Abstract
Background
Deciphering the mechanisms of tolerance represents a crucial aim of research in transplantation. We previously identified by DNA chip, IL-27 p28 and TGFβ1, as over-expressed in a model of rat cardiac allograft tolerance mediated by regulatory CD4+CD25+ T cells. The role of these two molecules on the control of the inflammatory response remains controversial. However, both are involved in the regulation of the Th17/Treg axis suggesting their involvement in tolerance.
Methods
We analyzed regulation of IL-27 and TGFβ1 expression in allograft response and their role in tolerance by using blocking anti-TGFβ antibody and by generating an adeno-associated virus encoding IL-27.
Results
Here, we confirmed the over-expression of IL-27 and TGFβ1 in tolerated cardiac allografts in two different rodent models. We observed that their expression correlates with inhibition of Th17 differentiation and with expansion of regulatory CD4+CD25+ T cells. We showed in rat that anti-TGFβ treatment abrogates infectious tolerance mediated by the transfer of regulatory CD4+CD25+ T cells. Moreover, over-expression of IL-27 by adeno-associated virus administration in combination with a short-term immuno-suppression allows prolongation of cardiac allograft survival and one tolerant recipient. We found that IL-27 over-expression did not induce Foxp3+CD4+CD25+ T cell expansion but rather IL10 expressing CD4+ T cells in the tolerant recipient.
Conclusions
Taken together, these data suggest that both TGFβ1 and IL-27 play a role in the mechanisms of tolerance. However, in contrast to TGFβ1, IL-27 seems not to be involved in regulatory CD4+CD25+ T cell expansion but rather in their mode of action.
Keywords: Tolerance, regulatory T cells, rodent
INTRODUCTION
IL-6/IL-12 family represents a group of factors remarkable for their pleiotropic effects on Th function, differentiation and development (1). One of the most recently described is the heterodimeric cytokine IL-27 that is composed of the EBV-induced 3 (EBI3) and IL-27 p28 subunits. IL-27 signals through the receptor subunits gp130 and WSX-1 that is restricted to T cells, NK cells and monocytes (1).
The role of IL-27 on Th differentiation and on inflammatory responses is controversial (2, 3). Initially, IL-27 was described to be involved in Th1 differentiation. This was suggested by its ability to induce the Th1 transcription factor, Tbet, in naïve CD4+ T cells (4-6). Then, IL-27 was shown to down-regulate effector Th function and particularly to suppress Th17 differentiation (7). Recently, an anti-inflammatory effect of IL-27 has been reinforced by its ability to regulate in vivo models of infectious and auto-immune diseases (3, 8, 9). Interestingly, IL-27 has recently been reported to generate IL-10 producing Tr1 cells (7, 10-12). IL-27 as other IL-12 family members have been shown to be produced mainly by antigen-presenting cells (APC) (13). However, some studies have demonstrated that regulatory CD4+ CD25+ T cells can express the IL-12 family cytokine IL-35, that is necessary for suppression (14, 15).
We previously identified by DNA chip, IL27p28 and TGFβ, as up-regulated in a experimental model of long-term allograft tolerance in rat in which we demonstrated the crucial role of CD4+CD25+ regulatory T cells (16-18). Since IL-6/IL-12 family members have been shown, together with TGFβ, to modulate effector and regulatory T cell development and function, we analyzed their expression and their role in allograft response.
RESULTS
Over-expression of IL-27 and TGFβ1 in cardiac allograft tolerance rodent models
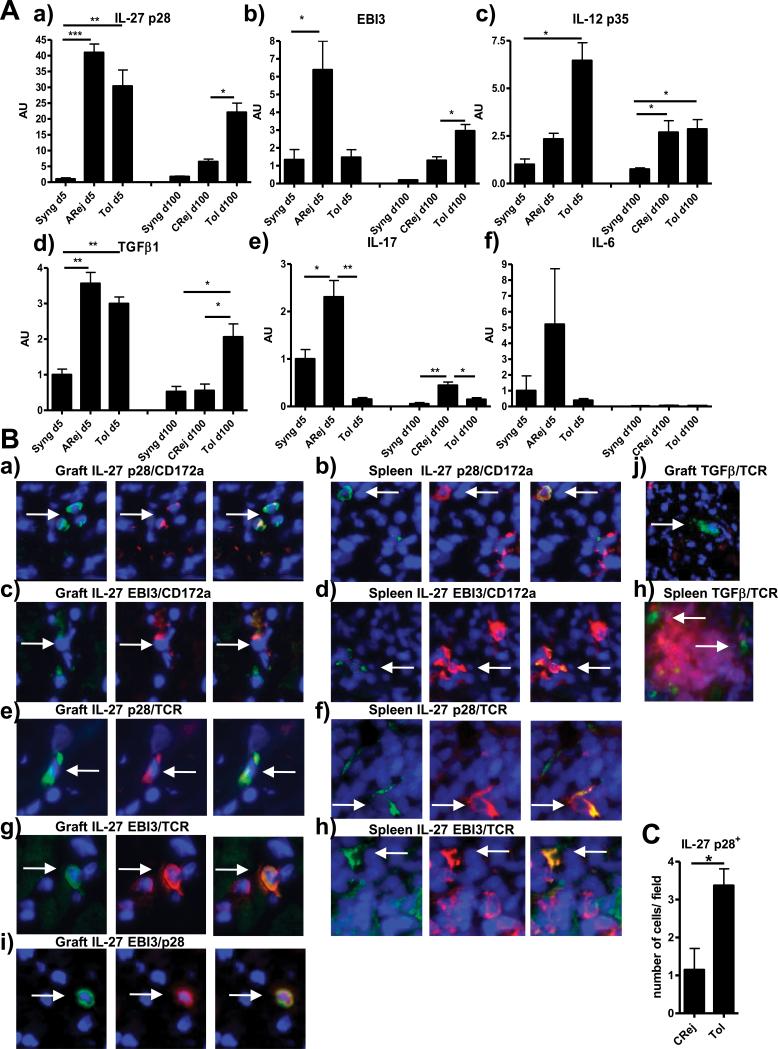
We previously described that a short-term treatment with the immuno-suppressor LF15-0195, a deoxyspergualin analog, induced MHC fully mismatched cardiac allograft tolerance in rat with no development of vascular lesions at long-term (16-18). We previously demonstrated the expansion of induced regulatory CD4+CD25+ T cells that are able to transfer allograft tolerance and that interplay with APCs for expression of tolerogeneic molecules (17, 19, 20). In order to identify new mediators of tolerance, we applied pan genomic DNA chip on whole tolerated allografts and compared with allografts that develop chronic rejection (induced in the same strain combination by donor-specific blood transfusion (DST)) (18). Among the genes up-regulated in tolerated allografts, we identified the IL-27 p28 chain and TGFβ1. We confirmed here by quantitative RT/PCR the over-expression of IL-27 p28 and EBI3 transcripts in long-term tolerated allografts (at day 100 after transplantation) compared to allografts that develop chronic rejection (Fig1A, a) and b) respectively). In order to determine whether the over-expression of IL-27 was also retrieved during the induction of tolerance, under the immuno-suppressive treatment, we analyzed the grafts at day 5 after transplantation. We observed a peak of expression of IL-27 p28 at day 5 after transplantation in tolerated allografts compared to syngeneic grafts but not of EBI3 suggesting that IL-27 is over-expressed only during maintenance of tolerance once regulatory CD4+ CD25+ T cells have expanded (Fig1A, b)). We analyzed also the expression of IL-12 p35 that forms with EBI3, the IL-35 cytokine. We observed a strong expression of IL-12 p35 during induction of tolerance that then partially decreases during maintenance of tolerance (Fig1A, c)). However, since EBI3 is not expressed during induction, the peak of IL-12 p35 expression does not correspond to IL-35. For TGFβ1, we observed a high-expression at both day 5 and 100 after transplantation (Fig1A, d)). Since TGFβ1 and IL-27 are both involved in the regulation of the Th17 differentiation axis, we analyzed the expression of IL-17 and IL-6. We observed that IL-17 and IL-6 expressions were inhibited in tolerated allografts at day 5 or 100 after transplantation compared to allografts that develop acute or chronic rejection (Fig1A, e) and f) respectively).
Fig 1. Expression of IL-27 p28, EBI3, IL-12 p35, TGFβ1, IL-17 and IL-6 in rat cardiac allograft model.
(A): mRNA expression of a) IL-27 p28, b) EBI3, c) IL-12 p35, d) TGFβ1, e) IL-17 and f) IL-6 was analyzed by quantitative RT-PCR in cardiac syngeneic grafts (Syng), acutely rejected allografts (ARej), chronically rejected allografts (CRej) or tolerated allografts (Tol) harvested at day 5 (d5) or 100 (d100) after transplantation (n=4). Results are expressed in AU of the gene/HPRT transcript ratio ± SEM and expressed as relative expression compared to the reference syngeneic grafts at day 5 (value=1), * p<0.05, ** p<0.01, ***p<0.001.
(B): Representative pictures of immuno-fluorescence merged staining by histology for DAPI (blue) and for: IL-27 p28 (green) or EBI3 (green) and CD172a (red) in respectively graft and spleen a), b) and c) d). IL-27 p28 (green) or EBI3 (green) and TCR (red) in respectively graft and spleen e), f) and g) h). EBI3 (green) and IL-27 p28 (red) in graft i). TGFβ (LAP complex) (green) and TCR (red) in respectively graft and spleen j) and h). Original magnification: ×600. Data are representative of three independent experiments on different rat tolerant recipients.
(C): Expression of IL-27 p28 protein was assessed by histology by counting the number of positive cells per field in chronically rejected (CRej) and tolerated allografts (Tol). Results are expressed in number of cells per field ± SEM, n=4,* p<0.05.
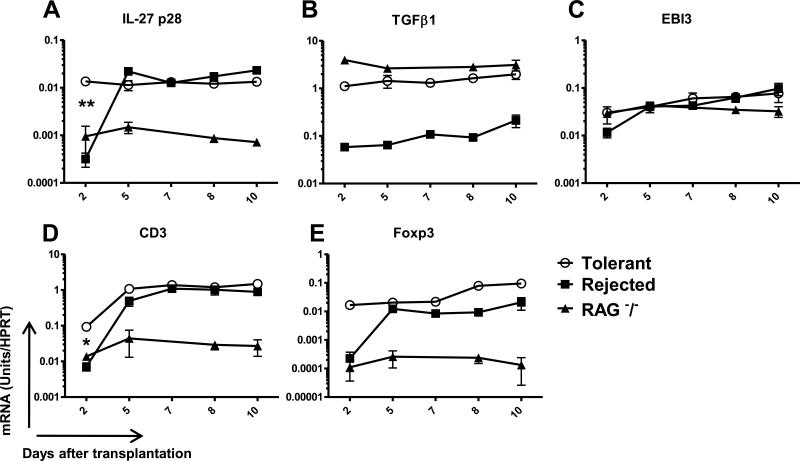
In an attempt to identify and characterize common mediators of tolerance within the RISET consortium, we decided to analyze mRNA expression of IL-27 and TGFβ1 in other tolerance models and regulatory cells. Interestingly, over-expression of IL-27 p28 and TGFβ1 was also observed in a cardiac allograft mouse model of tolerance that is induced by DST and anti-CD4 treatment and that also involved regulatory CD4+CD25+ T cells (21). An increase up to 45 fold for IL-27 p28 and of 19 fold for TGFβ1 was observed in tolerated grafts compared to rejecting ones (Fig3A and B respectively). EBI3 expression was also up-regulated over-time in tolerated allografts but at similar level than in rejecting grafts (Fig3C). Interestingly, IL-27 p28 expression correlates with the early regulatory Foxp3+ T cell infiltration (up to 10 fold in CD3 and 85 fold in Foxp3 expression) (Fig3D and E respectively) and is not observed in RAG−/− recipients lacking T cells. Therefore, IL-27 p28 over-expression may be induced by the regulatory Foxp3+ T cells that early infiltrate the allografts (Fig3A).
Fig 3. mRNA expression of IL-27 p28, TGFβ1, EBI3, CD3 and Foxp3 in cardiac allografts in mice.
mRNA expression of (A) IL-27 p28, (B) TGFβ1, (C) EBI3, (D) CD3 and (E) Foxp3 were analyzed by quantitative RT-PCR in cardiac untreated rejected allografts (Rejected), treated tolerant allografts (Tolerant) and allografts from RAG −/− recipients harvested at day 2, 5, 7, 8 or 10 after transplantation (n≥2). Results are expressed in mRNA Units/HPRT ratio ± SEM * p<0.05, ** p<0.01.
In tolerated allografts, IL-27 and TGFβ are expressed by myeloid cells
In order to determine which cells express IL-27 and TGFβ1 in long-term tolerant recipients, we analyzed allografts and spleen by histology. We observed protein expression of IL-27 p28 (Fig1B a) and b)) or EBI3 (Fig1B c) and d)) mostly by myeloid cells (CD172a+) respectively in the graft and spleen. Interestingly, we also observed some T cells (TCR+) expressing IL-27 p28 (Fig1B e), f)) or EBI3 (Fig1B g), h)) respectively in the graft and spleen. Some, but not all of EBI3+ cells, were co-stained with IL-27 p28 (Fig1B i) in the graft). Unfortunately, since the commercially available IL-12 p35 polyclonal antibody is not working in our hand, we were not able to determine whether the other EBI3+ cells represented IL-35+ cells. We counted more IL-27 p28+ cells in tolerated allografts than in allografts developing chronic rejection confirming the RT-PCR analysis (Fig1C). TGFβ1 (detected by anti-LAP-1 antibody) was expressed mostly by myeloid cells (non-T cells) (Fig1B j) in the graft and h) in red pulp of spleen).
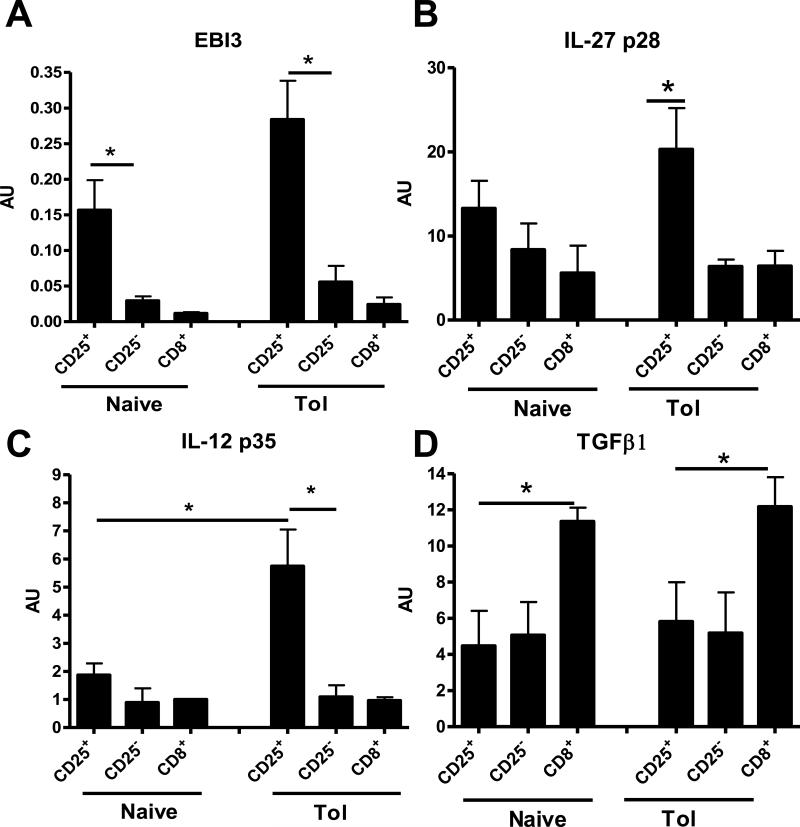
We also analyzed the mRNA expression of, EBI3, IL-27 p28, IL-12 p35 and TGFβ1 in highly purified regulatory CD4+CD25+ T cells that accumulated in the spleen of tolerant recipients (17). We compared with their CD25− counterparts and with both these populations from naive rats. Interestingly, EBI3, IL-27 p28 and IL-12 p35 transcripts were strongly expressed in CD4+CD25+ T cells from tolerant recipients compared to their CD25− counterparts whereas only EBI3 transcripts were up-regulated in CD4+CD25+ T cells from naive rats (Fig2 A, B and C respectively). These data suggest that IL-27 and IL-35 are over-expressed only in induced CD4+CD25+ T cells. No difference was observed in the expression of TGFβ1 in CD4+CD25+ T cells compared to their CD25− counterparts (Fig2D).
Fig 2. mRNA expression of EBI3, IL-27 p28, IL-12 p35 and TGFβ1 in rat CD4+CD25+ T cells.
mRNA expression of (A) EBI3, (B) IL-27 p28, (C) IL-12 p35 and (D) TGFβ1 was analyzed by quantitative RT-PCR in purified CD4+CD25+, CD4+CD25− and CD8+ T cells from naive rats (Naive) or from long-term tolerant recipients (Tol). Results are expressed in AU of the gene/HPRT transcript ratio ± SEM, n=4 * p<0.05.
Role of TGFβ1 and IL-27 in rat allograft tolerance
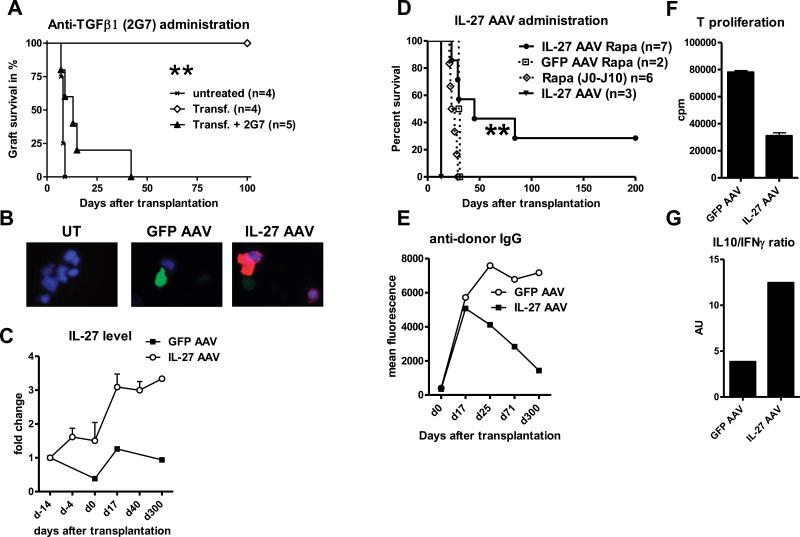
In order to determine the function of TGFβ1 in rat cardiac allograft tolerance, we administrated neutralizing anti-TGFβ antibody. We observed that anti-TGFβ treatment was not sufficient to abrogate long-term rat allograft tolerance (data not shown). However, when administered at the time of transfer of regulatory CD4+ T cells, anti-TGFβ treatment is able to abrogate transfer of tolerance (median survival time (MST) 13 days versus 100 days (Fig4A)). These data suggest a role for TGFβ in expansion and/or function of regulatory CD4+ T cells. To test the immuno-modulatory role of IL-27 in transplantation, we generated an AAV encoding rat IL-27. Recombinant AAV are among the most efficient gene delivery vehicles for therapeutic gene transfer in vivo especially because they can transduce many tissues with a safe, strong and long-term expression of the transgene (22-24). We demonstrated the efficiency of IL-27 AAV transduction and of IL-27 expression by transfecting in vitro HEK293 cells (Fig4B). We tested the effect of IL-27 over-expression on fully mismatched cardiac allograft survival. We administrated AAV to recipients by peripheral vein injection for systemic delivery and 3 weeks before transplantation to allow time for the transgene to be efficiently expressed. Intra-venous AAV administration targets all the blood-irrigated organs but this serotype 2/8 will particularly transduced hepatocytes (25, 26). Since IL-27 is a secreted protein, we should observe accumulation in the sera and a systemic effect. Indeed, we observed that IL-27 protein expression started to increase 3 weeks after administration in the sera of transfected recipients and remained stable over-time (up to 4 fold compared to basal level at day 300 after transplantation) (Fig4C). Administration of IL-27 AAV alone had moderate effect on allograft survival (MST=13 days versus 7 days for untreated recipients, Fig4D, and data not shown). However, when associated with a 10 day course of sub-optimal doses of rapamycin, IL-27 AAV administration was able to prolong allograft survival (MST=45 days) compared to control (GFP AAV plus rapamycin (MST=30 days) or rapamycin alone (MST=24 days) (Fig4D). One out of 7 rats treated with IL-27 AAV accepted its graft at long-term and displayed a low anti-donor response (alloantibody production and spleen T cell proliferation) compared to control recipient (Fig4E and 4F respectively). In addition, we noted a higher IL-10/IFNγ expression ratio by the alloantigen specific CD4+ T cells from this tolerant recipient (Fig4G).
Fig 4. Role of TGFβ and IL-27 in rat cardiac allograft tolerance.
A) Recipients that have been transferred with 20×106 of spleen CD4+T cells from long-term tolerant recipients (Transf.) were treated with neutralizing mouse anti-rat TGFβ mAb (clone 2G7, IgG2b) injected i.p (5 mg/kg) twice a week beginning the day of transplantation and until rejection (Transf.+2G7) n≥4, **p<0.01.
B) Representative pictures of immuno-fluorescence merged staining for IL-27 p28 (red), GFP (green) and DAPI (blue) of untransfected (UT) or GFP or IL-27 AAV transfected HEK 293 cells as described in Material and Methods. Original magnification: ×600.
C) IL-27 level was assessed by ELISA in the sera from GFP or IL-27 AAV transfected allograft recipients harvested at days −14, −4, 0, 17, 40, and 300 before or after transplantation. Results are expressed in fold change compared to the IL-27 basal level assessed from each recipient.
D) Rat cardiac allograft recipients were treated with GFP or IL-27 AAV (4.5×1011 vg) injected i.v. at recipients 3 weeks before transplantation. Alternatively, sub-optimal dose of rapamycin (Rapa) was given orally from day 0 to day 10 (0.4 mg/kg), n≥4, **p<0.01.
E) Assessment of anti-donor-specific IgG antibodies in the sera of IL-27 AAV tolerant recipient or GFP AAV treated recipient and harvested at days 0, 17, 25, 71 and 300 after transplantation. Data are expressed in Geometric Mean Fluorescence.
F) T cells from GFP or tolerant IL-27 AAV treated recipients (day 300 after transplantation) were stimulated by allogeneic (LEW.1W) APC for four days (MLR) and T cell proliferation was assessed in triplicates by 3H incorporation. Data are expressed in cpm ± SEM.
G) Ratio of IL-10/INFγ expression assessed by ELISA in the supernatant of MLR from stimulated CD4+ T cells from GFP or tolerant IL-27 AAV treated recipients. Data are expressed in arbitrary unit (AU).
DISCUSSION
We observed up-regulation of TGFβ1 and IL-27 in two experimental models of cardiac allograft tolerance mediated by regulatory CD4+CD25+ T cells reinforcing the idea that these two molecules may play a role in tolerance mechanisms. TGFβ1 is known to be implicated in many biological processes and to play pivotal functions in the immune system and notably in the Th17 and regulatory T cell differentiation (27). We found a high expression of TGFβ1 in our model of allograft tolerance in rat with no expression of IL-6 and IL-17 suggesting that the Th17 differentiation axis is totally inhibited and that TGFβ1 plays rather a role in the regulatory CD4+CD25+ T cell development or function. Indeed, we have demonstrated that TGFβ1, although not produced predominantly by regulatory CD4+CD25+ T cells, is required for the transfer of tolerance. Therefore, as previously demonstrated, regulatory CD4+CD25+ T cells require TGFβ1 to mediate their expansion and their suppression (28-31).
We found that induced regulatory CD4+CD25+ T cells from tolerant recipients expressed higher transcript level for IL-27 p28, EBI3 and IL-12 p35 chains than their CD25− counterparts suggesting that they could express IL-27 and IL-35 cytokines. This contrasts to naturally regulatory CD4+CD25+ T cells from naive rats that over-expressed only the EBI3 chain. Controversial studies have suggested that regulatory CD4+CD25+ T cells express IL-35 that is necessary for suppression (14, 15, 32, 33). In fact, IL-35 expression seems to depend of the activation state of the cells and whether they correspond to induced or natural suppressive cells (15, 34, 35). Unfortunately, due to lack of specific antibody, we have not been able to stain for IL-35 protein. We found IL-27 protein expression by some T cells in the graft and spleen of tolerant recipients. However, the majority was by myeloid cells suggesting that expression by regulatory CD4+CD25+ T cells may be rare and/or low. Nevertheless, we observed that IL-27 over-expression correlated with accumulation of regulatory CD4+CD25+ T cells in tolerated allografts in both rodent models. Therefore, although induced regulatory CD4+CD25+ T cells may not be the major source of IL-27, they promote directly or indirectly its expression in tolerated allografts.
To determine the role of IL-27 in transplantation, we generated an AAV encoding IL-27. AAV represents a promising safe strategy for gene therapy in clinic (22-24). We showed that, in combination with a short-term immuno-suppressive treatment, IL-27 over-expression allowed a significant prolongation of allograft survival and promoted tolerance in 1 out of 7 treated recipients. We observed no expansion of regulatory CD4+CD25+ T cells in IL-27 AAV treated recipients (data not shown). In this sense, a recent study have demonstrated that transgenic mice over-expressing IL-27, displayed limited differentiation of regulatory T cells due to a reduced production of IL-2 that is vital for regulatory CD4+CD25+ T cell maintenance (36). IL-27 is not expressed during induction of tolerance in our rat model but only during maintenance when regulatory CD4+CD25+ T cells expand (16, 17). This reinforces the idea that IL-27 is not necessary for induced regulatory CD4+CD25+ T cell development but rather for their mode of action. Interestingly, we observed more IL-10 expression by anti-donor CD4+ T cells in the IL-27 AAV treated tolerant recipient. Indeed, IL-27 has recently been shown to promote the development of induced Tr1 cells by inducing the ligand-activated transcription factor aryl hydrocarbon receptor to act in synergy with c-Maf (11, 12).
To conclude, although tolerance seems difficult to achieve in our fully mismatched cardiac allograft model, over-expressing IL-27 in combination with immune-suppression may represent a promising therapeutic strategy. It will be interesting, in the future to test the effect of this IL-27 AAV on other rat diseases notably on chronic rejection and to determine whether IL-27 is able to modulate the Th1, Th2 and Th17 responses that develop progressively in this model (18, 20, 37, 38).
MATERIALS AND METHODS
AAV generation and in vitro transduction
AAV encoding rat IL-27 (AAV2/8.CMV.WPRE including full length cDNA encoding IL-27p28 and EBI3 separated by IRES coding region) or AAV encoding GFP (AAV2/8.CMV WPRE.GFP) were generated as previously described (39), at the University Hospital of Nantes (http://www.vectors.nantes.inserm.fr). Human embryonic kidney cells (HEK 293) (0.25×106 cells/well) were transduced in 6-well plates in 600 μl of DMEM supplemented with 1% fetal bovine (FBS) by addition of IL-27 or GFP AAV (100 MOI) and null adenovirus (10 MOI) to enhance rapid transduction. Five hours after initial viral application, 3 ml of growth media (10% FBS) was added to each well. Cytospins and histology were performed 4 days following infection.
Animals and transplantation
Rodents are maintained in animal facilities under standard conditions according to institutional guidelines and studies were reviewed and approved by the appropriate institutional review committee.
Rats
Inbred 8-week-old male LEW.1W rats (RT1u) (‘Centre d’Elevage Janvier’ (Le Genest-Saint-Isle, France)) served as heart donors and LEW.1A (RT1a) as allograft recipients and heterotopic cardiac allografts were performed as previously described (40). Induction of tolerance in rat, by the short-term treatment with the deoxyspergualine analog, LF15-0195, and the model of development of chronic rejection were performed as previously described (16-18, 38). Graft of recipients was harvested at day 5 or 100 after transplantation. For in vivo transfer experiments, 20×106 of purified CD4+ T cells from tolerant recipients were injected i.v. into LEW.1A secondary syngeneic irradiated recipients as previously described (19). IL-27 or GFP AAV (4.5×1011 vg) were injected i.v. at recipients 3 weeks before transplantation. Rapamycin (Rapamune, Wyeth, Collegeville, PA) was given orally from day 0 to day 10 (0.4 mg/kg). A neutralizing mouse anti-rat TGFβ mAb (2G7; provided by Dr. K. Melief, Amsterdam, The Netherlands) was injected i.p (5 mg/kg) twice a week beginning the day of transplantation as previously described (41).
Mice
CBA.Ca (CBA; H2k) and C57BL/10 (B10; H2b) mice were originally purchased from Harlan Ltd. (Bicester, United Kingdom) and CBA.Ca RAG-1 knockout (CBA RAG−/−) a gift of Dr. D. Kioussis (Mill Hill, London, U.K.). Heterotopic cardiac transplants were performed as previously described in 6-12 week aged mice (42). Fully allogeneic B10 heart to CBA recipient are rejected with a median survival time (MST) of 10 days whereas in CBA.RAG−/−, MST was >100 days (43). For induction of tolerance, mice received 200□μg of anti-CD4 mAb (YTS177.9, H Waldmann, Oxford, U,K,) i.v. on days −28 and −27 and a DST (250□μL of donor blood) i.v. on day −27 (MST>100 days) as previously described (21). Graft of recipients was harvested at day 2, 5, 7, 8 or 10 after transplantation.
Cell purification and Flow cytometry
Total CD4+, CD4+CD25−, regulatory CD4+CD25+ and CD8+ T cells from naïve rats or tolerant recipients were purified by positive selection (with R73, W3/25, OX39 and Ox8 monoclonal antibodies) using a FACSAria flow cytometer (Becton Dickinson) as previously described (44). Purity was >99%. Fluorescent labeling of cells was measured using a FACS LSR II (BD Biosciences) and analyzed with FlowJo-R software (Tree Star, Inc., Ashland).
RNA extraction and real-time quantitative RT-PCR
Total RNA from grafts or cells were prepared and Real-time quantitative PCR were performed as previously described using a GenAmp 7700 Sequence Detection System and either SYBR® Green or qPCR Master mix (45, 46). Oligonucleotides used are described in Table 1. HPRT was used as an endogenous control gene to normalize for variations in the starting amount of RNA. Relative expression was calculated using the 2-ΔΔCt method (47).
Table I.
Oligo-nucleotides used in this study for rat (r) and mouse (m) genes. From 5′ to 3′-end.
| rHPRT | For CCTTGGTCAAGCAGTACAGCC |
| Rev TTCGCTGATGACACAAACATGA | |
| rIL-27 p28 | For AGCAGACCCCCTGAGCCT |
| Rev GTGGTAGCGAGGAAGCAGAGT | |
| rEBI3 | For CACTTACAGGCTCGGTGTGG |
| Rev CGGGCTTGATGATTCGTTC | |
| rTGFβ1 | For CTCAACACCTGCACAGCTCC |
| Rev ACGATCATGTTGGACAACTGCT | |
| rIL-17A | For TGCTGTTGCTGCTACTGAACC |
| Rev AACTTCCCCTCAGCGTTGAC | |
| rIFNγ | For TGGATGCTATGGAAGGAAAGA |
| Rev GATTCTGGTGACAGCTGGTG | |
| rIL-6 | For GCAAGAGACTTCCAGCCAGTT |
| Rev CATCATCGCTGTTCATACAATCA | |
| rIL-12 p35 | For TGATGATGAC CCTGTGCCTT |
| Rev GCATGGAGCA GGATACAGAGC | |
| rFoxp3 | For CCCAGGAAAGACAGCAACCTT |
| Rev CTGCTTGGCAGTGCTTGAGAA | |
| mHPRT | For ATCATTATGCCGAGGATTTGGAA |
| Rev TTGAGCACACAGAGGGCCA | |
| Probe TGGACAGGACTGAAAGACTTGCTCGAGATG | |
| mCD3 | For ATTGCGGGACAGGATGGAG |
| Rev CTTGGAGATGGCTGTACTGGTCA | |
| mIL-27 p28 | For ACAGCTTTGCTGAATCTCGATTG |
| Rev ACCGTAGTGGAGAGA CTGAGACT | |
| mEBI3 | For ACTGAAACAGCTCTCGTGGCTCTA |
| Rev AAGTAACGGTGA ATGTCCGAGC | |
| mTGFβ1 | For GGCTACCATGCCAACTTCTGTCT |
| Rev CCGGGTTGT GTTGGTTGTAGA | |
| Probe CACACAGTACAGCAAGGTCCTTGCCCT | |
| mFoxp3 | For CCC AGGAAAGACAGCAACCTT |
| Rev TTCTCACAACCAGGCCACTTG | |
| Probe ATCCTA CCCACTGCTGGCAAATGGAGTC |
Immuno-histology
Cryostat sections (7 μm) of cardiac and spleen tissues (snap-frozen) or cytospins of cells were fixed in acetone for 10 min. Sections were then labeled with anti-rat mouse IL-27 p28 and rabbit EBI3 antibodies that have been generated and kindly provided by Dr Taketoshi Taniguchi (Japan) (48), LAP (anti human Latency Associated Protein derived from the N-terminal region of the TGFβ gene product, RD system), R73 (anti-rat TCRα), with Ox41 (anti-rat CD172a) and with DAPI, mounted in Vectashield mounting medium (Vector Laboratories Inc., Burlingame, CA, USA) and observed by fluorescence microscopy (Axioskop2 plus-Carl Zeiss Inc., Göttingen, Germany). IL-27 p28+ cells were counted in 30 fields per graft.
Mixed Leucocyte Reaction (MLR)
APC-enriched cell populations from donor-type and responder spleen T cells were prepared and plated as previously described (19). [3H]TdR incorporation was measured for the last 8 h of the 96 h culture.
Cytokine assays
IFNγ and IL-10 were measured in MLR supernatants using ELISA from BD PharMingen OptEIA (San Diego, CA). IL-27 level was determined by ELISA (AbCys SA (Cusabio)) in sera harvested at day −14, −4, 0, +17, +40 and +300 after transplantation.
Assessment of circulating donor-specific antibodies
Donor-specific antibodies were assessed in the diluted sera (1/8) harvested from recipients at day 0, +17, +25, +71 and +300 after transplantation as previously described (49).
Statistical analysis
Data are expressed as mean ± SEM. Statistical evaluation was performed using the Student’s t-test for unpaired data, and results were considered significant if p values were <0.05. The Kaplan–Meier method was used to calculate the survival curves followed by Mantel–Cox log rank analysis.
ACKNOWLEDGMENTS
This work was supported in part by the European Union Framework Program 6 “Reprogramming the Immune System for the Establishment of Tolerance” (RISET) consortium (http://www.risetfp6.org).
We thank Dr Taketoshi Taniguchi, Medical research center, Kochi Medical School, Japan for providing mouse anti-rat IL-27 p28 and EBI3 antibodies.
Le Texier L. was supported by a fellowship from the “Ministere de l’Enseignement Superieur et de la Recherche”, Thebault P. by << Fondation Progreffe >> and Carvalho-Gaspar M by the Britich Heart Foundation Grant PG/07/089/23686.
L Le Texier, P Thebault, M Carvalho-Gaspar, V Vignard, E Merieau and C Usal participated in the performance of the research and data analysis. MC Cuturi participated in data analysis. K Wood participated in research design and data analysis. E Chiffoleau participated in research design, performance of the research, data analysis and writing of the paper.
ABBREVIATIONS
- Th
T helper
- GFP
Green Fluorescent Protein
- MOI
Multiplicity of infection
- VG
Vector genome
- HPRT
Hypoxanthine-guanine phosphoribosyltransferase
Footnotes
Authors have no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kastelein RA, Hunter CA, Cua DJ. Discovery and Biology of IL-23 and IL-27: Related but Functionally Distinct Regulators of Inflammation. Annual Review of Immunology. 2007;25(1):221. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg R, Zohar Y, Wildbaum G, Geron Y, Maor G, Karin N. Suppression of ongoing experimental autoimmune encephalomyelitis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173(10):6465. doi: 10.4049/jimmunol.173.10.6465. [DOI] [PubMed] [Google Scholar]
- 3.Kido MTS, Sugiyama N, Esaki H, Nakashima H, Yoshida H, Furue M. T cell-specific overexpression of interleukin-27 receptor α subunit (WSX-1) prevents spontaneous skin inflammation in MRL/lpr mice. Br J Dermatol. 2011;164(6):1214. doi: 10.1111/j.1365-2133.2011.10244.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Ghilardi N, Wang H, et al. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407(6806):916. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 5.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 6.Takeda A, Hamano S, Yamanaka A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170(10):4886. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 7.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney CMLR, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, Kelly PJ, Costelloe L, Tubridy N, Mills KH, Fletcher JM. IL-27 mediates the response to IFN-β therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav Immun. 2011;6:1170. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Pickens SRCN, Volin MV, Mandelin AM, 2nd, Agrawal H, Matsui M, Yoshimoto T, Shahrara S. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum. 2011;8:2289. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald DC, Zhang GX, El-Behi M, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8(12):1372. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 11.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8(12):1363. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 12.Apetoh L, Quintana FJ, Pot C, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2011;11(9):854. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocco C, Pistoia V, Airoldi I. Anti-leukemic properties of IL-12, IL-23 and IL-27: Differences and similarities in the control of pediatric B acute lymphoblastic leukemia. Crit Rev Oncol Hematol. doi: 10.1016/j.critrevonc.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi VCL, Guy CS, Workman CJ, Vignali DA. Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J Immunol. 2011;186(12):6661. doi: 10.4049/jimmunol.1100315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Chiffoleau E, Beriou G, Dutartre P, Usal C, Soulillou JP, Cuturi MC. Induction of donor-specific allograft tolerance by short-term treatment with LF15-0195 after transplantation. Evidence for a direct effect on T-cell differentiation. Am J Transplant. 2002;2:745. doi: 10.1034/j.1600-6143.2002.20808.x. [DOI] [PubMed] [Google Scholar]
- 17.Chiffoleau E, Beriou G, Dutartre P, Usal C, Soulillou JP, Cuturi MC. Role for thymic and splenic regulatory CD4+ T cells induced by donor dendritic cells in allograft tolerance by LF15-0195 treatment. J Immunol. 2002;168:5058. doi: 10.4049/jimmunol.168.10.5058. [DOI] [PubMed] [Google Scholar]
- 18.Heslan JM, Renaudin K, Thebault P, Josien R, Cuturi MC, Chiffoleau E. New evidence for a role of allograft accommodation in long-term tolerance. Transplantation. 2006;82(9):1185. doi: 10.1097/01.tp.0000236573.01428.f3. [DOI] [PubMed] [Google Scholar]
- 19.Thebault P, Condamine T, Heslan M, et al. Role of IFNgamma in allograft tolerance mediated by CD4+CD25+ regulatory T cells by induction of IDO in endothelial cells. Am J Transplant. 2007;7(11):2472. doi: 10.1111/j.1600-6143.2007.01960.x. [DOI] [PubMed] [Google Scholar]
- 20.Thebault P, Lhermite N, Tilly G, et al. The C-type lectin-like receptor CLEC-1, expressed by myeloid cells and endothelial cells, is up-regulated by immunoregulatory mediators and moderates T cell activation. J Immunol. 2009;183(5):3099. doi: 10.4049/jimmunol.0803767. [DOI] [PubMed] [Google Scholar]
- 21.Saitovitch D, Bushell A, Mabbs DW, Morris PJ, Wood KJ. Kinetics of induction of transplantation tolerance with a nondepleting anti-Cd4 monoclonal antibody and donor-specific transfusion before transplantation. A critical period of time is required for development of immunological unresponsiveness. Transplantation. 1996;61(11):1642. doi: 10.1097/00007890-199606150-00016. [DOI] [PubMed] [Google Scholar]
- 22.Bankiewicz KS, Forsayeth J, Eberling JL, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14(4):564. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Le Meur G, Stieger K, Smith AJ, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14(4):292. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 24.Niemeyer GP, Herzog RW, Mount J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113(4):797. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, MA K. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;1(79):214. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao G-P, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proceedings of the National Academy of Sciences. 2002;99(18):11854. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell P, Afzali B, Lombardi G, Lechler RI. The T helper 17-regulatory T cell axis in transplant rejection and tolerance. Curr Opin Organ Transplant. 2009;14(4):326. doi: 10.1097/MOT.0b013e32832ce88e. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194(5):629. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166(12):7282. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 30.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196(2):255. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daley SR, Ma J, Adams E, Cobbold SP, Waldmann H. A key role for TGF-beta signaling to T cells in the long-term acceptance of allografts. J Immunol. 2007;179(6):3648. doi: 10.4049/jimmunol.179.6.3648. [DOI] [PubMed] [Google Scholar]
- 32.Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. European Journal of Immunology. 2008;38(12):3282. doi: 10.1002/eji.200838373. [DOI] [PubMed] [Google Scholar]
- 33.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Herminé A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181(10):6898. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 34.Seyerl M, Kirchberger S, Majdic O, et al. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. European Journal of Immunology. 2010;40(2):321. doi: 10.1002/eji.200939527. [DOI] [PubMed] [Google Scholar]
- 35.Liu F, Tong F, He Y, Liu H. Detectable expression of IL-35 in CD4+ T cells from peripheral blood of chronic Hepatitis B patients. Clinical Immunology. 2011;139(1):1. doi: 10.1016/j.clim.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Wojno ED, Hosken N, Stumhofer JS, et al. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187(1):266. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koshiba T, Kitade H, Van Damme B, et al. Regulatory cell-mediated tolerance does not protect against chronic rejection. Transplantation. 2003;76(3):588. doi: 10.1097/01.TP.0000080980.26287.11. [DOI] [PubMed] [Google Scholar]
- 38.Ballet C, Renaudin K, Degauque N, et al. Indirect CD4+ TH1 response, antidonor antibodies and diffuse C4d graft deposits in long-term recipients conditioned by donor antigens priming. Am J Transplant. 2009;9(4):697. doi: 10.1111/j.1600-6143.2009.02556.x. [DOI] [PubMed] [Google Scholar]
- 39.Rabinowitz JE, Rolling F, Li C, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76(2):791. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ono K, Lyndsey ES. Improved technic of heart transplantation in rats. J. Thorac. Cardiovasc. Surg. 1968;57:225. [PubMed] [Google Scholar]
- 41.Josien R, Douillard P, Guillot C, et al. A critical role for transforming growth factor-b (TGF-b) in donor transfusion-induced allograft tolerance. J. Clin. Invest. 1998;102:1920. doi: 10.1172/JCI4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Bushell A, Niimi M, Morris PJ, Wood KJ. Evidence for immune regulation in the induction of transplantation tolerance: a conditional but limited role for IL-4. J Immunol. 1999;162(3):1359. [PubMed] [Google Scholar]
- 44.Chiffoleau E, Heslan JM, Heslan M, Louvet C, Condamine T, Cuturi MC. TLR9 ligand enhances proliferation of rat CD4+ T cell and modulates suppressive activity mediated by CD4+ CD25+ T cell. Int Immunol. 2007;19(2):193. doi: 10.1093/intimm/dxl136. [DOI] [PubMed] [Google Scholar]
- 45.Louvet C, Heslan JM, Merieau E, Soulillou JP, Cuturi MC, Chiffoleau E. Induction of Fractalkine and CX3CR1 mediated by host CD8+ T cells in allograft tolerance induced by donor specific blood transfusion. Transplantation. 2004;78(9):1259. doi: 10.1097/01.tp.0000140482.20336.77. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho-Gaspar M, Billing JS, Spriewald BM, Wood KJ. Chemokine gene expression during allograft rejection: comparison of two quantitative PCR techniques. J Immunol Methods. 2005;301(1-2):41. doi: 10.1016/j.jim.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.Saito F, Ohno Y, Morisawa K, Kamakura M, Fukushima A, Taniguchi T. Role of IL-27-producing dentritic cells in Th1-immunity polarization in Lewis rats. Biochemical and Biophysical Research Communications. 2005;338(4):1773. doi: 10.1016/j.bbrc.2005.10.149. [DOI] [PubMed] [Google Scholar]
- 49.Le Texier L, Thebault P, Lavault A, et al. Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant. 2010;11(3):429. doi: 10.1111/j.1600-6143.2010.03336.x. [DOI] [PubMed] [Google Scholar]