Abstract
AIM: To investigate the contribution of fucosyltransferase 2 (FUT2) variants to the genetic susceptibility and clinical heterogeneity of ulcerative colitis (UC) between Han and Uyghur patients in Xinjiang, China.
METHODS: A total of 102 UC patients (53 Han patients including 22 men and 31 women, and 49 Uyghur patients including 25 men and 24 women; aged 48 ± 16 years) and 310 age- and sex-matched healthy controls were enrolled from January 2010 to May 2011 in Xinjiang People’s Hospital of China. UC was diagnosed based on the clinical, endoscopic and histological findings following Lennard-Jones criteria. Blood samples were collected and genomic DNA was extracted by the routine laboratory methods. Polymerase chain reaction-sequence-based typing method was used to identify FUT2 variants rs281377, rs1047781, rs601338 and rs602662. Genotypic and allelic frequencies were documented and compared between the UC patients and the healthy controls. Genotypic frequencies were also compared between Han and Uyghur patients. Potential association of genetic variation and UC between Han and Uyghur patients was examined.
RESULTS: rs281377 was found significantly associated with UC in the Han population as compared with the controls (P = 0.011) while rs281377 was not associated with UC in the Uyghur population (P = 0.06). TT homozygous rs281377 frequencies were higher in the UC groups than in the controls (88.7% vs 68.7% and 55.1% vs 50.3%). rs1047781 was specifically associated with UC in the Uyghur population (P = 0.001), but not associated with UC in the Han population (P = 0.13). TT homozygous rs1047781 frequencies were lower in the UC groups than in the controls (9.5% vs 11.8% and 4.0% vs 6.7%). rs601338 was statistically related to UC in both populations (Han, P = 0.025; Uyghur, P = 8.33 × 10-5). AA homozygous rs601338 frequencies were lower in the UC groups than in the controls (0% vs 1.8% and 12.2% vs 13.4%). No association was found between rs602662 and UC in both Han and the Uyghur populations. Allelic analysis showed that rs281377 allele was significantly associated with UC in the Han population as compared with the controls [P = 0.001, odd ratio (OR) = 0.26], however, it was not associated with UC in the Uyghur population (P = 0.603, OR = 1.14), and rs1047781 allele was associated with UC in the Uyghur population (P = 0.001, OR = 0.029) while it was not associated with UC in the Han population (P = 0.074, OR = 0.62). Moreover, rs601338 was associated with UC in both Han (P = 0.005, OR = 0.1) and Uyghur populations (P = 0.002, OR = 0.43). Meta analysis showed that rs1047781 and rs601338 conferred risk of UC as compared with the controls [P = 0.005, OR = 0.47; P = 0.0003, OR = 0.35; 95% confidence interval (CI) = 0.31-0.72 and 0.21-0.58], but rs281377 and rs602662 showed no statistically significant differences between patients with UC and controls (P = 0.10, OR = 0.71; P = 0.68, OR = 0.09; 95% CI = 0.47-1.07 and 0.56-1.47).
CONCLUSION: Functionally relevant FUT2 gene variants are associated with UC, suggesting that they play a potential role in the pathogenesis of UC and may contribute to the clinical heterogeneity of UC between Han and Uyghur patients.
Keywords: Ulcerative colitis, Fucosyltransferase 2, Gene polymorphisms, Han, Uyghur
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease are often characterized as chronic inflammatory bowel diseases (IBD). Clinical features in both disorders include abdominal pain, diarrhea, weight loss and increased risk of developing colorectal cancer[1]. The etiology of UC is thought to be multifactorial, such as genetic, environmental, immune, and gut barrier factors[2]. Genetic factors involved in the regulation of the immune system are considered to play a significant role in the pathogenesis of IBD[3,4]. Much progress has been achieved in recent years regarding the genetic etiology of IBD. Studies have found that disease distribution and phenotypic appearance differ significantly between ethnic groups and even within populations. Apart from varying environmental factors that affect susceptible individuals, genetic heterogeneity between different populations itself plays an important role in IBD[5]. We previously compared the clinical characteristics of UC between the Han and Uyghur populations residing in the Xinjiang Uyghur Autonomous Region of China. We found differences between the Uyghur and Han populations living in the same region, where Uygur population had a higher prevalence of UC, a younger age of onset, an increased prevalence of the chronic persistence and acute outbreak type, more moderate and severe forms, a higher complication rate, and an increased frequency of positive anti-neutrophilic cytoplasmic antibodies[6,7]. However, the genetic heterogeneity related to UC between these two ethnic groups need to be investigated.
Secretor/non-secretor phenotypes [determined by the fucosyltransferase 2 (FUT2) gene] are associated with some metabolic and infectious diseases, and ABO glycosyltransferase activity has been shown to be involved in the pancreatic cancer risk[8]. Genetic variation in FUT2 has been implicated in susceptibility to Helicobacter pylori[9], Norovirus (Norwalk virus)[10,11], progression of human immunodeficiency virus[12], recurrent urinary tract infections[13], the development of vaginal candidiasis[14], gram-negative sepsis in infants[15] and cholera[16]. Carriers of non-secretor variants have higher plasma vitamin B12 levels than carriers of the secretor genotypes[17]. In addition to the genetic associations mentioned above, non-secretion of ABO blood group antigens into body fluids has been shown to be associated with rheumatic fever[18] and Crohn’s disease, but not associated with UC[19].
Earlier studies have shown differences in the intestinal microbiota between the UC patients and healthy subjects[20] and alterations in the microbiota of IBD patients are related to changes in the FUT2 genotype[21]. The FUT2 gene codes for an α (1,2)-fucosyltransferase and regulates the expression of ABH antigens in body secretions and the intestinal mucosa[22]. The FUT2 gene is located on chromosome 19q13.3.4 (chromosome 19: 49, 199, 228-49, 209, 207) and consists of two exons. The cDNA is 3.1 kb long and encodes a polypeptide of 332 amino acid residues. The gene determines the secretion status of the ABO antigens with secretors having at least one functional FUT2 allele (Se), whereas non-secretors are homozygous for nonfunctional FUT2 allele[23]. rs601338 nonsense mutation (Trp143stop) in the FUT2 gene of non-secretors has been reported in Caucasians (Europeans and Iranians) and Africans[24] and was found in approximately 1% of Chinese[25]. The frequency of non-secretors among east Asians is similar to Europeans and Africans, but east Asians are homozygous for a different weak-activity allele resulting from a rs1047781 missense mutation (Ile129phe)[26]. In Portuguese, two FUT2 polymorphisms, rs602662 and T839C, are associated with decreased or absent FUT2 enzyme activity[27]. rs281377 synonymous mutation (Asn-Asn) in the FUT2 gene of secretors is found all over the world with a higher frequency than the wild type allele (Se)[28]. Four variants (rs281377, rs1047781, rs601338 and rs602662) in the FUT2 genes have been identified in healthy Uyghur donors[29]. The rs1047781 was found in 54.7% of the Han populations with the most common alleles being rs281377, and rs281377 and rs1047781 were found in 94% of Chinese population[25]. rs281377 was reported in 71.2% and rs1047781 in 22.0% among the Uyghur populations[29]. In this study, we examined whether polymorphism of the FUT2 gene differed between the Han and Uyghur patients with UC.
MATERIALS AND METHODS
Patients and controls
A total of 102 consecutive patients with UC (53 Han and 49 Uyghur, 47 men and 55 women, aged 18-78 years) and 310 age- and sex-matched healthy controls (161 Han and 149 Uyghur, 137 men and 173 women, aged 18-75 years) were enrolled from January 2010 to May 2011 in the Department of Gastroenterology and the Physical Examination Center, Xinjiang People’s Hospital of China. UC was diagnosed based on the clinical, endoscopic and histological findings following the Lennard-Jones criteria[30]. The extent of the disease was assessed by colonoscopy at the initial diagnosis and during follow-up. Extensive colitis was defined as lesions located beyond the splenic flexure. Distal colitis was defined as lesions limited to the region distal to the spleen flexure[31,32]. The study protocol was approved by the Ethics Committee of Xinjiang People’s Hospital. Selected patients with UC and healthy controls are Chinese Han and Uyghur from Urumqi, Xinjiang, China. Informed consent was obtained from all the subjects.
Extraction of DNA
Genomic DNA was purified using a QIAamp DNA Blood Midikit (Qiagen, Germany). Purified genomic DNA from blood donors was stored frozen in micro-titre plates (10 ng/μL in TE buffer) until analyzed.
Polymerase chain reaction and sequencing primers
Primers for the polymerase chain reaction (PCR) amplification and sequencing were designed using conventional standard criteria. Primer specificity was checked with published nucleotide sequences using Basic Local Alignment Search Tool and analyzed for the risk of primer-dimer formation and secondary structures using Oligo 6.0 Software. The following pair of primers was used for the FUT2 (forward, 5′-AGCGCCCCGGGCCTCCATCTC C-3′; reverse, 5′-GGAACCATGTGTGCTTCTCATGCCCG-3′). The same forward and reverse primers were used for sequencing.
PCR and PCR product purification
Water of 17.3 μL, 0.5 μL 20 μmol/L forward primer, 0.5 μL 20 μmol/L reverse primer, 2.5 μL PCR buffer, 1 μL dNTPs, 2 μL MgCl2 and 0.2 μL TaqGold (Applied Biosystems, Branchburg, New Jersey, United States) were added to 1 μL DNA (≥ 50 ng). Amplification conditions for the FUT2 PCR were: one cycle of 5 min at 95 °C followed by 10 cycles of 20 s at 95 °C and 40 s at 78 °C and 23 cycles of 10 s at 95 °C, 10 s at 62 °C and 2 min at 72 °C, and finally one cycle of 5 min at 72 °C and 4 °C. The PCR was performed by a GeneAmp PCR system 9700 from the Applied Biosystems. PCR products were purified using the SAP-Exon method.
PCR product sequence
Twelve μL water, 1 μL 10 μmol/L forward primer, 2 μL BigDye sequencing buffer, 4 μL Bigdye (BigDye® Terminator v3.1Cycle Sequencing Kit) were added to 1 μL PCR product (≥ 50 ng). Cycle sequencing conditions for the FUT2 were: one cycle of 1 min at 96 °C followed by 25 cycles of 10 s at 96 °C, 5 s at 50 °C and 4 min at 60 °C and 4 °C. The PCR was performed by a GeneAmp PCR system 9700 from Applied Biosystems. DNA sequencing was performed using an automated sequencer (ABI 3730XL, Applied Biosystems). Chromas software version 2.0 was used to analyze the raw sequence data. The FUT2 genotyping method was established in accordance with the polymorphism (Figure 1). The frequencies of the genotypes were calculated by direct counting.
Figure 1.
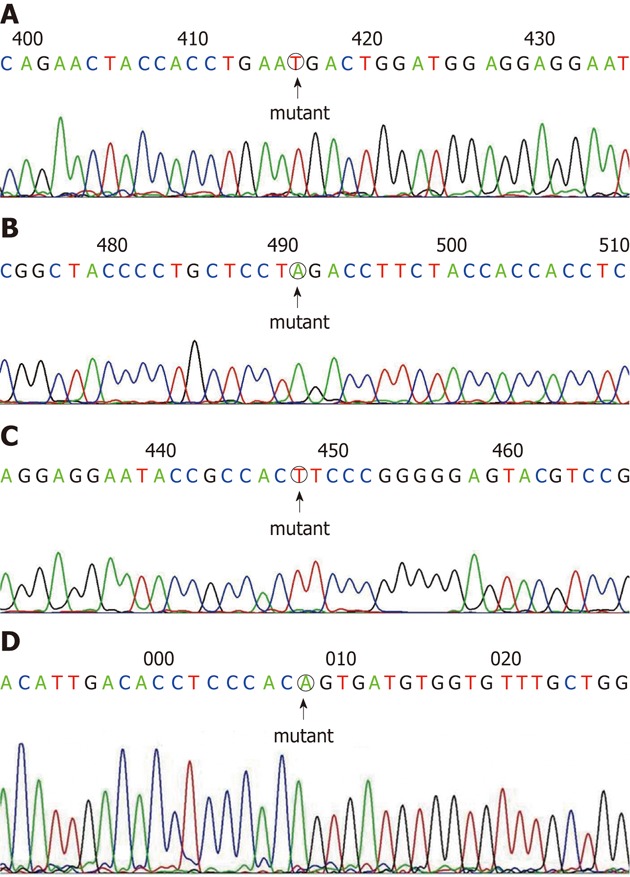
DNA sequence. A: Fucosyltransferase 2 (FUT2)-rs281377TT in a Han patient; B: FUT2- rs601338AA in a Uyghur patient; C: FUT2-rs1047781TT in a Han patient; D: FUT2-rs602662AA in a Uyghur patient.
Statistical analysis
Hardy-Weinberg disequilibrium was assessed with χ2 test and clinical records including age were analyzed with t test. Allelic and genotypic association analyses were performed using the Pearson’s 2 × 2 or 2 × 3 table χ2 test. Odd ratio (OR) and 95% confidential interval (95% CI) were also calculated by Pearson’s 2 × 2 χ2 test. To adjust for the multiple tests performed and obtain an empirical null distribution of the association test P values (P empirical), we conducted 10 000 permutations in the case-control samples. Tests for the heterogeneity of effect size between Han and Uyghur populations were carried out using Cochrane’s Q test, and P < 0.05 indicated that heterogeneity existed. When heterogeneity was found, random-effects model was applied using Cochran-Mantel-Hansel test to calculate the overall genetic effect of single nucleotide polymorphisms by combining the data from Han and Uyghur populations; otherwise, fixed-effects model was applied. The above analyses were performed using PLINK v.1.07 (http://pngu.mgh.harvard.edu/purcell/plink/).
RESULTS
Clinical characteristics of UC patients
DNA was obtained from 102 consecutive UC patients and 310 healthy individuals who were well matched by sex and age. The main clinical characteristics of the UC patients are summarized in Table 1. UC patients included 47 women and 55 men with 53 Han and 49 Uyghur, and their average age at onset was 48 ± 16 years. Among the 310 healthy controls, there were 161 Han and 149 Uyghur including 137 men and 173 women, and their average age was 47 ± 14 years. There were no significant differences in sex ratio and the average age between the UC patients and healthy controls (P = 0.74 and P = 0.801).
Table 1.
Clinical characteristics of the study subjects
| Items | Han population | Uyghur population | Total |
| UC patients | 53 | 49 | 102 |
| Gender (male/female) | 22/31 | 25/24 | 47/55 |
| Age of onset (yr) | 52 ± 15 | 46 ± 15 | 48 ± 16 |
| Extensive colitis | 20 (0.38) | 30 (0.61) | 50 (0.49) |
| Distal colitis | 33 (0.62) | 19 (0.39) | 52 (0.51) |
| Mild and moderate | 47 (0.89) | 40 (0.82) | 87 (0.85) |
| Severe | 6 (0.11) | 9 (0.18) | 15 (0.15) |
| Healthy controls | 161 | 149 | 310 |
| Gender (male/female) | 67/94 | 70/79 | 137/173 |
| Age of onset (yr) | 48 ± 15 | 49 ± 13 | 47 ± 14 |
Data are shown as mean ± SD or n (%). UC: Ulcerative colitis.
Comparison of FUT2 genotype and allelic variants between UC patients and healthy controls
The genotype frequencies of the FUT2 gene variants of the UC patients and the healthy controls are presented in Table 2. rs281377 was only associated with UC in Han population (P = 0.011), while rs1047781 was associated specifically with UC in Uyghur population (P = 0.001). rs601338 was statistically related to UC in both Han and Uyghur populations (Han, P = 0.025; Uyghur, P = 8.33 × 10-5). rs602662 was not associated with UC in both Han and Uyghur populations. TT homozygote of rs281377 was compared between the two ethnic UC groups and controls. Its frequencies were higher in the UC groups than in the controls (88.7% vs 68.7% and 55.1% vs 50.3%, respectively). TT homozygous rs1047781 frequencies (9.5% vs 11.8% and 4.0% vs 6.7%) and AA homozygous rs601338 frequencies (0% vs 1.8% and 12.2% vs 13.4%) were both lower than in the controls.
Table 2.
Genotypic association of four single nucleotide polymorphisms with ulcerative colitis in Han and Uyghur populations n (%)
| SNPs | Allele | Groups | UC cases | Controls | χ2 | P |
| rs281377 | C/T | Han | 0 (0)/6 (11.3)/47 (88.7) | 9 (5.6)/42 (26.1)/110 (68.4) | 9.091 | 0.011 |
| C/T | Uyghur | 10 (20.4)/12 (24.5)/27 (55.1) | 15 (10.1)/59 (39.6)/75 (50.3) | 5.633 | 0.060 | |
| rs1047781 | T/A | Han | 5 (9.5)/13 (24.5)/35 (66.0) | 19 (11.8)/61 (37.9)/81 (50.3) | 4.077 | 0.130 |
| T/A | Uyghur | 2 (4.0)/4 (8.2)/43 (87.8) | 10 (6.7)/50 (33.6)/89 (59.7) | 13.480 | 0.001 | |
| rs601338 | A/G | Han | 0 (0)/1 (1.9)/52 (98.1) | 3 (1.8)/23 (14.3)/135 (83.9) | 7.382 | 0.025 |
| A/G | Uyghur | 6 (12.2)/9 (18.4)/34 (69.4) | 20 (13.4)/76 (51.0)/53 (35.6) | 18.790 | 8.33 × 10-5 | |
| rs602662 | A/G | Han | 0 (0)/3 (5.7)/50 (94.3) | 0 (0)/9 (5.6)/152 (94.4) | NA | NA |
| A/G | Uyghur | 5 (10.2)/15 (30.6)/29 (59.2) | 11 (7.4)/61 (40.9)/77 (51.7) | 1.776 | 0.412 |
UC: Ulcerative colitis; SNPs: Single nucleotide polymorphisms; NA: Not available.
In allelic analysis as shown in Table 3 and Figure 2, rs281377 was found associated with UC only in the Han population (P = 0.001, OR = 0.26). rs1047781 was associated specifically with UC in Uyghur population (P = 0.001, OR = 0.29), whereas rs601338 was associated with UC in both Han and Uyghur populations (Han, P = 0.005, OR = 0.10; Uyghur, P = 0.002, OR = 0.43) and rs602662 was not associated with UC in both Han and Uyghur populations (Han, P = 0.985; Uyghur, P = 0.652, Figure 1).
Table 3.
Allelic association of four single nucleotide polymorphisms with ulcerative colitis in Han, Uyghur and combined populations
| SNPs | Groups | Risk allele | Frequency (case/control) | χ2 | Pobs | Pemp | OR (95% CI) | P (Q) | Poverall | OR (95% CI) |
| rs281377 | Han | C | 0.057/0.186 | 10.290 | 0.001 | 0.002 | 0.26 (0.11-0.63) | 0.003 | 0.100 | 0.71 (0.47-1.07) |
| Uyghur | C | 0.327/0.299 | 0.270 | 0.603 | 0.636 | 1.14 (0.70-1.86) | ||||
| rs1047781 | Han | T | 0.217/0.308 | 3.203 | 0.074 | 0.106 | 0.62 (0.37-1.05) | 0.100 | 0.005 | 0.47 (0.31-0.72) |
| Uyghur | T | 0.082/0.235 | 10.950 | 0.001 | 0.002 | 0.29 (0.13-0.63) | ||||
| rs601338 | Han | A | 0.009/0.090 | 7.954 | 0.005 | 0.102 | 0.10 (0.01-0.72) | 0.140 | 0.0003 | 0.35 (0.21-0.58) |
| Uyghur | A | 0.214/0.389 | 9.979 | 0.002 | 0.002 | 0.43 (0.25-0.73) | ||||
| rs602662 | Han | A | 0.028/0.028 | 0.000 | 0.985 | 1.000 | 1.01 (0.27-3.81) | 0.860 | 0.680 | 0.90 (0.56-1.47) |
| Uyghur | A | 0.255/0.279 | 0.204 | 0.652 | 0.699 | 0.89 (0.53-1.49) |
SNPs: Single nucleotide polymorphisms; Pobs: Observed P value, was calculated by 2 × 2 χ2 test; Pemp: Pempirical, empirical null distribution of the association test P values; P(Q): Heterogeneity test, was calculated by Cochrane's Q test; Poverall: Meta-analysis P value, was calculated by Mantel–Haenszel test under fixed-effects or random-effects model; OR: Odd ratio; 95% CI: 95% confidential interval.
Figure 2.
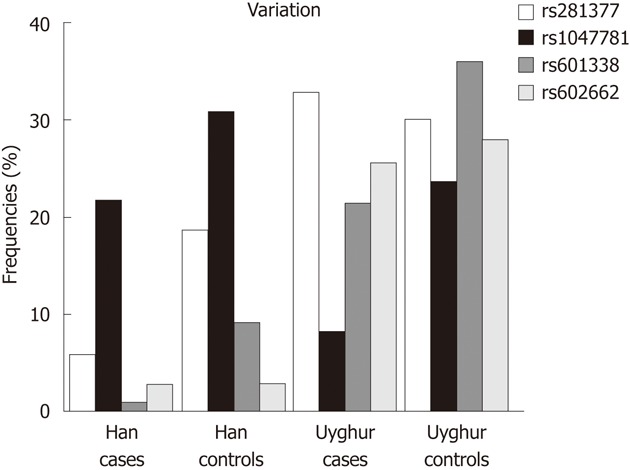
Allelic analysis of fucosyltransferase 2 variants and ulcerative colitis in Han and Uyghur populations.
The comparison between Han and Uyghur UC patients and controls revealed that genotype and allele rs281377 and rs601338 in the Han population were significantly associated with UC while in the Uyghur population, rs1047781 and rs601338 were significantly associated with UC.
Meta-analysis showed that rs1047781 and rs601338 conferred risk of UC as compared with the controls, (P = 0.005, OR = 0.47; P = 0.0003, OR = 0.35; 95% CI = 0.31-0.72 and 0.21-0.58) while rs281377 and rs602662 were not significantly different statistically between patients with UC and controls (P = 0.100, OR = 0.71; P = 0.680, OR = 0.90; 95% CI = 0.47-1.07 and 0.56-1.47).
DISCUSSION
UC is thought to fundamentally represent an altered interaction between the intestinal microbiota and the intestinal immune system[33]. The intestinal microbiota interacts with luminal enterocytes via host cell surface molecules, including oligosaccharides synthesized by glycosyltransferases[34]. Previous studies have shown that the allele polymorphism with a synonymous mutation rs281377, including the nonsense mutation rs601338, and missense mutation rs1047781 and rs602662, is almost inactive and is responsible for some instances of non-secretor statu[27]. Non-secretor phenotypes in the population occurring as the absence of particular carbohydrate molecules in the mucosa may have conferred protection against some pathogens. It is demonstrated that Fut2-null mice do not express fucosyl glycolipid FGA1 in the colon, whereas normal mice do[35]. Commensal bacteria and probiotics may exert their protective effects via preventing adherence, or even displacing pathogenic bacteria[36], which is consistent with the notion that FUT2 and non-secretor may affect the status of the gastrointestinal microbiota. Furthermore, changes in the microflora of IBD patients have been documented.
Our study indicated that the genetic polymorphisms of the FUT2 gene were correlated with UC, and the associations differed between Han and Uyghur people in China. We analyzed four variants of FUT2 gene, the frequency of three variants of the FUT2 genotype (rs281377, rs1047781 and rs601338) showed significant associations with UC (P < 0.05), however, the associations differed between the two ethnic groups. In the Han population, genotype and allele of rs281377 and rs601338 were significantly associated with UC whereas in the Uyghur population, genotype rs1047781 and rs601338 were associated with UC.
We also compared the TT homozygote of rs281377 between the two ethnic UC groups and controls. Its frequencies were higher in the UC groups than in the controls (88.7% vs 68.7 and 55.1% vs 50.3%, respectively). TT homozygous rs1047781 (9.5% vs 11.8 and 4.0% vs 6.7%) and AA homozygous rs601338 frequencies (0% vs 1.8% and 12.2% vs 13.4%) were both lower than in the controls.
A limitation of the present study was the relatively small number of patients included. In the subgroup analysis, population stratification may lower the sufficient number of correlation analysis and weaken the significance of our results. In order to overcome these inadequacies and confirm our results, further research on a larger number of participants will be needed in the future.
In conclusion, our study found that the functional FUT2 variants might not only play a role in the pathogenesis of UC, but also contribute to the different clinical manifestations of UC between Han and Uyghur patients in north-west China. The effects of the different genotypes on the intestinal microbiota of normal controls and patients with UC are still unknown and likely represent a potentially productive area for future research in the quest to better understand the pathogenesis and treatment of UC.
ACKNOWLEDGMENTS
The authors thank Professor David Graham for his assistance with English revision, and Professor Fan Wang for his technical assistance.
COMMENTS
Background
Earlier studies have shown differences in the intestinal microbiota between the ulcerative colitis (UC) patients and healthy subjects, and alterations in the microbiota of inflammatory bowel diseases (IBD) patients are related to changes in the fucosyltransferase 2 (FUT2) genotype. In this study, the authors examined whether polymorphism of the FUT2 gene differed between the Han and Uyghur patients with UC.
Research frontiers
FUT2 and non-secretor may affect the status of the gastrointestinal microbiota. Studies have found that disease distribution and phenotypic appearance differ significantly between ethnic groups and even within populations. The associations of the genetic heterogeneity of UC between two ethnic groups, Chinese Han and Uyghur, were investigated.
Innovations and breakthroughs
This study found that the functional FUT2 variants might not only play a role in the pathogenesis of UC, but also contribute to the different clinical manifestations of UC between Han and Uyghur patients in North-West China.
Applications
The effects of the different genotypes on the intestinal microbiota of normal controls and patients with UC are still unknown and likely represent a potentially productive area for future research in the quest to better understand the pathogenesis and treatment of UC.
Terminology
UC is a form of IBD. It is a refractory, chronic, and nonspecific disease which usually occurs in the rectum and the entire colon; FUT2 encodes the α (1,2) fucosyltransferase that determines blood group secretor status, and synthesizes the H-type 1 antigen in saliva and mucosa.
Peer review
The author investigated the contribution of FUT2 variants to the genetic susceptibility and clinical heterogeneity of UC between Han and Uyghur patients. This manuscript contains potentially interesting findings.
Footnotes
Supported by National Natural Science Foundation of China, No. 30871148 and No. 81160052; and Natural Science Foundation of Xinjiang Uyghur Autonomous Region of China, No. 2009211A26
Peer reviewers: Sung-Gil Chi, Professor, School of Life Sciences and Biotechnology, Korea University, No. 301, Nok-Ji Building, Seoul 136-701, South Korea; Espen Melum, MD, Medical Department, Rikshospitalet University Hospital, Sognsvannsvn. 20, Oslo 0027, Norway
S- Editor Lv S L- Editor Kerr C E- Editor Xiong L
.
References
- 1.Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. J Surg Oncol. 2010;101:706–712. doi: 10.1002/jso.21505. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Nava GM, Stappenbeck TS. Host genetic susceptibility, dysbiosis, and viral triggers in inflammatory bowel disease. Curr Opin Gastroenterol. 2011;27:321–327. doi: 10.1097/MOG.0b013e32834661b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nunes T, Fiorino G, Danese S, Sans M. Familial aggregation in inflammatory bowel disease: is it genes or environment? World J Gastroenterol. 2011;17:2715–2722. doi: 10.3748/wjg.v17.i22.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latiano A, Annese V. Genetics and ulcerative colitis: what are the clinical implications? Curr Drug Targets. 2011;12:1383–1389. doi: 10.2174/138945011796818171. [DOI] [PubMed] [Google Scholar]
- 5.Andersen V, Ernst A, Sventoraityte J, Kupcinskas L, Jacobsen BA, Krarup HB, Vogel U, Jonaitis L, Denapiene G, Kiudelis G, et al. Assessment of heterogeneity between European Populations: a Baltic and Danish replication case-control study of SNPs from a recent European ulcerative colitis genome wide association study. BMC Med Genet. 2011;12:139. doi: 10.1186/1471-2350-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Gao, Xing Liu, Ding Nu, Yue xian Li, Xue mei Jiang. Comparisons of clinical characteristics in patients with ulcerative colitis between Uygur and Han nationality in xinjiang. Zhonghua Xiaohua Neijing Zazhi. 2007;24:423–426. [Google Scholar]
- 7.Feng Gao, Zhi feng Wang. The diagnostic significance of antineutrophil cytoplasmic antibodies in patients of Uygur nationality and Hannationality with ulcerative colitis. Zhonghua Xiaohua Zazhi. 2001;21:611–613. [Google Scholar]
- 8.Wolpin BM, Kraft P, Xu M, Steplowski E, Olsson ML, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, et al. Variant ABO blood group alleles, secretor status, and risk of pancreatic cancer: results from the pancreatic cancer cohort consortium. Cancer Epidemiol Biomarkers Prev. 2010;19:3140–3149. doi: 10.1158/1055-9965.EPI-10-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magalhães A, Gomes J, Ismail MN, Haslam SM, Mendes N, Osório H, David L, Le Pendu J, Haas R, Dell A, et al. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology. 2009;19:1525–1536. doi: 10.1093/glycob/cwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gospodarek E, Zalas-Wiecek P. [Noroviruses--tactic of spread] Przegl Epidemiol. 2009;63:5–9. [PubMed] [Google Scholar]
- 11.Carlsson B, Kindberg E, Buesa J, Rydell GE, Lidón MF, Montava R, Abu Mallouh R, Grahn A, Rodríguez-Díaz J, Bellido J, et al. The G428A nonsense mutation in FUT2 provides strong but not absolute protection against symptomatic GII.4 Norovirus infection. PLoS One. 2009;4:e5593. doi: 10.1371/journal.pone.0005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kindberg E, Hejdeman B, Bratt G, Wahren B, Lindblom B, Hinkula J, Svensson L. A nonsense mutation (428G--& gt; A) in the fucosyltransferase FUT2 gene affects the progression of HIV-1 infection. AIDS. 2006;20:685–689. doi: 10.1097/01.aids.0000216368.23325.bc. [DOI] [PubMed] [Google Scholar]
- 13.Kinane DF, Blackwell CC, Brettle RP, Weir DM, Winstanley FP, Elton RA. ABO blood group, secretor state, and susceptibility to recurrent urinary tract infection in women. Br Med J (Clin Res Ed) 1982;285:7–9. doi: 10.1136/bmj.285.6334.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domino SE, Hurd EA, Thomsson KA, Karnak DM, Holmén Larsson JM, Thomsson E, Bäckström M, Hansson GC. Cervical mucins carry alpha(1,2)fucosylated glycans that partly protect from experimental vaginal candidiasis. Glycoconj J. 2009;26:1125–1134. doi: 10.1007/s10719-009-9234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrow AL, Meinzen-Derr J, Huang P, Schibler KR, Cahill T, Keddache M, Kallapur SG, Newburg DS, Tabangin M, Warner BB, et al. Fucosyltransferase 2 non-secretor and low secretor status predicts severe outcomes in premature infants. J Pediatr. 2011;158:745–751. doi: 10.1016/j.jpeds.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115:4635–4643. doi: 10.1182/blood-2010-01-261859. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Scheet P, Giusti B, Bandinelli S, Piras MG, Usala G, Lai S, Mulas A, Corsi AM, Vestrini A, et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. 2009;84:477–482. doi: 10.1016/j.ajhg.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haverkorn MJ, Goslings WR. Streptococci, ABO blood groups, and secretor status. Am J Hum Genet. 1969;21:360–375. [PMC free article] [PubMed] [Google Scholar]
- 19.McGovern DP, Jones MR, Taylor KD, Marciante K, Yan X, Dubinsky M, Ippoliti A, Vasiliauskas E, Berel D, Derkowski C, et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum Mol Genet. 2010;19:3468–3476. doi: 10.1093/hmg/ddq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 21.Rausch P, Rehman A, Künzel S, Häsler R, Ott SJ, Schreiber S, Rosenstiel P, Franke A, Baines JF. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci USA. 2011;108:19030–19035. doi: 10.1073/pnas.1106408108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrer-Admetlla A, Sikora M, Laayouni H, Esteve A, Roubinet F, Blancher A, Calafell F, Bertranpetit J, Casals F. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26:1993–2003. doi: 10.1093/molbev/msp108. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270:4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 24.Thorven M, Grahn A, Hedlund KO, Johansson H, Wahlfrid C, Larson G, Svensson L. A homozygous nonsense mutation (428G--& gt; A) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J Virol. 2005;79:15351–15355. doi: 10.1128/JVI.79.24.15351-15355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yip SP, Lai SK, Wong ML. Systematic sequence analysis of the human fucosyltransferase 2 (FUT2) gene identifies novel sequence variations and alleles. Transfusion. 2007;47:1369–1380. doi: 10.1111/j.1537-2995.2007.01280.x. [DOI] [PubMed] [Google Scholar]
- 26.Silva LM, Carvalho AS, Guillon P, Seixas S, Azevedo M, Almeida R, Ruvoën-Clouet N, Reis CA, Le Pendu J, Rocha J, et al. Infection-associated FUT2 (Fucosyltransferase 2) genetic variation and impact on functionality assessed by in vivo studies. Glycoconj J. 2010;27:61–68. doi: 10.1007/s10719-009-9255-8. [DOI] [PubMed] [Google Scholar]
- 27.Serpa J, Mendes N, Reis CA, Santos Silva LF, Almeida R, Le Pendu J, David L. Two new FUT2 (fucosyltransferase 2 gene) missense polymorphisms, 739G--& gt; A and 839T--& gt; C, are partly responsible for non-secretor status in a Caucasian population from Northern Portugal. Biochem J. 2004;383:469–474. doi: 10.1042/BJ20040803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koda Y, Soejima M, Kimura H. The polymorphisms of fucosyltransferases. Leg Med (Tokyo) 2001;3:2–14. doi: 10.1016/s1344-6223(01)00005-0. [DOI] [PubMed] [Google Scholar]
- 29.Wei TL, Su YQ, Wu GG, Cheng LH, Li DW. Study on the fUT2 gene structure of Xinjiang Uighur people of China. Zhonggu Shuxue Zazhi. 2004;17:306–307. [Google Scholar]
- 30.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6; discussion 16-19. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, Jiang Y, Lei Y, Zou K, Wang C, Huang S, Yi F, Xia B. Functional MICA-129 polymorphism and serum levels of soluble MICA are correlated with ulcerative colitis in Chinese patients. J Gastroenterol Hepatol. 2011;26:593–598. doi: 10.1111/j.1440-1746.2010.06524.x. [DOI] [PubMed] [Google Scholar]
- 32.Ye X, Jiang Y, Wang H, Chen L, Yuan S, Xia B. Genetic polymorphisms of glutathione S-transferases are associated with ulcerative colitis in central China. Cell Biochem Biophys. 2011;60:323–328. doi: 10.1007/s12013-011-9154-z. [DOI] [PubMed] [Google Scholar]
- 33.Harrison OJ, Maloy KJ. Innate immune activation in intestinal homeostasis. J Innate Immun. 2011;3:585–593. doi: 10.1159/000330913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng D, Newburg DS, Young C, Baker A, Tonkonogy SL, Sartor RB, Walker WA, Nanthakumar NN. Bacterial symbionts induce a FUT2-dependent fucosylated niche on colonic epithelium via ERK and JNK signaling. Am J Physiol Gastrointest Liver Physiol. 2007;293:G780–G787. doi: 10.1152/ajpgi.00010.2007. [DOI] [PubMed] [Google Scholar]
- 35.Iwamori M, Domino SE. Tissue-specific loss of fucosylated glycolipids in mice with targeted deletion of alpha(1,2)fucosyltransferase genes. Biochem J. 2004;380:75–81. doi: 10.1042/BJ20031668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collado MC, Meriluoto J, Salminen S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett Appl Microbiol. 2007;45:454–460. doi: 10.1111/j.1472-765X.2007.02212.x. [DOI] [PubMed] [Google Scholar]


