Abstract
Single-gene mutations that extend lifespan provide valuable tools for the exploration of the molecular basis for age-related changes in cell and tissue function and for the pathophysiology of age-dependent diseases. We show here that mice homozygous for loss-of-function mutations at the Pit1 (Snell dwarf) locus show a >40% increase in mean and maximal longevity on the relatively long-lived (C3H/HeJ × DW/J)F1 background. Mutant dwJ/dw animals show delays in age-dependent collagen cross-linking and in six age-sensitive indices of immune system status. These findings thus demonstrate that a single gene can control maximum lifespan and the timing of both cellular and extracellular senescence in a mammal. Pituitary transplantation into dwarf mice does not reverse the lifespan effect, suggesting that the effect is not due to lowered prolactin levels. In contrast, homozygosity for the Ghrhrlit mutation, which like the Pit1dw mutation lowers plasma growth hormone levels, does lead to a significant increase in longevity. Male Snell dwarf mice, unlike calorically restricted mice, become obese and exhibit proportionately high leptin levels in old age, showing that their exceptional longevity is not simply due to alterations in adiposity per se. Further studies of the Pit1dw mutant, and the closely related, long-lived Prop-1df (Ames dwarf) mutant, should provide new insights into the hormonal regulation of senescence, longevity, and late life disease.
Keywords: longevity, Pit1, Ghrhr, T lymphocytes, Snell dwarf
The analysis of single-gene mutations in flies (1) and nematode worms (2–4) has begun to yield important clues to the molecular basis of aging and genetic control of longevity in invertebrates. At present there are four examples of single gene mutations that extend longevity in mammals (5–8). The best documented of these is the Ames dwarf mutation, now known as Prop-1df, which in homozygous form has been shown to extend longevity by >50% in both males and females (5). Homozygous df/df mice show defects in embryonic development of the anterior pituitary that lead to an absence of cells responsible for the production of growth hormone (GH), thyroid-stimulating hormone, and prolactin (PRL). The small body size of these mice is apparent within the first 3 weeks of age, and young adults are approximately one-third of the size of +/df or +/+ littermates, which are themselves phenotypically indistinguishable from one another. Extended longevity (about 20%) and small body size also are seen in transgenic mice that express high brain levels of urokinase-type plasminogen activator (6); in this case the phenotypes are thought to reflect a loss of appetite and diminished food intake similar to that seen in genetically normal mice and rats subjected to involuntary food restriction (9). In a third instance, targeted deletion of the p66shc signal transduction protein has been shown to lead to increased lifespan presumably mediated by increased cellular resistance to apoptosis (7).
The fourth example, the Snell dwarf mutation Pit1dw and the coallelic mutation Pit1dwJ are the topic of this report. The Pit1 (pituitary-specific transcription factor 1) locus on mouse chromosome 16 encodes a transcription factor, detectable by day 14 of embryonic life, that is required for normal development of the anterior pituitary. Induction of Pit1 expression requires (10, 11) prior activation of the Prop1 pathway deficient in the Ames dwarf mouse, and the developmental and hormonal properties of the two dwarf mutants are thus very similar. The two spontaneous mutations Pit1dw and Pit1dwJ are noncomplementing, and compound heterozygotes resemble dw/dw and dwJ/dwJ homozygotes in growth rate and adult body size. A previous publication (8) has shown the preliminary results of an experiment comparing survival of (C3H/HeJ × DW/J)F1-dw/dwJ to control mice. At the time of this preliminary report, ≈60% of dwarf mice were still alive at an age, 1,200 days, at which all controls had succumbed, showing that Snell dwarf mice, like Ames dwarfs, were remarkably long-lived. Alterations in longevity alone, however, provide only a limited index of altered aging, because mutations that extend longevity might do so through an effect on specific lethal illnesses without a more general retardation of senescent processes (12). The report of Silberberg (13) that Snell dwarf mice exhibit diminished osteoarthritis of the knee joint provides preliminary evidence that the Pit1dw mutation might delay aspects of biological senescence, as does a previous report that this mutation prevents age-dependent splenomegaly and impaired splenic T cell proliferation in the DW/J stock (12). The current paper documents extended survival in two independent colonies of Snell dwarf mice, on two background genotypes, shows decelerated changes in age-sensitive assays of connective tissue and immune system status, explores the effects of altered PRL and GH axes on the longevity outcome, and contrasts the late-life obesity of the dw/dwJ mice to the lifelong leanness of long-lived calorically restricted (CR) mice. Thus these observations show that a single genetic difference can retard multiple indices of senescence as well as increasing longevity in a mammal and suggest that alterations in GH-dependent pathways play a critical role in the antigeric effects of the dwarfing mutations.
Methods
Mice.
Three separate groups of Snell dwarf (and control) mice were used in these studies. One population consisted of dwJ/dw compound heterozygotes bred and housed at the University of Michigan. These mice were the F1 progeny of C3H/HeJ-Pit1dwJ/+ females and DW/J-Pit1dw/+ males, designated (C3H/HeJ × DW/J)F1 mice. Nondwarf mice produced by this cross (+/+, +/dw, and dwJ/+) are phenotypically similar and for convenience are referred to as control animals. The second population was bred and housed at The Jackson Laboratory and consisted of the progeny of the same parental genotypes as the first group, except that the cross was in both directions; for convenience, all mice of this cross are designated (DW/J × C3H/HeJ)F1, because there were no apparent effects of parental strain or gender. The third population consisted of DW/J-Pit1dw/dw and control mice born and housed at The Jackson Laboratory. Dwarfs are designated in the text as dw/dw or dw/dwJ, and the normal controls are designated as +/?.
C57BL/6J-Ghrhrlit/lit mice (“little” mice) were produced by crossing heterozygote sires with homozygote dams. Heterozygote littermates of the little mice were used as controls; hetrozygosity at Ghrhr for lit does not affect body weight, compared with C57BL/6J mice, through at least 22 months of age (K.F., unpublished work).
Husbandry.
In the initial studies at The Jackson Laboratory, using the inbred DW/J strain of mice, dwarfs were always housed with 1–3 normal same-sex littermates to provide warmth; in subsequent studies with F1 hybrid dw/dwJ mice, both female and male dwarfs were housed with normal female littermates. DW/J-Pit1dw/dw dwarfs and controls were reared in a specific pathogen-free limited-access animal colony under positive air pressure with filtered air at 23 ± 2°C on a 12-h light cycle. Mice were kept in filter-hooded plastic cages and given an autoclaved diet (Emory Morse, Guilford, CT; Jackson Laboratory diet 96WA), 7% fat by weight, ad libitum, and chlorinated water, acidified to prevent the growth of Pseudomonas. Quarterly, mice from this colony were used for animal health assessment by The Jackson Laboratory's diagnostic laboratory. C57BL/6J-Ghrhrlit/lit and lit/+ mice were kept under the same conditions except they were housed without respect to genotype and the diet was autoclaved NIH-31 (Purina), 4% fat by weight. (DW/J × C3H/HeJ)F1 mice were kept within the same colony but in a separate room, which was kept at 27 ± 1°C and 45–55% humidity on a 14-h light/10-h dark cycle. They also were fed autoclaved NIH-31 (4% fat) ad libitum.
The (C3H/HeJ × DW/J)F1 hybrid mice used for the life table study at the University of Michigan were housed in a specific pathogen-free colony and given free access to Purina Mouse Chow and tap water. All mice were housed in the same room throughout the study period. Each cage was protected by a filter-paper bonnet to minimize the risks of airborne infection. The pathogen-free status of the colony was documented every 3 months by using a procedure in which spent bedding from the experimental mice was provided to sentinel mice of the outbred CD1 stock, and the sentinels later were tested for antiviral antibodies and parasites; all such tests were negative throughout the course of this study.
Measurement of Tail Tendon Break Time.
The procedure was as described (14). In brief, a collagen fiber was teased from a midtail section of the lateral tail tendon and tied to a 2-gm weight. The fiber was suspended in a 7 M urea bath at 45°C; the fiber breaking time was determined in quadruplicate for each mouse.
Assessment of T Cell Surface Markers by Flow Cytometry and Determination of Responder T Cell Frequencies by Limiting Dilution Analysis.
Pituitary Transplantation Experiments.
Mice aged 3–4 months were anesthetized with tribromoethanol. The left kidney was exposed, a small incision was made in the kidney capsule, and the capsule was separated from the kidney with a glass probe. A pituitary was removed from a 2-month-old +/? male mouse and placed beneath the kidney capsule of the recipient. +/? recipients received an entire pituitary, and dwarf mice received 1/2 of a pituitary. Sham-operated mice underwent the same procedure except that no tissue was grafted. Untreated +/? mice from the same cohort provided normative data for weight, tail length, and lifespan. Body weight and tail length were monitored for 36 weeks after grafting, until the mice were 12 months old. Mice were kept until natural death.
RIA for Leptin.
Circulating leptin was measured in duplicate 20-μl samples by using a mouse leptin RIA kit from Linco Research Immunoassay (St. Charles, MO). All samples were measured in a single run; all samples contained more leptin than the minimal sensitivity of 1.4 ng/ml. The intraassay coefficient of variation was 3.8%. Blood was collected either by retroorbital puncture using heparinized tubes or decapitation. Blood from decapitation was allowed to clot for 4–12 h at 4°C before the serum was separated. Serum and plasma samples were stored at −20°C.
Results
Effect on Lifespan.
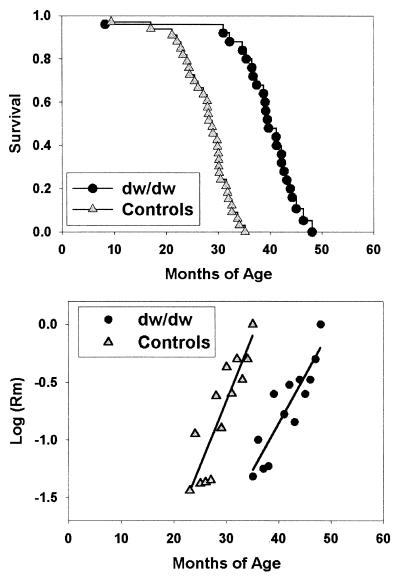
Fig. 1 shows the outcome of a survival study comparing (C3H/HeJ × DW/J)F1 controls to sibling dwJ/dw dwarf mice at the University of Michigan. Differences between male and female mice of either genotype were small and statistically insignificant, and data from both genders therefore were pooled for analysis. Mean survival was extended in the dwarf group by 42% (1,178 ± 235 days vs. 832 ± 158 days, P < 0.001). At the time of death of the longest-lived control at 1,060 days, 19 of the 25 dwarf mice were still alive, and the longest-lived dwarf died at 1,451 days of age. Mortality risks are shown in Fig. 1, starting for each genotype at the age at which the first 20% of the animals have died. The mortality curves are consistent with the interpretation that the effects of aging on lethal illness are delayed, in the dwJ/dw animals, by about 12 months, but that once initiated these effects increase at approximately the same rate in each genotype.
Figure 1.
Snell dwarf mice live longer than sibling controls. The triangles indicate control mice and the circles indicate mice of the dwJ/dw genotype on the (C3H/HeJ × DW/J)F1 background, housed at the University of Michigan. (Upper) Survival. Each symbol corresponds to a mouse dying at the age indicated. (Lower) Mortality rates, starting, for each genotype, as the age at which 20% of the mice have died. The lines are fitted by linear regression.
Effect on Collagen Cross-Linking.
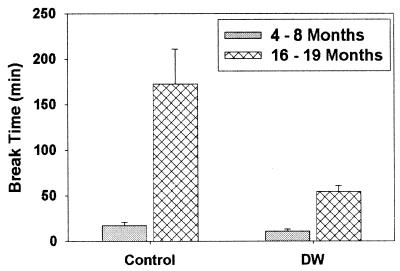
To see whether this increase in lifespan was accompanied by an alteration in the rates of aging for different biological systems, we examined tail collagen cross-linking as an index of age-dependent change in extracellular macromolecules and T lymphocyte status as a representative example of changes in an age-sensitive cellular compartment. Fig. 2 shows the results of tail tendon break testing for young and middle-aged mice of the (DW/J × C3H/HeJ)-dw/dwJ genotype and normal sibling controls. The wild-type animals show the expected (18) increase with age in resistance of tendons to breakage in a denaturing urea solution, but this change is much less dramatic in the dwarf mice, showing a highly significant 3.2-fold difference between dw/dwJ and wild-type collagen at 16–19 months of age. When analyzed by ANOVA after a square root transformation to minimize variance differences, the results indicated highly significant effects of age (P = 0.001) and genotype (P < 0.0001) and a significant (P = 0.008) gene × age interaction effect. Midtail temperature (mean = 29.4°C), taken using an infrared temperature gauge (Light Touch Pedi-Q, Exergen, Newton, MA), was not altered by the dw mutation, showing that the more rapid tail tendon collagen breaking time in dwarfs during aging does not result from in situ differences in temperature.
Figure 2.
Age-dependent change in collagen denaturation time is slowed in dwarf mice. The vertical axis represents time, in minutes, for breakage of a weighted tendon in a denaturing urea solution. Each bar shows mean and SEM for n = 5 mice on the (DW/J × C3H/HeJ)F1 background, housed at The Jackson Laboratory.
Effect on Age-Sensitive T Lymphocyte Subsets and T Cell Function.
Table 1 summarizes the analyses of memory T cell subsets in the spleen of female (DW/J × C3H/HeJ)F1 dwarf and control mice. Similar results (not shown) were obtained for male mice. In most mouse strains, aging leads to an increase in the proportion of both CD4 and CD8 cells that express the CD44 surface marker typical of the memory T cell population, referred to as CD4 M and CD8 M cells, respectively (17, 19). As expected, control mice of the (DW/J × C3H/HeJ)F1 background show an increase in the proportion of both splenic CD4 M and CD8 M cells as the mice age from 3–7 months to 27–29 months of age. Aged dwarf mice, however, have CD4 M and CD8 M cell levels that are similar to those seen in young control animals and significantly lower (P < 0.002) than those seen in aged control mice.
Table 1.
Age-sensitive T cell subsets in spleens of aged (DW/J × C3H/HeJ)F1 dwarf and control mice
| T cell subset | Units | Controls 3–7 months | Controls 27–29 months | Dwarf 27–29 months | Old control vs. old dwarf |
|---|---|---|---|---|---|
| CD4M | % of CD4 | 41 ± 3* | 67 ± 9 | 32 ± 6 | 0.002† |
| CD8M | % of CD8 | 19 ± 2 | 70 ± 5 | 14 ± 2 | 0.0001 |
| CD4P | % of CD4 | 37 ± 4 | 74 ± 2 | 43 ± 2 | 0.0001 |
| CD8P | % of CD8 | 67 ± 2 | 91 ± 1 | 64 ± 2 | 0.0001 |
Percentage of cells with the indicated phenotype, ± standard error of the mean, for n = 6 mice per group.
Significance level for difference between the old control and old dwarf group, calculated by using the Bonferroni/Dunn method after one-way ANOVA.
Aging also leads to an increase in the proportion of CD4 and CD8 cells that express cell-surface P-glycoprotein activity (15), referred to as CD4P and CD8P cells. CD4P cells are a subset of the CD4 M population, and within the CD4 M pool those cells that express P-glycoprotein activity are functionally anergic (20) and show defects in signal transduction (21). Interestingly, accumulation of CD4P cells in aged mice does not depend on contact with antigen, at least in the context of an immune system consisting largely of T cell receptor transgenic lymphocytes (22). As Table 1 illustrates, aging leads to the expected increase in numbers of CD4P and CD8P cells in control mice. In contrast, the proportion of both CD4P and CD8P cells in aged dwarf mice is significantly less than seen in old controls (P < 0.001) and similar to that seen in young control animals.
T cell function was tested by limiting dilution analysis to estimate the proportion of mitogen-stimulated T cells that can produce IL-2 (pHTL) and the proportion of cells that can respond to mitogen and IL-2 by proliferation and differentiation into cytotoxic effectors (pCTL). Previous work has shown that the pHTL assay detects mostly CD4 cell function and the pCTL test detects mostly CD8 cell activity (23); function in both tests declines with age (16). Because of significant interassay variation, the data are expressed as the percent of the value seen in young normal mice tested on the same day. As shown in Table 2, pHTL and pCTL frequencies have dropped, as expected, by ≈50% in DW/J control mice by 15–17 months of age compared with 3–7 month controls. In dw/dw dwarfs, however, the pHTL and pCTL frequencies at 15–17 months are, respectively, 95% and 81% of that seen in young control mice, and 87% or 102% of that seen in young dw/dw animals. Compared with young controls, only the pCTL values of the aged control mice are significantly depressed (P = 0.014 after Bonferroni correction). pHTL values also show a trend toward lower levels in aged control mice (P < 0.05), but this is not significant after Bonferroni correction. Thus all six tests of immune status are consistent with the hypothesis that the effects of aging are blunted in the dwarf mice compared with their nonmutant controls.
Table 2.
Limiting dilution assays for T cell function in spleens of DW/J Snell dwarf and control mice
| Assay | Units | dw/dw 3–7 months | +/? 15–17 months | dw/dw 15–17 months |
|---|---|---|---|---|
| pHTL | % of Y | 109 ± 26 (6)* | 55 ± 20 (5) | 95 ± 12 (3)† |
| pCTL | % of Y | 79 ± 15 (5) | 47 ± 10 (5) | 81 ± 18 (6) |
Values represent percent of response compared to young control mice run on the same day of assay, ± standard error of the mean; numbers in parenthesis show the number of mice tested.
One mouse in this group had a pHTL response 354% of the normal young control; this animal, whose response is >3 SDs above normal young controls, is omitted from the calculation.
Pituitary Grafting Experiments.
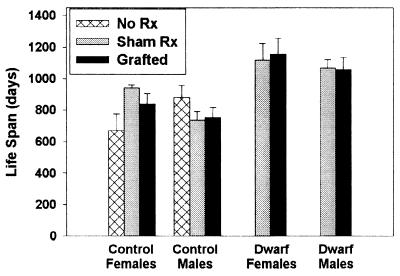
The phenotypes of the Pit1 mutations dw and dwJ are thought to be due to abnormally low production of GH, PRL, and thyroid-stimulating hormone, with resulting diminutions of thyroid hormones and insulin-like growth factor I (IGF-I). To see whether restoration of PRL would reverse the lifespan effect of the dwarf mutation, we examined the effect of pituitary gland transplantation, at 3 months of age, into normal and dwarf (DW/J × C3H/HeJ)F1 mice. Because prolactin is the only pituitary hormone known to be under tonic negative control by the hypothalamus, heterotopic pituitary transplants continuously synthesize and secrete prolactin, with a single transplanted pituitary typically maintaining circulating levels in the high physiological range indefinitely (24). GH is the only other pituitary hormone secreted from such pituitary transplants (25), and the circulating level is only 5% of normal (26). As expected (25), pituitary-transplanted dwarf mice grew slowly but continuously, doubling their weight in the 36 weeks after surgery, significantly higher than the 30% weight gain exhibited by sham-operated dwarf mice (P = 0.0005 by repeated-measures ANOVA with sexes combined), showing that the transplants were functionally active. In addition, tail length increased by 25% among grafted dwarf mice within the 12 weeks after surgery, significantly (P = 0.0001) greater than the 4% increase in tail length seen in sham-operated dwarf mice. The normal age-associated weight gain seen in nonmutant mice was not altered by either sham-operation or pituitary implantation nor was tail length altered by grafting in this genotype (not shown). The survival data, shown in Fig. 3, give no indication that the extended survival of the dw/dwJ mice can be reversed by PRL-producing pituitary implants, in either males or females. ANOVA of the lifespan results showed that there was no effect of the pituitary graft, compared with sham operation, either in control or in dw/dwJ mutant mice. Power analysis shows that this experimental design could have detected a decline in lifespan of as little as 7% (74 days), at 80% power using P < 0.05 for a one-tailed test with both sexes combined. It is noteworthy that this study, carried out at The Jackson Laboratory using (DW/J × C3H)F1 mice, produced mean longevity data that are essentially indistinguishable from that generated at the University of Michigan using mice of the (C3H/HeJ × DW/J)F1 background (Fig. 1); the lifespan extension phenomenon can therefore be replicated in at least two vivaria and including reciprocal breeding combinations.
Figure 3.
Lifespan of (DW/J × C3H/HeJ)F1-Pit1dw/dwJ mice is not diminished by pituitary graft implantation at 3 months of age. Each bar shows mean ± SEM for mice of the indicated gender, treatment, and genotype. The numbers of mice per group were (left to right) 3, 4, 5, 4, 4, 4, 3, 4, 3, and 4. ANOVA including all grafted and sham-operated animals showed a significant effect of genotype (P < 0.0001), but no significant effects for gender or grafting and no significant interaction effects.
Longevity Increases in Ghrhrlit/lit Mice.
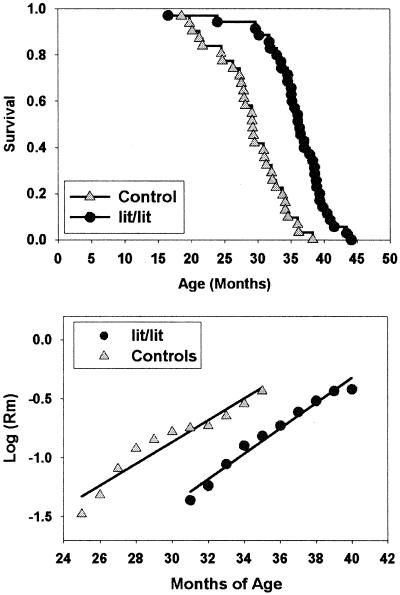
As a further guide to the hormonal basis for lifespan extension the dwarf mice, we measured longevity in male and female mice differing at the Ghrhr locus. Mice homozygous for the C57BL/6 mutation Ghrhrlit are defective in the response to the hypothalamic peptide GH releasing hormone (GHRH), have blood levels of GH that are 1% of normal, and are as young adults ≈two-thirds the weight of nonmutant controls. The results in Fig. 4 show that C57BL/6J mice of the lit/lit genotype are significantly longer lived than +/lit heterozygous controls. The longevity effect is 23% in males (1,093 ± 186 days, compared with 886 ± 148 days) and 25% in females (1,070 ± 127 days, compared with 857 ± 169 days). The effect of genotype is significant at P = 0.0001 by the log-rank test with genders pooled. Maximal lifespan, although difficult to estimate with any precision with such a small sample size, also increased by 15%, i.e., from 1,155 to 1,331 days. A total of 34% (12/35) of the lit/lit mice were still alive at the death of the last surviving wild-type animal (n = 31). Fig. 4 shows mortality rate curves with the first 20% of deaths in each genotype excluded; the plots are consistent with the hypothesis that the effects of age on mortality risk are delayed by about 6 months in the lit/lit mice, but then proceed at a pace similar to that seen in controls.
Figure 4.
Extended lifespan of Ghrhr-defective lit/lit mice. (Upper) The survival curve for 35 mice of the C57BL/6J-Ghrhrlit/lit genotype and 31 lit/+ littermate control mice; genders were not significantly different and are pooled. Each symbol represents one mouse. (Lower) Smoothed monthly mortality rates, starting, for each genotype, at the age at which 20% of the mice had died. The lines are fitted by linear regression.
Weight and Leptin Levels in Aged Dwarf Mice.
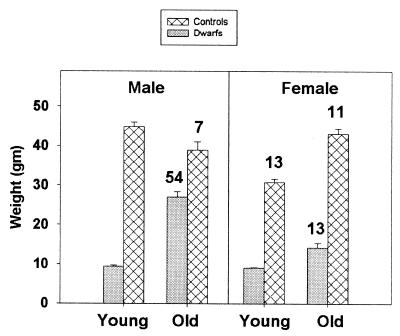
Experimental restriction of caloric intake leads to lifespan extension in many strains of genetically normal mice and rats (9). CR mice and rats are exceptionally lean throughout life. In contrast, Snell dwarf mice, particularly (DW/J × C3H/HeJ)F1 males, become relatively obese at later ages. Fig. 5 shows body weight and serum leptin values for dwarf and control mice in two age ranges: 3–6 months, and 22–30 months. The dramatic increase in weight of the male dwarf mice seen at The Jackson Laboratory is accompanied by an increase in serum leptin levels consistent with the increase in amounts of adipose tissue noted at necropsy in these mice. In contrast, circulating leptin in 27-month old CR B6C3F1 mice in the same colony was 6 ± 2 ng/ml, i.e., half the level seen in old B6C3F1 ad libitum-fed controls and in aged dwarf (DW/J × C3H/HeJ)F1 females, and almost 10-fold lower than in (DW/J × C3H/HeJ)F1 males.
Figure 5.
Body weight and serum leptin concentrations in (DW/J × C3H/HeJ)F1 dwarf and control mice at two age ranges. The bars indicate mean weight ± SEM; n = 7–20 mice for each point. Values written over the bars show leptin concentrations in ng/ml; standard errors were 2–3 ng/ml except for the aged dwarf males, which had a standard error of 9 ng/ml. n = 7–11 mice/point except n = 3 for the aged control males. For the leptin data, two mice have been omitted (one control male and one dw/dwJ female, both old) because they had leptin levels 6 and 19 standard deviations above the mean for the group as a whole. Excluding the outlier, the difference in leptin levels between old dwarf and old control mice is significant (P = 0.007) in males but not in females.
At the University of Michigan colony, both male and female dwarf mice become strikingly obese in later life (not shown); the basis for this disparity in obesity of aged female dwarf mice is unknown, but seems likely to reflect differences in experimental diets between the two vivaria.
Discussion
The remarkable increase in mean and maximal longevity seen in the Snell dwarf mice is consistent with published data on the phenotypically similar Ames dwarf (5) model. Extended longevity in Pit1 and Prop1 mutant mice has now been documented in three laboratories (Southern Illinois, University of Michigan, The Jackson Laboratory) and on three genetic backgrounds—Ames stock, DW/J, and the (C3H × DW)F1 hybrid—among which the F1 hybrid provides excellent survival even in nonmutant controls. It thus seems reasonable to conclude that the effect of these dwarfing mutations does not reflect merely the correction of some hypothetical life-shortening abnormality present in a specific control mouse stock.
The demonstration that a specific genetic or environmental intervention extends lifespan provides prima facie evidence, but not compelling proof, that the effect represents alteration in aging rate per se. The gradual realization that caloric restriction not only extended lifespan but also decelerated age-dependent changes in multiple organs, cell types, and intracellular and extracellular processes (9) was a critical step in the widespread adoption of the CR rodent as a model for decelerated aging. Delayed senescence in Snell dwarf mice has been reported previously for knee joint pathology (13) and age-related splenomegaly and T cell proliferative responses (12). Here we have shown that Snell dwarf mice show slower than normal change in tail tendon collagen cross-linking, an index of aging in extracellular macromolecules, and in six age-sensitive indices of immunological development and function. These data thus suggest that the ability of the dwarfing mutations to postpone fatal diseases and extend lifespan is accompanied by, and probably caused by, a generalized deceleration or delay in a wide range of age-dependent processes.
Snell and Ames dwarf mice show primary defects in at least three endocrine axes: thyroid-stimulating hormone stimulation of thyroid hormone production, GH stimulation of IGF-I, and PRL effects. Our data suggest that among these, the changes in GH and IGF-I action may be particularly important. The extended longevity of the lit/lit mice show that alteration in the GH/IGF-I axis can be sufficient to lead to lifespan extension, and add a fifth member to the catalog of single gene mutations that extend lifespan in mammals. We note that previous studies of longevity in colonies of lit/lit mice (12) showed small and inconsistent effects. Our current study of lit/lit mice made use of a diet containing only 4% fat content, which we speculate may have helped to extend longevity in the lit/lit animals by preventing the obesity seen at older ages in mice of this genotype on diets containing higher lipid levels (K.F., unpublished work). Our data showing that pituitary transplantation does not diminish longevity in dw/dwJ dwarfs is consistent with the idea that lower circulating PRL levels are not a critical factor in this model; heterotopically transplanted pituitary glands continuously produce PRL at high physiologic levels (24), but GH is secreted only at very low levels (26), and there is no evidence for secretion of other hypophyseal hormones (24, 25). A preliminary report (27) of extended longevity in knockout mice in which the GH receptor has been rendered nonfunctional lends additional support to the hypothesis that GH/IGF-I deficiency is the key element in the anti-aging effect of dw and df mutations. It is also noteworthy that size disparities among breeds of dogs, which are very strongly associated (R2 = 56%) with interbreed differences in longevity (8), have in both of the studied instances (28, 29) been shown to reflect alterations in production of IGF-I, in each case smaller, long-lived breeds having lower IGF-I levels. Genetically heterogeneous mouse stocks selected for differential growth rates in the first 10–56 days of life also differ greatly in mean and maximal lifespan (30), with smaller body size strongly associated (R2 = 48%, P < 0.004) with superior longevity, although in this instance it is not yet known to what extent the stocks differ in production of or response to GH and IGF-I. The smaller impact of the lit mutation, with its relatively pure GH/IGF-I effect, compared with the effect size of the dw and df mutations, suggests that impaired thyroid hormone production also may play a role in extending lifespan in the later mutant, and studies in which GH and thyroid hormone levels are independently altered will be informative. Thyroid hormone-deficient animals are cold sensitive and might be expected to have diminished core body temperatures. It therefore will be useful to study lifespan and age-sensitive phenotypes in dw/dw mice in which body temperature is kept within normal limits either by exogenous treatment with thyroxine or by housing them in a warmer room.
Snell dwarf mice share several characteristics with CR mice, including small body size and long lifespan. Food consumption is also low in both models, although for different reasons: CR mice eat less because of extrinsic limitations of food supply, whereas Snell and Ames dwarf mice eat less because their small body size requires less fuel usage for maintenance of body temperature and metabolic activities. Young adult Ames dwarf mice have been shown to consume more food calories per g body weight per day than sibling controls (31), consistent with the metabolic demands imposed by a high ratio of surface area to body mass. Our observations that aged dw/dwJ mice become obese and maintain high circulating leptin levels also clearly distinguish these mice from CR rodents, which remain exceptionally lean throughout life. Compiling a catalog of similarities and differences between these and other models of decelerated aging in mice will help to narrow down the list of possible mechanisms for the longevity extension seen in these models.
Our data also help address the controversial issue of whether the multiple degenerative processes seen in aging mammals are synchronized by some underlying control system. Conventional wisdom (32, 33), noting the very wide range of age-dependent changes in cellular, extracellular, and intercellular traits, views aging as a collection of independently regulated changes that work in parallel to diminish organismic homeostasis and increase vulnerability. In contrast, the ability of caloric restriction to retard, in parallel, age-dependent changes in mitotic and nonmitotic cell types, extracellular macromolecules, and intercellular control pathways is difficult to reconcile with models based on multiple clocks, and thus supports models in which a very small number of fundamental timing processes acts to speed up or slow down age-dependent change in multiple domains. Because age at death is changed only slightly by changes in the risk of individual forms of disease (34), it seems unlikely that single gene mutations that extend longevity do so by an influence on a single form of disease; extensive data on terminal pathology, not yet available for any genetic model of longevity, are needed to address this question explicitly. Single gene mutations that extend lifespan thus provide prima facie evidence for the idea that multiple late-life processes—including multiple forms of illness—can be decelerated in parallel by some underlying control mechanism. Our data, showing that the dw/dwJ genotype not only extends lifespan but also delays the effects of aging on T cell subsets and tail tendon collagen cross-linking, adds support to these “single clock” models of aging. Further studies of these mutants, and of other mutants that affect the GH/IGF-I axis, seem likely to yield important clues to the molecular basis of aging and disease in mammals.
Acknowledgments
We thank Maggie Vergara, Gretchen Buehner, and Keith Oliver for excellent technical assistance. This work was supported by National Institutes of Health Grants AG08808 (to R.A.M.), AG05429 (to K.F.), AG11643 (to D.E.H.), and AG16622 (to J.P. and D.E.H.), a core grant from the National Cancer Institute to The Jackson Laboratory (CA34190), and a grant from Empire Pharmaceuticals.
Abbreviations
- GH
growth hormone
- PRL
prolactin
- Pit1
pituitary-specific transcription factor 1
- CR
calorically restricted
- IGF-I
insulin-like growth factor I
- GHRH
GH-releasing hormone
References
- 1.Lin Y J, Seroude L, Benzer S. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 2.Lithgow G J, White T M, Melov S, Johnson T E. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin K, Dorman J B, Rodan A, Kenyon C. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 4.Kimura K D, Tissenbaum H A, Liu Y, Ruvkun G. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 5.Brown-Borg H M, Borg K E, Meliska C J, Bartke A. Nature (London) 1996;384:33–33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 6.Miskin R, Masos T. J Gerontol A Biol Sci Med Sci. 1997;52:B118–B124. doi: 10.1093/gerona/52a.2.b118. [DOI] [PubMed] [Google Scholar]
- 7.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi P P, Lanfrancone L, Pelicci P G. Nature (London) 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 8.Miller R A. J Gerontol Biol Sci. 1999;54:B297–B307. doi: 10.1093/gerona/54.7.b297. [DOI] [PubMed] [Google Scholar]
- 9.Weindruch R, Walford R L. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas; 1988. [Google Scholar]
- 10.Gage P J, Brinkmeier M L, Scarlett L M, Knapp L T, Camper S A, Mahon K A. Mol Endocrinol. 1996;10:1570–1581. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- 11.Sornson M W, Wu W, Dasen J S, Flynn S E, Norman D J, O'Connell S M, Gukovsky I, Carriere C, Ryan A K, Miller A P, et al. Nature (London) 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 12.Flurkey K, Harrison D E. In: Genetic Effects on Aging II. Harrison D E, editor. Caldwell, NJ: Telford; 1990. pp. 437–456. [Google Scholar]
- 13.Silberberg R. Pathol Microbiol. 1972;38:417–430. doi: 10.1159/000162458. [DOI] [PubMed] [Google Scholar]
- 14.Harrison D E, Archer J R. Exp Gerontol. 1978;12:75–82. doi: 10.1016/0531-5565(78)90033-5. [DOI] [PubMed] [Google Scholar]
- 15.Witkowski J M, Miller R A. J Immunol. 1993;150:1296–1306. [PubMed] [Google Scholar]
- 16.Miller R A. J Immunol. 1984;132:63–68. [PubMed] [Google Scholar]
- 17.Miller R A. Mech Aging Dev. 1997;96:181–196. doi: 10.1016/s0047-6374(97)01893-9. [DOI] [PubMed] [Google Scholar]
- 18.Harrison D E, Archer J R, Sacher G A, Boyce F M I. Exp Gerontol. 1978;12:63–73. doi: 10.1016/0531-5565(78)90032-3. [DOI] [PubMed] [Google Scholar]
- 19.Miller R A. In: Handbook of Physiology: Section 11, Physiology of Aging. Masoro E, editor. New York: Oxford Univ. Press; 1995. pp. 555–590. [Google Scholar]
- 20.Bining N, Miller R A. J Gerontol A Biol Sci Med Sci. 1997;52:B137–B145. doi: 10.1093/gerona/52a.3.b137. [DOI] [PubMed] [Google Scholar]
- 21.Eisenbraun M D, Tamir A, Miller R A. J Immunol. 2000;164:6105–6112. doi: 10.4049/jimmunol.164.12.6105. [DOI] [PubMed] [Google Scholar]
- 22.Linton P J, Haynes L, Klinman N R, Swain S L. J Exp Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller R A. J Immunol. 1983;131:2864–2868. [PubMed] [Google Scholar]
- 24.Kwa H G, van der Gugten A A, Sala M, Verhofstad F. Eur J Cancer. 1977;8:39–54. doi: 10.1016/0014-2964(72)90082-5. [DOI] [PubMed] [Google Scholar]
- 25.Mohlbock O, Boot L M. Cancer Res. 1959;19:402–412. [PubMed] [Google Scholar]
- 26.Paneri A E, Gil-Ad L, Cocchi D, Locatelli V, Rossi G L, Muller E E. J Endocrinol. 1977;72:301–311. doi: 10.1677/joe.0.0720301. [DOI] [PubMed] [Google Scholar]
- 27.Kopchick J J, Laron Z. Mol Genet Metab. 2000;68:232–236. doi: 10.1006/mgme.1999.2890. [DOI] [PubMed] [Google Scholar]
- 28.Eigenmann J E, Amador A, Patterson D F. Acta Endocrinol. 1988;118:105–108. doi: 10.1530/acta.0.1180105. [DOI] [PubMed] [Google Scholar]
- 29.Eigenmann J E, Patterson D F, Froesch E R. Acta Endocrinol. 1984;106:448–453. doi: 10.1530/acta.0.1060448. [DOI] [PubMed] [Google Scholar]
- 30.Miller R A, Chrisp C, Atchley W R. J Gerontol Biol Sci. 2000;55:B455–B461. doi: 10.1093/gerona/55.9.b455. [DOI] [PubMed] [Google Scholar]
- 31.Mattison J A, Wright C, Bronson R T, Roth G S, Ingram D K, Bartke A. J Am Aging Assoc. 2000;23:9–16. doi: 10.1007/s11357-000-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masoro E J. In: Handbook of Physiology: Section 11, Aging. Masoro E J, editor. New York: Oxford Univ. Press; 1995. pp. 3–21. [Google Scholar]
- 33.Holliday R. Understanding Aging. Cambridge: Cambridge Univ. Press; 1999. [Google Scholar]
- 34.Olshansky S J, Carnes B A, Cassel C. Science. 1990;250:634–640. doi: 10.1126/science.2237414. [DOI] [PubMed] [Google Scholar]