Abstract
AIM: To investigate whether expressing biliary phenotype predicted poor outcome after the surgical treatment in primary liver cancers.
METHODS: Out of 204 patients that underwent liver resection due to hepatocellular carcinoma (HCC), liver specimens of 70 patients with HCC were evaluated for biliary components by cytokeratin (CK) 19 immunostain (CK19- HCC and CK19+ HCC). CK19 positivity was defined as membranous and/or cytoplasmic expression in ≥ 5% of tumor cells with moderate or strong intensity. Patients with other primary liver cancers, such as combined HCC and cholangiocarcinoma (cHCC-CC), intrahepatic cholangiocarcinoma (ICC) who received curative liver resection, were also included in the study. Clinical characteristics of CK19- HCC and CK19+ HCC patients, including survival outcome after curative liver resection, were compared with that of cHCC-CC and ICC patients.
RESULTS: The overall survival (OS) rate of CK19- HCC (n = 49) after the curative surgical treatment was 90.7%, and 80.4% at 1 and 5 years after the resection. OS rate of CK19+ HCC (n = 21) was 74.3%, 28.9% and OS rate of cHCC-CC (n = 22) was 66.7%, 32.2% at 1 and 5 years after the surgery. For ICC (n = 19), 1 and 5-year-OS rate was 50.2% and 14.3% after the curative resection. The OS rates of CK19+ HCC and cHCC-CC were significantly lower than that of CK19- HCC, but higher than the OS rate of ICC (P = 0.000). There was no statistically significant difference in OS rate between CK19+ HCC and cHCC-CC. The disease free survival (DFS) rate of CK19- HCC was 72.0% and 54.5% at 1 and 3 years after the surgical treatment. DFS rate of CK19+ HCC was 53.3%, 34.3% and DFS rate of cHCC-CC was 51.5%, 39.2% at 1 and 3 years after the resection. For ICC, 1 and 3-year-DFS rate was 28.0% and 14.0% after the curative resection. DFS rate of CK19- HCC was significantly higher than that of ICC (P = 0.017), but marginally higher than DFS rate of either CK19+ HCC or cHCC-CC (P = 0.097, P = 0.089, respectively). Predictors of outcome after the surgery of primary liver cancer were pathology of the resected mass, existence of microvascular invasion and accompanying satellite nodule.
CONCLUSION: Primary liver cancers with biliary components tended to show poorer surgical outcome. This suggested that immuno-phenotype of liver cancers was as important as their morphological classification.
Keywords: Cytokeratin 19, Hepatocellular carcinoma, Intrahepatic cholangiocarcinoma, Liver cancers, Hepatectomy
INTRODUCTION
Proposed treatment strategy for solid tumor is usually guided by tumor stage. In case of hepatocellular carcinoma (HCC), Barcelona Clinic Liver Cancer (BCLC) staging system, that has come to be widely accepted in clinical practice[1], carefully allocates HCC patients according to tumor stage and liver function as well as physical status. Despite the careful selection of candidates for each treatment option, HCC patients with similar BCLC grade that underwent the same mode of treatments may show very different outcome. It has been suggested that HCC heterogeneity in the molecular and morphological features may be related to the treatment outcome[2,3]. Recently, efforts have been made to classify HCC histopathologically, encompassing subpopulations of HCC according to their distinct molecular features[4].
While the World Health Organization (WHO) still classifies primary liver cancer into HCC, intrahepatic cholangiocarcinoma (ICC), and combined hepatocellular and cholangiocarcinoma (cHCC-CC)[5], there has been a report on HCCs whose cells of some proportion displayed cholangiolar differentiation and which showed highly aggressive behavior[6]. The authors claimed that this type of HCC with biliary differentiation must be different from cHCC-CC since it displayed typical HCC morphological features, and referred it as dual-phenotype HCC. On the other hands, according to the WHO classification, cHCC-CC is characterized by having unequivocal, intimately mixed elements of both HCC and ICC components within a single nodule[5], and it is also reported to show much worse prognosis than HCC[7-11].
Meanwhile, it has recently been proposed that HCC with biliary differentiation should be categorized as HCC with stem/progenitor cell immunophenotype whereas cHCC-CC should be included as mixed hepatobiliary carcinoma, classical type[4].
Since both HCC with biliary differentiation and cHCC-CC appeared to be the primary liver cancer displaying biliary phenotype, we hypothesized that the outcome of curative resection in primary liver cancer would be grave as the cancer expressed biliary phenotype rather than hepatic phenotype. In order to test this hypothesis, we compared the surgical outcome of classical HCC without biliary differentiation, with that of HCC with biliary differentiation, defined as having cytokeratin (CK) 19 positive cells, along with cHCC-CC and ICC.
MATERIALS AND METHODS
Case selection and histopathologic analysis
Liver specimens, surgically resected between January 2000 and August 2010 at Inha University Hospital (Incheon, South Korea) were reviewed and selected from pathologic files. Out of 204 patients who underwent liver resection due to HCC, 70 patients were assessed for CK19 positivity. These patients had well preserved liver function, evaluated by Child-Pugh scoring system. None of the selected cases experienced preoperative cancer treatment including transarterial chemoembolization (TACE). HCC was diagnosed under hematoxylin and eosin (HE) stain according to the morphologic criteria as defined by the WHO. Edmondson and Steiner Grading System (E-S, I-IV) was used to evaluate the degree of differentiation[5]. The primary antibody for CK19 (monoclonal, DAKO, Glostrup, Denmark, 1:50) was applied in order to detect CK19 positivity in HCC. CK19 positivity was defined as membranous and/or cytoplasmic expression in ≥ 5% of tumor cells with moderate or strong intensity.
Twenty-two cases of cHCC-CC were analyzed. Combined HCC-CC was diagnosed when there were evidences of both HCC and CC differentiation demonstrated within the same nodule[12]. Nineteen cases of ICC were included as the control.
Medical records of the selected patients were reviewed for etiology of underlying liver disease and laboratory data for liver function and tumor markers. The patients were regularly followed up. Liver function tests, acrylic fixed prosthesis measurements were done every 2-3 mo. Dynamic CT was regularly performed with the interval no longer than 6 mo. All patients were followed until the time of death or for at least 12 mo. Recurrence was defined as the appearance of a new lesion with the radiologic features of HCC. Early recurrence was defined as any recurrence within 12 mo after the initial hepatectomy.
Histopathologic analysis was also performed for tumor size, differentiation, presence of multiple tumors, microvascular and major vessel invasion, lymph node metastasis and existence of background liver cirrhosis.
Statistical analysis
Statistical analysis was performed using SPSS software version 12.0 (SPSS, Chicago, IL). Clinicopathologic characteristics among different liver cancers were compared using χ2 test. Overall survival (OS) was calculated using Kaplan-Meier method, and differences in survival rate were compared using Log-rank test. Univariate analysis for factors affecting prognosis of primary liver cancer after curative resection was evaluated using Log-rank test. All variables that appeared to be associated with the prognosis were put under multivariate analysis using Cox proportional hazards model. Statistical significance and marginal significance were assumed when P < 0.05 and P < 0.01, respectively.
RESULTS
Pathological evaluation
Diagnosis of HCC was made under HE stain in all 70 cases. Forty-nine HCC patients had CK19 immunostain negative (CK19- HCC), and identified as HCC without biliary differentiation (Figure 1). Another 21 cases out of 70 HCC cases showed CK19 positivity (CK19+ HCC) and classified as HCC with biliary differentiation (Figure 1).
Figure 1.
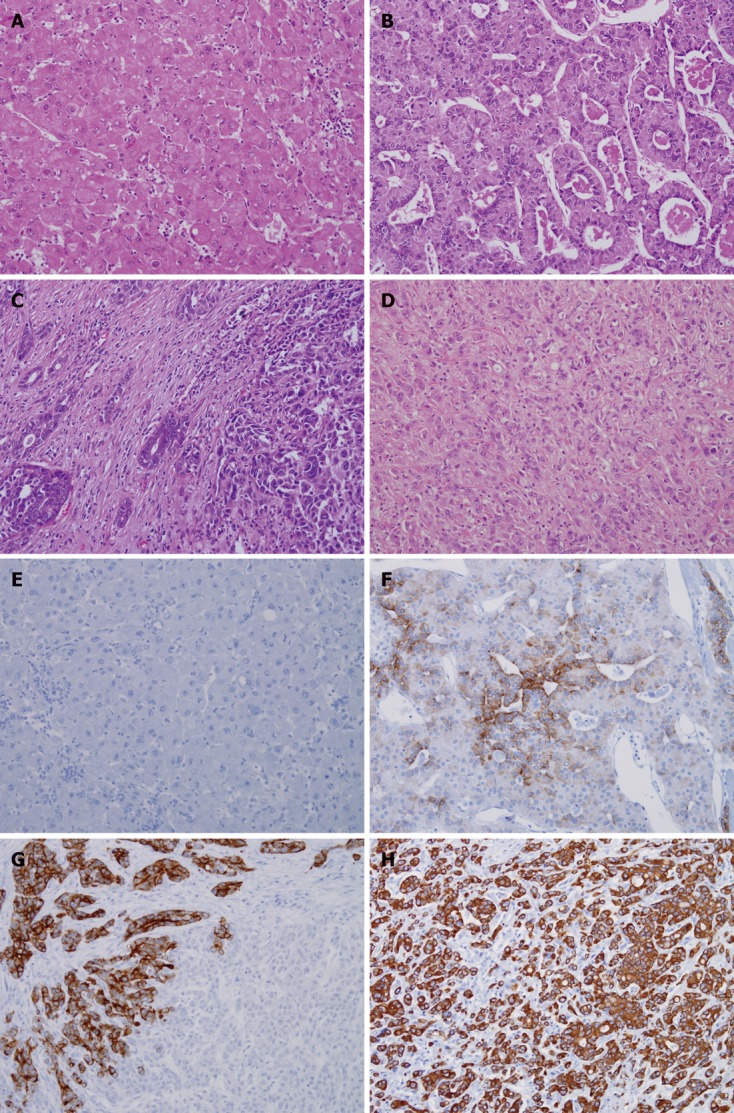
Pathological features of primary liver cancer. A-D: Hematoxylin and eosin (HE) stain for cytokeratin 19 negative hepatocellular carcinoma (CK19- HCC) (A), cytokeratin 19 positive hepatocellular carcinoma (CK19+ HCC) (B), combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) and intrahepatic cholangiocarcinoma (ICC); E-H: Immunohistochemical stain results of CK19 stain for CK19- HCC (E), CK19+ HCC (F), cHCC-CC (G) and ICC (H). All in original magnification × 200.
Clinical features
All the patients underwent curative liver resection of primary liver cancer. There were no statistically significant differences in clinical findings of CK19- HCC (n = 49), CK19+ HCC (n = 21), cHCC-CC (n = 22) and ICC (n = 21) except in gender composition, etiologies of liver disease, and proportion of patients having liver cirrhosis (Table 1). In cases of CK19- and CK19+ HCC, as well as cHCC-CC, male gender was the predominant population, whereas in ICC, there was no gender predisposition (Table 1). There was significantly lower number of patients with liver cirrhosis in the ICC group.
Table 1.
Clinical characteristics of patients and treatment modality of recurrent with primary liver cancer n (%)
| CK19- HCC (n = 49) | CK19+ HCC (n = 21) | cHCC-CC (n = 22) | ICC(n = 19) | P value | |
| Age (yr) | 0.43 | ||||
| < 45 | 11 (22.4) | 4 (19.0) | 4 (18.2) | 1 (5.3) | |
| ≥ 45 | 38 (77.6) | 17 (81.0) | 18 (81.8) | 18 (94.7) | |
| Gender | 0.014a | ||||
| Male | 41 (83.7) | 14 (66.7) | 18 (81.8) | 9 (47.4) | |
| Female | 8 (16.3) | 7 (33.3) | 4 (18.2) | 10 (52.6) | |
| Underlying liver disease | 0.004a | ||||
| None | 0 (0.0) | 0 (0.0) | 3 (13.6) | 12 (63.2) | |
| HBV | 38 (79.2) | 18 (85.7) | 17 (77.3) | 2 (10.5) | |
| HCV | 4 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Others | 6 (12.5) | 3 (14.3) | 2 (9.1) | 5 (26.3) | |
| Cirrhosis | 0.024a | ||||
| No | 22 (44.9) | 10 (47.6) | 10 (45.5) | 16 (84.2) | |
| Yes | 27 (55.1) | 11 (52.4) | 12 (54.5) | 3 (15.8) | |
| Tumor number | 0.313 | ||||
| Single | 41 (83.7) | 17 (81.0) | 16 (72.7) | 18 (94.7) | |
| Multiple | 8 (16.3) | 4 (19.0) | 6 (27.3) | 1 (5.3) | |
| Satellite nodule | 0.063 | ||||
| Absent | 43 (87.8) | 19 (90.5) | 15 (68.2) | 18 (94.7) | |
| Present | 6 (12.2) | 2 (9.5) | 7 (31.8) | 1 (5.3) | |
| Microvascular invasion | 0.836 | ||||
| Absent | 21 (42.9) | 10 (47.6) | 11 (55.0) | 9 (47.4) | |
| Present | 28 (57.1) | 11 (52.4) | 9 (45.0) | 10 (52.6) | |
| Macrovascular invasion | 0.119 | ||||
| Absent | 45 (91.8) | 17 (81.0) | 19 (95.0) | 14 (73.7) | |
| Present | 4 (8.2) | 4 (19.0) | 1 (5.0) | 5 (26.3) | |
| Lymph node metastasis | 0.086 | ||||
| Absent | 47 (95.9) | 20 (95.5) | 21 (95.5) | 15 (78.9) | |
| Present | 2 (4.1) | 1 (4.8) | 1 (4.5) | 4 (21.1) | |
| Distant metastasis | 0.521 | ||||
| Absent | 46 (93.9) | 21 (100) | 21 (95.5) | 17 (89.5) | |
| Present | 3 (6.1) | 0 (0.0) | 1 (4.5) | 2 (10.5) | |
| Differentiation | 0.301 | ||||
| E1, E2 or well, moderate | 15 (30.6) | 7 (33.3) | 9 (47.4) | 9 (52.9) | |
| E3, E4 or poorly | 34 (69.4) | 14 (66.7) | 10 (52.6) | 8 (47.1) | |
| Treatment modality | |||||
| Number of patience with recurrence | 19/49 (38.8) | 12/21 (57.1) | 15/22 (68.2) | 11/19 (57.9) | |
| Conservative treatment | 2/19 (10.5) | 1/12 (8.3) | 3/15 (20.0) | 8/11 (72.7) | |
| Transarterial chemoembolization | 15/19 (78.9) | 11/12 (91.7) | 12/15 (80.0) | 0/11 (0.0) | |
| Systemic chemotherapy | 0/19 (0.0) | 0/12 (0.0) | 0/15 (0.0) | 2/11 (3.5) | |
| Others | 2/19 (10.5) | 0/12 (0.0) | 0/15 (0.0) | 3/11 (5.3) |
P < 0.05 among 4 groups. CK19- HCC: Cytokeratin 19 negative hepatocellular carcinoma; CK19+ HCC: Cytokeratin 19 positive hepatocellular carcinoma; cHCC-CC: Combined hepatocellular carcinoma and cholangiocarcinoma; ICC: Intrahepatic cholangiocarcinoma; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
Comparison of clinical prognosis
The OS rate of CK19- HCC after the curative surgical treatment was 90.7% and 80.4% at 1 and 5 years after the resection, respectively. OS rate of CK19+ HCC was 74.3%, 28.9%, and OS rate of cHCC-CC was 66.7%, 32.2% at 1 and 5 years after the surgery. In cases of ICC, 1 and 5-year-OS rate was 50.2% and 14.3% respectively after the curative resection. The OS rates of CK19+ HCC and cHCC-CC were significantly lower than that of CK19- HCC, but higher than the OS rate of ICC (P = 0.000) (Figure 2A). There was no statistically significant difference in OS rate between CK19+ HCC and cHCC-CC (Figure 2A).
Figure 2.
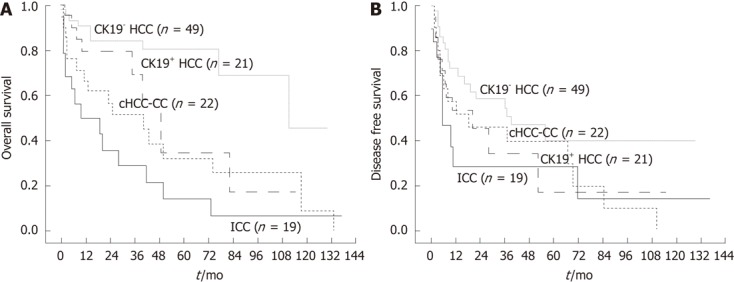
Clinical outcome after the curative liver resection of primary liver cancers. A: Overall survival (OS) rates of different groups of primary liver cancer after the curative resection. The OS rates of cytokeratin 19 positive hepatocellular carcinoma (CK19+ HCC) and combined hepatocellular carcinoma and cholangiocarcinoma (cHCC-CC) were significantly lower than that of cytokeratin 19 negative hepatocellular carcinoma (CK19- HCC), but higher than the OS rate of intrahepatic cholangiocarcinoma (ICC) (P < 0.05). There was no statistically significant difference in OS rate between CK19+ HCC and cHCC-CC (P = 0.245); B: Disease free survival (DFS) rate. The DFS rate of CK19- HCC was significantly higher than that of ICC (P = 0.017), but marginally higher than DFS rate of either CK19+ HCC or cHCC-CC (P = 0.097, 0.089, respectively).
The disease free survival (DFS) rate of CK19- HCC was 72.0% and 54.5% at 1 and 3 years after the surgical treatment. DFS rate of CK19+ HCC was 53.3%, 34.3%, and DFS rate of cHCC-CC was 51.5%, 39.2% at 1 and 3 years after the resection. In cases of ICC, 1 and 3-year-DFS rate was 28.0% and 14.0% respectively after the curative resection. The DFS rate of CK19- HCC was significantly higher than that of ICC (P = 0.017), but marginally higher than DFS rate of either CK19+ HCC or cHCC-CC (P = 0.097, P = 0.089, respectively) (Figure 2B). However there was no statistically significant difference in DFS rate among CK19+ HCC, cHCC-CC and ICC (Figure 2B). The percentage of patients that received treatment for the recurred cancer was much smaller in cases of ICC whereas the majority of HCC regardless of CK19 expression, and cHCC-CC patients underwent TACE for the recurred mass (Table 1).
Prognostic factors for primary liver cancer after the curative resection
In cases of resectable primary liver cancer in functionally well preserved liver, surgical resection is usually recommended regardless of its pathologic diagnosis. Univariate analysis of prognostic factors after the curative resection in primary liver cancer was performed. The tested factors included pathologic diagnosis of the liver mass, age at the operation, gender, existence of liver cirrhosis, tumor size, tumor number, coexisting satellite nodule, existence of micro and macro vascular invasion, as well as lymph node and distant metastasis. Pathologic diagnosis of the resected mass, tumor size, accompanying satellite nodule, existence of microvascular, macrovascular and lymph node invasion were possible prognostic factors forecasting survival after the curative surgery (Table 2). On multivariate pathologic diagnosis of the resected mass, existence of microvascular invasion and accompanying satellite nodule affected the prognosis of primary liver cancer after the surgical resection (Table 2).
Table 2.
Univariate and multivariate analysis of predictors of survival
|
Univariate analysis |
Multivariate analysis |
|||
| Variable | P value | Hazard ratio | 95% CI | P value |
| Pathologc diagnosis | 0.000 | 0.000 | ||
| CK19- HCC | ||||
| CK19+ HCC | 3.055 | 1.235-7.558 | 0.016 | |
| cHCC-CC | 4.588 | 1.953-10.781 | 0.000 | |
| ICC | 6.986 | 2.997-16.284 | 0.000 | |
| Age (yr) | 0.672 | |||
| < 45 vs ≥ 45 | ||||
| Gender | 0.197 | |||
| Cirrhosis | 0.542 | |||
| Tumor size (cm) | 0.000 | |||
| < 5 vs ≥ 5 | ||||
| Tumor number | 0.179 | |||
| Single vs multiple | ||||
| Satellite nodule | 0.001 | 1.997 | 1.010-3.949 | 0.047 |
| Microvascular invasion | 0.005 | 2.884 | 1.491-5.581 | 0.002 |
| Macrovascular invasion | 0.000 | |||
| Lymph node metastasis | 0.002 | |||
| Distant metastasis | 0.111 | |||
CK19- HCC: Cytokeratin 19 negative hepatocellular carcinoma; CK19+ HCC: Cytokeratin 19 positive hepatocellular carcinoma; cHCC-CC: Combined hepatocellular carcinoma and cholangiocarcinoma; ICC: Intrahepatic cholangiocarcinoma; 95% CI: 95% confidence interval.
DISCUSSION
This study suggested that the outcome of the surgical treatment in the primary liver cancer would be worse as the resected tumor expressed more components with biliary phenotypes.
Although current WHO classification of primary liver cancer is still guided by the morphologic features of the tumor[13], new classification emphasizing morpho-phenotypic categories with patho-genetic implications is rapidly emerging[4]. Demand for the new type of primary liver cancer classification came from heterogeneous treatment outcome within the liver mass that had similar morphological features. Several studies supported that there would be subpopulation of HCCs, expressing biliary phenotype as being CK19 positive, and that this subpopulation would demonstrate aggressive behavior[6,14-17]. Our study also showed that morphologically similar HCC might exhibit very different outcome after the curative treatment if their malignant cells contain components of biliary phenotype detected by CK19 immunochemical study.
Out of 70 patients who had CK19 immunochemical study, 21 patients had CK19 positive. These 70 patients underwent immunochemical study due to atypical pathologic features that made diagnosis ambiguous without immunochemical evaluation. Therefore, the proportion of CK19+ HCC was relatively higher than that of other studies[6,14]. Even though HCC patients in this study had resectable liver mass, they had higher Edmonson grade in more than 60% regardless of CK19 immunostain result. It is very likely that HCC with ambiguous morphology under HE stain might have poorer differentiation and thus might demonstrate less favorable surgical outcome. However, it appeared that CK19- HCC had more favorable outcome compared with that of CK19+ HCC independent of Edmonson grade.
It is beyond the scope of this study addressing the origin of this biliary component in HCC. However, since CK19 is known as a stem cell marker, it can be contemplated that CK19 positive phenotype reflected an origin from a progenitor cell. Studies suggesting primary liver cancer of progenitor cell origin used CK19 as one of liver stem cell markers[18-22]. Moreover, one recent study reported that CK19 was well correlated with tumor aggressiveness, compared to other stemness-related markers[20,23]. In addition CK19 being a stem cell marker, a report suggested that expression of CK19 was related with epidermal growth factor thereby increasing the growth abilities of HCC[24-26].
Most of the studies on CK19 in liver cancer performed immunohistochemical analysis in order to evaluate its expression[15-19]. Some studies that assessed CK19 gene expression along with its protein expression demonstrated that protein expression well correlated gene expression[20,27].
Meanwhile, origin of cancer cells in cHCC-CC also needs to be verified. By definition, cHCC-CC has areas of typical HCC and areas of typical cholangiocarcinoma. It was reported that cholangiocarcinoma components in cHCC-CC were usually positive for CK19, and careful examination usually revealed intermediate morphology at the interface between HCC and cholangiocarcinoma areas[4].
Clinically, studies consistently reported that prognosis of cHCC-CC worse than that of HCC in general[7,8,21]. In this study, when CK19+ HCC was compared with cHCC-CC, we found their clinical features somewhat similar, as both cell types demonstrated unfavorable prognosis than CK19- HCC. Regarding OS, both CK19+ HCC and cHCC-CC showed lower OS rates than CK19- HCC, and higher rates than ICC. However, when we compared DFS rates, although CK19- HCC still showed higher DFS rate than any other types of primary liver cancer, there were no significant differences in DFS rates of CK19+ HCC, cHCC-CC and ICC. This discrepancy in OS and DFS rates might be resulted from not having effective means of treating recurrent ICC when HCC and cHCC-CC could partly be controlled by the non-surgical method such as TACE.
Recently suggested histopathological classification of primary liver cancer put CK19+ HCC as cancer of hepatocellular phenotype with stem/progenitor cell immunophenotype, whereas cHCC-CC as cancer of mixed hepatobiliary carcinoma, classical type. According to this proposed classification, these two subpopulations of liver cancer resided between the spectrum of HCC and ICC, where CK19+ HCC closer to HCC and cHCC-CC being closer to cholangiocarcinoma[4]. In accordance with this classification, our study showed that clinical outcome of CK19+ HCC and cHCC-CC after the curative liver resection was also placed between that of HCC and ICC where HCC side showing good prognosis and ICC side displaying poor prognosis. It seemed that primary liver cancer that possessed more components with biliary phenotype resulted in poor prognosis after the curative surgical treatment. However, since our study was limited by relatively small number of patients, especially in CK19+ HCC and cHCC-CC group, another study over larger number of patients should be able to verify this suggestion.
COMMENTS
Background
Hepatocellular carcinoma (HCC) of similar clinical stage may produce heterogeneous treatment outcome, and immuno-phenotypical information of HCC might facilitate more appropriate stratification of the tumor.
Research frontiers
Efforts have been made to classify HCC histopathologically according to their distinct molecular features. Several studies suggested that there would be subpopulation of HCCs, expressing biliary phenotype as being cytokeratin (CK) 19 positive, and that this subpopulation would demonstrate aggressive behavior.
Innovations and breakthroughs
New classification emphasizing morpho-phenotypic categories with patho-genetic implications is rapidly emerging. This histopathological classification of primary liver cancer put cytokeratin 19 positive hepatocellular carcinoma (CK19+ HCC) as cancer of hepatocellular phenotype with stem/progenitor cell immunophenotype, whereas combined hepatocellular and cholangiocarcinoma (cHCC-CC) as cancer of mixed hepatobiliary carcinoma, classical type. According to this proposed classification, these two subpopulations of liver cancer resided between the spectrum of HCC and intrahepatic cholangiocarcinoma, where CK19+ HCC closer to HCC and cHCC-CC being closer to cholangiocarcinoma.
Applications
Primary liver cancer that possessed more components with biliary phenotype, detected by CK19 immunochemical study, might predict poor outcome after the curative surgical treatment. Therefore, alternative treatments or more vigorous adjuvant therapy after the surgical treatment could be recommended in such cases.
Terminology
Studies on primary liver cancer of progenitor cell origin used CK19 as one of liver stem cell markers along with other markers such as cluster of differentiation 133, c-kit and epithelial cell adhesion molecule. Among many stemness-related markers, a recent study reported that CK19 was well correlated with tumor aggressiveness.
Peer review
The authors examined three different types of primary liver cancers for its biliary phenotype and gave a vital conclusion that primary liver cancer that showed more of biliary components resulted in poor prognosis after the surgery. This work provides useful information on immuno-phenotype of primary liver cancers might be as important as their morphological classification with interesting results.
Footnotes
Peer reviewer: Min-Hsiung Pan, PhD, Professor, Department of Seafood Science, National Kaohsiung Marine University, No.142 Haijhuan Rd., Nanzih District, Kaohsiung 81143, Taiwan, China
S- Editor Gou SX L- Editor A E- Editor Xiong L
.
References
- 1.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 2.Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 3.Katoh H, Ojima H, Kokubu A, Saito S, Kondo T, Kosuge T, Hosoda F, Imoto I, Inazawa J, Hirohashi S, et al. Genetically distinct and clinically relevant classification of hepatocellular carcinoma: putative therapeutic targets. Gastroenterology. 2007;133:1475–1486. doi: 10.1053/j.gastro.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Roncalli M, Park YN, Di Tommaso L. Histopathological classification of hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S228–S234. doi: 10.1016/S1590-8658(10)60510-5. [DOI] [PubMed] [Google Scholar]
- 5.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC; 2010. [Google Scholar]
- 6.Lu XY, Xi T, Lau WY, Dong H, Zhu Z, Shen F, Wu MC, Cong WM. Hepatocellular carcinoma expressing cholangiocyte phenotype is a novel subtype with highly aggressive behavior. Ann Surg Oncol. 2011;18:2210–2217. doi: 10.1245/s10434-011-1585-7. [DOI] [PubMed] [Google Scholar]
- 7.Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040–2046. doi: 10.1002/cncr.10392. [DOI] [PubMed] [Google Scholar]
- 8.Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005;189:120–125. doi: 10.1016/j.amjsurg.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Wachtel MS, Zhang Y, Xu T, Chiriva-Internati M, Frezza EE. Combined hepatocellular cholangiocarcinomas; analysis of a large database. Clin Med Pathol. 2008;1:43–47. doi: 10.4137/cpath.s500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, Yoon JH, Lee HS, Yi NJ, Suh KS, et al. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011;45:69–75. doi: 10.1097/MCG.0b013e3181ce5dfa. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Choi KH, Choi SB, Choi JW, Kim do Y, Ahn SH, Kim KS, Choi JS, Han KH, Chon CY, et al. Clinicopathological characteristics in combined hepatocellular-cholangiocarcinoma: a single center study in Korea. Yonsei Med J. 2011;52:753–760. doi: 10.3349/ymj.2011.52.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh MM. Pathology of combined hepatocellular-cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1485–1492. doi: 10.1111/j.1440-1746.2010.06430.x. [DOI] [PubMed] [Google Scholar]
- 13.Walther Z, Jain D. Molecular pathology of hepatic neoplasms: classification and clinical significance. Patholog Res Int. 2011;2011:403929. doi: 10.4061/2011/403929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uenishi T, Kubo S, Yamamoto T, Shuto T, Ogawa M, Tanaka H, Tanaka S, Kaneda K, Hirohashi K. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci. 2003;94:851–857. doi: 10.1111/j.1349-7006.2003.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V, Roskams T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 16.Aishima S, Nishihara Y, Kuroda Y, Taguchi K, Iguchi T, Taketomi A, Maehara Y, Tsuneyoshi M. Histologic characteristics and prognostic significance in small hepatocellular carcinoma with biliary differentiation: subdivision and comparison with ordinary hepatocellular carcinoma. Am J Surg Pathol. 2007;31:783–791. doi: 10.1097/01.pas.0000213421.53750.0a. [DOI] [PubMed] [Google Scholar]
- 17.Yang XR, Xu Y, Shi GM, Fan J, Zhou J, Ji Y, Sun HC, Qiu SJ, Yu B, Gao Q, et al. Cytokeratin 10 and cytokeratin 19: predictive markers for poor prognosis in hepatocellular carcinoma patients after curative resection. Clin Cancer Res. 2008;14:3850–3859. doi: 10.1158/1078-0432.CCR-07-4338. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004;40:298–304. doi: 10.1016/j.jhep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, Cho JY, Yoo JE, Choi JS, Park YN. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54:1707–1717. doi: 10.1002/hep.24559. [DOI] [PubMed] [Google Scholar]
- 21.Ariizumi S, Kotera Y, Katagiri S, Nakano M, Yamamoto M. Combined hepatocellular-cholangiocarcinoma had poor outcomes after hepatectomy regardless of Allen and Lisa class or the predominance of intrahepatic cholangiocarcinoma cells within the tumor. Ann Surg Oncol. 2012;19:1628–1636. doi: 10.1245/s10434-011-2150-0. [DOI] [PubMed] [Google Scholar]
- 22.Andersen JB, Loi R, Perra A, Factor VM, Ledda-Columbano GM, Columbano A, Thorgeirsson SS. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology. 2010;51:1401–1409. doi: 10.1002/hep.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai X, Zhai J, Kaplan DE, Zhang Y, Zhou L, Chen X, Qian G, Zhao Q, Li Y, Gao L, et al. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology. 2012:Jun 9; Epub ahead of print. doi: 10.1002/hep.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoneda N, Sato Y, Kitao A, Ikeda H, Sawada-Kitamura S, Miyakoshi M, Harada K, Sasaki M, Matsui O, Nakanuma Y. Epidermal growth factor induces cytokeratin 19 expression accompanied by increased growth abilities in human hepatocellular carcinoma. Lab Invest. 2011;91:262–272. doi: 10.1038/labinvest.2010.161. [DOI] [PubMed] [Google Scholar]
- 25.Höpfner M, Sutter AP, Huether A, Schuppan D, Zeitz M, Scherübl H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J Hepatol. 2004;41:1008–1016. doi: 10.1016/j.jhep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Poon RT, Shao W, Sun X, Chen H, Kok TW, Fan ST. Blockage of epidermal growth factor receptor by quinazoline tyrosine kinase inhibitors suppresses growth of human hepatocellular carcinoma. Cancer Lett. 2007;248:32–40. doi: 10.1016/j.canlet.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Van Hul NK, Abarca-Quinones J, Sempoux C, Horsmans Y, Leclercq IA. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology. 2009;49:1625–1635. doi: 10.1002/hep.22820. [DOI] [PubMed] [Google Scholar]


