Abstract
AIM: To study and provide data on the evolution of medical procedures and outcomes of patients suffering from perforated midgut diverticulitis.
METHODS: Three data sources were used: the Medline and Google search engines were searched for case reports on one or more patients treated for perforated midgut diverticulitis (Meckel’s diverticulitis excluded) that were published after 1995. The inclusion criterion was sufficient individual patient data in the article. Both indexed and non-indexed journals were used. Patients treated for perforated midgut diverticulitis at Vestfold Hospital were included in this group. Data on symptoms, laboratory and radiology results, treatment modalities, surgical access, procedures, complications and outcomes were collected. The Norwegian patient registry was searched to find patients operated upon for midgut diverticulitis from 1999 to 2007. The data collected were age, sex, mode of access, surgical procedure performed and number of patients per year. Historical controls were retrieved from an article published in 1995 containing pertinent individual patient data. Statistical analysis was done with SPSS software.
RESULTS: Group I: 106 patients (48 men) were found. Mean age was 72.2 ± 13.1 years (mean ± SD). Age or sex had no impact on outcomes (P = 0.057 and P = 0.771, respectively). Preoperative assessment was plain radiography in 53.3% or computed tomography (CT) in 76.1%. Correct diagnosis was made in 77.1% with CT, 5.6% without (P = 0.001). Duration of symptoms before hospitalization was 3.6 d (range: 1-35 d), but longer duration was not associated with poor outcome (P = 0.748). Eighty-six point eight percent of patients underwent surgery, 92.4% of these through open access where 90.1% had bowel resection. Complications occurred in 19.2% of patients and 16.3% underwent reoperation. Distance from perforation to Treitz ligament was 41.7 ± 28.1 cm. At surgery, no peritonitis was found in 29.7% of patients, local peritonitis in 47.5%, and diffuse peritonitis in 22.8%. Peritonitis grade correlated with the reoperation rate (r = 0.43). Conservatively treated patients had similar hospital length of stay as operated patients (10.6 ± 8.3 d vs 10.7 ± 7.9 d, respectively). Age correlated with hospital stay (r = 0.46). No difference in outcomes for operated or nonoperated patients was found (P = 0.814). Group II: 113 patients (57 men). Mean age 67.6 ± 16.4 years (range: 21-96 years). Mean age for men was 61.3 ± 16.2 years, and 74.7 ± 12.5 years for women (P = 0.001). Number of procedures per year was 11.2 ± 0.9, and bowel resection was performed in 82.3% of patients. Group III: 47 patients (21 men). Patient age was 65.4 ± 14.4 years. Mean age for men was 61.5 ± 17.3 years and 65.3 ± 14.4 years for women. Duration of symptoms before hospitalization was 6.9 d (range: 1-180 d). No patients had a preoperative diagnosis, 97.9% of patients underwent surgery, and 78.3% had multiple diverticula. Bowel resection was performed in 67.4% of patients, and suture closure in 32.6%. Mortality was 23.4%. There was no difference in length of history or its impact on survival between Groups I and III (P = 0.241 and P = 0.198, respectively). Resection was more often performed in Group I (P = 0.01). Mortality was higher in Group III (P = 0.002).
CONCLUSION: In cases with contained perforation, conservative treatment gives satisfactory results, laparoscopy with lavage and drainage can be attempted and continued with a conservative course.
Keywords: Intestinal, Small bowel, Jejunum, Ileum, Perforation, Diverticulitis, Conservative treatment
INTRODUCTION
Perforated midgut diverticulitis is an uncommon, acquired condition that is only sporadically observed and treated in surgical departments[1]. This could be the reason for the confusion that still exists when clinical manifestations, diagnosis and treatment options are concerned. The evidence at hand does not seem to be satisfactory to provide the answers needed. Furthermore, there are no data on the use of minimally invasive procedures in the treatment of perforated midgut diverticulitis. A previously published review by Chendrasekhar et al[2] in 1995 provided individual patient data for all case reports previously published. This article was chosen to be the landmark for this study because of its extensive data,firm grasp of the problem up to 1995, prior to important developments in diagnostics and treatment.
The aim of this study is to provide new data on the evolution of medical procedures and outcomes in patients with perforated midgut diverticulitis based on individual patient data derived from published case reports, original data and the Norwegian patient registry.
MATERIALS AND METHODS
The data obtained in this study came from three different sources: (Group I) review of published case reports in the literature after 1995 combined with original data; (Group II) data from the Norwegian patient registry; and (Group III) case reports from the literature prior to 1995 as historical controls[2].
Case reports and original data (Group I)
Search strategy: Two independent researchers with a medical degree performed the search. The Medline database (http://www.ncbi.nlm.nih.gov/PubMed/) was searched for articles published after 1995. An effort to incorporate all available articles not included in Medline was made through the use of the Google search engine (http://www.google.com/). The following key search strategy was used: ("perforated" or "perforation") and [("jejunum" or "jejunal") or ("intestine, small") or ("intestine" and "small") or ("small intestine") or ("small" and "bowel") or ("small bowel")] and ("diverticulum" or "diverticulitis") for medline databases; and ("perforated" or "perforation") and [("jejunum" or "jejunal") or ("intestine" and "small") or ("small intestine" or "intestine") and ("bowel" or "small bowel")] and ("diverticulum" or "diverticulitis") not sigmoid for Google. References did not have to have abstracts. There were no restrictions by language or publication type. English, French, Spanish, Portuguese, Italian, German and the Scandinavian and Slavic languages were translated by the authors, while help was acquired for translations of Turkish and Korean. All references matching the search were identified and screened for retrieval. Duplicate references were removed by a manual search. The retrieved references were scanned by the authors independently.
Selection: The inclusion criterion was original articles or case reports with any study design including at least one patient undergoing any kind of treatment for perforated midgut diverticulitis (Meckel’s diverticulitis excluded), containing pertinent individual patient data. Perforated midgut diverticulitis was defined either as histopathologically confirmed in the surgical specimen (operated patients) or where clear radiological evidence was provided in conservatively treated patients. This evidence was usually computed tomography (CT)-based (localized inflammation or thickened wall of the midgut with/without air bubbles, pseudotumor or abscess in the midgut mesentery), or confirmed with a small bowel series, and in some cases, through radiography with contrast medium after abscess drainage. The population selected were 18 years or older.
Data abstraction: Individual data for each patient included the following: age; sex; surgical procedure (if operated); access (laparoscopic/open/combined); number of diverticula (single/multiple); distance from the ligament of Treitz; diverticula in other segments of the gut (jejunum/ileum/colon); length of symptoms prior to hospitalization; fever; peripheral blood leucocytes and C-reactive protein (CRP) at admission; method of diagnosis; hospital stay; complications (general and surgical); reoperation; and outcome. Details of the intervention included any type of treatment (conservative or surgical). Surgical procedure was classified as resection, suture closure or exploration, laparoscopic or open. Other complications of midgut diverticulitis (bleeding, bacterial overgrowth) were not studied.
Vestfold hospital patient data: Patients treated at Vestfold hospital for perforated midgut diverticulitis from March 2008 to December 2010 were included in this series. All patients had an abdominal CT scan at hospitalization. Indication for surgery was made through the evaluation of abdominal tenderness, rebound tenderness, bowel sounds, blood work-up and CT scan. Antibiotics were administered. Patients who were operated upon had laparoscopy that confirmed the diagnosis, and thereafter a precisely placed minilaparotomy 4-5 cm in length, through which a limited small bowel resection and end-to-end hand-sewn anastomosis was performed. Further investigations (gastroscopy, colonoscopy and small bowel series) were done several weeks after the patient was discharged from the hospital.
Norwegian patient registry (Group II)
A comprehensive search of the Norwegian patient registry was performed. The time limit was from 1999 (introduction of the ICD-10 coding system in Norway) to 2007. The search criteria: K57.0 (midgut diverticulitis with perforation or abscess), K57.4 (small and large bowel diverticulitis with perforation or abscess), K57.8 (bowel diverticulitis, unspecified with perforation or abscess), Q43.0 (Meckel’s diverticulum) excluded, crossed with JFB00 (small bowel resection), JFB01 (laparoscopic small bowel resection), JFA70 (enterorrhaphy), JFA71 (laparoscopic enterorrhaphy). Patients under the age of 18 years were excluded. The data requested were age, sex, mode of access, surgical procedure performed, and number of patients per year.
Historical controls (Group III)
The data on historical controls was retrieved from Chendrasekhar et al[2]. Patients under the age of 18 years, and patients that did not have data on age were deleted and the remaining data were copied into the statistical software and used for further analysis.
Statistical analysis
Analysis was done using SPSS statistical software (Chicago, IL, United States). Distribution of data was checked with the Shapiro-Wilks test. Nonparametric values were compared using the χ2 test and the Mann-Whitney test, while parametric values were compared using Student’s t test. Correlations were tested with Spearman’s test. The P values given were two sided, P < 0.05 was considered as the limit of significance.
RESULTS
Case reports and patients treated in Vestfold Hospital (Group I )
The search strategy (Figure 1) provided 356 articles from the Medline database, and 1700 documents were retrieved through the Google search engine. After manual removal of duplicate references, 104 articles were retrieved. Thirty of these did not contain pertinent patient data and were, therefore, excluded. The end result was 74 articles with 103 case reports containing detailed and pertinent individual patient data mostly retrieved through Medline[3-59], while 22.9% of these came from Google alone[60-76]. The individual patient data collected from the three patients treated for perforated midgut diverticulitis at Vestfold Hospital were added to this group, making a total of 106 patients (48 men and 58 women). The mean age of the group was 72.2 ± 13.1 years, and the age distribution was not normal (P = 0.001). The mean age for men was 73.7 ± 12.2 years and the mean age for women was 71.1 ± 13.8 years, with non-normal distribution for both sexes, P = 0.005 and P = 0.001, respectively. Sex distribution according to age was not significantly different in this group (P = 0.296). Patient age or sex did not have an impact on outcome (P = 0.057 and P = 0.771, respectively).
Figure 1.
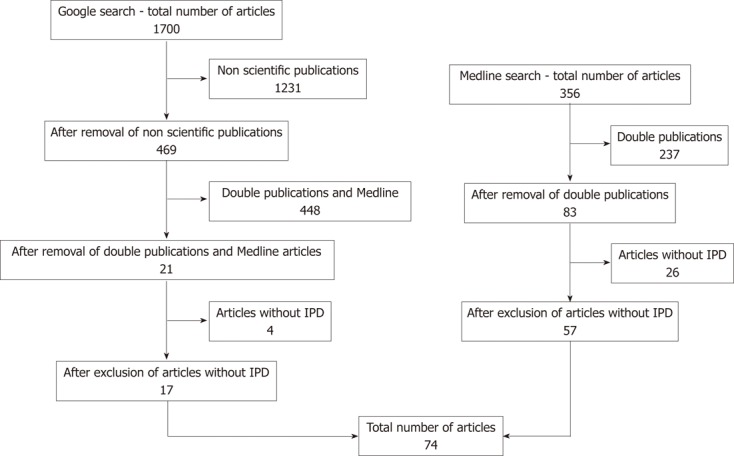
Schematic of the search strategy. IPD: individual patient data.
Preoperative assessment was described as plain radiography in 49 patients (53.3%) or CT in 70 (76.1%) in a total of 92 (86.8%) patients. Case reports did not include preoperative assessment in 14 (13.2%) patients. Where CT was performed, the correct preoperative diagnosis was made in 54 (77.1%) patients, while the probability of detecting perforated midgut diverticulitis without CT was 5.6% (two patients), both patients were previously known to have midgut diverticulosis. The difference in diagnosis with/without CT was statistically significant (P = 0.001).
Data on the duration of symptoms prior to hospitalization were present for 88 (83.0%) patients. The mean duration of symptoms prior to hospitalization was 3.6 d (range: 1-35 d). A longer duration of symptoms did not result in a poor outcome (P = 0.748). Data on fever were listed in 49 (46.2%) patients. Patients most often presented with moderate fever 23 (46.9%), while no fever or high fever was present in 26.5% each. Leukocyte and CRP levels were 13.8 ± 3.6 × 109/L and 155.4 ± 100.6 mg/L, respectively. Ninety-two (86.8%) patients underwent surgery, and 14 (13.2%) received conservative treatment. The surgical procedure most often performed was small bowel resection (83, 90.1%), followed by suture closure (5, 5.5%). Two patients (2.2%) underwent complex procedures that included multiple resections and 2 (2.2%) underwent surgical exploration with drainage. Access was through open surgery in 85 (92.4%) patients, while laparoscopic access was chosen in 5 (5.5%) and converted in 3 (60%). Two procedures (2.2%) were completed through laparoscopy. Data on postoperative complications were accessible in 73 patients. Complications occurred in 19.2% (Table 1). Fifteen (16.3%) patients underwent reoperation.
Table 1.
Overview of complications in Group I (case reports)
| Complications | Frequency | Present |
| General1 | 5 | 4.7 |
| Surgical2 | 8 | 7.5 |
| Both | 3 | 2.9 |
| None | 59 | 55.7 |
| Not available | 31 | 29.2 |
| Total | 106 | 100 |
Acute myocardial infarction, pulmonary embolism, pneumonia, deep vein thrombosis, urinary tract infection and stroke;
wound infection/dehiscence, anastomotic leakage, small bowel obstruction, intra-abdominal abscess and incisional hernia.
The operative report contained data on the distance of the perforated diverticulum to the ligament of Treitz in 29 patients, setting the mean distance to 41.7 ± 28.1 cm (range: 4-110 cm). Data on peritonitis was missing in five (4.7%) patients. There was no peritonitis or abscess in 30 (29.7%) patients, local peritonitis was present in 48 (47.5%), and 23 (22.8%) had diffuse peritonitis. Two (2.0%) patients had a mesenteric abscess. A reasonable correlation between the grade of peritonitis and reoperation rate was noted (r = 0.43, Spearman’s test).
Seventeen (16.8%) patients had a solitary midgut diverticulum. Multiple jejunal diverticula were noted in 84 (83.2%) patients, while no data were provided for 5 (4.7%). Data on location and number of diverticula throughout the digestive tube were listed for 46 (43.4%) patients. Concomitant ileal diverticulosis was found in 18 (39.1%), while 31 (67.4%) had colon diverticulosis. In all, 21 (45.6%) patients had diverticulosis throughout the intestinal tract.
Mean hospital stay was 10.7 ± 7.9 d. Conservatively treated patients did not have a longer hospital stay than operated patients (10.6 ± 8.3 d vs 10.7 ± 7.9 d, respectively). Reoperated patients had a longer hospital stay 13.6 ± 8.4 d. There was a reasonable correlation between patient age and length of hospital stay (r = 0.46). Overall mortality was 5.7%. There was no statistically significant difference in outcomes for operated or nonoperated patients (P = 0.814).
Norwegian patient registry (Group II)
The analysis of the Norwegian patient registry provided data on 113 patients, 57 men and 56 women. Patient mean age was 67.6 ± 16.4 years (range: 21-96 years). The age distribution within this group was not normal (P = 0.04). Mean age for men was 61.3 ± 16.2 years, while that for women was 74.7 ± 12.5 years, with non-normal distribution for both sexes, P = 0.027 and P = 0.013, respectively. The difference in age between the sexes was highly significant (P = 0.001) (Figure 2). The mean frequency of surgical procedures per year was 11.2 ± 0.9. The procedures most often performed were small bowel resection in 93 (82.3%) patients and enterorrhaphy in 17 (15%). Equivalent procedures performed through laparoscopic access were performed in 2 (1.8 %) and 1 (0.9 %) patients, respectively.
Figure 2.
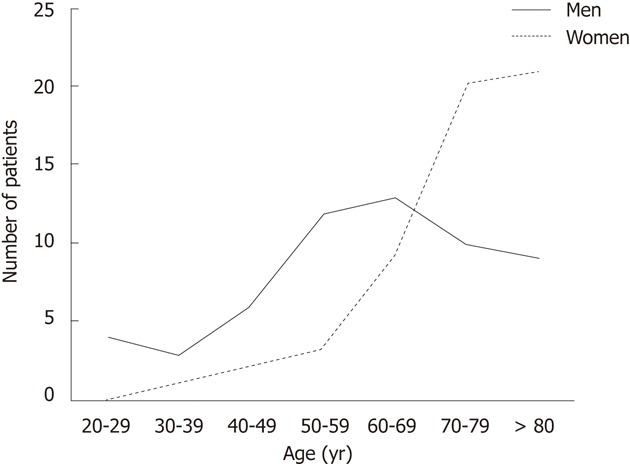
Correlation between sex and age for patients in the Norwegian patient registry.
Historical controls (Group III)
After the exclusion of patients under the age of 18 years, the group consisted of 47 patients (21 men and 26 women). Patient mean age was 65.4 ± 14.4 years. The age distribution within this group was normal (P = 0.18). Mean age for men was 61.5 ± 17.3 years, while that for women was 65.3 ± 14.4 years, with normal distribution for both sexes, P = 0.29 and P = 0.76, respectively.
Duration of symptoms prior to hospitalization was 6.9 d (range: 1-180 d). No patients had a preoperative diagnosis. Only one (2.1%) patient was treated conservatively, while the remaining 46 (97.9%) underwent surgery. Peroperative data were available for 24 (51.1%) patients, and cited as perforation in all, without grade of peritonitis. Twenty-three (48.9%) patients had data on number of diverticula, 18 (78.3%) had multiple and 5 (21.7%) had solitary diverticula. Small bowel resection was performed in 31 (67.4%) patients and suture closure in 15 (32.6%). Mortality in this group was 23.4%.
Comparison between patient groups
When length of history was concerned there was no difference between Groups I and III (P = 0.241). Furthermore, there was no impact of length of history on survival between Groups I and III (P = 0.198). No significant difference was detected in outcomes of conservative and surgical treatment (P = 0.23). However, there was a significant difference noted for type of surgical procedure, in favor of resection in Group I compared to Group III (P = 0.01), as well as the number of diverticula (P = 0.001). Mortality was significantly higher in Group III compared to Group I (P = 0.002).
DISCUSSION
One of the objective difficulties that a researcher encounters when investigating rare conditions is the absence of evidence and data. Data from the Norwegian patient registry show that a trial on a national level would prove to be difficult, including at most 10 patients per year. In these circumstances, one chooses the next best solution, reanalysis of small case series or case reports that contain sufficient individual patient data. A tendency to not publish case reports or small case series in indexed journals is noted, making this task even more demanding, hence, the contribution of the Google search engine in locating almost 20% of these case reports. We are well aware of the shortcomings of such a study; however, the use of abductive, probabilistic reasoning[77] allows for some valuable conclusions to be drawn.
The main finding of the present study is a transition in treatment, diagnosis and outcomes of patients suffering from perforated midgut diverticulitis. There seems to be a shift towards conservative treatment when properly diagnosed, a much higher percentage of accurate diagnosis, and small bowel resection is usually performed with lower mortality rates. This implies that elderly patients suffering from acute abdominal symptoms with elevated infection parameters should undergo a CT scan. Hence, the surgeon should have a high level of suspicion that the patient is suffering from perforated midgut diverticulitis after the initial CT scan is performed. Moreover, it seems that a substantial number of patients that could have been treated conservatively underwent surgery, because the majority of operated patients suffered from contained perforation. Surgical treatment should be avoided not only because of the burden of age and comorbidity in this patient group, but rather to avoid the risk of extensive resection in patients with multiple diverticula[1,2,78]. The fact that laparoscopy was so seldom used is even more surprising, because laparoscopic exploration and evaluation do not represent advanced minimally invasive surgical procedures.
The present study provides an opportunity to recommend the intensive use of laparoscopic exploration and evaluation in the emergency surgical setting. Nowadays, it seems that laparoscopic exploration provides at least as useful evaluation of abdominal pathology as open surgery does. Apart from the obvious benefits, laparoscopic exploration allows us to abandon the abdomen after lavage and drainage, and continue a conservative course[79]. A heavy proximal (within 1 m from the Treitz ligament) midgut mesentery with fibrin deposits is typical for the condition, and if generalized peritonitis is not present, our results seem to show that a conservative approach is feasible. The rationale is the fact that length of history prior to surgery does not seem to affect treatment outcome; there was no increase in mortality or morbidity between the groups. Even length of hospital stay was equal between patients operated upon and those conservatively treated. On the other hand, generalized peritonitis had a significant correlation with length of hospitalization and mortality, and should be treated surgically. The affected loop can be exteriorized through a mini-laparotomy after laparoscopic mobilization, and limited resection performed. Suture closure does not seem to have a place in the treatment of perforated midgut diverticulitis.
Such an approach would minimize the frequency of midgut resections in this high-risk population, while allowing the correct choice of patients for conservative treatment. A similar approach has recently had an impact on the treatment of sigmoid diverticulitis. Laparoscopic lavage and drainage have been advocated for generalized peritonitis due to perforated sigmoid diverticulitis. In this study, only 8% of patients underwent conversion and surgical resection, while the vast majority were handled conservatively after lavage and drainage[79].
It seems that correct diagnosis, together with the recognition of the immunological and physiological advantages of minimally invasive surgery, modern antibiotics and critical care could contribute to even better outcomes.
In summary, technological developments have led to better diagnosis of patients with perforated midgut diverticulitis. However, these have as yet not been applied in the operating room, where open surgery is still dominant over laparoscopy. When properly diagnosed, a transition in treatment is noticed towards conservative treatment. Only generalized peritonitis seems to have a significant correlation with length of hospitalization and mortality, and should be treated surgically. In these patients, laparoscopy and small bowel resection should be performed.
COMMENTS
Background
Perforated midgut diverticulitis is an uncommon, acquired condition that is only sporadically observed and treated in surgical departments. Most of the literature consists of case reports, and this could be the reason for the confusion that still exists when clinical manifestations, diagnosis and treatment options are concerned. In addition, developments in surgery, radiology and other branches of medicine have occurred since the last review that has changed both surgical and conservative treatment.
Research frontiers
As demonstrated in the results this is a condition occurring in the elderly, and therefore has its challenges in choice of conservative treatment over surgical in the individual patient. Minimally invasive surgery would seem to have a place in this setting as well as the development of innovative surgical techniques to tackle this specific setting.
Innovations and breakthroughs
A transition to conservative treatment due to a correct preoperative diagnosis is apparent. Laparotomy continues to be dominant and the use of minimally access surgery is negligible. Small bowel resection is most often performed; suture closure is abandoned.
Applications
All elderly patients presenting with fever, infection and abdominal pain should have a computed tomography (CT) scan at admission. Surgery should be considered only in cases with generalized peritonitis. Conservative treatment should have a main role in treatment. Innovative minimally invasive procedures are required.
Terminology
Midgut is a segment of bowel that extends from the apex of the duodenal loop to the last third of the transverse colon. The midgut is supplied with blood by the superior mesenteric artery and innervated by the vagus nerve.
Peer review
The paper described a transition in diagnosis, treatment (a shift toward conservative treatment when properly diagnosed with CT), and outcomes (lower mortality rates) of patients suffering from perforated midgut diverticulitis. This topic will be of interest to WJG readers.
Footnotes
Peer reviewer: Naoki Ishii, MD, Department of Gastroenterology, St. Luke’s International Hospital, 9-1 Akashi-cho, Chuo-ku, Tokyo 104-8560, Japan
S- Editor Cheng JX L- Editor Kerr C E- Editor Xiong L
.
References
- 1.Tsiotos GG, Farnell MB, Ilstrup DM. Nonmeckelian jejunal or ileal diverticulosis: an analysis of 112 cases. Surgery. 1994;116:726–731; discussion 731-732. [PubMed] [Google Scholar]
- 2.Chendrasekhar A, Timberlake GA. Perforated jejunal diverticula: an analysis of reported cases. Am Surg. 1995;61:984–988. [PubMed] [Google Scholar]
- 3.Rosing MA, Amory S. Perforated ileal diverticulitis. An atypical presentation with definitive diagnosis by laparoscopy. Surg Endosc. 1995;9:522–524. doi: 10.1007/BF00206842. [DOI] [PubMed] [Google Scholar]
- 4.Koger KE, Shatney CH, Dirbas FM, McClenathan JH. Perforated jejunal diverticula. Am Surg. 1996;62:26–29. [PubMed] [Google Scholar]
- 5.Alvarez OA, Mejia A, Ostrower VS, Lee M. Jejunal diverticulitis manifesting with abdominal wall abscess. Am J Gastroenterol. 1995;90:2060–2062. [PubMed] [Google Scholar]
- 6.Costa G, Mancini R, Di Castro A, Capaldi M, Sciacca P, Ialongo P. [Perforated jejunal diverticulum: a rare cause of acute abdomen] Chir Ital. 2005;57:521–525. [PubMed] [Google Scholar]
- 7.Benini B, Scocchera F, Giorgiano F, Manfroni S, Cataldi C, Antonellis D. [Perforated jejunal diverticulitis: A case report] Chir Ital. 2008;60:745–748. [PubMed] [Google Scholar]
- 8.Albu E, Parikh V, Alankar S, Gerst PH. Perforated solitary jejunal diverticulum. South Med J. 1995;88:575–576. doi: 10.1097/00007611-199505000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Kleemann M, Kujath P, Stellmacher F, Gellissen J, Bruch HP, Eckmann Ch. [Perforated jejunal divertikula - a rare differential diagnosis of acute abdominal pain] Zentralbl Chir. 2006;131:521–524. doi: 10.1055/s-2006-955454. [DOI] [PubMed] [Google Scholar]
- 10.De Peuter B, Box I, Vanheste R, Dymarkowski S. Small-bowel diverticulosis: imaging findings and review of three cases. Gastroenterol Res Pract. 2009;2009:549853. doi: 10.1155/2009/549853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veen M, Hornstra BJ, Clemens CH, Stigter H, Vree R. Small bowel diverticulitis as a cause of acute abdomen. Eur J Gastroenterol Hepatol. 2009;21:123–125. doi: 10.1097/MEG.0b013e328303bfdb. [DOI] [PubMed] [Google Scholar]
- 12.Quiles AM, Rodríguez-Hermosa JI, Ortiz MR, Febrer M. [Perforated jejunal diverticulitis] Gastroenterol Hepatol. 2006;29:432–433. doi: 10.1157/13091458. [DOI] [PubMed] [Google Scholar]
- 13.Gotian A, Katz S. Jejunal diverticulitis with localized perforation and intramesenteric abscess. Am J Gastroenterol. 1998;93:1173–1175. doi: 10.1111/j.1572-0241.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirbaş I, Yildirim E, Harman A, Başaran O. Perforated ileal diverticulitis: CT findings. Diagn Interv Radiol. 2007;13:188–189. [PubMed] [Google Scholar]
- 15.Sakurai Y, Tonomura S, Yoshida I, Masui T, Shoji M, Nakamura Y, Matsubara T, Uyama I, Komori Y, Ochiai M. Abdominal wall abscess associated with perforated jejunal diverticulitis: report of a case. Surg Today. 2005;35:682–686. doi: 10.1007/s00595-004-2972-5. [DOI] [PubMed] [Google Scholar]
- 16.Yağmur Y, Aldemir M, Büyükbayram H, Taçyildiz I. Multiple jejunal diverticulitis with perforation in a patient with systemic lupus erythematosus: report of a case. Surg Today. 2004;34:163–166. doi: 10.1007/s00595-003-2653-9. [DOI] [PubMed] [Google Scholar]
- 17.Eriguchi N, Aoyagi S, Nakayama T, Emi Y, Saku M, Yoshida K. Ileo-abdominal wall fistula caused by diverticulum of the ileum. J Gastroenterol. 1998;33:272–275. doi: 10.1007/s005350050082. [DOI] [PubMed] [Google Scholar]
- 18.Klee FE, Osswald BR, Wysocki S. Severe abdominal pain and thrombocytopenia--typical symptoms of occult jejunal diverticulum perforation? J Gastroenterol. 1997;32:246–250. doi: 10.1007/BF02936376. [DOI] [PubMed] [Google Scholar]
- 19.Habib NA, Enslin RC. Perforated jejunal diverticula. Br J Clin Pract. 1984;38:431, 433. [PubMed] [Google Scholar]
- 20.Kubota T. Perforated jejunal diverticulitis. Am J Surg. 2007;193:486–487. doi: 10.1016/j.amjsurg.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 21.Fass G, Colonval P. Perforation and abscess formation of a solitary jejunal diverticulum. Acta Chir Belg. 2007;107:222–224. [PubMed] [Google Scholar]
- 22.Tankova L, Berberova M, Purvanov P, Tsankov Ts, Gegova A. Complicated small bowel diverticulosis--a case report and literature review. Chirurgia (Bucur) 2007;102:603–606. [PubMed] [Google Scholar]
- 23.Abboud B, Aouad R, Jaoude JB, Ghorra C. [A rare cause of acute abdomen: jejunal diverticulitis] Presse Med. 2008;37:416–419. doi: 10.1016/j.lpm.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 24.Iannelli A, Piche T, Novellas S, Gugenheim J. Small bowel diverticulitis of the Roux loop after gastric bypass. Obes Surg. 2006;16:1249–1251. doi: 10.1381/096089206778392248. [DOI] [PubMed] [Google Scholar]
- 25.Schock J, Mainster H. Perforation of acquired small bowel diverticulum. J Am Osteopath Assoc. 1999;99:113–115. doi: 10.7556/jaoa.1999.99.2.113. [DOI] [PubMed] [Google Scholar]
- 26.Buis CI, Hofker HS, Nieuwenhuijs VB. Diverticulitis of the jejunum, an uncommon diagnosis. Dig Surg. 2008;25:83–84. doi: 10.1159/000121445. [DOI] [PubMed] [Google Scholar]
- 27.Kassahun WT, Fangmann J, Harms J, Bartels M, Hauss J. Complicated small-bowel diverticulosis: a case report and review of the literature. World J Gastroenterol. 2007;13:2240–2242. doi: 10.3748/wjg.v13.i15.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sibille A, Willocx R. Jejunal diverticulitis. Am J Gastroenterol. 1992;87:655–658. [PubMed] [Google Scholar]
- 29.Cross MJ, Snyder SK. Laparoscopic-directed small bowel resection for jejunal diverticulitis with perforation. J Laparoendosc Surg. 1993;3:47–49. doi: 10.1089/lps.1993.3.47. [DOI] [PubMed] [Google Scholar]
- 30.Morgenstern L. Acute diverticulitis of the jejunum; report of two cases. Calif Med. 1954;80:403–404. [PMC free article] [PubMed] [Google Scholar]
- 31.Donald JW. Major complications of small bowel diverticula. Ann Surg. 1979;190:183–188. doi: 10.1097/00000658-197908000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenstein S, Jones B, Fishman EK, Cameron JL, Siegelman SS. Small-bowel diverticulitis: CT findings. AJR Am J Roentgenol. 1986;147:271–274. doi: 10.2214/ajr.147.2.271. [DOI] [PubMed] [Google Scholar]
- 33.Novak JS, Tobias J, Barkin JS. Nonsurgical management of acute jejunal diverticulitis: a review. Am J Gastroenterol. 1997;92:1929–1931. [PubMed] [Google Scholar]
- 34.McCourtney JS, Karim S, Rahilly M, Dalling R. Fatal complication of coincidental operative finding. Postgrad Med J. 1999;75:625–627. doi: 10.1136/pgmj.75.888.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang M, Agha S, Lee R, Culpepper-Morgan J, D’Souza A. Perforation of jejunal diverticulum: case report and review of literature. Conn Med. 2000;64:7–10. [PubMed] [Google Scholar]
- 36.Zager JS, Garbus JE, Shaw JP, Cohen MG, Garber SM. Jejunal diverticulosis: a rare entity with multiple presentations, a series of cases. Dig Surg. 2000;17:643–645. doi: 10.1159/000051978. [DOI] [PubMed] [Google Scholar]
- 37.Matteoni R, Lolli E, Barbieri A, D’Ambrosi M. Perforated jejunal diverticulitis: personal experience and diagnostic with therapeutical considerations. Ann Ital Chir. 2000;71:95–98. [PubMed] [Google Scholar]
- 38.Franzen D, Gürtler T, Metzger U. [Multiple recurrent perforated jejunal diverticulitis] Chirurg. 2002;73:1218–1220. doi: 10.1007/s00104-002-0538-x. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham SC, Gannon CJ, Napolitano LM. Small-bowel diverticulosis. Am J Surg. 2005;190:37–38. doi: 10.1016/j.amjsurg.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Lempinen M, Salmela K, Kemppainen E. Jejunal diverticulosis: a potentially dangerous entity. Scand J Gastroenterol. 2004;39:905–909. doi: 10.1080/00365520410006288. [DOI] [PubMed] [Google Scholar]
- 41.García LJ, Otero J, Santamaría L, Pérez A. [Jejunal diverticulitis. A rare cause of acute abdomen] Cir Esp. 2005;77:357–358. doi: 10.1016/s0009-739x(05)70870-8. [DOI] [PubMed] [Google Scholar]
- 42.Al-Samarrai AY. Perforation of jejunal diverticulum. Saudi J Gastroenterol. 2002;8:62–63. [PubMed] [Google Scholar]
- 43.Aksoy F, Demirel G, Bilgiç T, Güngör IG, Ozçelik A. A previously diagnosed mitochondrial neurogastrointestinal encephalomyopathy patient presenting with perforated ileal diverticulitis. Turk J Gastroenterol. 2005;16:228–231. [PubMed] [Google Scholar]
- 44.Ahlman H, Björck S, Jonsson O, Gamklou R. Perforated jejunal diverticula. Report on two cases. Acta Chir Scand. 1980;146:79–80. [PubMed] [Google Scholar]
- 45.Priestley J, Patiniotis T. Gram-negative sepsis as a presentation of jejunal diverticular disease. ANZ J Surg. 2004;74:701. doi: 10.1111/j.1445-1433.2004.03125.x. [DOI] [PubMed] [Google Scholar]
- 46.De Raet J, Brugman T, Geukens A. Non-Meckel’s ileal diverticulitis with perforation: a rare cause of acute right lower quadrant pain. Acta Chir Belg. 2010;110:90–92. doi: 10.1080/00015458.2010.11680574. [DOI] [PubMed] [Google Scholar]
- 47.Slaninka I, Páral J, Chobola M, Motycka V, Ferko A, Bláha V. [Peritonitides caused by gastrointestinal perforations--analysis of an elderly patient group] Rozhl Chir. 2009;88:656–661. [PubMed] [Google Scholar]
- 48.Basile G, Buffone A, Boscarelli G, Maria S, Santagati G, Corsaro A, Cirino E. [Perforated jejunal diverticulum. A case report] Ann Ital Chir. 2008;79:53–56. [PubMed] [Google Scholar]
- 49.Papaziogas B, Koutelidakis J, Dragoumis D, Atmatzidis S, Giakoustidis A, Atmatzidis K. Perforated jejunal diverticulum presenting as acute abdomen. Chirurgia (Bucur) 2010;105:119–121. [PubMed] [Google Scholar]
- 50.Graña L, Pedraja I, Mendez R, Rodríguez R. Jejuno-ileal diverticulitis with localized perforation: CT and US findings. Eur J Radiol. 2009;71:318–323. doi: 10.1016/j.ejrad.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 51.Prost A La Denise J, Douard R, Berger A, Cugnenc PH. Small bowel diverticulosis complicated by perforated jejunal diverticula: conservative and/or surgical management? Hepatogastroenterology. 2008;55:1657–1659. [PubMed] [Google Scholar]
- 52.Jarral OA, Purkayastha S, Darzi A, Zacharakis E. Education and Imaging. Gastrointestinal: Enterolith-induced perforation on a background of jejunal diverticulum. J Gastroenterol Hepatol. 2010;25:429. doi: 10.1111/j.1440-1746.2010.06261.x. [DOI] [PubMed] [Google Scholar]
- 53.França M, Certo M, Silva D, Peixoto C, Varzim P. Elderly patient with acute, left lower abdominal pain: perforated jejunal diverticulitis (2010: 7b) Eur Radiol. 2010;20:2541–2545. doi: 10.1007/s00330-010-1726-6. [DOI] [PubMed] [Google Scholar]
- 54.Lacz NL, Zurlo JV. Small bowel diverticulitis: an often overlooked cause of acute abdomen. Emerg Radiol. 2010;17:497–501. doi: 10.1007/s10140-010-0896-5. [DOI] [PubMed] [Google Scholar]
- 55.Colvin HS, Kuenfoo C, Rajab TK, Sayadatas T. Non-surgical management of recurrent perforation of a jejunal diverticulum following previous segmental bowel resection: a case report. J Med Case Rep. 2009;3:7318. doi: 10.4076/1752-1947-3-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reeve K, Hotouras A, Manghat M, Pillai S. Catheter balloon mimicking incarcerated femoral hernia and co-existing small bowel diverticular perforation: a case report. Cases J. 2009;2:8755. doi: 10.4076/1757-1626-2-8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel VA, Jefferis H, Spiegelberg B, Iqbal Q, Prabhudesai A, Harris S. Jejunal diverticulosis is not always a silent spectator: a report of 4 cases and review of the literature. World J Gastroenterol. 2008;14:5916–5919. doi: 10.3748/wjg.14.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staszewicz W, Christodoulou M, Proietti S, Demartines N. Acute ulcerative jejunal diverticulitis: case report of an uncommon entity. World J Gastroenterol. 2008;14:6265–6267. doi: 10.3748/wjg.14.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butler JS, Collins CG, McEntee GP. Perforated jejunal diverticula: a case report. J Med Case Rep. 2010;4:172. doi: 10.1186/1752-1947-4-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juchems MS, Brams HJ, Pauls S. Small bowel diverticulitis. European J of Rad Extra. 2006;59:119–121. [Google Scholar]
- 61.Kim KH, Kim JH, Her MY, Lee SH, Paik BL, Jee SR, Park ET, Seol SY, Chung JM. A case of ileal diverticulitis causing small bowel stenosis. Korean J Gastrointest Endosc. 2004;28:193–196. [Google Scholar]
- 62.Choi JW, Kim KH, Lee JE, Kim JH, Jang BI. A case of jejunal diverticulitis with perforation combined with intussusception caused by infalammatory fibroid polyp. Yeungnam Univ J Med. 2005;22:113–118. [Google Scholar]
- 63.Perea García J, Turégano Fuentes F, Pérez Díaz MD, Sanz Sánchez M. Diverticulitis De Yeyuno Perforada. Gastroenterol Hepatol. 2002;25:526–527. [PubMed] [Google Scholar]
- 64.Koyuncu A, Turan M, Sozeri S, Kivanc F, Gokgoz S. Perforated jejunal diverticulum: A case report. Çukurova Üniversitesi Tıp Fakültesi Dergisi. 2002;25:35–37. [Google Scholar]
- 65.Goshtasby P, Tiruchelvam V, Nicholson T. Perforated Jejunal Diverticulitis. Surgical Rounds. 2006;29:361–364. [Google Scholar]
- 66.Cegla J, Chudasama P, Agarwal T, Chaudhary S. A perforated Jejunal Diverticulum. Grand Rounds. 2007;7:5–8. [Google Scholar]
- 67.Das DN, Walia J, Gone SR, Scobie DJ. Surgical options in proximal jejunal diverticulum perforation and review of literature. SMJ. 2007;52:53. [Google Scholar]
- 68.Woods AQ, Ly JQ, Binstock A, Beall DP. Radiological case: Jejunal diverticulitis. Appl Radiol. 2008;37:7. [Google Scholar]
- 69.Stout CL, Moore AB, Woodyard T. Emergency medicine quiz: Can you diagnose the cause of this patientt’s abdominal pain? RSP. 2008 Available from: http://www.hcplive.com/publications/Resident-and-Staff/2008/2008-WW/2008-06_W1. [Google Scholar]
- 70.Pelaez MC, Rodriguez FR, Tato G, Quintela A. Perforated Jejunal Diverticulitis. Publicado en Cir Esp. 2001;69:627–628. [Google Scholar]
- 71.Lee BG, Park SB, Byun CG, Koh YT, Suh DY, Park DS, Kang MJ, Lee KJ. Localized peritonitis due to perforation of multiple jejunal diverticula. J Korean Surg Soc. 2004;67:75–78. [Google Scholar]
- 72.Siyamek NM, Terlizzi J, Mills C, Steele J. Jejunal diverticulitis masked by subhepatic collection. Internet J Surg. 2009;19:9. [Google Scholar]
- 73.Ozdemir A, Ulas M, Karaman K, Teke Z, Ozer I, Ercan M, Ozogul YB, Seven , MC Acute abdomen caused by a jejuna diverticulum perforation: A rare clinical entity. Anatol J Clin Investig. 2010:4: 168–170. [Google Scholar]
- 74.Eisold S, Guenther KH, Reith HB. Diverticulitis of the small bowel. Pol Przegl Chir. 2007;79:385–388. [Google Scholar]
- 75.Mortimer A, Harding J, Roach H, Callaway M, Virjee J. Jejunal diverticulitis: an unusual cause of an intra-abdominal abscess - coronal Computed Tomography reconstruction can aid the diagnosis. J Radiol Case Rep. 2008;2:15–18. doi: 10.3941/jrcr.v2i5.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tilakaratne S, Coomaraswamy W, Ratnapala SD. A rare case of jejunal diverticulitis. Galle Medical Journal. 2009;14:79–80. [Google Scholar]
- 77.Haig BD. Scientific method, abduction, and clinical reasoning. J Clin Psychol. 2008;64:1013–1018. doi: 10.1002/jclp.20505. [DOI] [PubMed] [Google Scholar]
- 78.Vallicelli C, Coccolini F, Catena F, Ansaloni L, Montori G, Di Saverio S, Pinna AD. Small bowel emergency surgery: literature’s review. World J Emerg Surg. 2011;6:1. doi: 10.1186/1749-7922-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Myers E, Hurley M, O’Sullivan GC, Kavanagh D, Wilson I, Winter DC. Laparoscopic peritoneal lavage for generalized peritonitis due to perforated diverticulitis. Br J Surg. 2008;95:97–101. doi: 10.1002/bjs.6024. [DOI] [PubMed] [Google Scholar]


