Abstract
AIM: To evaluate the efficacy and safety of emergency transcatheter arterial embolization (ETAE) for patients with acute massive duodenal ulcer hemorrhage.
METHODS: Twenty-nine consecutive patients with acute massive bleeding of duodenal ulcer were admitted to our hospital from 2006 to 2011. Superselective angiography of the celiac and gastroduodenal arteries was performed to find out the bleeding sites before ETAE, then, embolotherapy was done with gelatin sponge particles or microstrips via a 5 French angiographic catheter or 3 French microcatheter. After ETAE, further superior mesenteric arteriography was undertaken in case collateral circulation supplied areas of the duodenal ulcer. Technical and clinical success rates were analyzed. Changes in the mucous membrane were observed using endoscopy following ETAE.
RESULTS: Angiography showed active bleeding with extravasation of contrast medium in seven cases with a 24% positive rate of celiac artery bleeding, and in 19 cases with a 65.5% rate of gastroduodenal artery bleeding. There were no angiographic signs of bleeding in three patients who underwent endoscopy prior to ETAE. Twenty-six patients achieved immediate hemostasis and technical success rate reached 90%. No hemostasis was observed in 27 patients within 30 d after ETAE and clinical success rate was 93%. Recurrent hemorrhage occurred in two patients who drank a lot of wine who were treated by a second embolotherapy in the same way. Five patients underwent transient ischem with light abdominal pain under xiphoid, spontaneous restoration without special treatment. No mucous necrosis happened to 29 cases for ischem of gastroduodenal arteries embolized.
CONCLUSION: ETAE is an effective and safe measure to control acute massive bleeding of duodenal ulcer.
Keywords: Transcatheter embolization, Massive bleeding, Duodenal ulcer, Angiography
INTRODUCTION
Acute massive hemorrhage of duodenal ulcer is a common emergency. Emergency endoscopic hemostasis for duodenal ulcer is regarded as the first-choice therapy and considered to be the gold standard treatment[1-3]. However, endoscopic therapy does not successfully control bleeding or rebleeding in 8%-25% of patients[4-6]. Surgical treatment is a second choice after failure of endoscopic hemostatic therapy, and improves efficacy in these patients, but there is no decrease in mortality rate[7]. Most patients, especially, treated by endoscopy but rebleeding occurred, are physically weak, the indications of life are not stable, thus it is difficult to receive a second endoscopic or surgical treatment. Transcatheter arterial embolization has been performed for at least two to three decades and has been shown to be effective at controlling hemorrhage and decreasing mortality[7,8-12]. With the development of interventional radiology, emergency transcatheter arterial embolization (ETAE) has also been used for treatment of massive bleeding of duodenal ulcer because it is minimally invasive, repeatable, effective and safe for patients with advanced age, weakness and unstable blood pressure[3,13,14].
MATERIALS AND METHODS
Materials
We collected and retrospectively analyzed data from 29 consecutive patients with acute massive bleeding of duodenal ulcer, and a transfusion requirement of at ≥ 4 units of packed red blood cells per 24 h[3,15-17], who were admitted to our hospital from January 2006 to August 2011. There were 18 male and 11 female patients, aged 16-67 years, with an average age of 36 years. All cases were diagnosed by endoscopy, and 10 patients received endoscopy, before receiving angiography and artery embolization, which clearly indicated the existence of duodenal ulcer. Electrocoagulation was carried for treatment, including three patients who suffered from rebleeding after surgery, and then ETAE was conducted. Nineteen patients presented with massive bleeding or hemodynamic instability (hypotension with systolic pressure < 100 mmHg or clinical shock secondary to blood loss) that did not respond to conservative treatment combined with volume replacement and proton pump inhibitors. They were sent directly from the emergency room for interventional radiological angiographic diagnosis and ETAE. Three to five days after treatment, endoscopic diagnosis clearly indicated the presence of duodenal ulcer once again. Six to eleven units of blood were transfused before treatment and 2-4 units were transfused after ETAE, which aimed to correct severe anemia, instead of correcting blood volume and restoring blood pressure.
Operating methods and procedures of ETAE
Under digital subtraction angiography, we used the improved Seldinger technique to puncture the right or left femoral artery, inserted a 6 F arterial catheter sheath (Japan Terumo), for elderly patients with significant tortuosity of the femoral artery, and used a 6 F anti-bending catheter sheath to open the tortuous blood vessels, to facilitate the superselective intubation of the catheter. We used a 5 F Rosch or Yasiro radiography catheter to conduct celiac artery angiography, and a 0.035-inch ultra-smooth black guide wire, to superselect the radiography catheter for gastroduodenal artery angiography. For patients with significant tortuous vessels, w used a coaxial catheter technique to superselect the 3F SP microcatheter (Japan Terumo), under the support of the radiography catheter, for angiography of the gastroduodenal artery or its branches. For branch arteries with clear bleeding or for the bleeding branch arteries that the microcatheter could reach, we used the microcatheter technique. Microcatheters were used for embolization of 17 patients, 14 of whom underwent embolization of bleeding the branch arteries, and three of whom underwent embolization of duodenal arteries; 5 F catheters were used for 12 patients. After the bleeding sites were identified, the catheter or microcatheter was placed in the proximal end of the bleeding artery, and then gelatin sponge particles or sponge strips were injected via the catheter or microcatheter. The specifications of the sponge particles were: 1 mm × 1 mm × 1 mm; the specification of the sponge strips were 5 mm × 1 mm × 1 mm. Under continuous fluoroscopy, we used contrast agent to inject the embolic agent, stop injecting the sponge particles or the sponge strips once the contrast agent in corresponding bleeding artery or bleeding gastroduodenal artery slow flow or the contrast agent is held up. Superior mesenteric artery angiography should be carried out after gastroduodenal artery embolization, to prevent the establishment of collateral arterial blood supply of the branches from the superior mesenteric artery: the pancreaticoduodenal anterior inferior arcuate artery and pancreaticoduodenal posterior inferior arcuate artery. If collateral blood supply is found by radiography, there is a possibility of rebleeding, and arterial embolization must be carried out using a microcatheter. Conservative treatment is recommended after embolization: to give drugs for inhibition of gastric acid secretion and gastric mucosal protective agents and hemostasis, and inject fluid to correct hypovolemia. For patients who did not have preoperative endoscopy, 3-5 d after bleeding stopped, when they had stable blood pressure and partial recovery from weakness, we carried out endoscopy and Helicobacter pylori (H. pylori)-related examination. Patients who were H. pylori-positive were treated with oral antibacterial medication. Before 2008, follow-up was by outpatient visit and letter, and after that time, follow-up was by outpatient visit and telephone interview for 3-6 mo.
Evaluation of efficacy and safety
The bleeding artery after ETAE is occluded by angiography, or contrast agent extravasation disappears, and the blood pressure is stable; no further bleeding occurs within 30 d after ETAE, and the occult blood test of fecal within the 3-6 mo follow-up period is negative. The serious complications of ETAE are duodenal infarction or ischemic necrosis perforation, which are indicated by persistent upper abdominal (xiphoid) pain, peritoneal irritation, or muscle guarding sign.
Note for vascular grading: the 1st-grade-artery is abdominal aortic artery, the 2nd-grade-artery is celiac artery, the 3rd-grade-artery is gastroduodenal artery, and the 4th-grade-artery is branch arteries of gastroduodenal artery (the grading of blood vessels is defined by the authors, pending further discussion).
RESULTS
Among the 29 patients, there were three cases with multiple organ failure and seven elderly patients. Angiography with a celiac artery catheter (opening of the 2nd grade vessel) found bleeding in seven cases, with a positive rate of 24%; gastroduodenal arteriography (opening of the 3rd grade vessel) found signs of bleeding in 19 patients, with a positive rate of 66%; and there were three cases with no significant bleeding, with a negative proportion of 10%. Manifestations of hemorrhage were as follows: angiography of most celiac aorta hemorrhage was shown as the contrast agent gathering in small areas or with no imaging; angiography of gastroduodenal artery bleeding was shown as the contrast agent gathering into a lake-like area, with a large amount smearing the duodenal wall (Figures 1 and 2). There were 26 cases of immediate hemostasis after ETAE, with a technical success rate of 90%. The three cases that showed no significant escape of contrast agent on angiography were not included in the calculation of the technical success rate. Twenty-nine patients had stable blood pressure after ETAE. Four patients had a hemoglobin concentration of 4.5-5.0 g/L due to a large amount of bleeding before ETAE, and to correct anemia, 2-4 units of red blood cell suspension were infused. The amount of red blood cell suspension infused was significantly lower compared to 4-11 units before ETAE. The clinical success rate was 93% (27/29), and there were only two cases of rebleeding due to ulcers caused by heavy drinking. Angiography was carried out again and treated with the same procedure for embolization. After 3-6 mo follow-up, there was only one case of recurrence, and ETAE treatment was conducted once again. The first angiography of all the 3 cases of rebleeding showed positive bleeding.
Figure 1.
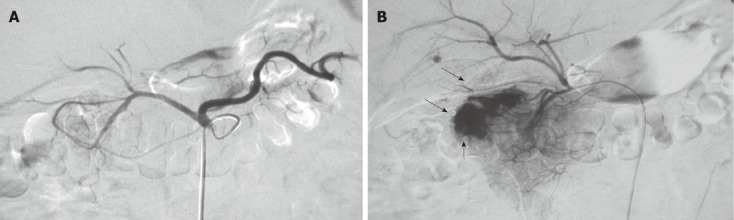
Impact of catheter position on the signs of bleeding. A: When the catheter tip was in the celiac artery, radiography failed to show signs of bleeding in the gastroduodenal artery; B: When the catheter super-select to the beginning site of the gastroduodenal artery for angiography, and the exo-contrast agent and smearing of the duodenal mucosa (arrows) are shown.
Figure 2.
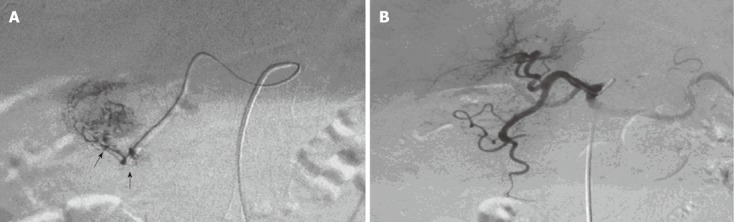
Angiographic changes before and after embolization of the gastroduodenal branch by microcatheter. A: Micro-catheter super-select to the gastroduodenal artery branch for angiography, and the exo-contrast agent is shown (indicated by the arrows); B: After embolization by micro-catheter, angiography showed that the bleeding had stopped; the distal end of the bleeding artery was kept open, to avoid widespread ischemia of normal tissue.
Five patients had showed transient upper abdominal pain due to acute ischemia after ETAE, and the symptoms relieved spontaneously without treatment. There was no occurrence of persistent upper abdominal pain, muscle guarding and other peritoneal irritation caused by severe gastroduodenal artery infarction, ischemia, or perforation.
Nineteen patients received endoscopy after ETAE. Seven of the 10 patients who had received endoscopy before ETAE received endoscopy again after treatment. Endoscopy showed that the normal duodenal mucosa in the embolization area showed pale change, and there was no case of necrosis. We distinguished complicated mucosal defects from previous duodenal ulcer by histological representations under endoscopic examination, which showed that complicated mucosal defects were not only around the areas near the duodenal ulcer but also far removed from them, distributed along the embolized artery.
Twenty-eight cases of duodenal ulcer were certainly related to H. pylori infection, and only in one case could the cause of duodenal ulcer not be identified. All patients who were H. pylori-positive took oral antibacterial medication during follow-up.
DISCUSSION
Efficacy of medical conservative therapy for upper gastrointestinal hemorrhage caused by duodenal ulcer is poor. Endoscopy and endoscopic hemostasis is the recognized method for first-line treatment[1-3,18,19]. The incidence of rebleeding after initial endoscopic treatment is about 14.1%, and in 15%-25% of cases, the bleeding cannot be controlled endoscopically or rebleeding requires alternative treatment[20,21], and surgery has become such an alternative[22,23]. However, for elderly patients with other associated diseases[24], and patients after surgery, rebleeding is a poor prognostic factor for massive hemorrhage of duodenal ulcer, with mortality of 44%-60%[3]. In recent years, interventional radiology has played an increasingly important role in the emergency treatment of the disease. Angiography cannot only quickly and clearly identify the bleeding site, but ETAE has become an effective alternative treatment for patients with recurrence after endoscopic and surgical treatment[12,25]. It has even been reported that ETAE can be the preferred treatment for emergency gastrointestinal hemorrhage[7,26,27].
The efficacy of interventional treatment is largely influenced by the treatment devices and methods. From the mid-1980s to mid-1990s, the diameter of the catheter used ranged between 5 and 6 F, and the texture of the catheter was coarse. Therefore, it was difficult to carry out super-selective catheterization, which meant that most patients with gastroduodenal bleeding due to arterial rupture used transcatheter perfusion of vasopressin to shrink arterial lesions, to stop the bleeding. However, local perfusion of drugs still achieved a good technical success rate. Miller et al[28] have reported that there were 267 patients who received perfusion therapy between 1979 and 1984; the success rate was 70%-80%, but the rebleeding rate was still nearly 20%, and for perfusion patients with difficulty in hemostasis, the rebleeding rate was up to 40%. The reason was that some elderly patients had hypertension, coronary atherosclerosis, or myocardial infarction and other underlying diseases, even partial use of vasopressin, its usage amount and continuous drug use time were still limited. In addition, the rich collateral circulation still provide much more blood supply for the bleeding duodenal intestinal. In recent years, with improvements of the technical skills of catheter, guide wire and other devices and the extensive use of coax system of micro-catheter (2.4-2.6 French size)[29], and arterial intubation by super-selection to the 3rd-grade or more above and the embolization have been skillfully used in most units, which has ensured the accuracy and effectiveness of ETAE, and has ensured blood supply of normal tissues. From our ETAE results of 29 patients, the success rate and clinical success rate were 90% and 93%, respectively. The results basically agreed with the technical success rate of 95% and clinical success rate of 52%-95% reported previously for ETAE treatment of upper gastrointestinal hemorrhage[29,30]. Earlier reports have shown that only two of seven cases of embolization had rebleeding, and four of six patients with conservative treatment had rebleeding. Toyoda et al[31] have reported control of bleeding after endoscopic treatment of 11 patients with duodenal ulcer. The bleeding was stopped in 10 patients treated with ETAE, with an efficacy of 91%. The remaining patient had atherosclerosis and embolization could only be applied to the proximal gastroduodenal artery, and as the embolization of the distal artery has established collateral circulation which led to rebleeding.
The patients with hemorrhage of gastroduodenal ulcer who had been clearly diagnosed by endoscopy and treated showed intermittent bleeding, while angiography could not clearly indicate the signs of bleeding, and there were three such patients in this group. Are such patients treated with ETAE? Although they were not included in calculating the technical success rate of ETAE, we believe that stopping bleeding or negative performance in angiography is only an illusion, which is related to low blood volume, vasoconstrictor drugs, or short-term thrombosis caused by hemostatic agents. If not handled, once the blood pressure is corrected, the contracted blood vessel expands again, or thrombolysis occurs, and rebleeding occurs. Under such circumstances, ETAE should still be applied[13], and the three patients treated by ETAE achieved clinical success.
For patients with multiple organ failure, although there were only two cases in this group of patients, randomized controlled studies could not be carried out. ETAE treatment effectively controlled massive bleeding caused by arterial rupture, and both of the two cases achieved technical and clinical success. There were another seven elderly patients, and only one had rebleeding after ETAE treatment, due to arteriosclerosis and tortuous vessels. Only the proximal gastroduodenal artery was embolized, and the branch of the superior mesenteric artery - pancreatic collateral arteries was open. Hemostasis was achieved after super-selective embolization of the collateral branch of the superior mesenteric artery. At the same time, the patient’s situation reminded us that angiography of the superior mesenteric artery can be used as a routine procedure before ETAE, by which rebleeding caused by collateral circulation of the duodenum can be avoided. In general, second endoscopic treatment, subsequent reoperation and unfavorable prognosis do not adversely affect the effective implementation of ETAE; on the contrary, ETAE can be repeatedly used for partial treatment of rebleeding due to its minimally invasive nature. It can be rapidly implemented because there is no need for preoperative preparation, and it can effectively control bleeding because it is a real-procedure and it accurately shows the bleeding site.
From the results of this group of patients, we can conclude that the treatment of duodenal massive bleeding using ETAE is safe, without severe ischemic complications such as duodenal infarction[32,33]. After ETAE treatment, some patients showed short-term duodenal ischemic symptoms, xiphoid pain or secret anguish, but these were relieved spontaneously without treatment. We initially selected, without experience, small particles as embolic agents, with a diameter of 300-500 µm. This resulted in mucosal defects far removed from the immediate area of the duodenal ulcer, which were distributed extensively along the embolized artery. For a few patients, endoscopy after ETAE showed that there were mild ischemic changes in the duodenal mucosa, manifested as the normal mucosa surrounding the ulcers was pale, but there were no ulceration and necrosis of normal mucosa after arterial embolization. Literature reported 28 patients with gastroduodenal distal artery embolized, and 7 cases had duodenal stenosis caused by ischemia, and for the 29 cases with arterial proximal embolized, only two cases showed the aforementioned pathological changes. The reason is related to mixed use of various embolic agents, including liquid embolic agents, at the same as ETAE treatment. There were 32 cases reported in another group, ETAE caused two cases of duodenal necrosis and one of new ulcer formation[34]. This was related to the use of gelatin sponge powder. Both liquid embolic agents and gelatin sponge powder can cause damage to the submucosal microvascular network, which can lead to loss of the compensatory blood supply of the mucosa.
We used large gelatin particles or strips of gelatin sponge for embolization of the proximal gastroduodenal artery. even the embolization of small bleeding artery is also under the premise that the bleeding targeting is a very clear, to implement the micro-catheter super-selective ETAE, that is on the two levels of the diameter of embolic agent and embolization range, to ensure the submucosal microvascular network is not damaged, thus the complications reported in the literature did not occur. In terms of safety, we do not agree with the approach proposed by Lee et al[35] in choosing the type of embolic agents. They have used the liquid embolic agent N-butyl-2-cyanoacrylate to embolize 16 patients, and two patients showed a large area of thrombosis and multiple gastric ulcers. Therefore, choosing embolic agents of the right size and superselective catheterization and controlling the embolization range can guarantee ETAE safety.
In conclusion, ETAE is an effective, safe and fast emergency procedure for the treatment of massive bleeding of duodenal ulcer, which is especially suitable for frail elderly patients or those with multiorgan dysfunction with bleeding duodenal ulcer.
COMMENTS
Background
Acute massive hemorrhage of duodenal ulcer is a life-threatening condition. Emergency endoscopic hemostasis for duodenal ulcer is regarded as first-line therapy and considered to be the gold standard treatment. Surgery is usually considered for secondary intervention after failure of endoscopic hemostasis, or rebleeding. Transcatheter endovascular embolization is an alternative method of surgical treatment for stopping bleeding.
Research frontiers
Emergency transcatheter arterial embolization (ETAE) is an alternative to hemostasis after failure of endoscopic treatment. In the area of hemostasis for massive bleeding of duodenal ulcer, the research hotspot is to evaluate the efficacy and success rate of ETAE, and whether it becomes a first-line therapy.
Innovations and breakthroughs
Transcatheter arterial embolization has become a mature and effective method of hemostasis for arterial bleeding, but it is still controversial whether it can become the first choice for massive gastrointestinal hemorrhage. It has been successful in treating peptic ulcer bleeding. The innovations of this study were that the authors adopted the supra-selected arterial angiography to increase the positive rate of diagnosis and identify sites of ulcer bleeding. They also used it as the first-line treatment for some particular patients, such as older patients, the extra-weakness and the unstable-pressure with acute massive ulcer hemorrhage.
Applications
The results suggest that ETAE via supraselection angiography is an effective and safe therapy and could be an alternative, even first-choice therapy, for endoscopic hemostasis in some patients with acute massive hemorrhage of duodenal ulcer.
Terminology
Transcatheter arterial embolization: In peptic ulcer, trauma and other injury to the arterial system results in arterial rupture and massive hemorrhage, a catheter is inserted into the target artery, and contrast agent is injected to locate the ruptured vessel. Other agents can then be injected via the catheter to block the artery.
Peer review
This was a case series of 29 patients who received ETAE for acute massive hemorrhage of duodenal ulcer. The success rate of hemostasis was 90%. Recurrent hemorrhage occurred in two patients. Severe complications were not found. In conclusion, transcatheter arterial embolization is an effective and safe procedure to control acute massive bleeding of duodenal ulcer. It has some minor problems.
Footnotes
Peer reviewer: Mototsugu Kato, MD, Department of Endoscopy, Hokkaido University Hospital, Nishi-5, Kita-14, Kita-ku, Sapporo 060-8648, Japan
S- Editor Cheng JX L- Editor Kerr C E- Editor Xiong L
.
References
- 1.Liou TC, Lin SC, Wang HY, Chang WH. Optimal injection volume of epinephrine for endoscopic treatment of peptic ulcer bleeding. World J Gastroenterol. 2006;12:3108–3113. doi: 10.3748/wjg.v12.i19.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson CB, Mitchell RM. Nonvariceal upper gastrointestinal bleeding: standard and new treatment. Gastroenterol Clin North Am. 2005;34:607–621. doi: 10.1016/j.gtc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Ichiro I, Shushi H, Akihiko I, Yasuhiko I, Yasuyuki Y. Empiric transcatheter arterial embolization for massive bleeding from duodenal ulcers: efficacy and complications. J Vasc Interv Radiol. 2011;22:911–916. doi: 10.1016/j.jvir.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Lau JY, Sung JJ, Lam YH, Chan AC, Ng EK, Lee DW, Chan FK, Suen RC, Chung SC. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751–756. doi: 10.1056/NEJM199903113401002. [DOI] [PubMed] [Google Scholar]
- 5.Chiu PW, Lam CY, Lee SW, Kwong KH, Lam SH, Lee DT, Kwok SP. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: a prospective randomised trial. Gut. 2003;52:1403–1407. doi: 10.1136/gut.52.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong SK, Yu LM, Lau JY, Lam YH, Chan AC, Ng EK, Sung JJ, Chung SC. Prediction of therapeutic failure after adrenaline injection plus heater probe treatment in patients with bleeding peptic ulcer. Gut. 2002;50:322–325. doi: 10.1136/gut.50.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rösch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102:303–306. doi: 10.1148/102.2.303. [DOI] [PubMed] [Google Scholar]
- 8.Funaki B. Endovascular intervention for the treatment of acute arterial gastrointestinal hemorrhage. Gastroenterol Clin North Am. 2002;31:701–713. doi: 10.1016/s0889-8553(02)00025-0. [DOI] [PubMed] [Google Scholar]
- 9.Encarnacion CE, Kadir S, Beam CA, Payne CS. Gastrointestinal bleeding: treatment with gastrointestinal arterial embolization. Radiology. 1992;183:505–508. doi: 10.1148/radiology.183.2.1561358. [DOI] [PubMed] [Google Scholar]
- 10.Lang EV, Picus D, Marx MV, Hicks ME. Massive arterial hemorrhage from the stomach and lower esophagus: impact of embolotherapy on survival. Radiology. 1990;177:249–252. doi: 10.1148/radiology.177.1.2399325. [DOI] [PubMed] [Google Scholar]
- 11.Ljungdahl M, Eriksson LG, Nyman R, Gustavsson S. Arterial embolisation in management of massive bleeding from gastric and duodenal ulcers. Eur J Surg. 2002;168:384–390. doi: 10.1080/110241502320789050. [DOI] [PubMed] [Google Scholar]
- 12.Holme JB, Nielsen DT, Funch-Jensen P, Mortensen FV. Transcatheter arterial embolization in patients with bleeding duodenal ulcer: an alternative to surgery. Acta Radiol. 2006;47:244–247. doi: 10.1080/02841850600550690. [DOI] [PubMed] [Google Scholar]
- 13.Poultsides GA, Kim CJ, Orlando R, Peros G, Hallisey MJ, Vignati PV. Angiographic embolization for gastroduodenal hemorrhage: safety, efficacy, and predictors of outcome. Arch Surg. 2008;143:457–461. doi: 10.1001/archsurg.143.5.457. [DOI] [PubMed] [Google Scholar]
- 14.Loffroy R, Guiu B. Role of transcatheter arterial embolization for massive bleeding from gastroduodenal ulcers. World J Gastroenterol. 2009;15:5889–5897. doi: 10.3748/wjg.15.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii Y, Shimada H, Endo I, Yoshida K, Matsuo K, Takeda K, Ueda M, Morioka D, Tanaka K, Togo S. Management of massive arterial hemorrhage after pancreatobiliary surgery: does embolotherapy contribute to successful outcome? J Gastrointest Surg. 2007;11:432–438. doi: 10.1007/s11605-006-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loffroy R, Rao P, Ota S, De Lin M, Kwak BK, Geschwind JF. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010;33:1088–1100. doi: 10.1007/s00270-010-9829-7. [DOI] [PubMed] [Google Scholar]
- 17.Goldman ML, LAND WC, Bradley EL, Anderson RT. Transcatheter therapeutic embolization in the management of massive upper gastrointestinal bleeding. Radiology. 1976;120:513–521. doi: 10.1148/120.3.513. [DOI] [PubMed] [Google Scholar]
- 18.Defreyne L, De Schrijver I, Decruyenaere J, Van Maele G, Ceelen W, De Looze D, Vanlangenhove P. Therapeutic decision-making in endoscopically unmanageable nonvariceal upper gastrointestinal hemorrhage. Cardiovasc Intervent Radiol. 2008;31:897–905. doi: 10.1007/s00270-008-9320-x. [DOI] [PubMed] [Google Scholar]
- 19.Chung IK, Kim EJ, Lee MS, Kim HS, Park SH, Lee MH, Kim SJ, Cho MS, Hwang KY. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy. 2001;33:969–975. doi: 10.1055/s-2001-17951. [DOI] [PubMed] [Google Scholar]
- 20.Larssen L, Moger T, Bjørnbeth BA, Lygren I, Kløw NE. Transcatheter arterial embolization in the management of bleeding duodenal ulcers: a 5.5-year retrospective study of treatment and outcome. Scand J Gastroenterol. 2008;43:217–222. doi: 10.1080/00365520701676443. [DOI] [PubMed] [Google Scholar]
- 21.Song JS, Kwak HS, Chung GH. Nonvariceal upper gastrointestinal bleeding: the usefulness of rotational angiography after endoscopic marking with a metallic clip. Korean J Radiol. 2011;12:473–480. doi: 10.3348/kjr.2011.12.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung FK, Lau JY. Management of massive peptic ulcer bleeding. Gastroenterol Clin North Am. 2009;38:231–243. doi: 10.1016/j.gtc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Abe N, Takeuchi H, Yanagida O, Sugiyama M, Atomi Y. Surgical indications and procedures for bleeding peptic ulcer. Dig Endosc. 2010;22 Suppl 1:S35–S37. doi: 10.1111/j.1443-1661.2010.00966.x. [DOI] [PubMed] [Google Scholar]
- 24.Khanna A, Ognibene SJ, Koniaris LG. Embolization as first-line therapy for diverticulosis-related massive lower gastrointestinal bleeding: evidence from a meta-analysis. J Gastrointest Surg. 2005;9:343–352. doi: 10.1016/j.gassur.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 25.Wong TC, Wong KT, Chiu PW, Teoh AY, Yu SC, Au KW, Lau JY. A comparison of angiographic embolization with surgery after failed endoscopic hemostasis to bleeding peptic ulcers. Gastrointest Endosc. 2011;73:900–908. doi: 10.1016/j.gie.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Venclauskas L, Bratlie SO, Zachrisson K, Maleckas A, Pundzius J, Jönson C. Is transcatheter arterial embolization a safer alternative than surgery when endoscopic therapy fails in bleeding duodenal ulcer? Scand J Gastroenterol. 2010;45:299–304. doi: 10.3109/00365520903486109. [DOI] [PubMed] [Google Scholar]
- 27.Burris JM, Lin PH, Johnston WF, Huynh TT, Kougias P. Emergent embolization of the gastroduodenal artery in the treatment of upper gastrointestinal bleeding. The experience from a surgeon-initiated interventional program. Am J Surg. 2009;198:59–63. doi: 10.1016/j.amjsurg.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 28.Miller M, Smith TP. Angiographic diagnosis and endovascular management of nonvariceal gastrointestinal hemorrhage. Gastroenterol Clin North Am. 2005;34:735–752. doi: 10.1016/j.gtc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Loffroy RF, Abualsaud BA, Lin MD, Rao PP. Recent advances in endovascular techniques for management of acute nonvariceal upper gastrointestinal bleeding. World J Gastrointest Surg. 2011;3:89–100. doi: 10.4240/wjgs.v3.i7.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schenker MP, Duszak R, Soulen MC, Smith KP, Baum RA, Cope C, Freiman DB, Roberts DA, Shlansky-Goldberg RD. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12:1263–1271. doi: 10.1016/s1051-0443(07)61549-8. [DOI] [PubMed] [Google Scholar]
- 31.Toyoda H, Nakano S, Takeda I, Kumada T, Sugiyama K, Osada T, Kiriyama S, Suga T. Transcatheter arterial embolization for massive bleeding from duodenal ulcers not controlled by endoscopic hemostasis. Endoscopy. 1995;27:304–307. doi: 10.1055/s-2007-1005697. [DOI] [PubMed] [Google Scholar]
- 32.Shapiro N, Brandt L, Sprayregan S, Mitsudo S, Glotzer P. Duodenal infarction after therapeutic Gelfoam embolization of a bleeding duodenal ulcer. Gastroenterology. 1981;80:176–180. [PubMed] [Google Scholar]
- 33.Milosavljevic T, Kostić-Milosavljević M, Jovanović I, Krstić M. Complications of peptic ulcer disease. Dig Dis. 2011;29:491–493. doi: 10.1159/000331517. [DOI] [PubMed] [Google Scholar]
- 34.Keeling WB, Armstrong PA, Stone PA, Zweibel BR, Kudryk BT, Johnson BL, Back MR, Bandyk DF, Shames ML. Risk factors for recurrent hemorrhage after successful mesenteric arterial embolization. Am Surg. 2006;72:802–806; discussion 802-806. [PubMed] [Google Scholar]
- 35.Lee CW, Liu KL, Wang HP, Chen SJ, Tsang YM, Liu HM. Transcatheter arterial embolization of acute upper gastrointestinal tract bleeding with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2007;18:209–216. doi: 10.1016/j.jvir.2006.12.003. [DOI] [PubMed] [Google Scholar]


