Summary
Background
Robust evidence of the effectiveness of task shifting of antiretroviral therapy (ART) from doctors to other health workers is scarce. We aimed to assess the effects on mortality, viral suppression, and other health outcomes and quality indicators of the Streamlining Tasks and Roles to Expand Treatment and Care for HIV (STRETCH) programme, which provides educational outreach training of nurses to initiate and represcribe ART, and to decentralise care.
Methods
We undertook a pragmatic, parallel, cluster-randomised trial in South Africa between Jan 28, 2008, and June 30, 2010. We randomly assigned 31 primary-care ART clinics to implement the STRETCH programme (intervention group) or to continue with standard care (control group). The ratio of randomisation depended on how many clinics were in each of nine strata. Two cohorts were enrolled: eligible patients in cohort 1 were adults (aged ≥16 years) with CD4 counts of 350 cells per μL or less who were not receiving ART; those in cohort 2 were adults who had already received ART for at least 6 months and were being treated at enrolment. The primary outcome in cohort 1 was time to death (superiority analysis). The primary outcome in cohort 2 was the proportion with undetectable viral loads (<400 copies per mL) 12 months after enrolment (equivalence analysis, prespecified difference <6%). Patients and clinicians could not be masked to group assignment. The interim analysis was blind, but data analysts were not masked after the database was locked for final analysis. Analyses were done by intention to treat. This trial is registered, number ISRCTN46836853.
Findings
5390 patients in cohort 1 and 3029 in cohort 2 were in the intervention group, and 3862 in cohort 1 and 3202 in cohort 2 were in the control group. Median follow-up was 16·3 months (IQR 12·2–18·0) in cohort 1 and 18·0 months (18·0–18·0) in cohort 2. In cohort 1, 997 (20%) of 4943 patients analysed in the intervention group and 747 (19%) of 3862 in the control group with known vital status at the end of the trial had died. Time to death did not differ (hazard ratio [HR] 0·94, 95% CI 0·76–1·15). In a preplanned subgroup analysis of patients with baseline CD4 counts of 201–350 cells per μL, mortality was slightly lower in the intervention group than in the control group (0·73, 0·54–1.00; p=0·052), but it did not differ between groups in patients with baseline CD4 of 200 cells per μL or less (0·94, 0·76–1·15; p=0·577). In cohort 2, viral load suppression 12 months after enrolment was equivalent in intervention (2156 [71%] of 3029 patients) and control groups (2230 [70%] of 3202; risk difference 1·1%, 95% CI −2·4 to 4·6).
Interpretation
Expansion of primary-care nurses' roles to include ART initiation and represcription can be done safely, and improve health outcomes and quality of care, but might not reduce time to ART or mortality.
Funding
UK Medical Research Council, Development Cooperation Ireland, and Canadian International Development Agency.
Introduction
Since 2006, efforts to increase access to antiretroviral therapy (ART) in Africa have emphasised task shifting—ie, delegation of clinical tasks from doctors to other health-care workers.1 However, robust evidence of its effectiveness is scarce. A 2010 systematic review of task shifting in care of patients with HIV infection2 showed that it is effective and can provide high-quality care, but of 25 original studies reviewed, only 11 made comparisons with alternatives, and only two of those were randomised trials. Neither trial assessed the effect of task shifting on mortality in people awaiting ART, which in both was initiated by doctors.3,4
In South Africa, a major obstacle to ART expansion has been the shortage of doctors available to initiate treatment, because of an absolute shortfall and also because doctors spend much of their time represcribing ART. Delayed ART initiation has resulted in high mortality rates in patients who are eligible for ART but waiting for treatment.5,6 Thus, evidence from randomised trials is needed on whether other health workers can effectively and safely identify patients eligible for ART, start treatment, and then follow up and represcribe. In South Africa, nurses provide most primary care for the general population.
Streamlining Tasks and Roles to Expand Treatment and Care for HIV (STRETCH) is a complex health systems intervention with educational and organisational components. It trains nurses to assume responsibility for ART initiation and represcribing. It combines an educational outreach training model (Practical Approach to Lung Health in South Africa; PALSA and PALSA PLUS)7–9—previously shown to effectively improve care for respiratory disorders, tuberculosis, and HIV before ART is used—with additional organisational components. STRETCH is intended to rationalise ART and other services for people with HIV infection, to treat patients already stabilised on ART at clinics close to their homes, to increase the number of clinics in which ART can be initiated, and to raise the number of clinics and nurses providing high-quality pretreatment care. The purpose of our study was to assess the effects of STRETCH on mortality, viral suppression, and other health outcomes and quality indicators, compared with the present system in which only doctors can prescribe ART.
Methods
Study design and participants
We undertook a pragmatic, parallel, cluster-randomised trial in the Free State province of South Africa.10 The province's public-sector ART programme started in 2004, in designated nurse-led primary-care clinics and doctor-led hospital outpatient departments. Patients are assessed and prepared for ART by nurses and referred to doctors for initiation and represcriptions. High mortality of patients awaiting treatment initiation by doctors5 caused the provincial health department to introduce STRETCH and to commission us to assess its effect on outcomes for patients.
We enrolled patients from all 31 clinics participating in the ART programme between Jan 28, 2008, and June 30, 2009, and completed follow-up on June 30, 2010. We enrolled two cohorts to allow us to simultaneously assess the effect of the intervention when patients became eligible for ART initiation, and for individuals on long-term ART. Patients in cohort 1 were adults (aged ≥16 years) with CD4 counts of 350 cells per μL or less who had not yet started ART. They were either eligible for ART (CD4 ≤200 cells per μL) or likely to become eligible during the trial (CD4 201–350 cells per μL). They were followed up for at least 12 months. Patients in cohort 2 were adults who had already received ART for at least 6 months and were being treated at the time of enrolment. In clinics with more than 100 patients eligible for cohort 2, a random sample was taken electronically (sample size proportional to total number of eligible patients); in other clinics, all eligible patients were included. We excluded patients from both cohorts if they did not return to their clinic after enrolment, because they needed to visit a clinic more than once to initiate ART after obtaining CD4 results in cohort 1 or to be potentially exposed to the intervention in cohort 2.
The trial protocol was approved by the research ethics committees of the faculties of health sciences at the University of Cape Town (Cape Town, South Africa) and the University of the Free State (Bloemfontein, South Africa). Clinic managers provided written informed consent to take part in the trial. Informed consent was not requested from patients because the intervention was educational and managerial, and was aimed at entire clinics and their staff, not at individual patients, so all patients in the same clinic would be exposed to the same intervention irrespective of whether they consented to participate.10,11 Patients in intervention clinics were given written information about the trial and the intervention. We adhered to ethical principles for use of medical records for research without patients' consent:10,12 the research had clear public benefit; we obtained approval for the study from lead doctors and nurses managing the programme; use of data for research did not affect individuals' care; data were already being used by the research team for programme assessment on behalf of the provincial health department; and data confidentiality was strictly enforced. Only specific data managers had access to personal identifiers. Anonymised data were provided only to the principal investigators (LF, MOB), the lead statistician, and a health economist. With hundreds of patients in each clinic, individuals could not be identified from clinic names.
Randomisation and masking
Clinics and their patients were randomly assigned to either of two parallel groups. Randomisation was done within nine strata—one for each referral hospital in the province—to avoid confounding of outcomes by variation in care provided by doctors in each hospital. One stratum contained four clinics and another two clinics; the even numbers meant that randomisation could be done in a 1:1 ratio. The other seven strata contained odd numbers of clinics and were randomly allocated to have either one more or one less intervention clinic than control clinics with simple random sampling in nQuery Advisor. Six strata each had three clinics. Three of those were randomised with a ratio of two intervention clinics to one control clinic. The remaining three were randomised with a ratio of one intervention clinic to two control clinics. The last stratum had seven clinics, and was randomised with a ratio of four intervention clinics to three control clinics. Within each stratum, clinics were randomly assigned to intervention or control according to sequences of random numbers in a random number table (even numbers for control and odd numbers for intervention), with separate sequences for each stratum. In total, we had 16 intervention clinics and 15 control clinics. The trial statistician (CL) undertook the randomisation before the trial started. Masking of patients and clinicians was not possible because implementation of the intervention was obvious. The interim analysis was blind, but data analysts were not masked after the database was locked for final analysis.
Procedures
The model of care in the control clinics was the standard of HIV and ART care in provincial health services of the Free State before the trial, and was continued during the trial (appendix). It was consistent with public sector health services in most parts of South Africa. Patients diagnosed with HIV infection were referred to designated nurse-led clinics to establish whether they were eligible for ART. According to treatment protocols at the time, adults were eligible for ART when their CD4 count was less than 200 cells per μL, they had had stage IV HIV infection (AIDS),13 or were pregnant with a CD4 count of less than 350 cells per μL.14 Patients not yet eligible for ART received routine care, such as regular CD4 testing, until they became eligible. Patients eligible for ART were referred to ART treatment sites in hospital outpatient departments for initiation of treatment and review of ART prescriptions every 3–6 months, both done by a doctor. To comply with national regulations that require ART to be dispensed by or under the supervision of pharmacists, who were not always located in clinics (appendix), drugs were dispensed at treatment sites in patient-named packages, then delivered to clinics where nurses issued them to patients every month in-between doctor visits. In some remote areas, visiting doctors provided ART initiation and on-site represcription in nurse-led clinics. Nurses in both control and intervention clinics continued to receive educational outreach training in the use of PALSA PLUS, which includes management of HIV infection and AIDS but not ART prescribing. Control clinics continued to receive routine managerial support and monitoring.
Intervention clinics implemented STRETCH (appendix).15 Care of patients with HIV infection differed from control clinics in several ways. Prescribing nurses received at least four educational outreach training sessions about ART prescribing and side-effects with a special edition of the PALSA PLUS guidelines, which included algorithms to start and to monitor patients on ART, and to identify those needing referral to a doctor.15 Patients had to meet certain criteria for nurse initiation and represcription of ART (appendix). Patients who did not meet these criteria were referred to doctors who, unlike nurses, were authorised to initiate tailored regimens, to change prescriptions, and to prescribe second-line drugs.
Nurse middle managers, who had already been trained as outreach trainers for PALSA PLUS, participated in an additional 2·5 day training course about STRETCH and delivered STRETCH educational outreach training to all nursing staff at every intervention clinic. After training, 103 nurses in intervention clinics were registered with the Free State's Pharmaceutical Services Department and authorised to initiate first-line ART drugs and repeat ART prescriptions during the trial. 24 doctors who supported these nurses were familiarised with the guidelines by the STRETCH trial co-ordinator (a doctor experienced in care of patients with HIV infection; KU).
The intervention was implemented in three phases to give nurses time to gain confidence with ART. First, training was delivered and the STRETCH trial coordinator visited every intervention clinic to establish a STRETCH team who were responsible for support of phased decentralisation of care. Second, nurses assumed responsibility for represcribing ART for patients already receiving treatment. The care for all stable patients given ART was consolidated in their clinic, so they did not need to travel to another treatment site for represcriptions. Third, nurses began to initiate ART in eligible patients. The rate of implementation was set by clinic staff, allowing well functioning clinics to progress rapidly, while others took longer times. Implementation was phased between January, and December, 2008.
All 16 intervention clinics successfully implemented phases one and two; two clinics could not implement phase three because of difficulties with staffing and drug distribution, but remained in the trial. In each randomisation stratum, the first date on which patients in intervention and control clinics were enrolled in the trial was the date that the last intervention clinic started implementation of phase three to ensure that patients in both groups were enrolled at similar times. In the two strata with the intervention clinics unable to proceed to phase three, enrolment of patients in intervention and control clinics started on Dec 1, 2008.
Data for individual patients were obtained from routine electronic medical records that had been implemented as part of the treatment programme in 27 clinics. At every clinical visit, information was written on paper forms by clinicians and entered into the province's computer system by clerks in the clinic. At the four clinics without electronic records, research fieldworkers entered specific variables from paper forms in patients' folders into an electronic database.
We identified deaths from programme data and by linkage with the national mortality register with national identity numbers. The national mortality register is based on death certification and records 90% of all deaths,16 including those that occur at home or in hospital that are not noted by the ART programme. We linked individuals' medical record data with the provincial health department's laboratory, hospital admission, and tuberculosis databases.
Data were downloaded to a central database every week. We implemented routine checks of data quality to minimise missing and unreliable data, prioritising variables used to assess eligibility and primary outcome measures. For example, patients with missing national identity numbers, or numbers that did not match their recorded date of birth or did not conform to the standard identity number algorithm used in South Africa were identified every 2–4 weeks. Eight research fieldworkers travelled to the trial clinics and searched for missing information in patients' paper records. A dedicated database manager co-ordinated data collection, management, and linkage, and reported weekly on enrolment, follow-up, and data quality.
The primary outcome for cohort 1 was time from enrolment to death. Each patient's follow-up was censored 12–18 months after enrolment, depending on whether patients were enrolled towards the end or beginning of the process, to ensure comparable follow-up across clinics that started implementation early or late. Secondary outcomes were measures of health status (changes in weight and CD4 cell counts, viral loads, hospital admissions, and inpatient days) and indicators of quality of care (ART initiation, time from enrolment to start of ART, detection of tuberculosis, co-trimoxazole provision, programme retention 1 year after enrolment, baseline CD4 cell count in patients who started ART, and clinic consultations with nurses and doctors).
For cohort 2, the primary outcome was the proportion with undetectable viral loads (<400 copies per mL) 1 year after enrolment. Secondary outcomes were measures of health status (time to death censored 12–18 months after enrolment, changes in weight and CD4 cell counts, hospital admissions, and inpatient days) and indicators of quality of care (programme retention, diagnosis of tuberculosis, co-trimoxazole provision, switching of ART regimens, and clinic consultations with nurses and doctors).
Statistical analysis
For cohort 1, sample size was calculated for a superiority trial, because we hoped that STRETCH would increase access to ART and thus reduce mortality in the intervention group. We analysed previous programme data for patients with initial CD4 counts of 350 cells per μL or less in the trial clinics between 2004, and 2007, and noted that 29% of patients followed up for at least 1 year died within that year, with an intra-clinic correlation coefficient (ICC) of 0·01. A sample size of 6000 (3000 per group) would provide 90% power to detect a 6% difference in 1-year mortality (24% vs baseline frequency of 30%) at the 5% significance level (two-sided), assuming ICC was 0·01. On the basis of a 10% dropout rate in our previous trial,7 the sample size was increased to 7400. This increased sample size was a conservative adjustment with the denominator (1–[rate of loss to follow-up])2—ie, 6400/(0·92)—which accounts for the dropout in the intervention group and the proportion receiving the standard of care.17 We planned for, and completed, an interim analysis 1 year after recruitment started. Neither of the prespecified stopping rules (difference between groups in either primary outcome with p<0·001)10 for either cohort were met, and the trial monitoring committee recommended that the trial continue. However, the analysis of pooled data showed that the 1-year mortality rate was lower than had been previously assumed; therefore, more patients were enrolled into cohort 1 than was originally planned.
For cohort 2, sample size was calculated for an equivalence trial, because we hoped to show that nurse-led ART would be as effective in maintenance of viral suppression as is doctor-led treatment. In previous programme data, 82% of patients who had received ART for 12 months had undetectable viral loads, with an ICC of 0·005. A sample size of 4000 (2000 per group) would provide 90% power to show equivalence between groups with a 6% equivalence limit, with 5% significance and an ICC of 0·005. To allow for 10% dropout, the sample size was increased to 4900 (ie, 4000/[0·92]). This 6% equivalence limit was smaller than was the 9% equivalence limit for viral suppression used in the Jinja trial.3 The interim analysis of pooled data showed that the proportion of patients with a measured viral load measured after 1 year was lower than had been previously assumed; therefore more patients were enrolled into cohort 2 than was originally planned.
Effects of the intervention were estimated by comparisons of patients in the intervention and control groups with multiple regression models and Huber-White robust adjustment of errors for intra-cluster correlation of outcomes; they were stratified by randomisation strata with Stata (version 11.1). All clinics and patients were analysed in the treatment group to which they were randomly assigned (intention-to-treat). Time from enrolment to death was analysed with Cox proportional hazards models. Time from enrolment to ART initiation was analysed by competing risks regression,6 with death as a competing risk. For these time-to-event analyses, follow-up was censored on June 30, 2010, or 18 months after enrolment, whichever was earlier, thus providing 12–18 months of follow-up.
For the preplanned subgroup analysis of patients in cohort 1, we included an allocation-subgroup interaction term in the Cox model to separately estimate the effect of the intervention on survival in patients with CD4 counts higher and lower than 200 cells per μL at enrolment. We used binomial regression to estimate differences in proportions of patients with suppressed viral loads, and other secondary outcomes in cohort 2. We calculated risk ratios for secondary binary outcomes for cohort 1. We used linear regression to compare changes in CD4 count and weight in both cohorts, by comparing values at the end of follow-up while adjusting for the corresponding baseline values (ANCOVA).18 Poisson regression was used to estimate incidence rate ratios for count outcomes such as clinic visits, accounting for individuals' duration of follow-up. Secondary analyses further adjusted for potentially confounding baseline characteristics such as presence of a national identification number (potentially affecting ascertainment of death), baseline CD4 cell count, age, and sex.
This trial is registered, number ISRCTN46836853.
Role of the funding source
The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, and LF and MOB share final responsibility for the decision to submit for publication.
Results
Figures 1 and 2 show the trial profiles for each cohort. All 31 clinics completed the trial. Median follow-up was 16·3 months (IQR 12·2–18·0) in cohort 1 and 18·0 months (18·0–18·0) in cohort 2. In cohort 1 (figure 1), the median number of patients enrolled per clinic was 210 in the intervention group (IQR 154–323) and 260 (143–345) in the control group. More patients were enrolled in the intervention group than in the control group because it had one more clinic and, by chance, two intervention clinics had high numbers of eligible patients (1377 and 959 compared with a maximum of 596 in control clinics). Median duration of follow-up in this cohort was 16·4 months (IQR 12·4–18·0) in the intervention group and 16·3 months (11·5–18·0) in the control group. In cohort 2 (figure 2), the median number of patients enrolled per clinic was 176 (97–251) in the intervention group and 134 (96–349) in the control group. Median duration of follow-up in cohort 2 was 18·0 months (18·0–18·0) in both groups. Control group clinics tended to be smaller and were more likely to have on-site doctor support: 8/15 (53%) of control clinics and 5/16 (31%) of intervention clinics had a full or part time doctor available for on-site ART initiation. Table 1 shows patients' baseline characteristics.
Figure 1.
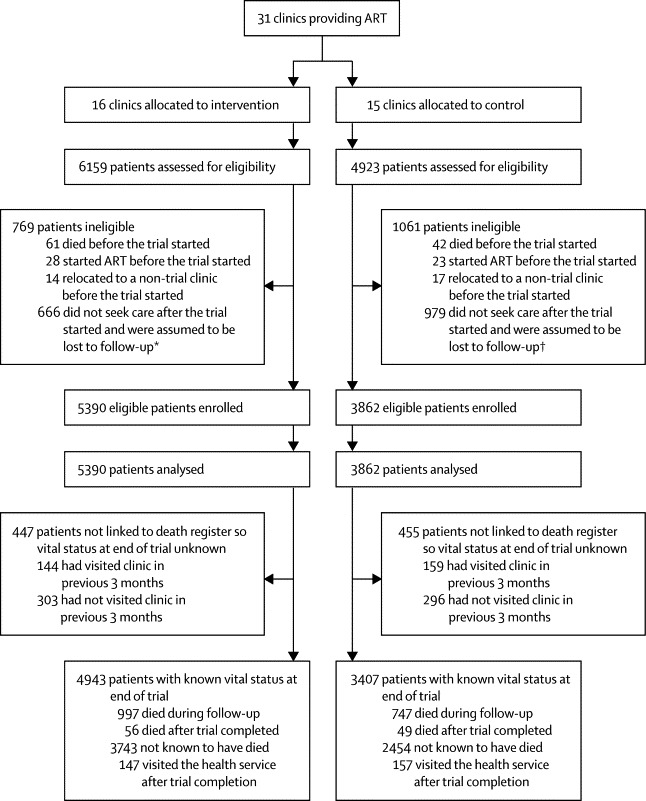
Trial profile for cohort 1
ART=antiretroviral therapy. *105 of these patients died after the trial started. †119 of these patients died after the trial started.
Figure 2.
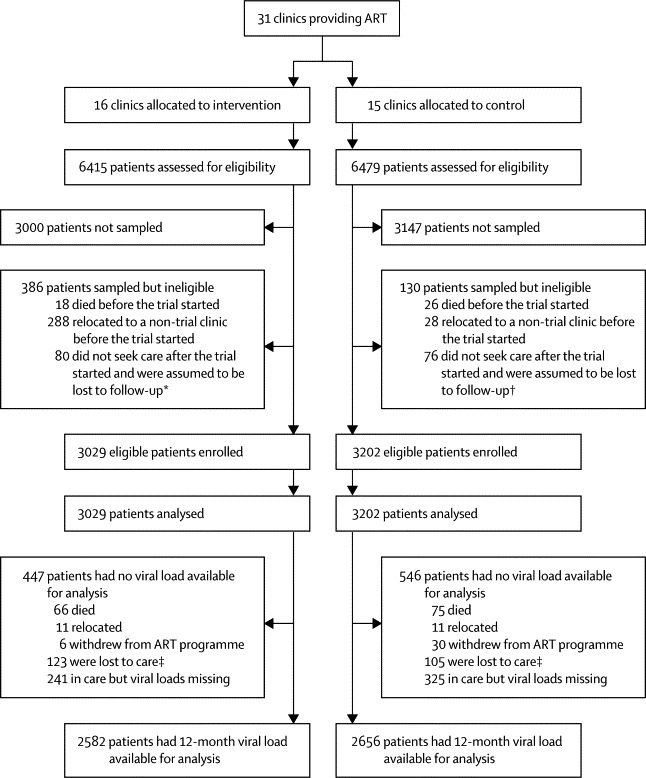
Trial profile for cohort 2
ART=antiretroviral therapy. *22 of these patients died after the trial started. †22 of these patients died after the trial started. ‡After 12 months of follow-up, patients had been recorded as withdrawn or relocated, or they had had no clinic visit or laboratory test in the previous 6 months, and we had no documentation of death.
Table 1.
Baseline characteristics by cohort
| Intervention group | Control group | ||
|---|---|---|---|
| Cohort 1 | |||
| Number of patients | 5390 | 3862 | |
| Women | 3604 (67%) | 2681 (69%) | |
| Age (years) | 36 (30–43) | 35 (29–42) | |
| National identity number recorded | 4767 (88%) | 3184 (82%) | |
| CD4 (cells per μL) | 141 (70–201) | 137 (70–197) | |
| 0–49 | 934 (17%) | 678 (18%) | |
| 50–99 | 949 (18%) | 720 (19%) | |
| 100–199 | 2141 (40%) | 1547 (40%) | |
| 200–350 | 1366 (25%) | 917 (24%) | |
| WHO stage recorded* | 3057 (57%) | 1719 (45%) | |
| Stage I | 1582/3057 (52%) | 551/1719 (32%) | |
| Stage II | 637/3057 (21%) | 470/1719 (27%) | |
| Stage III | 725/3057 (24%) | 653/1719 (38%) | |
| Stage IV | 113/3057 (4%) | 45/1719 (3%) | |
| Weight recorded | 4400 (82%) | 2875 (74%) | |
| Weight (kg) | 59 (14) | 58 (14) | |
| Present tuberculosis | 301 (6%) | 200 (5%) | |
| Admitted in the year before enrolment | 392 (7%) | 313 (8%) | |
| Cohort 2 | |||
| Number of patients | 3029 | 3202 | |
| Women | 2113 (70%) | 2332 (73%) | |
| Age (years) | 38 (32–44) | 38 (32–45) | |
| National identity number recorded | 2859 (94%) | 2958 (92%) | |
| Duration on ART (months) | 13·9 (6·8–21·7) | 13·7 (7·3–22·3) | |
| ART regimen | |||
| First line (stavudine, lamivudine, efavirenz) | 1846 (61%) | 2056 (64%) | |
| First line (stavudine, lamivudine, nevirapine) | 1012 (33%) | 1011 (32%) | |
| Second line (zidovudine, didanosine, lopinavir) | 37 (1%) | 28 (1%) | |
| Other | 109 (4%) | 100 (3%) | |
| Not known | 25 (1%) | 7 (<1%) | |
| Viral load <400 copies per mL | 2378 (79%) | 2507 (78%) | |
| Weight recorded | 2886 (95%) | 3128 (98%) | |
| Weight (kg) | 61 (13) | 62 (13) | |
| Present tuberculosis | 241 (8%) | 186 (6%) | |
| Admitted in the year before enrolment | 282 (9%) | 299 (9%) | |
Data are n (%), median (IQR), n/N (%), or mean (SD). ART=antiretroviral theraoy.
Staged just before initiation of ART, usually after enrolment.
In cohort 1, 997 (20%) of 4943 patients with known vital status at the end of the trial analysed in the intervention group and 747 (19%) of 3862 analysed in the control group died (ICC 0·008; figure 1). Time to death did not differ between groups (table 2, figure 3). Adjustment for baseline characteristics did not change this result (table 2, appendix). The preplanned subgroup analysis10 showed that intervention-group patients with CD4 counts of 201–350 cells per μL at enrolment had a 27% lower risk of death than did those in the control group, but this difference was not significant; we recorded no difference between groups in patients with CD4 counts of 200 cells per μL or less at enrolment (table 2, figure 3). In patients with CD4 counts of 201–350 cells per μL, adjustment for characteristics strengthened the association between the intervention and mortality (table 2).
Table 2.
Effect of the intervention on time from enrolment to death in cohort 1
|
Intervention group |
Control group |
Hazard ratio (95% CI) | p value | Adjusted hazard ratio (95% CI)† | Adjustedp value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of deaths | Person-years at risk | Hazard of death per 100 person-years at risk (95% CI)* | Number of deaths | Person-years at risk | Hazard of death per 100 person-years at risk (95% CI)* | |||||
| Primary analysis (n=9252) | 997 | 74 256 | 1·34 (1·26–1·43) | 747 | 51 861 | 1·44 (1·34–1·55) | 0·94 (0·76–1·15) | 0·532 | 0·92 (0·76–1·12) | 0·401 |
| Subgroup analysis: baseline CD4 count 201–350 cells per μL (n=2283) | 102 | 20 710 | 0·06 (0·03–0·10) | 90 | 13 224 | 0·68 (0·55–0·84) | 0·73 (0·54–1·00)‡ | 0·052 | 0·70 (0·52–0·95)§¶ | 0·020 |
| Subgroup analysis: baseline CD4 count ≤200 cells per μL (n=6969) | 895 | 53 546 | 1·67 (1·56–1·78) | 657 | 38 637 | 1·70 (1·57–1·83) | 1·00 (0·80–1·24) | 0·999 | 0·94 (0·77–1·16) | 0·577 |
Binomial exact confidence intervals.
Adjusted for patients' age, sex, CD4 cell count at enrolment, and record of an identity number.
Interaction between group and CD4 cell count stratum p=0·050.
Adjusted for patients' age, sex, and record of an identity number.
Interaction term between group and CD4 cell count stratum p=0·049.
Figure 3.
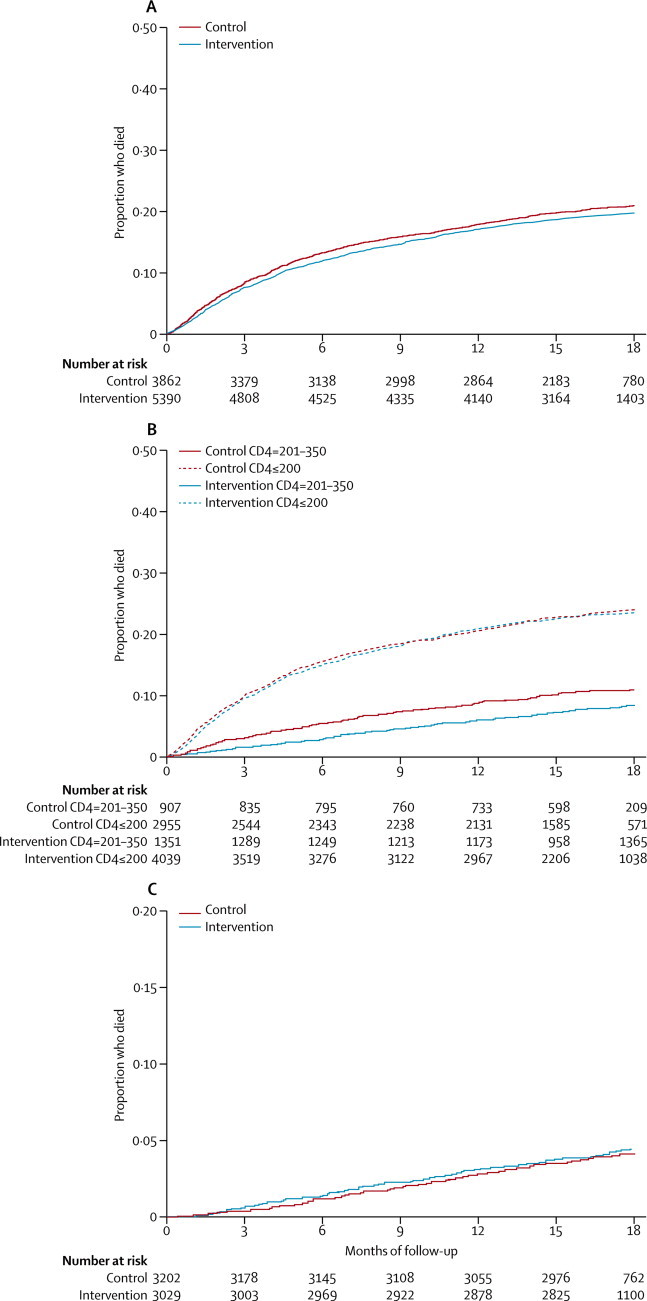
Kaplan-Meier curves of time to death
(A) Cohort 1. (B) CD4 subgroups of cohort 1. (C) Cohort 2.
With pooling of patients in intervention and control groups in cohort 1, ART was associated with a 47% lower risk of death than no treatment (hazard ratio [HR] 0·53, 95% CI 0·42–0·68). The strength of this association did not differ between intervention and control groups (data not shown). Detection of tuberculosis, programme retention, and CD4 cell count at the end of follow-up were higher in the intervention group than in the control group (table 3). In the intervention group, 965 (26%) of 3712 ART initiations were by a nurse; in the control group, none were.
Table 3.
Secondary outcomes in cohort 1
| Intervention group | Control group |
Effect estimate* |
p value | Intra-cluster correlation coefficient | Regression model* | ||
|---|---|---|---|---|---|---|---|
| Type | Estimate (95% CI) | ||||||
| Started on ART | 3712/5390 (69%) | 2418/3862 (63%) | Risk ratio | 1·24 (0·88 to 1·73) | 0·218 | 0·065 | Binomial |
| Time to ART†‡ | .. | .. | Subdistribution hazard ratio | 1·14 (0·92 to 1·43) | 0·232 | 0·065 | Competing risk |
| New tuberculosis diagnosis | 1057/5390 (20%) | 510/3862 (13%) | Risk ratio | 1·46 (1·18 to 1·81) | 0·001 | 0·051 | Binomial |
| Received co-trimoxazole prophylaxis | 3899/5390 (72%) | 2767/3862 (72%) | Risk ratio | 1·03 (0·93 to 1·13) | 0·608 | 0·149 | Binomial |
| Programme retention§ | 3373/5390 (63%) | 2254/3862 (58%) | Risk ratio | 1·10 (1·04 to 1·16) | <0·001 | 0·019 | Binomial |
| Baseline CD4 cell count of patients starting ART | 132 (82); n=3470 | 131 (82); n=2083 | Difference in means | 0·102 (−13·1 to 13·4) | 0·988 | 0·030 | Linear |
| Suppressed viral load in patients who started ART¶ | 1706/2375 (72%) | 1062/1449 (73%) | Risk ratio | 0·97 (0·90 to 1·03) | 0·324 | 0·040 | Binomial |
| Proportion with a missing viral load in patients who started ART | 1274/3712 (34%) | 945/2219 (43%) | Risk ratio | 0·86 (0·71 to 1·04) | 0·120 | 0·014 | Binomial |
| Weight at follow-up (kg) | 62·6 (14·0); n=2712 | 62·4 (13·7); n=1503 | Difference in means | 0·10 (−1·35 to 1·56) | 0·884 | 0·019 | Linear |
| CD4 count at follow-up (cells per μL) | 161·3 (175·2); n=2345 | 141·7 (161·6); n=1544 | Difference in means | 22·3 (3·6 to 40·9) | 0·021 | 0·026 | Linear |
Data are n/N (%) or mean (SD), unless otherwise stated. ART=antiretroviral therapy.
Regression models adjusted for randomisation strata and intra-cluster correlation of outcomes.
Follow-up censored, so no mean time to ART listed.
Adjusted for the competing risk of death.
Patients were judged to be retained by the programme when after 12 months they were alive, were not known to have withdrawn or relocated, and had documentation of a clinic visit or laboratory test in the previous 6 months (if started ART or last known CD4 count was less than 200 cells per μL) or in the past 9 months (if they had not started ART and last known CD4 count was more than 200 cells per μL).
Patients with at least 6 months of ART and viral load results available.
In cohort 2, viral suppression a year after enrolment did not differ between intervention and control patients, and the prespecified equivalence limit of 6% was met (table 4). Adjustment for patients' baseline characteristics did not change this result (appendix). Gains in CD4 cell count and weight (data not shown), and probability of switching ART drugs (table 4), were higher in the intervention group than in the control group. 47 (77%) of 61 patients in the intervention group who switched regimens had documentation of detectable viraemia beforehand, compared with 20 (74%) of 27 patients in the control group, suggesting that the increased numbers of switches in the intervention group were appropriate. Time to death did not differ between groups (figure 3).
Table 4.
Primary and secondary outcomes in cohort 2
| Intervention group | Control group |
Effect estimate* |
p value | Intra-cluster correlation coefficient | Regression model* | ||
|---|---|---|---|---|---|---|---|
| Type | Estimate (95% CI) | ||||||
| Primary outcome | |||||||
| Suppressed viral load† | 2156/3029 (71%) | 2230/3202 (70%) | Risk difference | 1·1% (−2·3 to 4·6) | 0·534 | 0·010 | Binomial |
| Secondary outcomes | |||||||
| Time to death‡ | .. | .. | Hazard ratio | 1·05 (0·84 to 1·31) | 0·684 | 0·005 | Cox |
| Programme retention§ | 2733/3029 (90%) | 2926/3202 (91%) | Risk difference | −0·3% (−2·1 to 1·54) | 0·758 | 0·013 | Binomial |
| New tuberculosis diagnosis | 119/3029 (4%) | 113/3202 (4%) | Risk difference | 0·21% (−0·40 to 0·84) | 0·487 | 0·019 | Binomial |
| Received co-trimoxazole prophylaxis | 2143/3029 (71%) | 2578/3202 (81%) | Risk difference | 9·8% (−33·7 to 14·2) | 0·424 | 0·477 | Binomial |
| Change in ART drugs during trial | 161/3029 (5%) | 57/3202 (2%) | Risk difference | 1·25% (0·65 to 1·86) | <0·001 | 0·044 | Binomial |
| Weight at follow-up (kg) | 63·0 (13·5); n=2136 | 63·2 (14·1); n=2271 | Difference in means | 0·62 (0·01 to 1·23) | 0·045 | 0·010 | Linear |
| CD4 count at follow-up (cells per μL) | 438·8 (219·5); n=1733 | 418·4 (201·8); n=1691 | Difference in means | 24·2 (7·2 to 41·3) | 0·007 | 0·007 | Linear |
Data are n/N (%) or mean (SD), unless otherwise stated.
Regression models adjusted for randomisation strata and intra-cluster correlation of outcomes.
All patients enrolled in the trial were included in the denominator; of these 2308/3029 (76%) of patients in intervention group and 2499/3202 (78%) in control group had been receiving ART for more than 2 years when viral load was measured; 1084/3029 (36%) patients in intervention group and 1125/3202 (35%) in control group had been receiving ART for more than 3 years.
Follow-up censored, so no mean time to time to death listed.
Patients were judged to be retained by the programme when after 12 months they were alive, not known to have withdrawn or relocated, and had documentation of a clinic visit or laboratory test in the previous 6 months.
Patients in the intervention group visited nurses more often than did those in the control group in both cohorts, and doctors in cohort 1 (appendix). Adverse events of interest were deaths and admissions to hospital (figures 1, 2, appendix).
Discussion
We have shown that task shifting of the primary responsibility for ART from doctors to primary-care nurses in a large-scale public sector programme did not improve survival of patients not yet taking ART with CD4 counts of 350 cells per μL or less, but did in patients with CD4 counts of 201–350 cells per μL, although the difference was not significant. It did achieve its second primary goal of equivalent viral load suppression in patients already taking ART at enrolment. The 95% CI for the comparison of viral load suppression were more precise in our study than in the Jinja trial of ART task shifting,3 because our sample size was larger. Additionally, the STRETCH intervention improved several other health outcomes and quality indicators. No outcomes were worse in intervention groups than in control ones.
Our encouraging evidence supports task shifting of ART from doctors to nurses and other health workers, which seems essential for ART expansion in South Africa and elsewhere in Africa. Since our trial ended in 2010, South African national policy has changed to promote nurse initiation and management of ART.19 However, if such a strategy is implemented without sufficient clinical and management support, it could be less effective than the STRETCH programme was in our trial.
Our trial was done to a high standard, with enrolment exceeding our planned sample sizes, and with data for primary outcomes available for 94% of participants. With two cohorts of patients, we could simultaneously assess effects on both short-term and long-term care. Linkage of electronic clinical, laboratory, hospital, and mortality data made the examination of a wide range of health outcomes and indicators of care quality possible for large and generalisable samples of patients. However, our study was limited by the restriction of follow-up to 18 months. Furthermore, we were missing data for weight and CD4 cell count in both cohorts, and for viral load after 12 months of ART in cohort 1.
Outside of the trial, the Free State Health Department attempted to improve access to doctors so as to accelerate ART provision from July, 2008. Doctors who were part of the ART programme were instructed to visit specific clinics to review problem cases, to complete represcriptions, and to initiate patients on ART. This change in programme might have unintentionally favoured the control group if these doctors thereby provided more intensive and expert care than would otherwise have been available. Intervention clinics were less likely to have doctor-provided ART initiation on site at the start of the trial and by the middle of the study, this disparity had increased with on-site doctors at 11 (73%) of 15 control clinics and seven (44%) of 16 intervention clinics. Therefore, a clinic's ability to expedite ART initiation in patients whom nurses thought needed to be assessed by a doctor might have been reduced in intervention groups.
This pragmatic trial realistically shows practical problems with large-scale implementation of ART in Africa. Several reasons could explain why the intervention did not accelerate ART initiation or reduce mortality in cohort 1, and why only a quarter of patients who started ART had treatment initiated by nurses. First, our qualitative research showed that many STRETCH nurses were initially hesitant to initiate ART when they had the option of referrals to doctors. Second, allocation of increased numbers of doctors to control clinics during the trial probably placed intervention clinics at a comparative disadvantage. Third, difficulties with funding and delivery of ART to clinics reduced STRETCH nurses' ability to initiate ART promptly. For example, initiation of ART was suspended for 3 months from November, 2008, to February, 2009, because the provincial health department temporarily exhausted its ART budget,20 as in other countries when donor funding has decreased.21 Fourth, during the trial several clinics that were not in the study started to provide ART. This change could have reduced the workload of trial clinics, thus decreasing the extent to which STRETCH could accelerate ART initiation compared with control clinics. The favourable results for cohort 1 patients with CD4 counts of 201–350 cells per μL at enrolment, and for cohort 2 patients, suggest that nurses in intervention clinics could competently build on what they had done before—ie, preparation of patients for ART initiation and monitoring of those already on ART. However, the subgroup analysis of mortality in cohort 1 should be interpreted with caution, because the difference in effects between subgroups was moderate, the intervention–subgroup interaction was marginally significant, and the subgroups defined by CD4 cell count were not precisely defined in advance.
Nurses in the intervention group had little trouble with task shifting of represcriptions from doctors, which relieved doctors of a heavy burden and enabled them to focus on referred patients who were seriously ill. Biological evidence that intervention patients received more effective treatment than did those in the control group included the large increase in CD4 cell count in both cohorts and weight gain in cohort 1. In cohort 1, patients in the intervention group were more likely to remain in the programme and to have tuberculosis identified than were those in control clinics, and in cohort 2, switching of regimens occurred more in intervention than control clinics, indicating that STRETCH training and guidelines improved the delivery of appropriate care. Increased regimen switching could have resulted from nurses having good awareness of adverse treatment effects or drug resistance, leading to referrals to doctors authorised to switch regimens.
Our trial is unique because we included and followed up patients who had not yet started ART, and because the intervention included nurse initiation of ART. In the two most similar trials of ART task shifting—the CIPRA trial in South Africa4 and the Jinja trial in Uganda3 (panel)—treatment was initiated by doctors but followed up by nurses and non-medical field officers, respectively. These trials3,4 also provided substantially more training than we did in our trial, and patients who had not yet started ART were not followed up. However, our finding that outcomes were no worse in intervention than in control groups is in keeping with their results.
Panel. Research in context.
Systematic review
We searched PubMed and Google Scholar for randomised trials assessing task shifting of antiretroviral therapy (ART) published at any time before Oct 31, 2011, with the search terms “antiretroviral”, “task-shifting” or “nurse” or “community health worker”, and “trial”. We identified three randomised trials of ART task shifting: cluster-randomised trials from Uganda3 and Kenya,22 and the individually randomised CIPRA trial from South Africa.4 Another trial from Uganda23 was excluded because it investigated the addition of community-based follow-up to clinic-based care, and so did not entail task shifting as defined by WHO.1 The Ugandan3 and Kenyan22 trials compared clinic-based with community-based ART follow-up. The CIPRA trial4 compared ART follow-up by nurses with follow-up by doctors, both provided at clinics. After ART initiation in one clinic, the Ugandan study3 was based in 44 geographical areas, but the Kenyan one22 was in only one clinic and CIPRA4 was in two. All three trials had similar outcomes (viral load suppression, CD4 cell counts, and loss to follow-up) for patients on ART. In the Ugandan3 and South African4 trials, ART was initiated by doctors. None of these trials enrolled patients who had not yet started ART but were eligible or would soon be eligible. All three trials showed no significant difference in outcomes.
Interpretation
Our results are in keeping with the Ugandan,3 Kenyan,22 and CIPRA4 trials and support provision of ART follow-up care by non-physicians. The generalisability and feasibility of implementation of our programme are supported by its basis in many clinics throughout a province. The suppression of viral load in patients who were already receiving ART at enrolment is similar to that reported for the Ugandan trial.3 However, our trial provides original evidence of the effectiveness of a nurse-led system on the clinically challenging task of ART initiation, including for patients recently or newly enrolled in the treatment programme. We have shown that expansion of nurses' roles to include ART initiation can be done safely and can improve health outcomes and quality of care, but that time to ART initiation or mortality did not change. Several observational studies support the role of non-physician clinicians in provision of ART care, but few are of programmes in which ART initiation is led by non-physician clinicians.2 Taken together, our study and the others we have identified suggest that the present approach of non-physician clinicians expanding ART programmes in resource-constrained environments is safe and feasible.
The high mortality of cohort 1 patients is of concern, although it is lower than the proportion who died within 1 year of enrolment in these clinics before 2008 (29%), and much lower than the proportion who died in the province before 2006 (87%),5 continuing the trends of decreasing mortality in South African ART programmes over time.24
STRETCH is thus an effective and feasible method of rapidly expanding ART provision in South Africa and other countries where shortages of doctors restrict access to ART. The increased rates of clinic visits to both doctors and nurses in cohort 1 could constrain implementation, although the lower rates and duration of admission in the intervention group than in the control group in cohort 1, and the task shifting of clinic visits from doctors to nurses in cohort 2, indicate that resources are used more efficiently with the programme than without. The cost-effectiveness of the intervention will be reported separately. The suitability of this approach in countries where access to physicians is even more restricted than in South Africa or is non-existent should be assessed in a separate trial. Our results are relevant to other countries in Africa because they show that non-physician health workers can provide comprehensive ART care, including ART initiation, after just four additional short training sessions. Our training methods and guideline design have been previously assessed and are already being implemented in The Gambia and Malawi.25,26
Acknowledgments
Acknowledgments
Funding for this trial was provided by the UK Medical Research Council, Development Cooperation Ireland, and the Canadian International Development Agency. We thank intervention clinic nurses who were willing to take on additional clinical responsibilities despite their tremendous workloads; doctors, clinic managers, pharmacists and pharmacy assistants at ART sites and primary-care services; Pat Mayers and our STRETCH trainers; local area and district managers; district ART coordinators; the Free State Department of Health's Pharmaceutical and Therapeutics Committee; Wendy Adolph, Elsie Pretorius and our team of data clerks; senior managers from the department including Sipho Kabane, Moeder Khokho, Lache Katzen, Portia Shai-Mhathu, Yolisa Tsibolane, Sam Boleme, Roeleen Booi, Lydia van Turha, Hettie Marais, and Palesa Santho; the National Health Laboratory Service for providing electronic blood result data; members of our trial monitoring and steering committees (George Swingler, Victor Lithlakanyane, Chris Butler, Margaret May, Dave Sackett, Douglas Wilson, David Kalambo, Elizabeth Corbett, and Nokhewzi Hoboyi); Garry Barton; and the patients who participated, especially those who died before they could be started on ART.
Contributors
LF, MOB, CL, MZ, AB, RCo, CvV, DS, and EB designed the protocol. LF, MOB, and RCh obtained funding. LF, KU, GF, and RCo developed and implemented the intervention. LF, VT, KU, and EK oversaw data collection, data cleaning, merging of datasets, and preparation of extracts for analysis. CL led the analysis with assistance from LF and MOB. All authors contributed to interpretation of findings and preparation of the report, and have approved the final version.
Conflicts of interest
The Knowledge Translation Unit of the University of Cape Town Lung Institute (to which LF, VT, KU, DG, GF, RCo, BD, and EB are affiliated) provides training in PALSA PLUS and STRETCH to the South African and Western Cape Departments of Health. The other authors declare that they have no conflicts of interest.
Supplementary Material
References
- 1.WHO Task shifting: global recommendations and guidelines. 2008. http://www.who.int/workforcealliance/knowledge/resources/taskshifting_guidelines/en/index.html (accessed June 25, 2012).
- 2.Callaghan M, Ford N, Schneider H. A systematic review of task-shifting for HIV treatment and care in Africa. Hum Resour Health. 2010;8:8. doi: 10.1186/1478-4491-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffar S, Amuron B, Foster S. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in Jinja, southeast Uganda: a cluster-randomised equivalence trial. Lancet. 2009;374:2080–2089. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanne I, Orrell C, Fox MP. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fairall LR, Bachmann MO, Louwagie G. Effectiveness of antiretroviral treatment in a South African program: cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 6.Ingle S, May M, Uebel K. Outcomes in patients waiting for antiretroviral treatment in the Free State Province, South Africa: prospective linkage study. AIDS. 2010;24:2717–2725. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairall L, Zwarenstein M, Bateman ED. Educational outreach to nurses improves tuberculosis case detection and primary care of respiratory illness: a pragmatic cluster randomised controlled trial. BMJ. 2005;331:750–754. doi: 10.1136/bmj.331.7519.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwarenstein M, Fairall LR, Lombard C. Outreach education integrates HIV/AIDS/ART and tuberculosis care in South African primary care clinics: a pragmatic cluster randomized trial. BMJ. 2011;342:d2022. doi: 10.1136/bmj.d2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann MO, Fairall LR, Lombard C. Effect on tuberculosis outcomes of educational outreach to South African clinics during two randomised trials. Int J Tuberculosis Lung Dis. 2010;14:311–317. [PubMed] [Google Scholar]
- 10.Fairall LR, Bachmann MO, Zwarenstein MF. Streamlining tasks and roles to expand treatment and care for HIV: randomised controlled trial protocol. Trials. 2008;9:21. doi: 10.1186/1745-6215-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medical Research Council Cluster Randomised Trials: methodological and ethical considerations. Nov 11, 2002. http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC002406 (accessed Aug 10, 2011).
- 12.Haines A, Ashcroft R, Coggon D. Personal information in medical research. Oct 9, 2000. http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC002452 (accessed Aug 10, 2011).
- 13.South African National Department of Health National antiretroviral treatment guidelines: 1st edn. 2004. http://www.doh.gov.za/docs/facts/2004/intro.pdf (accessed June 25, 2012).
- 14.Knowledge Translation Unit. University of Cape Town Lung Institute . Practical Approach to Lung Health and HIV/AIDS (PALSA PLUS) Guidelines. Knowledge Translation Unit; Cape Town: 2006. [Google Scholar]
- 15.Uebel KU, Fairall LR, van Rensburg HCJ. Task shifting and integration of HIV care into primary care in South Africa: the development and content of the streamlining tasks and roles to expand treatment and care of HIV (STRETCH) intervention. Implement Sci. 2011;6:86. doi: 10.1186/1748-5908-6-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statistics South Africa . Mortality and causes of death in South Africa, 1997–2003: findings from death notification. Statistics South Africa; Pretoria: 2005. [Google Scholar]
- 17.Donner A. Approaches to sample size estimation in the design of clinical trials—a review. Stat Med 198; 3: 199–214. [DOI] [PubMed]
- 18.Vickers AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323:1123–1124. doi: 10.1136/bmj.323.7321.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colvin CJ, Fairall L, Lewin S. Expanding access to ART in South Africa: the role of nurse initiated treatment. S Afr Med J. 2010;100:210–211. doi: 10.7196/samj.4124. [DOI] [PubMed] [Google Scholar]
- 20.Bateman C. Free State ARV crisis—central government blamed. S Afr Med J. 2009;99:284–287. [PubMed] [Google Scholar]
- 21.Boseley S. Crisis looms as Global Fund forced to cut back on AIDS, malaria and TB grants. Nov 23, 2011. http://www.guardian.co.uk/society/sarah-boseley-global-health/2011/nov/23/aids-tuberculosis (accessed April 11, 2012).
- 22.Selke HM, Kimaiyo S, Sidle JE. Task-shifting of antiretroviral delivery from health care workers to persons with HIV/AIDS: clinical outcomes of a community-based program in Kenya. J Acquir Immune Defic Syndr. 2010;55:483–490. doi: 10.1097/QAI.0b013e3181eb5edb. [DOI] [PubMed] [Google Scholar]
- 23.Chang LW, Kagaayi J, Nakigozi G. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomised trial. PLoS One. 2010;5:e10923. doi: 10.1371/journal.pone.0010923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornell M, Grimsrud A, Fairall L. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS. 2010;24:2263–2270. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schull MJ, Cornick R, Thompson S. From PALSA PLUS to PALM PLUS: adapting and developing a South African guideline and training intervention to better integrate HIV/AIDS care with primary care in rural health centers in Malawi. Implement Sci. 2011;6:82. doi: 10.1186/1748-5908-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schull MJ, Banda H, Kathyola D. Strengthening health human resources and improving clinical outcomes through an integrated guideline and educational outreach in resource-poor settings: a cluster-randomized trial. Trials. 2010;11:118. doi: 10.1186/1745-6215-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


