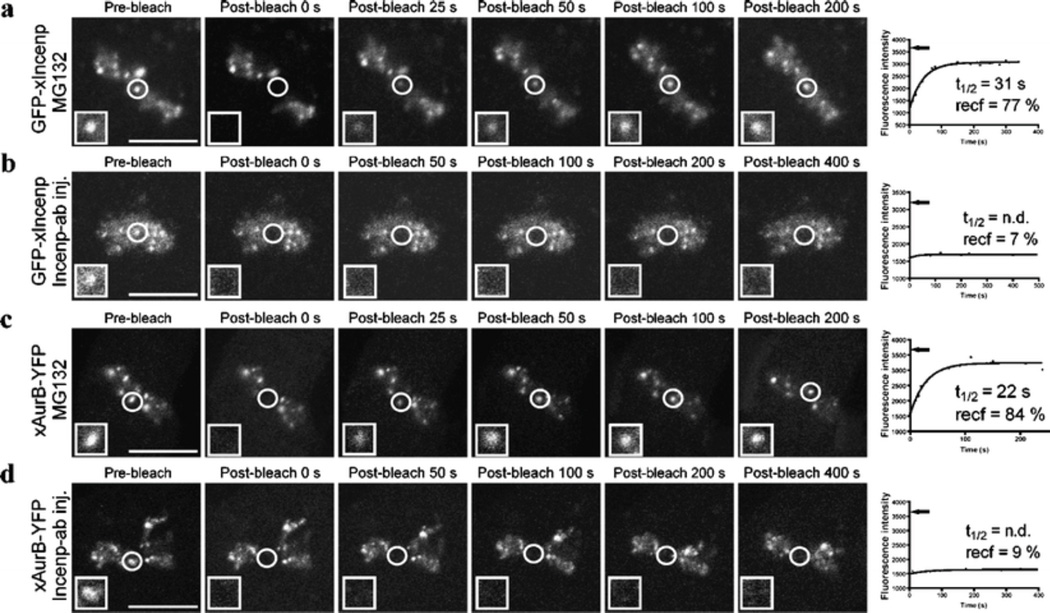
Fig. 3.
Incenp−ab prevents the dynamic exchange of Incenp and Aurora B at inner centromeres. a, c FRAP analyses of GFP-xIncenp and xAurora B-YFP turnover at the inner centromeres of Xeno S3 cells in the presence of MG132. b, d GFP-xIncenp and xAurora B-YFP turnover after Incenp−ab injection in the presence of MG132. Introduction of Incenp−ab significantly limits the recovery of both fusion proteins to the photobleached areas. Fluorescent images show fusion protein signal before and at different time points after photobleaching. White squares show higher magnification views of the target areas. The recovery half-time (t1/2) and total recovery of fluorescence (recf) are determined from the recovery curves that correspond to the still images. Black arrows indicate the level of fluorescence intensity of the target area prior to photobleaching. Scale bars = 10 µm. Videos corresponding to still images in panels a–d (Electronic supplementary material, Videos 4, 5, 6, 7) are available as supplementary material
Cell fixation and immunofluorescence labeling
Xeno S3 cells growing on coverslips were fixed for 15 min in PHEM, 2% paraformaldehyde, 0.2% glutaraldehyde (added only in case of fixation for tubulin staining), and 0.5% Triton X-100. The coverslips were rinsed in 10 mM MOPS, pH 7.4, 150 mM NaCl, 0.05% Tween-20 (MBST), and blocked for 1 h in 20% boiled normal goat serum in MBST. To image microtubules, coverslips were stained with rat anti-tubulin primary antibody (Abcam) followed by Cy5-conjugated secondary anti-rat antibody (Jackson Immunoresearch). Coverslips were incubated in FITC-conjugated goat anti-rabbit secondary antibody (Jackson Immunoresearch) to detect Incenp−ab injected cells, or antibody was injected together with FITC-dextran (Invitrogen). Cy3-conjugated T95-pMCAK was used at dilution 1:300 (Zhang et al. 2007). DNA was counter stained with DAPI before mounting in Vectashield (Vector Laboratories).
Results
Introduction of Incenp−ab into early mitotic cells perturbs normal chromosome alignment and SAC signaling
The polyclonal rabbit Incenp−ab was raised against His-tagged Incenp677–874 encoding the C-terminal fragment of Xenopus Incenp (Bolton et al. 2002). Immunostaining of Xeno S3 cells with Incenp−ab showed a staining pattern that corresponds to the reported subcellular localization of Incenp (Cooke et al. 1987). Bright signals were detected at inner centromeres from prophase to metaphase, at midzone microtubules in anaphase, and at the midbody of telophase cells (Electronic supplementary material, Fig. S1A). On western blot, the antibody detected a specific protein of 135 kDa in Xenopus egg and tissue culture cell extracts corresponding to the expected size of Incenp (Bolton et al. 2002). Importantly, when injected into metaphase (Electronic supplementary material, Fig. S1B) or anaphase Xeno S3 cells (Electronic supplementary material, Fig. S1C), Incenp−ab rapidly located to the same regions as the endogenous protein, the inner centromeres, and the spindle midzone, respectively, suggesting that the antibody targets Incenp at its natural locations.
To temporally dissect Incenp or CPC functions in mitosis, we first introduced Incenp−ab or control IgG into Xeno S3 cells at early stages of mitosis and imaged the cells using time-lapse microscopy as they progressed through cell division. In Incenp−ab-injected prophase cells, chromosomes rapidly made initial attachments to microtubules upon nuclear envelope breakdown (NEB) as indicated by fast poleward chromosome movements (Fig. 1a and b; Electronic supplementary material, Videos 1 and 2). However, most of the chromosomes were unable to achieve metaphase alignment within the same time the control cells had formed metaphase plates (approximately 20 min after NEB; Fig. 1c). The speed of the chromosome movements appeared reduced in the Incenp−ab-injected prometaphase cells compared to controls. These observations are in agreement with previous studies that reported chromosome alignment defects after silencing or genetic perturbation of Incenp (Adams et al. 2001; Mackay et al. 1998). Together, these data suggest that Incenp−ab does not prevent formation of microtubule– kinetochore attachments but interferes with the cells’ ability to resolve erratic interactions, a process that is required for normal chromosome congression.
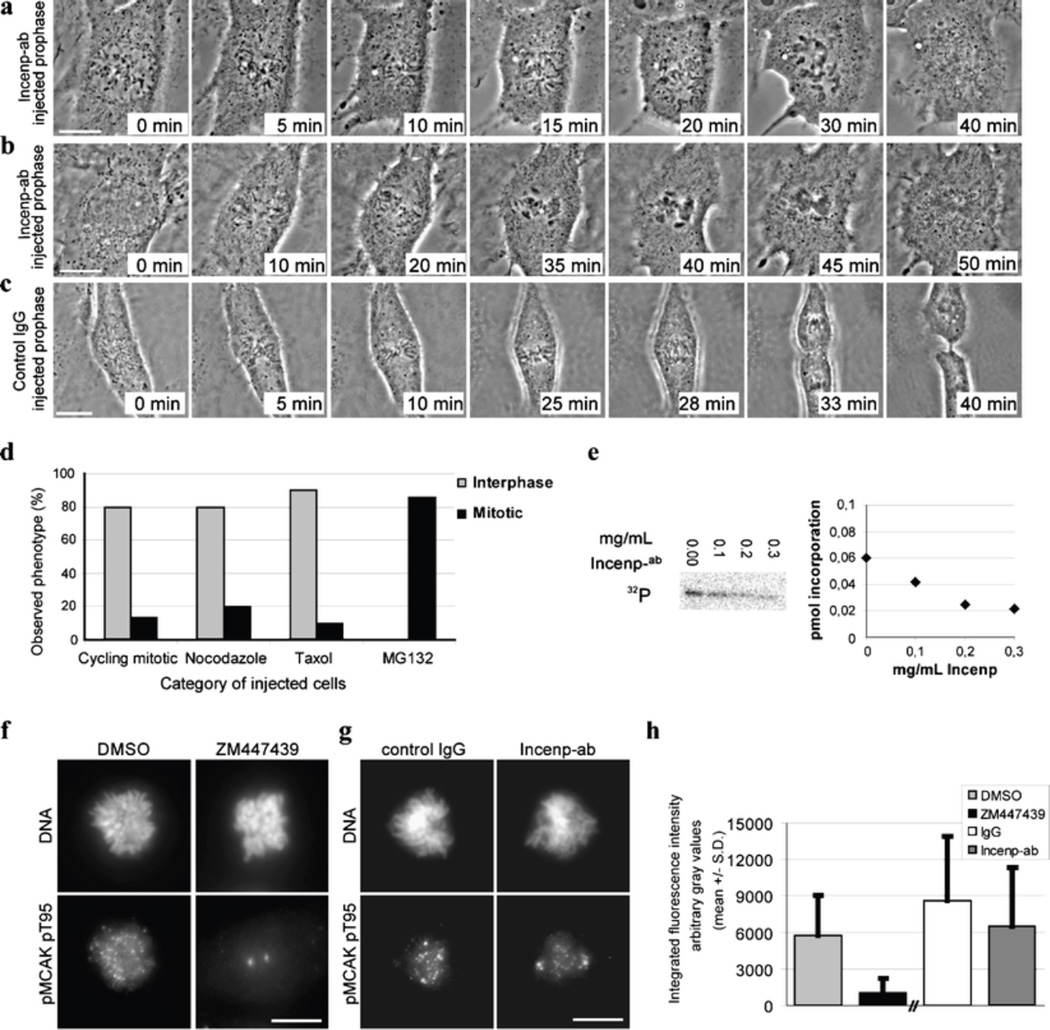
Fig. 1.
Injection of Incenp−ab into early mitotic cells results in chromosome congression defects and override of SAC. a, b Xeno S3 cells injected with Incenp−ab at late prophase. NEB and initial chromosome capture by microtubules appear normal but many chromosomes fail to move to the spindle equator within the time period the chromosomes in control IgG-injected cells (c) form a metaphase plate. Chromosomes of the Incenp−ab-injected cells undergo premature decondensation and cells exit mitosis without execution of cytokinesis. d Mitotic phenotypes of Xeno S3 cells pretreated with various chemicals prior to introduction of Incenp−ab. Cells are categorized as interphase (grey bar) or mitotic (black bar) cells based on their nuclear morphology after the microinjection and subsequent fixation. Most cycling, nocodazole, or taxol-treated cells exit mitosis aberrantly and form polyploid cells with fragmented nuclei indicating forced mitotic exit, whereas MG132-treated cells remain arrested in mitosis. Apoptotic cells are excluded from the grouping. e Aurora B kinase activity in vitro, in the presence of different Incenp−ab concentrations. Incorporation of 32P to histone H3 is moderately reduced indicating partial kinase inhibition. f−h Aurora B kinase activity towards pT95 on MCAK. Prometaphase Xeno S3 cells treated with DMSO or Aurora inhibitor ZM447439 (f), or injected in prophase with control IgG or Incenp−ab (g). h Integrated fluorescence intensity of pMCAK at the centromere-kinetochore regions. ZM447439 abolishes and Incenp−ab partially reduces phospho-MCAK staining at kinetochores. Scale bar = 10 µm. Time-lapse movies corresponding to the still images in panels a and b (Electronic supplementary material, Videos 1 and 2) are available as supplementary material
The majority of Xeno S3 cells injected with Incenp−ab between prophase and metaphase (75%, n = 12) prematurely decondensed their chromosomes and subsequently exited M-phase without cytokinesis leading to generation of polyploidy (Fig. 1a and b; Electronic supplementary material, Videos 1 and 2). Notably, the time between NEB and precocious chromosome decondensation in the Incenp−ab-injected prophase cells (37 ± 7 min, mean ± SD, n = 5) was not statistically different from the length of NEB-to-anaphase period of control IgG-injected cells (43 ± 5 min, mean ± SD, n = 5). The rest of the Incenp−ab-injected early mitotic cells (25%) underwent cytokinesis but exhibited defective sister chromatid movements (data not shown). The data demonstrated that most Incenp−ab-injected cells failed to delay mitosis despite the presence of many unaligned chromosomes, indicating that SAC was weakened or inactivated in these cells.
Cell lines with a robust SAC remain arrested in mitosis for several hours upon treatment with microtubule drugs such as nocodazole (depolymerizes microtubules) or taxol (stabilizes microtubules; Stukenberg and Burke 2004). To analyze if Incenp−ab injection causes an override of hyperactivated SAC, Xeno S3 cells were pretreated for 1 h with nocodazole or taxol or MG132 (a proteasome inhibitor that causes metaphase arrest downstream of the SAC), followed by Incenp−ab injection. After 2-h incubation in the continued presence of the drug, the cells were fixed and categorized according to their nuclear morphology (Fig. 1d). The majority of the Incenp−ab-injected cells pretreated with microtubule drugs (35 out of 40 injected cells) or without any drug treatment (12 out of 15 injected cells) showed a forced mitotic exit without completion of cytokinesis. In contrast, most cells pretreated with MG132 prior to Incenp−ab injection (12 out of 14 cells) remained arrested at M-phase. Supplemental figures (Electronic supplementary material, Fig. 1d–f) show still images from time-lapse movies of representative cells. These data confirm the notion that introduction of Incenp−ab into early mitotic cells results in premature SAC inactivation leading to proteasome-dependent forced mitotic exit.
The above-described cell cycle errors resemble those reported after small molecule inhibition of Aurora B kinase (Gadea and Ruderman 2005; Hauf et al. 2003). To investigate if introduction of Incenp−ab has an effect on the activity of Aurora B kinase, we performed an in vitro kinase assay (Fig. 1e) using a range of Incenp−ab concentrations selected on the basis of the estimated intracellular antibody concentration in the injected cells. At the average intracellular concentration of 0.12 mg/ml, Incenp−ab inhibited Aurora B activity by 40% as indicated by the reduced incorporation of 32P to histone H3 (Fig. 1e). Next, we determined the effect of Incenp−ab on Aurora B kinase activity in vivo using immunofluorescent detection of an antibody that recognizes the Aurora B target phoshoepitope pT95 on MCAK (Zhang et al. 2007). As reported earlier (Zhang et al. 2007), the pT95 antibody stained chromosome arms and centromeres (Fig. 1f). This staining was abolished near to background levels in cells co-treated with Aurora B inhibitor ZM447439 and MG132 for 30 min (Fig. 1f and h; P < 0.0001) indicating that the phospho-MCAK antibody responds to the loss of Aurora B activity as expected. The average integrated intensity of pT95 staining at the centromere-kinetochore region of Incenp−ab-injected cells was reduced by 28% compared to control IgG-injected cells (Fig. 1g and h; P = 0.01) 20 min after the injections. Therefore, Incenp−ab caused partial inhibition of Aurora B kinase activity, which likely contributes to the observed early mitotic defects.
Incenp−ab interferes with normal microtubule–kinetochore associations
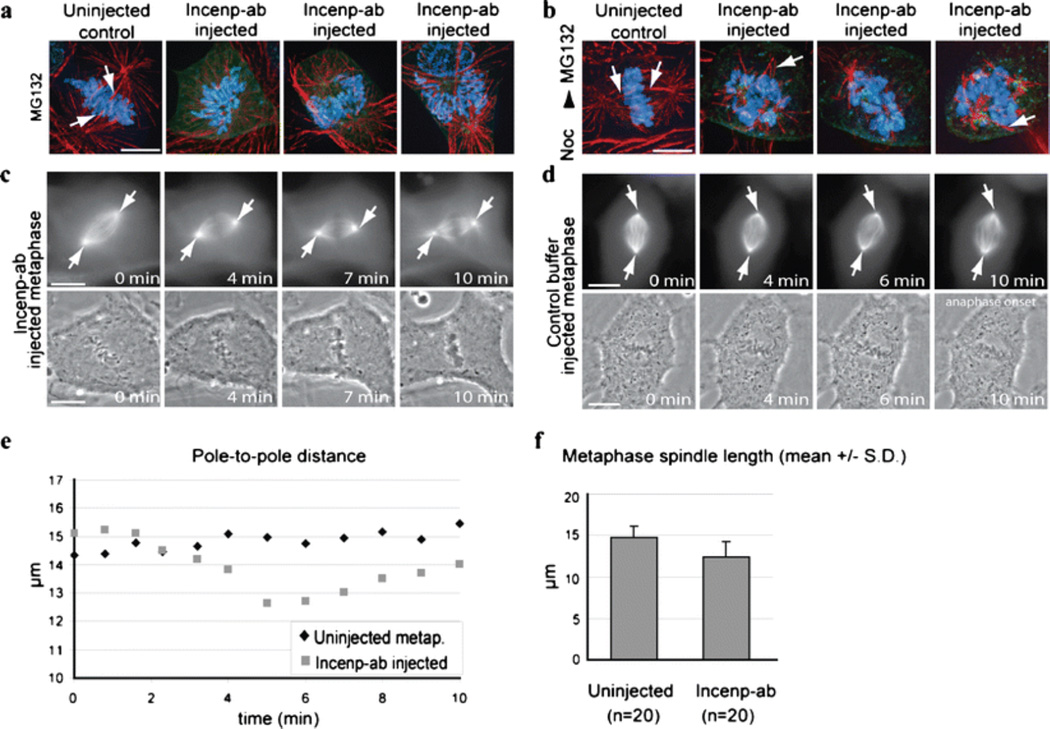
To explore the possibility that the observed chromosome alignment defects in Incenp−ab-injected cells could be an indication of spindle damage we analyzed the microtubule network of Incenp−ab-injected cells. Prophase cells were incubated in the presence of MG132 before Incenp−ab injection to prevent premature mitotic exit. The cells were fixed 1 h after the injection and stained for tubulin (Fig. 2a). Control cells treated with MG132 had all chromosomes aligned in a metaphase configuration with thick kinetochore microtubule bundles clearly visible (Fig. 2a). In sharp contrast, Incenp−ab-injected cells exhibited unorganized spindle morphology characterized by fewer and thinner kinetochore microtubule bundles (Fig. 2a). As expected, these cells were incapable of aligning their chromosomes to the spindle equator (Fig. 2a). The spindle defects were even more pronounced when the cells were first preincubated with nocodazole for 1 h to depolymerize all existing microtubules followed by Incenp−ab injection and incubation in MG132-containing medium without nocodazole for 1 h to allow spindle reformation (Fig. 2b). Notably, microtubule arrays emanating from the spindle poles of these cells were greatly reduced in number and microtubules were without clear interactions with the chromosomes (Fig. 2b). In contrast, control cells had reformed bipolar spindles with thick kinetochore–microtubule bundles and spread astral microtubule sheets and had all chromosomes aligned at the metaphase plate (Fig. 2b). To analyze the effects of Incenp−ab on spindle morphology and microtubule function in cells with stable microtubule–kinetochore interactions, we introduced Incenp−ab or control buffer into metaphase Xeno S3 cells expressing GFP-tubulin and followed the cells with time-lapse microscopy (Fig. 2c and d). As a result of Incenp−ab introduction, the chromosomes first underwent reduced oscillation and then started to decondense but maintained their metaphase orientation indicating that connections to microtubules were not lost (Fig. 2c). The spindle apparatus exhibited rapid morphological changes; the spindle became shorter while astral microtubules extended compared to controls (Fig. 2, c–e, Electronic supplementary material Video 3). In addition, the kinetochore microtubule bundles appeared to depolymerize from their minus ends during the forced mitotic exit (Electronic supplementary material, Video 3, 10 min). To quantify these changes we measured the length of metaphase spindles in control buffer and Incenp−ab-injected cells that were kept in MG132 and fixed 1 h after the injection (Fig. 2f). The average spindle length of Incenp−ab injected cells (12.4 ± 1.9 µm, mean ± SD, n = 20) was significantly shorter compared to control cells (14.7 ± 1.4 µm, mean ± SD, n = 20, P < 0.001). These results demonstrate that Incenp−ab perturbs the proper assembly of the spindle when injected into prophase cells and interferes with the maintenance of normal spindle morphology when injected into metaphase cells.
Fig. 2.
Incenp−ab interferes with the establishment of the mitotic spindle and maintenance of normal spindle length. a, b Images of uninjected control and Incenp−ab-injected cells after fixation and staining for microtubules (red), Incenp−ab (green), and DNA (blue, DAPI). Prior to fixation the cells were (a) treated with MG132 and injected with Incenp−ab or (b) treated with nocodazole, injected with Incenp−ab, and washed out of nocodazole into MG132 containing medium. Incenp−ab-injected cells exhibit unorganized spindle morphology characterized by fewer and thinner kinetochore microtubule bundles compared to control cells, which show normal spindle structure with robust kinetochore microtubule bundles (white arrows). c, d Time-lapse images of Incenp−ab (c) and control buffer (d) injected metaphase Xeno S3 cells expressing GFP-tubulin (arrows mark spindle poles). e Pole-to-pole distance in cells from panel c vs. panel d plotted over time. f Quantification of spindle length from Incenp−ab and control buffer injected cells after fixation. Scale bars = 10 µm. Video corresponding to still images in panel c (Electronic supplementary material, Video 3) is available as supplementary material
Incenp−ab prevents the dynamic turnover of Incenp and Aurora B at inner centromeres
SAC signaling is thought to be based on the fast turnover of regulatory centromere or kinetochore proteins (Howell et al. 2004; Kallio et al. 2002a; Shah et al. 2004). The observed rapid override of SAC by Incenp−ab prompted us to investigate whether the antibody affects the association of CPC subunits with the inner centromeres. We first examined the turnover of GFP-tagged Incenp at the inner centromeres of Xeno S3 and LLC-PK cells transiently expressing Xenopus (GFP-xIncenp) or human (GFP-hIncenp) GFP-Incenp. To determine the protein turnover halftime (t1/2,) and the mobile protein fraction (recf), we utilized the FRAP technique. The average t1/2 of GFP-xIncenp at the centromeres of Xeno S3 cells in metaphase was 53 ± 19 s (Table 1). Similar turnover values were obtained for GFP-hIncenp at the centromeres of HeLa (data not shown) and LLC-PK cells in metaphase (the average t1/2 = 100 ± 37 s; Electronic supplementary material, Table S1). The centromere GFP-xIncenp signals recovered close to the prebleach levels in metaphase cells (recf = 83 ± 8%) suggesting that the immobile protein fraction is very small. Removal of microtubules by nocodazole treatment did not cause any significant changes to GFP-xIncenp turnover or mobile fraction (t1/2 = 69 ± 21 s, recf = 86 ± 10, Table 1) indicating that Incenp exchanges at inner centromeres with a constant rate independently of microtubule–kinetochore attachments or SAC activity. FRAP results from LLC-PK cells were in agreement with the Xenopus cell data (Electronic supplementary material, Table S1).
Table 1.
Turnover of Incenp and Aurora B in Xeno S3 cells after various drug treatments
| Mitotic phase/location | Treatment | Number | T1/2(s)a | Recf (%)a |
|---|---|---|---|---|
| XenoS3 (GFP-xIncenp) | ||||
| Metaphase/inner centromere | – | 8 | 53 ± 19 | 83 ± 8 |
| Prometaphase/inner centromere | nocodazole | 6 | 69 ± 21 | 86 ± 10 |
| Metaphase/inner centromere | MG132 | 8 | 38 ± 9 | 80 ± 13 |
| Metaphase/inner centromere | MG132 + inj. | 4 | n.d. | 9 ± 2 |
| XenoS3 (xAurB-YFP) | ||||
| Metaphase/inner centromere | – | 3 | 43 ± 7 | 88 ± 18 |
| Metaphase/inner centromere | MG132 | 5 | 31 ± 5 | 69 ± 11 |
| Metaphase/inner centromere | MG132 + inj. | 4 | n.d. | 10 ± 4 |
Mean ± SDt 1/2 (s) Protein turnover half-time in seconds, Recf mobile protein fraction, Inj. Injected with Incenp−ab
Next, we measured the dynamics of Aurora B to compare its turnover to that of Incenp and to resolve the contradicting results of previous reports regarding the mobility of the kinase (Delacour-Larose et al. 2004; Murata-Hori and Wang 2002). xAurora B-YFP recovered to the photobleached centromeres of Xeno S3 cells with t1/2 and recf of 43 ± 7 s and 88 ± 18%, respectively (Table 1). Similar dynamics was observed in HeLa cells expressing hAurora B-GFP (data not shown). The results demonstrate that Incenp exchanges with moderately rapid turnover at the inner centromeres of early mitotic cells. Moreover, our results support the notion that Aurora B is turning over at centromeres with moderate dynamics (Murata-Hori et al. 2002) rather than being stably associated (Delacour-Larose et al. 2004; Delacour-Larose et al. 2007).
To test if Incenp−ab changes the centromere binding dynamics of CPC subunits, we determined t1/2 and recf of Incenp or Aurora B in uninjected control and Incenp−ab injected Xeno S3 cells expressing GFP-xIncenp or xAurora B-YFP. The cells were incubated in the presence of MG132 to prevent Incenp−ab-induced forced exit from mitosis. MG132 slightly increased Incenp turnover (t 1/2 = 38 ± 9; Table 1; Fig. 3a) compared to untreated cells in metaphase (t1/2 = 53 ± 19 s; Table 1). In MG132-treated control cells, the turnover of GFP-xIncenp and xAurora B-YFP occurred with similar dynamics (Fig. 3a and c; Electronic supplementary material, Videos 4 and 6; Table 1). In contrast, the introduction of Incenp−ab dramatically reduced the recovery of GFP-xIncenp and xAurora B-YFP at the inner centromeres (Fig. 3b and d; Electronic supplementary material, Videos 5 and 7; Table 1). The average recf of GFP-xIncenp and xAurora B-YFP were 9 ± 2% (n = 4 cells) and 10 ± 4% (n = 4 cells) in the Incenp−ab-injected cells compared to 80 ± 13% (n = 8 cells) and 69 ± 11% (n = 5 cells) in control cells (n = 4), respectively (P < 0.0001). These data indicate that Incenp−ab renders the CPC immobile. Due to the very low recf values, protein half-lives could not be determined in Incenp−ab-injected cells.
In summary, our results demonstrate that Incenp and Aurora B molecules undergo moderate turnover at the inner centromeres. Interestingly, Incenp−ab strongly inhibited the turnover of both Incenp and Aurora B. This was mainly due to the inability of Incenp and Aurora B to dissociate from the centromeres as we detected normal centromere association in early mitotic cells that were injected into nucleus with Incenp−ab at prophase (data not shown). Interestingly, Incenp and Aurora B exhibit slower dynamics (t1/2 = 30−100 s depending on the cell line) compared to Survivin that exhibits rapid turnover with t1/2 of 5−7 s (Beardmore et al. 2004).
Defective Incenp function leads to errors in anaphase chromatid movements, midzone microtubule organization and cytokinesis
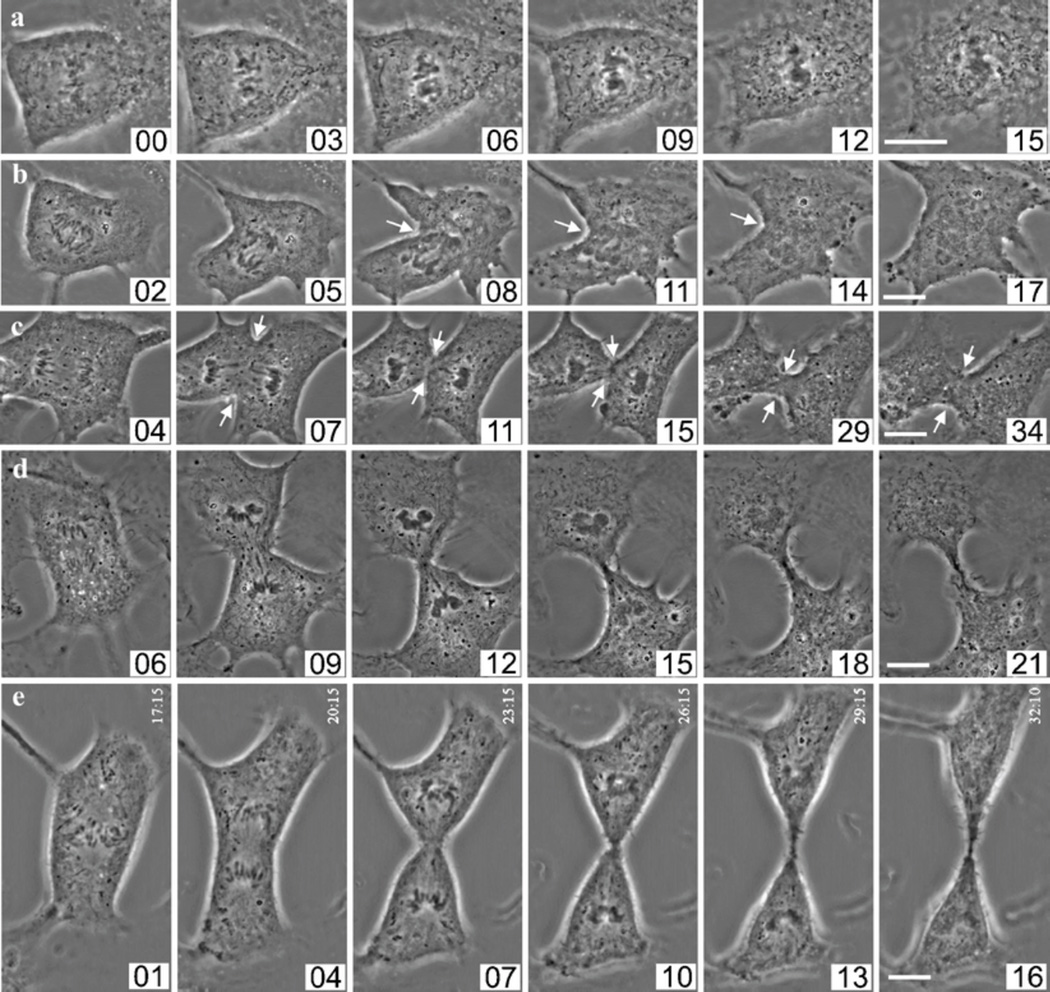
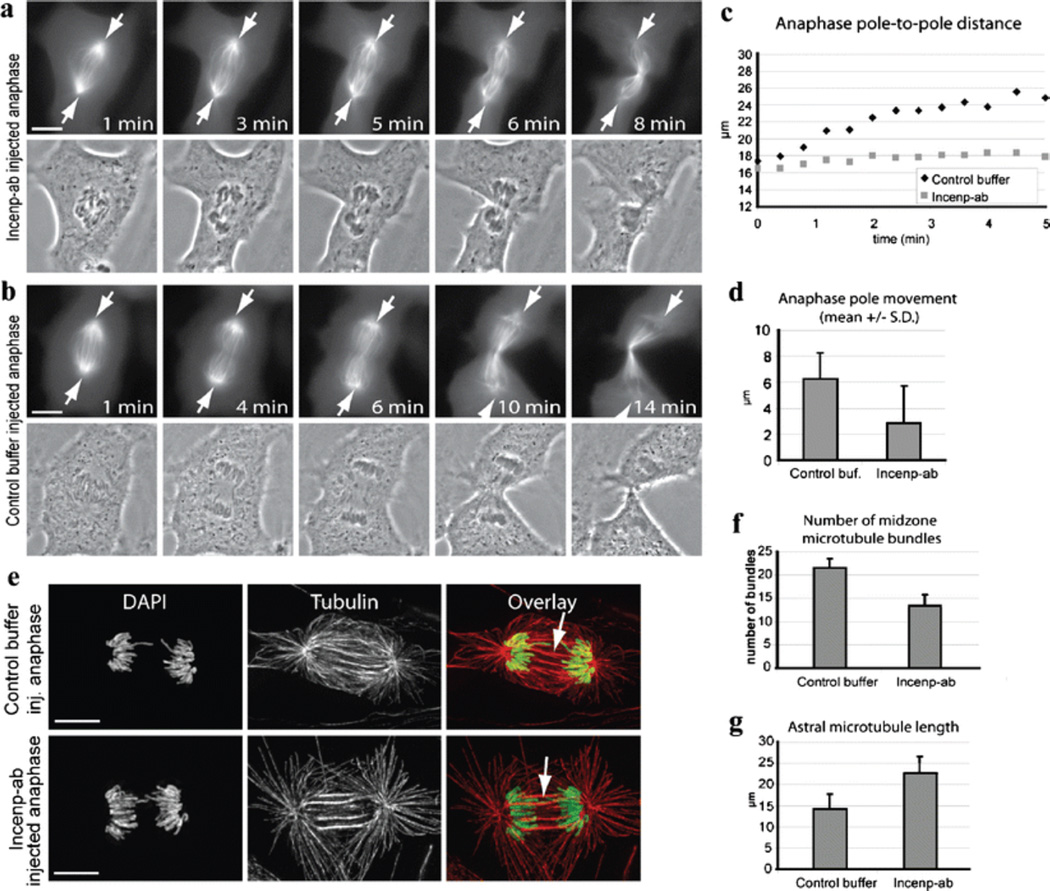
The microinjection technique allowed us to perturb Incenp/CPC in anaphase cells without disturbing earlier mitotic functions of the CPC. We introduced Incenp−ab into anaphase cells at 1-min intervals after the first visible signs of sister chromatid separation. Surprisingly, blocking Incenp function within 3 min after anaphase onset (n = 6) prevented normal sister chromatid movements (Fig. 4a and b). Injection around anaphase onset resulted in nearly stationary sister chromatids (Fig. 4a; Electronic supplementary material, Video 8). Usually, incomplete sister chromatid separation was followed by failure in the execution of cytokinesis (Fig. 4a and b), but occasionally, cells underwent cytokinesis despite the failure of the sisters to separate (Fig. 5a). Typically, cleavage furrow ingression was initiated but the furrow regressed (Fig. 4b; Electronic supplementary material, Video 9). Injection 3–4 min after anaphase onset (n = 3) resulted in a failure in the abscission of the daughter cells and abnormal midbody formation (Fig. 4c; Electronic supplementary material, Video 10). Cells injected with Incenp−ab five or more minutes after anaphase onset (n = 4) showed normal sister chromatid separation and cytokinesis (Fig. 4d; Electronic supplementary material, Video 11). All control cells injected with IgG 1–2 min after anaphase onset (n = 5) underwent normal anaphase and cytokinesis within 15 min (Fig. 4e). One plausible explanation for the observed mistakes in anaphase sister chromatid separation is spindle malfunction. To analyze the effect of Incenp−ab on anaphase spindle microtubules, we introduced Incenp−ab or control buffer into early anaphase Xeno S3 cells expressing GFP-tubulin and imaged the cells using time-lapse microscopy as they exited mitosis. The time-lapse films demonstrated that Incenp−ab-disturbed spindle elongation and the separation of the two spindle poles compared to controls (Fig. 5a–d; Electronic supplementary material, Videos 12 and 13). The average pole-to-pole distance was notably shorter after the first 5 min of anaphase in Incenp−ab-injected cells (2.9 ± 2.9 µM, n = 5) compared to control cells (6.3 ± 2.0 µM, n = 5, P = 0.03). A morphological comparison of anaphase spindle structure in fixed cells showed that microtubule bundles at the spindle midzone were thicker, and their number was significantly reduced from an average of 22 ± 2 of control cells (n = 5) to 13 ± 2 in Incenp−ab-injected cells (n = 5, P < 0.001, Fig. 5e and f). We also noted that astral microtubules (Fig. 5g) were significantly longer in the Incenp−ab-injected cells (22.8 ± 3.9 µm, n = 5) compared to controls (14.2 ± 3.6 µm, n = 5, P < 0.001).
Fig. 4.
Interference with Incenp function after anaphase onset disrupts sister chromatid separation and cytokinesis. a Xeno S3 cell injected with Incenp−ab at the onset of anaphase showing perturbed sister chromatid separation and cytokinesis. b Xeno S3 cell injected with Incenp−ab 1 min after the onset of anaphase showing incomplete sister chromatid separation and failure of cytokinesis. Cleavage furrow formation (white arrows) is initiated but the furrow soon regresses and the cell exits mitosis forming a polyploid progeny cell. c Xeno S3 cell injected with Incenp−ab 3 min after the onset of anaphase showing incomplete sister chromatid separation and spindle elongation. Also the cleavage furrow regresses (white arrows) and midbody is not formed. d Xeno S3 cell injected with Incenp−ab 5 min after the onset of anaphase undergoes normal anaphase and telophase. e Xeno S3 cell injected with control IgG 30 s after the onset of anaphase undergoes normal anaphase and telophase. Scale bars = 10 µm. The first image in each row shows the first frame after the injection except in panel a where the first image was taken right before the injection. The numbers correspond to minutes after the onset of anaphase. Scale bars = 10 µm. Videos corresponding to still images in panels a–d (Electronic supplementary material, Videos 8, 9, 10, 11) are available as supplementary material
Fig. 5.
Incenp−ab prevents normal spindle elongation and induces abnormal bundling of midzone microtubules. a GFP-tubulin expressing Xeno S3 cell injected with Incenp−ab 1 min after the onset of anaphase shows defects in spindle elongation, spindle pole separation, and anaphase sister chromatid movements (arrows mark spindle poles). b Xeno S3 cell injected with buffer 1 min after anaphase onset shows normal sister chromatid separation and cytokinesis. The first image in panel a and b shows the first frame after injection. The numbers correspond to minutes after anaphase onset. c Spindle pole movements of the cells in panels a and b plotted over time. d Quantification of spindle pole separation in live Incenp−ab (n = 5) and control buffer (n = 5) injected anaphase cells within 5 min from injection. e Incenp−ab and control buffer injected cells after fixation and staining for microtubules (red) and DNA (DAPI, green). Incenp−ab-injected cell exhibits a narrower anaphase spindle with reduced number of microtubule bundles in the spindle midzone (white arrows) and elongated astral microtubules compared to control. f, g Quantification of the number of midzone microtubule bundles and astral microtubule length in Incenp−ab (n = 5) and control buffer injected cells (n = 5). Scale bars = 10 µm. Videos corresponding to still images in panels a and b (Electronic supplementary material, Videos 12 and 13) are available as supplementary material
Together, the data show that blocking Incenp function in early anaphase prevents the normal separation of sister chromatids and spindle poles. Normal Incenp function was also required for cytokinesis and the abscission of the midbody agreeing with previously reported roles of CPC members in these processes (Ruchaud et al. 2007).
Discussion
Many mitotic processes are controlled by the activities of the CPC in a timely coordinated manner (Ruchaud et al. 2007). Erroneous CPC function is a potential source of numerical chromosome changes (aneuploidy) and polyploidy, both being hallmarks of cancer. The members of Aurora kinase family, including Aurora B, are overexpressed in many human cancers (Katayama et al. 2003) pointing to a possible role for the kinases in tumorigenesis. Inhibition of the CPC, especially via suppression of Aurora B kinase activity, has raised interest as a potential therapeutic target in the treatment of cancer (Girdler et al. 2006). Our results demonstrated that perturbation of Incenp function with function blocking antibodies results in a similar phenotype as inhibition of Aurora B with antibody injection or small molecules (Harrington et al. 2004; Hauf et al. 2003; Kallio et al. 2002b). Blocking the function of either protein in early mitosis causes chromosome misalignment followed by a forced mitotic exit through SAC inactivation. Aurora B kinase is generally thought to function in the tension-sensitive branch of the SAC as cells treated with Aurora B inhibitor in combination with taxol escape mitotic arrest, whereas cells treated with inhibitor and nocodazole remain in mitosis (Ditchfield et al. 2003; Hauf et al. 2003). Furthermore, cells depleted of Survivin and, thus, without centromeric Aurora B escape taxol but not nocodazole induced mitotic block (Carvalho et al. 2003; Lens et al. 2003). Notably, Incenp−ab caused exit also in the presence of nocodazole, which was unexpected but in line with earlier findings using anti-Aurora B antibody (Kallio et al. 2002b) or Aurora B RNAi (Ditchfield et al. 2003) that showed escape from nocodazole-induced mitotic block. It is likely that the antibody interferes with other aspects of CPC functions apart from effects on Aurora B kinase activity such as protein–protein interactions that are necessary for SAC activity in the absence of microtubule attachment.
Introduction of Incenp−ab caused a partial inhibition of Aurora B kinase in vitro and in vivo at the intracellular concentrations used. The Incenp−ab targets the C-terminus of Incenp where Aurora B binds (Bolton et al. 2002). Therefore, the antibody may prevent normal contact between the two proteins, which is needed for the two-step process leading to full activation of Aurora B (Bishop and Schumacher 2002; Bolton et al. 2002; Honda et al. 2003; Sessa et al. 2005). We speculate that Incenp−ab binding blocks phosphorylation of a conserved Ser-Ser motif near the C terminus of Incenp required for maximum Aurora B activation (Sessa et al. 2005). The reduced but not totally abolished Aurora B kinase activity by Incenp−ab in vitro and in vivo is in line with the notion that in the presence of Incenp−ab Aurora B and Incenp associate but are in a conformation that does not elicit full kinase activity.
Two Aurora B substrates, whose dysfunction at early mitosis as a result of lowered Aurora B kinase activity may explain the observed phenotypes, are the microtubule-depolymerizing kinesin MCAK, which has a central role in converting erroneous kinetochore–microtubule interactions into stable bipolar attachments (Lan et al. 2004) and Hec1, which has been proposed to mediate the establishment of correct and robust kinetochore–microtubule attachments (DeLuca et al. 2006). The observation that soon after NEB, the Incenp−ab-injected prophase cells formed initial kinetochore–microtubule attachments (which often are erratic monotelic or syntelic attachments) but later failed to move the chromosomes to the spindle equator favors the notion that MCAK function is affected in the Incenp−ab-injected cells. Furthermore, Incenp−ab injection caused similar defects as observed after anti-Hec1 antibody injections targeting the N-terminus of the molecule (DeLuca et al. 2006). After anti-Hec1 or Incenp−ab injections, the pole-to-pole distance shortened, chromosome oscillations were reduced and the kinetochore microtubules were pulled away from their normal position at the edge of the centrosome. Antibody injection against the N-terminus of Hec1 blocked plus-end microtubule dynamics at the kinetochore but did not effect minus-end depolymerization at the pole, which may explain why both antibodies reduced spindle length. Taken together, these findings suggest that Incenp−ab may block the regulation of the N-terminus of Hec1, which is phosphorylated by Aurora B in vitro on residues that are important in chromosome alignment in vivo (DeLuca et al. 2006).
Our FRAP data showed that Incenp and Aurora B both exchange at the inner centromeres as was previously reported for Survivin (Beardmore et al. 2004; Delacour-Larose et al. 2004). Incenp and Aurora B, however, exhibit slower dynamics compared to Survivin. These results contradict earlier studies, which reported that Survivin is the only mobile CPC component at inner centromeres (Delacour-Larose et al. 2004; Delacour-Larose et al. 2007). After careful analysis of three different cell lines, we conclude that Incenp and Aurora B both recover at photo-bleached centromeres of mitotic cells with a moderately fast turnover of 30–100 s. The injection of Incenp−ab abolished this mobility resulting in stably bound centromeric Incenp and Aurora B. We propose that CPC members shuttle on and off the inner centromeres in a dynamic process that is required for rapid SAC signaling or maintenance of Aurora B activity. Our data suggest that Incenp and Aurora B exchange at the inner centromeres as a complex whereas at least part of the Survivin protein pool turns over independently. We speculate that the observed rapid exit from nocodazole and taxol induced M-phase arrest upon introduction of Incenp−ab is primarily caused by immobilization of Incenp and Aurora B.
In addition to the known CPC-dependent tasks in early mitosis, we identified a new function for Incenp-Aurora B in moving the separated sister chromatids at anaphase. In cells injected with Incenp−ab 20–60 s after the onset of anaphase, the poleward movement of sister chromatids halted completely, and cytokinesis was blocked. Careful examination of the time-lapse films proposes that introduction of Incenp−ab at early anaphase did not break microtubule–kinetochore associations as the chromatids remained oriented towards the poles during the mitotic exit but rather that the microtubules lost their ability to promote poleward chromatid motions. This suggests that in very early anaphase, Incenp−ab prevented normal depolymerization of kinetochore fibers needed for sister chromatid separation during anaphase A (Sharp and Rogers 2004). In Drosophila embryos, anaphase A is facilitated by at least two Kin I microtubule destabilizing enzymes, KLP59C and KLP10A, that drive Pacman and poleward flux-based anaphase chromatid motility, respectively (Sharp and Rogers 2004). However, it remains to be elucidated if CPC directly controls anaphase functions of vertebrate homologs of KLP59C and KLP10A. Importantly, the observed mistakes in anaphase chromatid separation were not caused by defective dissolution of cohesion between sister chromatids as proposed by Kaitna et al., who reported that in C. elegans embryos depleted of ICP-1, a protein related to vertebrate Incenp, both chromosome segregation and cytokinesis were abnormal due to the failed dissolution of cohesion between sister chromatids rather than erroneous kinetochore or spindle function (Kaitna et al. 2000). Because in our experiments, Incenp−ab was introduced into the cells after the onset of anaphase, the failed sister chromatid separation cannot be due to the persistence of cohesion but rather implicates Incenp-Aurora B directly in the microtubule-mediated anaphase processes. Our observations regarding cytokinesis defects are in agreement with others (Adams et al. 2001; Kaitna et al. 2000).
Interestingly, spindle elongation that drives chromatid movements in anaphase B was defective in Incenp−ab-injected cells. In most cells, cytokinesis was incomplete as the abscission of the daughter cells by the cleavage furrow failed. We speculate that the observed anaphase B errors are a consequence of the inability of spindle midzone microtubule bundles to undergo normal elongation and/or sliding motions needed for spindle pole separation during anaphase B (Brust-Mascher et al. 2004; Kwon et al. 2004; Sharp et al. 2000). According to a recent model (Kwon et al. 2004; Kwon and Scholey 2004), at least four motor proteins (bipolar kinesin KLP61F, chromokinesin KLP3A, KLP10A, and dynein) are implicated in the elongation process of anaphase spindles. For example, KLP61F drives the sliding filament mechanism required for spindle elongation as the Drosophila embryos injected with function neutralizing anti-KLP61F antibodies exhibit diminished anaphase-B elongation (Sharp et al. 2000). The requirement of CPC for central spindle organization and function is well characterized (Giet and Glover 2001; Kaitna et al. 2000; Wheatley et al. 2001), but direct links between CPC components and these motor proteins have not been established. Our study suggests that CPC indirectly controls anaphase chromatid motions through midzone-located motor proteins. Furthermore, our results strengthen a proposed role for Aurora B kinase in the regulation of cytokinesis through astral microtubule dynamics (Miyauchi et al. 2007). Finally, Aurora B is known to phoshorylate centralspindlin subunit ZEN-4/MKLP1 that is implicated in the completion of cytokinesis (Guse et al. 2005). Therefore, the observed defects in cytokinesis by Incenp−ab may be attributed to the inability of the centralspindlin complex to associate or function normally at the midzone when CPC activities are perturbed. It is noteworthy that blocking the CPC with antibody injection at 5 min after anaphase onset, or later, did not prevent cytokinesis or midbody formation proposing that the continued function of the CPC was not required at this late stage of mitosis. However, we cannot rule out the possibility that this observation was due to the time it took for the antibody to take its effect.
In summary, the data underline the notion that Incenp is an essential component of the CPC and that perturbation of Incenp with an antibody targeting its C-terminus inhibits CPC activity. The data suggest that allosteric inhibition of the Incenp-Aurora B complex could be utilized as an alternative therapeutic opportunity to facilitate the inactivation of SAC. The results extend previous analyses of Incenp-Aurora B functions and suggest that their mobility at centromeres plays a role in SAC activity and that CPC controls anaphase sister chromatid separation after centromeric cohesion has been resolved.
Supplementary Material
Acknowledgements
This work was supported by grants to MJK (Marie Curie EXT 002697, Academy of Finland 8206930), to PTS (R01GM063045-06), and Turku Graduate School of Biomedical Sciences. We thank Erich Nigg and Ulf Klein for sending GFP-hIncenp plasmid, David Wotton for providing pCS2 + YFP, and AstraZeneca Pharmaceuticals for providing ZM447439. We thank Jouko Sandholm at the Turku Centre for Biotechnology for his kind help with the photobleaching experiments.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00412-008-0178-0) contains supplementary material, which is available to authorized users.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1 Turnover of GFP-hIncenp in LLC-PK cells after various drug treatments (DOC 11.5 KB)
Fig. S1 a Immunofluorescence images of fixed Xeno S3 cells stained with Incenp-ab. The antibody stains inner centromeres from prophase to metaphase, spindle microtubules in anaphase, and the midbody in telophase. When injected into metaphase (b) or anaphase cells (c), the Incenp−ab binds to inner centromeres or midzone microtubules, respectively. Cells were injected, fixed, and stained for tubulin (red in the overlay, one selected focal plane is shown), Incenp-ab (green), and DNA (blue, DAPI). Insets show magnified views of the metaphase plate and the spindle midzone. (d–e) Incenp−ab-injected Xeno S3 cells undergo a forced mitotic exit despite nocodazole or taxol-induced mitotic arrest. f Incenp−ab-injected Xeno S3 cells remain arrested at M phase in the presence of proteasome inhibitor MG312. Scale bars = 10 µm (JPEG 1.73 MB)
Video 1 Xeno S3 cell injected with Incenp−ab at prophase (MOV 8.07 MB).
Video 2 Xeno S3 cell injected with Incenp−ab at prophase (MOV 4.61 MB).
Video 3 GFP-tubulin expressing Xeno S3 cell injected with Incenp−ab at metaphase (MOV 619 KB).
Video 4 Recovery of photobleached GFP-xIncenp at the centromeres of a Xeno S3 cell in the presence of MG132 (MOV 1.36 MB).
Video 5 Recovery of photobleached GFP-xIncenp at the centromeres of a Xeno S3 cell after Incenp−ab injection in the presence of MG132 (MOV 1.30 MB).
Video 6 Recovery of photobleached xAurora B-YFP at the centromeres of a Xeno S3 cell in the presence of MG132 (MOV 2.70 MB).
Video 7 Recovery of photobleached xAurora B-YFP at the centromeres of a Xeno S3 cell after Incenp−ab injection in the presence of MG132 (MOV 2.08 MB).
Video 8 Xeno S3 cell injected with Incenp−ab at the onset of anaphase (MOV 1.56 MB).
Video 9 Xeno S3 cell injected with Incenp−ab 1 min after the onset of anaphase (MOV 1.28 MB).
Video 10 Xeno S3 cell injected with Incenp−ab 3 min after the onset of anaphase (MOV 3.23 MB).
Video 11 Xeno S3 cell injected with Incenp−ab 5 min after the onset of anaphase (MOV 3.67 MB).
Video 12 GFP-tubulin expressing Xeno S3 cell injected with Incenp−ab at early anaphase (MOV 506 KB).
Video 13 GFP-tubulin expressing Xeno S3 cell injected with control buffer at early anaphase (MOV 662 KB).
References
- Adams RR, Wheatley SP, Gouldsworthy AM, Kandels-Lewis SE, Carmena M, Smythe C, Gerloff DL, Earnshaw WC. INCENP binds the Aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr Biol. 2000;10:1075–1078. doi: 10.1016/s0960-9822(00)00673-4. [DOI] [PubMed] [Google Scholar]
- Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardmore VA, Ahonen LJ, Gorbsky GJ, Kallio MJ. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J Cell Sci. 2004;117:4033–4042. doi: 10.1242/jcs.01242. [DOI] [PubMed] [Google Scholar]
- Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton MA, Lan W, Powers SE, McCleland ML, Kuang J, Stukenberg PT. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol Biol Cell. 2002;13:3064–3077. doi: 10.1091/mbc.E02-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust-Mascher I, Civelekoglu-Scholey G, Kwon M, Mogilner A, Scholey JM. Model for anaphase B: role of three mitotic motors in a switch from poleward flux to spindle elongation. Proc Natl Acad Sci U S A. 2004;101:15938–15943. doi: 10.1073/pnas.0407044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol. 2002b;12:900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- Carvalho A, Carmena M, Sambade C, Earnshaw WC, Wheatley SP. Survivin is required for stable checkpoint activation in taxol-treated HeLa cells. J Cell Sci. 2003;116:2987–2998. doi: 10.1242/jcs.00612. [DOI] [PubMed] [Google Scholar]
- Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Cooke CA, Heck MM, Earnshaw WC. The inner centromere protein (INCENP) antigens: movement from inner centromere to midbody during mitosis. J Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour-Larose M, Molla A, Skoufias DA, Margolis RL, Dimitrov S. Distinct dynamics of Aurora B and Survivin during mitosis. Cell Cycle. 2004;3:1418–1426. doi: 10.4161/cc.3.11.1203. [DOI] [PubMed] [Google Scholar]
- Delacour-Larose M, Thi MN, Dimitrov S, Molla A. Role of survivin phosphorylation by aurora B in mitosis. Cell Cycle. 2007;6:1878–1885. doi: 10.4161/cc.6.15.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea BB, Ruderman JV. Aurora kinase inhibitor ZM447439 blocks chromosome-induced spindle assembly, the completion of chromosome condensation, and the establishment of the spindle integrity checkpoint in Xenopus egg extracts. Mol Biol Cell. 2005;16:1305–1318. doi: 10.1091/mbc.E04-10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R, Glover DM. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol. 2001;152:669–682. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler F, Gascoigne KE, Eyers PA, Hartmuth S, Crafter C, Foote KM, Keen NJ, Taylor SS. Validating Aurora B as an anti-cancer drug target. J Cell Sci. 2006;119:3664–3675. doi: 10.1242/jcs.03145. [DOI] [PubMed] [Google Scholar]
- Gorbsky GJ. The mitotic spindle checkpoint. Curr Biol. 2001;11:R1001–R1004. doi: 10.1016/s0960-9822(01)00609-1. [DOI] [PubMed] [Google Scholar]
- Guse A, Mishima M, Glotzer M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr Biol. 2005;15:778–786. doi: 10.1016/j.cub.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, Su M, Golec JM, Miller KM. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–267. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr Biol. 2004;14:953–964. doi: 10.1016/j.cub.2004.05.053. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Klein UR, Lindner D, Ebert J, Nigg EA, Conti E. Structure of a Survivin-Borealin-INCENP core complex reveals how chromosomal passengers travel together. Cell. 2007;131:271–285. doi: 10.1016/j.cell.2007.07.045. [DOI] [PubMed] [Google Scholar]
- Kagey MH, Melhuish TA, Wotton D. The polycomb protein Pc2 is a SUMO E3. Cell. 2003;113:127–137. doi: 10.1016/s0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Kaitna S, Mendoza M, Jantsch-Plunger V, Glotzer M. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr Biol. 2000;10:1172–1181. doi: 10.1016/s0960-9822(00)00721-1. [DOI] [PubMed] [Google Scholar]
- Kallio MJ, Beardmore VA, Weinstein J, Gorbsky GJ. Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J Cell Biol. 2002a;158:841–847. doi: 10.1083/jcb.200201135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Brinkley WR, Sen S. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22:451–464. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of borealin, survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17:2547–2558. doi: 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M, Scholey JM. Spindle mechanics and dynamics during mitosis in Drosophila. Trends Cell Biol. 2004;14:194–205. doi: 10.1016/j.tcb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Kwon M, Morales-Mulia S, Brust-Mascher I, Rogers GC, Sharp DJ, Scholey JM. The chromokinesin, KLP3A, dives mitotic spindle pole separation during prometaphase and anaphase and facilitates chromatid motility. Mol Biol Cell. 2004;15:219–233. doi: 10.1091/mbc.E03-07-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Lens SM, Wolthuis RM, Klompmaker R, Kauw J, Agami R, Brummelkamp T, Kops G, Medema RH. Survivin is required for a sustained spindle checkpoint arrest in response to lack of tension. Embo J. 2003;22:2934–2947. doi: 10.1093/emboj/cdg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AM, Ainsztein AM, Eckley DM, Earnshaw WC. A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J Cell Biol. 1998;140:991–1002. doi: 10.1083/jcb.140.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi K, Zhu X, Foong C, Hosoya H, Murata-Hori M. Aurora B kinase activity is required to prevent polar cortical ingression during cytokinesis. Cell Cycle. 2007;6:2549–2553. doi: 10.4161/cc.6.20.4817. [DOI] [PubMed] [Google Scholar]
- Murata-Hori M, Wang YL. Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J Cell Biol. 2002;159:45–53. doi: 10.1083/jcb.200207014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Hori M, Tatsuka M, Wang YL. Probing the dynamics and functions of aurora B kinase in living cells during mitosis and cytokinesis. Mol Biol Cell. 2002;13:1099–1108. doi: 10.1091/mbc.01-09-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Pinsky BA, Kung C, Shokat KM, Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol. 2006;8:78–83. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Shah JV, Botvinick E, Bonday Z, Furnari F, Berns M, Cleveland DW. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr Biol. 2004;14:942–952. doi: 10.1016/j.cub.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Rogers GC. A Kin I-dependent Pacman-flux mechanism for anaphase A. Cell Cycle. 2004;3:707–710. [PubMed] [Google Scholar]
- Sharp DJ, Brown HM, Kwon M, Rogers GC, Holland G, Scholey JM. Functional coordination of three mitotic motors in Drosophila embryos. Mol Biol Cell. 2000;11:241–253. doi: 10.1091/mbc.11.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenberg PT, Burke DJ. Analyzing the spindle checkpoint in yeast and frogs. Methods Mol Biol. 2004;280:83–98. doi: 10.1385/1-59259-788-2:083. [DOI] [PubMed] [Google Scholar]
- Wheatley SP, Carvalho A, Vagnarelli P, Earnshaw WC. INCENP is required for proper targeting of Survivin to the centromeres and the anaphase spindle during mitosis. Curr Biol. 2001;11:886–890. doi: 10.1016/s0960-9822(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Yuen KW, Montpetit B, Hieter P. The kinetochore and cancer: what’s the connection? Curr Opin Cell Biol. 2005;17:576–582. doi: 10.1016/j.ceb.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lan W, Ems-McClung SC, Stukenberg PT, Walczak CE. Aurora B Phosphorylates Multiple Sites on Mitotic Centromere-associated Kinesin to Spatially and Temporally Regulate Its Function. Mol Biol Cell. 2007;18:3264–3276. doi: 10.1091/mbc.E07-01-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.