Abstract
CD73 is a cell surface enzyme that suppresses T-cell mediated immune responses, by producing extracellular adenosine. Increasing evidence suggest that targeting CD73 in cancer may be useful for an effective therapeutic outcome. Here, we demonstrate that administration of a specific CD73 inhibitor, APCP, to melanoma-bearing mice induced a significant tumor regression, by promoting the release of Th1- and Th17-associated cytokines in the tumor microenvironment. CD8+T cells were increased in melanoma tissue of APCP-treated mice. Accordingly, in nude mice APCP failed to reduce tumor growth. Importantly, we observed that after APCP administration the presence of B cells into the melanoma tissue was higher than control. This was associated with production of immunoglobulin (Ig)G2b within the melanoma. Depletion of CD20+ B cells partially blocked the anti-tumor effect of APCP and significantly reduced the production of IgG2b induced by APCP, implying a critical role for B cells in the anti-tumor activity of APCP. Our results also suggest that APCP could influence B cells activity to produce IgG, through IL-17A which significantly increased in the tumor tissue of APCP-treated mice. In support of this, we found that in melanoma-bearing mice receiving anti-IL-17A mAb the anti-tumor effect of APCP was ablated. This correlated with a reduced capacity of APCP-treated mice to mount an effective immune response against melanoma since neutralization of this cytokine significantly affected both the CD8+ T cell- and the B cell-mediated responses. In conclusion, we demonstrate that both T-cells and B cells play a pivotal role in the APCP-induced anti-tumor immune response.
Keywords: APCP, melanoma, Tumor immunity, B cells, IL-17A
Introduction
Cancer cells are able to escape immune-surveillance through multiple mechanisms, including the production of immunosuppressive factors in the tumour microenvironment that can impair immune cell function (1). Adenosine plays an important role in the mechanism of tumour escape (2, 3). Adenosine is an ATP-derived nucleoside, highly released during hypoxic conditions, typical of tumour microenvironment (4). In this context, cancer cells rapidly degrade ATP into adenosine which, in turn, accumulates in the tumour mass (5). Adenosine inhibits T cell proliferation (6) and critically impairs the cytokine production and the cytotoxicity of activated T cells (7, 8), protecting the tumour from immune-mediated destruction (2). Adenosine, thus, represents an important immunosuppressive molecule in the tumor microenvironment, that limits the activation of immune system to eradicate cancer cells (3, 9).
Extracellular adenosine is produced from the cells by two ectonucleotidases; CD39 (nucleoside triphosphate diphosphohydrolase) which hydrolyzes ATP and adenosine diphosphate (ADP) into adenosine monophosphate (AMP) and CD73 (ecto-5′-nucleotidase) that catalyses AMP conversion into adenosine. CD73 is the rate-limiting enzyme in this process (10) and is expressed on different cell types, including endothelial and epithelial cells (11), subsets of leukocytes (12) and T-regulatory cells (Foxp3+ Treg) (13). Interestingly, CD73 is up-regulated in several types of cancers (14) and increasing evidence suggests that CD73 plays a crucial role in the control of tumor progression. Indeed, it has been demonstrated that inhibition of CD73 activity (15) or CD73 knockdown on tumor cells (16) inhibit tumor growth by enhancing the anti-tumor T cell response. More recently, by using CD73 deficient mice, it has been shown that CD73 on hematopoietic cells (including Foxp3+ Treg cells) impair the anti-tumor T-cell-mediated immune response (17, 18). These effects are attributed to the regulation of extracellular adenosine generated by CD73 within the tumor microenvironment (17, 18).
In the current study, we determined the therapeutic anti-tumor efficacy of a specific inhibitor of CD73, APCP. Here, we provide new insights into the mechanism(s) underlying the anti-tumor activity of APCP in a mouse model of melanoma. Our results indicate that administration of APCP inhibited tumor growth, by promoting a Th1- and Th17-like immune response in the tumor environment. These effects are correlated with a higher presence of tumor-infiltrating CD8+ T cells. Moreover, we show that B cells are also required for the anti-tumor effects induced by APCP, as immunoglobulin-producing cells. Indeed, depletion of CD20+ B cells significantly reduced the anti-tumor effects of APCP and the production of APCP-induced IgG2b. Furthermore, we found that the anti-tumor activity of APCP is dependent on IL-17A, which in turn affects the APCP-induced cytotoxic immune response and the levels of IgG2b within the melanoma tissue.
Materials and Methods
Mice
C57Bl/6j and Athymic Nude-Foxn1nu mice were purchased from Harlan Laboratories (Udine, Italy). Mice were maintained in the National Cancer Institute “G.Pascale” Animal Facility (Naples, Italy), according to Institutional animal care guidelines, Italian D.L. no.116 of 27 January 1992 and European Communities Council Directive of 24 November 1986 (86/609/ECC).
Cell culture and CD19+ B cell isolation
B16-F10 mouse melanoma cells were from American Type Culture Collection (LGC Standards S.r.l., Milan, Italy) and K1735 mouse melanoma cells were kindly provided by Dr. Silvio Hemmi (University of Zuerich, Switzerland). Cells were cultured in complete DMEM, containing 10% FBS, 2mM L-Glutamine, 100U/ml penicillin and 100U/ml streptomycin (Sigma-Aldrich, Milan Italy).
CD19+ B cells were purified from the spleens of naïve C57Bl6j mice by magnetic separation using a CD19+ cell isolation kit, according to the manufacturer’s instructions (EasySep Stem Cell, Voden, Milan, Italy). Purity of CD19+ B cells was checked by flow cytometry by using anti-CD19 and anti-B220 antibodies (eBioscience, CA, USA) and was routinely around 90%. CD19+ B cells were cultured in RPMI 1640 enriched with 10% FBS and treated with APCP (5μM; Sigma-Aldrich, Milan Italy) for 24h. Supernatants were analyzed for cytokine production by ELISA and cells were stained with the following markers: MHC class I, MHC class II and CD20 and analyzed by FACS.
Animal studies
Mice (female at 6-8 weeks old) were injected subcutaneously (s.c.) on the right flank with 3 × 105 B16-F10 cells or with 5 × 105 K1735 cells. Adenosine 5′-(α,β-methylene) diphosphate (APCP 400μg/mouse) was delivered to the mice by the peritumoral (p.t.) route on day 10 and day 12 after tumor injection. This time point was selected in preliminary studies as it achieved optimal anti-tumor effects. Tumor volume was monitored with digital calipers and calculated using the formula: V= 4/3 π × (D/2) × (d/2)2, where V = volume (mm3), D = long diameter (mm) and d = short diameter (mm). Mice were sacrificed on day 13 after tumor cell implantation and melanoma tissues and proximal lymph nodes were isolated for further analyses. In some experiments an anti-CD73 mAb (TY/23, 10μg/mouse, p.t.) was administered to melanoma-bearing mice as described for APCP.
In some experiments an anti-CD20 monoclonal antibody (mAb) (rat IgG, 250μg/mouse in 100μl PBS; eBioscience, San Diego, CA, USA) (19, 20) was injected i.p. on the same day mice received APCP (day 10) and sacrificed on day 13. The anti-CD20 mAb depleted splenic CD20+ B cells by 90% compared with IgG, as previously demonstrated in our laboratory (20).
In other experiments, a neutralizing monoclonal antibody against IL-17A (Clone:eBioMM17F3; mouse IgG, 20μg/mouse, eBioscience, San Diego, CA, USA) was injected i.p. every day starting from day 10 until day 13. The anti-IL17A mAb reduced IL-17A release in the melanoma tissue by ~95% compared with IgG (data not shown).
Cell analysis
Tumors, lymph nodes and spleens were digested with 1U/ml collagenase A (Sigma-Aldrich, Milan, Italy). Cell suspensions were passed through 70-μm cell strainers and red blood cells were lysed. The cells were used for flow cytometric analyses (BD FacsCalibur, Milan, Italy). The following antibodies were used: CD8-PE, CD3-PeCy5.5, CD4-FITC, CD25-PE, Foxp3-PeCy5.5, NK1.1-PE, CD11c-FITC, CD19-PeCy5.5, B220-PE (eBioscience, San Diego, CA, USA). Further characterization was performed by using the following Abs: CD3-PeCy5.5, CD8-APC CD4-APC, IFNγ-PE, IL-17-PE (eBioscience).
ELISA
IL-17A, TNF-α, IFN-γ, IL-10, TGF-β and IgG2b were detected in melanoma tissue homogenates by using mouse specific ELISA kits (eBioscience, San Diego, CA, USA; R&D System, Abingdon, UK; Bethyl Laboratories, Montgomery, TX).
Immunohistochemistry
For histological analysis, melanoma tissues were fixed in OCT medium (Pella, Milan, Italy) and cut in 7μm cryosections. Frozen sections were stained with Ki67 (Abcam, Cambridge, UK) or Bcl2 (Santa Cruz Biotechnology, Inc., DBA, Milan, Italy) and detected with FITC anti-rabbit or FITC-anti mouse secondary antibodies, respectively. In all staining experiments, isotype-matched IgG and omission of the primary antibody was used as negative controls. Slides were analyzed by a fluorescence microscope (Carl Zeiss, Milan, Italy) by means of Axioplan Imaging Programme (Carl Zeiss).
Immunoblot analysis
Tumor tissues were homogenized in RIPA Buffer (Radioimmunoassay Precipitation Buffer). Anti Bcl-2 (Santa Cruz Biotechnology, DBA, Italy) or anti-tubulin antibodies (Sigma Aldrich, Rome, Italy) were used. Immunoreactive proteins were quantified by densitometry analysis (GelDoc Instrument).
Statistical analysis
Results are expressed as mean ± SEM. All statistical differences were evaluated by either Student’s t test or one way ANOVA, followed by Bonferroni’s post test as appropriate. P values less than 0.05 were considered statistically significant.
Results
APCP-induced tumor regression is associated with increased release of Th17- and Th1-like cytokines
To investigate the effect of CD73 blockade on tumor growth, we used 5′-(α,β-methylene) adenosine diphosphate (APCP), successfully used in various murine models, including those for cancer (16, 18, 21).
C57Bl6j mice were subcutaneously injected with 3 × 105 B16-F10 cells and 10 days later mice were treated with APCP (400μg/mouse, p.t.). The administration of APCP significantly reduced tumor growth in melanoma-bearing mice compared with PBS-treated mice (APCP: 254.4±65.8 versus PBS: 816.2±259.2 mm3; p<0.01) (Figure 1A). To verify the effect of APCP on melanoma growth we also evaluated the expression of Ki67, a proliferation marker (22). We observed a significant reduction in cells staining for Ki67 when mice were treated with APCP (Figure 1B and 1C). In addition, Bcl2 expression, an anti-apoptotic protein (23), was reduced in tissues section harvested from mice treated with APCP compared with PBS (Figure 1D and 1E). Thus, mice receiving APCP exhibited reduced tumor growth compared with control, consistent with previous studies (16, 18, 21). This effect was associated with a reduction in the number of proliferating cells within the tumor and increased susceptibility of cells to apoptosis.
Figure1. APCP administration significantly reduced tumor growth in melanoma-bearing C57Bl6j mice.
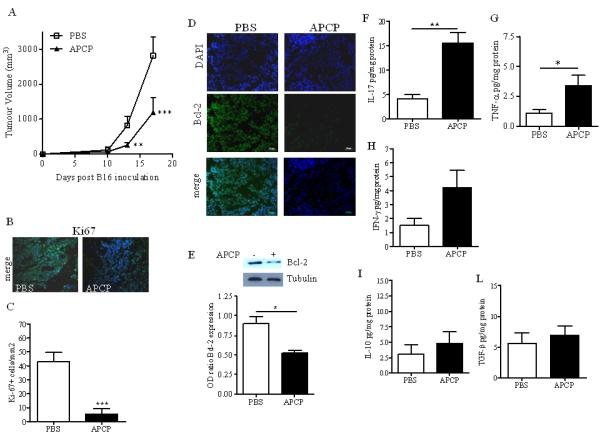
A) Tumor volume (mm3) was significantly reduced in mice receiving APCP (400μg/mouse, p.t.) compared with control mice (PBS) (n=13). Ki67 expression was determined by immunoflorescence staining in melanoma cryosections harvested from PBS- and APCP-treated mice (magnification:x20) (B) and quantified as number of Ki67 positive cells per mm2 of melanoma section by using ImageJ Software (NIH, USA) (n=6) (C). D) and E) The expression of the anti-apoptotic protein Bcl-2 in melanoma cryosections by immunofluorescence staining and in tissue lysates by Western blotting, respectively, of mice treated with PBS or APCP. Pictures are representative of n=5. F), G),H), I) and L) Levels of IL-17A, TNF-α, IFN-γ, IL-10 and TGF-β respectively, measured in the tissue homogenate of mice treated with PBS or APCP (n=13). Results are from three independent experiments and are expressed as mean ± S.E.M. Statistical differences, denoted by *, ** indicating p<0.05 and p<0.01 respectively, were determined by one way ANOVA or Student’s t test, as appropriate.
CD73-derived adenosine can modulate the inflammatory response (24); therefore, we analysed the levels of cytokines (IFN-γ, TNF-α, IL-17A, IL-10, TGF-β) in the homogenates of melanoma tissue harvested from the APCP-treated mice described above. Interestingly, we found that the levels of IL-17A, a pro-inflammatory cytokine, were significantly increased in the tumor tissue after APCP treatment (Figure 1F). Mice receiving APCP also showed increased release of the Th1-associated cytokines TNF-α and IFN-γ (Figures 1 G and 1H, respectively); whilst the levels of both IL-10 and TGF-β were not elevated in the tissue of mice treated with APCP (Figure 1I and 1L, respectively). APCP is a well-known CD73 inhibitor and the possibility of off-target effects in vivo cannot rule out. However, similar results were obtained in mice administered with the anti-CD73 mAb TY/23 (Figure 2A, B and C).
Figure 2.
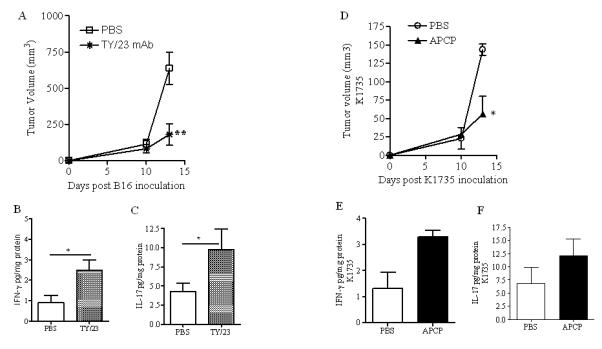
Administration of anti-CD73 mAb, TY/23 (10μg/mouse, p.t.), reduced tumor growth in B16-F10 melanoma-bearing mice (A) and increased the levels of IFN-γ (B) and IL-17 (C) in the tumor tissue. APCP treatment reduced tumor volume in mice bearing K1735 tumors (D) and increased the levels of IFNγ (E) and IL-17 (F) in the tumor tissue. Results are expressed as mean ± S.E.M (n=5). Statistical differences, denoted by *, ** indicating p<0.05 and p<0.01 respectively, were determined by one way ANOVA or Student’s t test, as appropriate.
The anti-tumor activity of APCP was also evaluated in K1735 tumor model. C57Bl6j mice were s.c. injected with K1735 melanoma cells and 8 days later, APCP was administered as previously described. APCP treatment significantly reduced tumor growth (Figure 2D). This effect was associated with increased levels of IFN-γ (Figure 2E) and IL-17A (Figure 2F) in the tumor tissue. These results indicate that the anti-tumor effect of APCP in melanoma-bearing C57Bl6j mice was accompanied by high production of Th1- and Th17-like cytokines within tumor tissue.
APCP treatment increased tumor-infiltrating B cells
Previous studies showed that tumor growth is inhibited in CD73-deficient mice, due to the improved T cell-mediated response (17, 18). Our results described above show that inhibition of tumor growth by APCP administration in melanoma-bearing mice correlated to cytokines associated with Th17- and Th1-like immune responses in the melanoma. Consistent with previous reports (18), the percentage of tumor-infiltrating CD3+CD8+ T cells was increased after APCP treatment (Figure 3A and 3B); whilst the percentage of CD4+ T cells, NK1.1+ cells, NKT cells and Foxp3+Treg cells were not altered (Supplementary Figure 1A, B, C and D). Surprisingly, we found that APCP increased the number of infiltrating B cells (CD19+B220+ cells) within the melanoma tissue (Figure 3C and 3D). This was associated with increased levels of the immunoglobulin (Ig)G2b in the tumor tissue (Figure 3E); whereas the levels of IgM (PBS: 0.155±0.02 versus APCP: 0.113±0.01 ng/mg protein, n=11), IgG2a (PBS: 1.38±0.21 versus APCP: 1.41±0.35 ng/mg protein, n=7) were unaltered, and IgG1 and IgG3 not detectable.
Figure 3. PCP administration promotes both the recruitment of CD8+ T cells and B cells within tumor lesion.
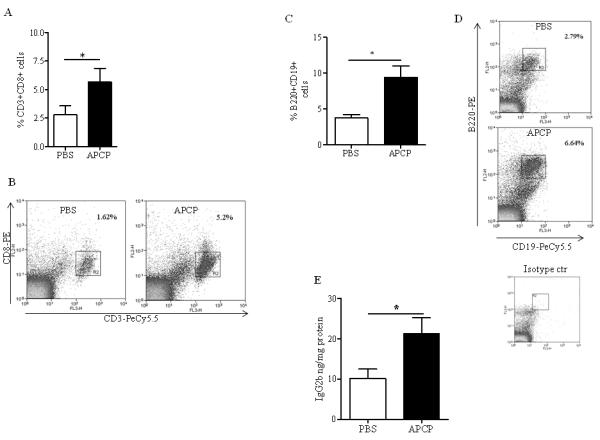
Percentage of CD8+ T cells (A) and B cells (C) in tumor tissue by gating on CD3+CD8+ T cells and CD19+B220+, respectively. Representative dot plots are shown in panels B and D. E) IgG2b levels detected by means of ELISA in tumor tissue homogenates. Results are from three independent experiments and are expressed as mean ± S.E.M, n=10. Statistical differences, denoted by * indicating p<0.05, were determined by Student’s t test.
These results indicate that the tumor regression observed in mice receiving the CD73 inhibitor APCP is associated with an increased percentage of tumor-infiltrating CD8+ T cells. Moreover, the data suggest that APCP administration increased B cells and the production of the immunoglobulin IgG2b within the melanoma tissue.
B cells contribute to the anti-tumor effects induced by APCP in melanoma-bearing mice
To determine whether administration of APCP could directly regulate B cell function, we performed in vitro experiments on isolated B cells. CD19+ B cells were isolated from spleen of naïve C57Bl6j mice and cultured for 24h with APCP (5μM) or PBS. APCP treatment did not affect either B-cell production of IL-10, IL-17A and TNF-α (Supplementary Figure 2A, B and C, respectively) or MHC class I, MHC class II and CD20 expression on B cells in vitro (Supplementary Figure 2D, E and F, respectively). Thus, although APCP was unable to directly influence B cell function in vitro, data obtained in mice indicate that inhibition of CD73 in the tumor environment may affect the humoral immune response.
To assess the role of B cells in the anti-tumor effect of APCP in vivo, we treated mice with APCP or PBS (on day 10 and 12 after B16-F10 injection) after B cell depletion using an anti-CD20 mAb injected i.p. on day 10 (Figure 4A). The anti-CD20 mAb treatment alone did not significantly affect tumor growth in melanoma-bearing mice (Figure 4B). The anti-tumor effect of APCP was partially reduced in CD20+ B cell-depleted mice, compared to IgG + APCP-treated mice (mAb anti-CD20+APCP: 617.75±107.1 versus IgG+APCP: 299.54.24±71.37 mm3; p<0.01) (Figure 4B). To further examine the effect of APCP in CD20+ B cell-depleted mice we analyzed the tumor-infiltrating cells. Neither the percentage of APCP-induced IFN-γ +CD8+ T cells (Figure 4C) nor IFN-γ levels (Figure 4D) were significantly affected in B-cell depleted mice after APCP administration. The percentage of IFN-γ+CD4+T cells, that was similar in all groups, are also shown (Figure4C). CD20+ B cell depletion (black bar), however, prevented APCP-induced levels of IgG2b within the melanoma compared with IgG-treated mice (white bar) (Figure 4E). This suggests that IgG2b-producing B cells significantly contributed to the anti-tumor effects induced by APCP in melanoma-bearing mice.
Figure 4. Depletion of CD20+ B cells reduced the anti-tumor effect of APCP in melanoma-bearing mice.
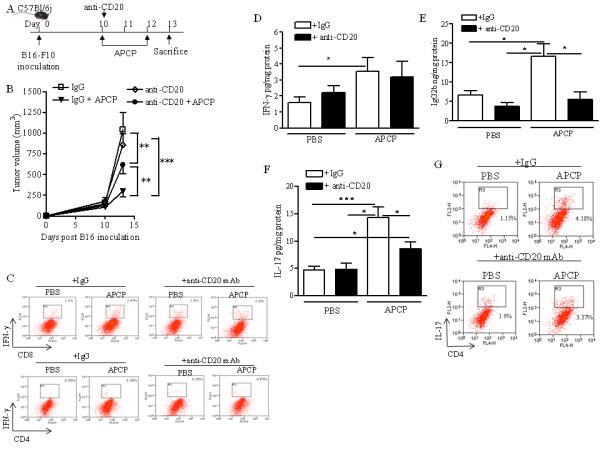
A) Experimental protocol: anti-CD20 mAb (250μg/mouse, i.p.) was administered on day 10 after B16-F10 tumor cell implantation, when mice received APCP (400μg/mouse). B) Tumor volume (mm3) in mice receiving anti-CD20 mAb or isotype control IgG after APCP or PBS administration. Representative dot plots of IFN-γ+ cells gated on CD3+CD8+ T cells or CD3+CD4+ T cells are shown (C). D), E) and F) Levels of IFN-γ, IgG2b and IL-17, respectively, in the melanoma tissue of mice receiving anti CD20 mAb (black bar) or IgG (white bar) after APCP or PBS administration. G) Representative dot plots for IL17+ gated on CD3+CD4+T cells in the tumor tissue are shown. Results are from three independent experiments and are expressed as mean ± S.E.M, n=10. Statistical differences, denoted by *, **, *** indicating p<0.05, p<0.01 and p<0.001 respectively, were determined by one way ANOVA.
In B-cell depleted animals we observed that, although anti-CD20 mAb treatment can affect IL-17A production (25, 26), APCP treatment increased the levels of IL-17 in the tumor tissue (Figure 4F). Moreover, we found that APCP-treated mice had increased tumor-infiltrating IL17+CD4+ T cells (Figure 4G). The number of tumor-infiltrating IL17+CD8+T cells was similar in all treated groups (Supplementary Figure 3).
APCP -induced anti-tumour effect is dependent on IL-17A
To understand the role of IL-17A in APCP-induced tumor growth regression, B16-F10-implanted C57Bl6j mice were injected with a neutralizing antibody for IL-17A (20 μg/mouse, i.p.) or IgG control (mouse IgG) every day starting from the day 10 after tumor cell implantation (Figure 5A). Mice were treated with APCP or PBS on day 10 and 12 and sacrificed on day 13 as described above (Figure 5A). Administration of the IL-17A mAb did not alter tumour growth in melanoma-bearing mice (Figure 5B). In contrast, IL-17A neutralization significantly blocked the anti-tumor effect of APCP (mAb anti-IL-17A+APCP: 704.18±98.4 versus IgG+APCP: 379.73±78.9 mm3; p<0.05) (Figure 5B). In addition, both the percentage of tumor-infiltrating CD8+ T cells (Figure 5C) and the production of IFN-γ within tumor tissue (Figure 5D and Supplementary Figure 4) were significantly reduced after APCP treatment in IL-17A-depleted mice. Blockade of IL-17A also significantly reduced both the percentage of B cells (Figure 5E) and the levels of APCP-induced IgG2b within the tumor mass (Figure 5F).
Figure 5. The anti-tumor effect of APCP in melanoma-bearing C57Bl6j mice is IL-17A-mediated.
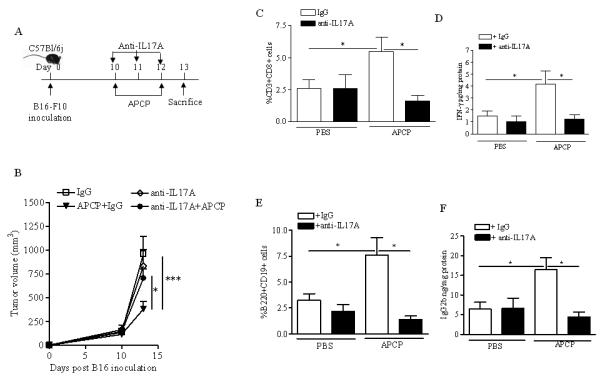
A) Experimental protocol: anti-IL17A mAb (20μg/mouse, i.p.) was administered every day starting from day 10 after B16-F10 tumor cell implantation, when mice received APCP (400μg/mouse). B) Tumor volume (mm3) in mice receiving anti-IL-17A mAb or isotype IgG control, and treated with PBS or APCP. Percentage of CD8+T cells recruited in the tumor tissue (C) and levels of IFN-γ in the tumor mass (D) of mice receiving isotype IgG control (white bar) or anti-IL-17A mAb (black bar), and treated with PBS or APCP. In E and F are reported the percentage of tumor-infiltrating B cells and the tissue levels of IgG2b, respectively, in mice receiving isotype IgG control (white bar) or anti-IL-17A mAb (black bar). Results are from three independent experiments and are expressed as mean ± S.E.M, n=9. Statistical differences, denoted by *, **, indicating p<0.05 and p<0.01 respectively, were determined by one way ANOVA.
Together these results suggest that blockade of CD73 is associated with high IL-17A production into the tumor environment. Moreover, this cytokine is critical for the observed anti-tumor effect of APCP. Indeed, the results suggest that APCP-induced IL-17A could positively influence both CD8+T cell- and B cell–mediated responses within the tumor.
APCP did not affect tumour growth in nude mice
We further investigated the effect of APCP on tumor growth in athymic nude mice, which lack T cells. Nude mice were injected with B16-F10 cells and 10 days later, mice were administered twice with APCP as described above for C57Bl6j mice. Tumor growth in nude mice was not affected by APCP treatment (APCP: 990.4±414.7 versus PBS: 1066.8±520.4 mm3) (Figure 6A). These results confirm that T cells are required for the APCP-induced regression of melanoma. Additionally, APCP treatment did not modulate B cell activation or IgG2b levels in nude mice (Figure 6B). These data further support the concept that APCP could indirectly influence B cells activity to produce IgG by inducing inflammatory T cell-associated cytokines, such as IL-17A, which we could not detect in these mice (data not shown).
Figure 6. APCP administration did not affect tumor growth in nude mice.
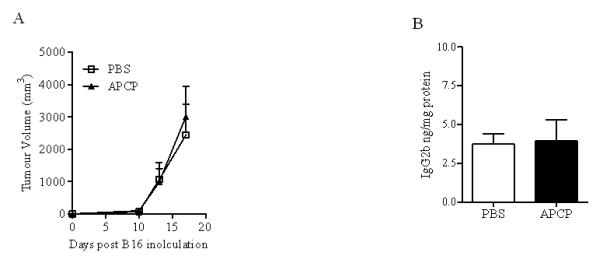
Athymic nude mice bearing melanoma B16 cells were treated with APCP 10 days later tumor cell implantation. A) Tumor growth after APCP or PBS treatment. B) IgG2b levels in the tumor tissue harvested from nude mice treated with APCP or PBS. Results are from two independent experiments and are expressed as mean ± S.E.M, n=6.
Discussion
In this study we provided new insights into the mechanism underlying the anti-tumor activity of APCP, a CD73 inhibitor, in a mouse model of melanoma. Administration of APCP facilitated a local Th1- and Th17-associatedcytokine release, which in turn affects tumor cell growth. Similar results were observed using an anti-CD73 mAb. Importantly, we observed that the anti-tumor activity of APCP in mice is mediated, at least in part, by B cells-producing immunoglobulin IgG2b within the tumor lesion.
Several studies have shown that CD73 via adenosine generation can promote tumor growth in mice. Adenosine derived from CD39 in concert with CD73, expressed both on tumor cells and on host cells (including Treg cells), accumulates within tumor tissue dampening anti-tumor T cell immunity (13, 15, 16). Moreover, tumor-associated Treg cells, which highly express CD39 and CD73, inhibit Th17 cell development through the adenosinergic pathway (27). The tumor resistance of CD73-deficient mice is associated with an increased influx of CD8+ T cells (18) and low numbers of Tregs within the tumor (21). Of note, anti-CD73 mAb therapy or blockade of CD73 significantly inhibit tumor growth (15, 18) and enhances the efficacy of adoptive T cell therapy (18). In our study we found that the anti-tumor effect of APCP was associated with a greater presence of melanoma-infiltrating CD8+ T cells. These data further indicate that the anti-tumor activity of APCP in immune-competent mice, bearing B16-F10 melanoma, is T cell-dependent. Accordingly, in nude mice APCP failed to reduce tumor growth. This study is the first, to our knowledge, to demonstrate that B cells are also involved in the anti-tumor effect of APCP in mice.
Several studies have shown that B cells play an important role in the anti-tumor immunity. For example, B cell deficient mice (28, 29) or mice depleted of B cells (30-32) are protected from tumor proliferation. These results may be due to the activation status of B cells (33) and/or the immune-regulatory function of B cells (B10 cells), that produce IL-10 (34). In contrast, recent studies demonstrate that B cells facilitate T-mediated responses, which in turn impair tumor development (19, 20). These observations indicate that B cells can significantly contribute to control tumor growth. In addition, activated B cells can mediate significant tumor regression in a IgG2b-dependent manner (20, 35). These latter studies highlight the effector function of B cells as a source of IgG2b which are highly cytotoxic toward tumor cells (35).
Many studies report that the majority of B cells do not express CD73 (12), although some authors have demonstrated that CD73 is expressed on a subset of memory B cells (36), suggesting that CD73-derived adenosine could regulate B cell function (36, 37). However, as yet the role of CD73 in regulating B cell function has not been clearly defined. In the present study, we observed increased numbers of B cells into the melanoma tissue of APCP-treated mice. This result is associated with enhanced production of IgG2b in the tumor mass. Depletion of CD20+ B cells markedly reduced the anti-tumor effect of APCP and the level of IgG2b enhanced by APCP, further supporting the notion that B cells mediated the activity of APCP in reducing tumor growth as immunoglobulin-producing cells. Further work is needed to assess the importance of the IgG2b-mediated response in the therapeutic activity of APCP. APCP could indirectly affect the in vivo B cell activity to produce IgG, by inducing the release of cytokines, such as IL-17, into the tumor microenvironment. IL-17A is a pro-inflammatory cytokine implicated in the pathogenesis of autoimmunity (38); however, the role of IL-17A in tumor immunity is controversial, since both pro- and anti-tumor effects have been described. In immune-deficient mice IL-17A over-expressed in tumor cells enhanced tumor growth, by promoting angiogenesis (39). Similar results have been obtained in IL17a-/- mice (40). In contrast, other studies demonstrated that IL-17A inhibits tumor growth in immune-competent mice through enhanced anti-tumor immunity (41, 42). Recent studies also show that Th17 cells protect mice from tumor proliferation, by facilitating the activation of CD8+ T cells and NK cells (43, 44). Similarly, IL-17 produced by cytotoxic CD8+T cells (Tc17) inhibit B16-F10 melanoma growth (45).
The present study shows that APCP administration leads to enhanced production of T-cell derived IL-17A within tumor tissue, suggesting that inhibition of CD73 could condition CD4+T cell polarization toward Th17-producing cells. This hypothesis is supported by previous data on adenosine-induced suppression of Th17 development. It has been reported that hydrolysis of ATP to adenosine or adenosine analogs reduce IL-17 production by CD4+ T cells (46). Notably, Th17 cells in the tumor microenvironment are negatively associated with the presence of Treg cells, which suppress Th17 cells through adenosine induction (27). Inhibition of ectonucleotidases, highly expressed on Treg cells, recovered T-cell IL-17 production (27). .
We found that IL-17A blockade prevents the ability of APCP to inhibit tumor growth. This effect was correlated with a reduced presence of CD8+ T cell and reduced IFN-γ production in the melanoma tissue of APCP-treated mice. Although previous data indicate that IL-17A drives T-cell recruitment (43), the effects on proliferation and/or survival may also be important. Interestingly, our results also suggest that APCP-induced IL17A facilitates the presence of B cells within the tumor tissue, and the production of IgG2b. Recent data indicates that IL-17A can positively regulate the humoral immune response. IL-17A promotes germinal center formation and class switch recombination to IgG subclasses (47, 48). Moreover, IL-17A sustains the proliferation of B cells and their differentiation into immunoglobulin-secreting cells in systemic lupus erythematosus (49). This supports our concept that APCP-induced IL17A-within the tumor tissue is essential for the regulation of the local B cell response. It is currently unclear what the relative role of the other cytokines such as TNFα and IFNγ, which were elevated in melanoma tissue, in comparison to that of IL-17A in regulating the T- and B-cell recruitment, proliferation and survival in response to APCP treatment in this model. Further work in this area is needed to elucidate these aspects of the anti-tumor effect of APCP.
In conclusion, our data demonstrates that, in addition to T-cells, B cells also contribute to the anti-tumor activity of APCP in mice via an IL-17A-mediated process. Thus, pharmacological inhibition of CD73 in the tumor tissue exerts a beneficial therapeutic effect, by mounting a protective B- and T-cell mediated anti-tumor response.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by PRIN 2008 Ministero della Universita' e Ricerca Scientifica, Italy (in favor of A.P.). RS is supported by University of Salerno fellowship. IMA is supported by the Wellcome Trust, BBSRC, The MRC and The Royal Society.
Footnotes
Conflict of interest: The authors have no conflicting financial interests.
References
- 1.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 2.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103(35):13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29(39):5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 4.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57(13):2602–2605. [PubMed] [Google Scholar]
- 5.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3(7):e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Conrad DM, Butler JJ, Zhao C, Blay J, Hoskin DW. Adenosine acts through A2 receptors to inhibit IL-2-induced tyrosine phosphorylation of STAT5 in T lymphocytes: role of cyclic adenosine 3′,5′-monophosphate and phosphatases. J Immunol. 2004;173(2):932–944. doi: 10.4049/jimmunol.173.2.932. [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90(4):1600–1610. [PubMed] [Google Scholar]
- 8.Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J Immunol. 2009;183(9):5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 9.Spychala J. Tumor-promoting functions of adenosine. Pharmacol Ther. 2000;87(2-3):161–173. doi: 10.1016/s0163-7258(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 10.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 11.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2(2):351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita Y, Hooker SW, Jiang H, Laurent AB, Resta R, Khare K, et al. CD73 expression and fyn-dependent signaling on murine lymphocytes. Eur J Immunol. 1998;28(10):2981–2990. doi: 10.1002/(SICI)1521-4141(199810)28:10<2981::AID-IMMU2981>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res. 2010;70(16):6407–6411. doi: 10.1158/0008-5472.CAN-10-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107(4):1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70(6):2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71(8):2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121(6):2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184(7):4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorrentino R, Morello S, Forte G, Montinaro A, De Vita G, Luciano A, et al. B cells contribute to the antitumor activity of CpG-oligodeoxynucleotide in a mouse model of metastatic lung carcinoma. Am J Respir Crit Care Med. 2011;183(10):1369–1379. doi: 10.1164/rccm.201010-1738OC. [DOI] [PubMed] [Google Scholar]
- 21.Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemelä J, Laurila JP, Elima K, et al. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol. 2011;41(5):1231–1241. doi: 10.1002/eji.201041292. [DOI] [PubMed] [Google Scholar]
- 22.Gimotty PA, Van Belle P, Elder DE, Murry T, Montone KT, Xu X, et al. Biologic and prognostic significance of dermal Ki67 expression, mitoses, and tumorigenicity in thin invasive cutaneous melanoma. J Clin Oncol. 2005;23(31):8048–8056. doi: 10.1200/JCO.2005.02.0735. [DOI] [PubMed] [Google Scholar]
- 23.Sorrentino R, Morello S, Luciano A, Crother TR, Maiolino P, Bonavita E, et al. Plasmacytoid dendritic cells alter the antitumor activity of CpG-oligodeoxynucleotides in a mouse model of lung carcinoma. J Immunol. 2010;185(8):4641–4650. doi: 10.4049/jimmunol.1000881. [DOI] [PubMed] [Google Scholar]
- 24.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, Timmermans K, Di Padova F, Koenders MI, et al. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum. 2011;63(6):1507–1516. doi: 10.1002/art.30314. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Wang Z, Graner MW, Yang L, Liao M, Yang Q, et al. B cell infiltration is associated with the increased IL-17 and IL-22 expression in the lungs of patients with tuberculosis. Cell Immunol. 2011;270(2):217–223. doi: 10.1016/j.cellimm.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4(5):627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 29.Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU, et al. Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells. Int J Cancer. 2005;117(4):574–586. doi: 10.1002/ijc.21177. [DOI] [PubMed] [Google Scholar]
- 30.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I. Immunity to a chemically induced tumor. J Immunol. 1978;121(1):359–362. [PubMed] [Google Scholar]
- 31.Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF, Jr, Feng L, et al. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother.; 2000;48(10):541–549. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Fridlender ZG, Dunn R, Kehry MR, Kapoor V, Blouin A, et al. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. J Immunother. 2008;31(5):446–457. doi: 10.1097/CJI.0b013e31816d1d6a. [DOI] [PubMed] [Google Scholar]
- 33.Watt V, Ronchese F, Ritchie D. Resting B cells suppress tumor immunity via an MHC class-II dependent mechanism. J Immunother. 2007;30(3):323–332. doi: 10.1097/CJI.0b013e31802bd9c8. [DOI] [PubMed] [Google Scholar]
- 34.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66(15):7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE. In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol. 2009;183(5):3195–3203. doi: 10.4049/jimmunol.0803773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204(9):2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur J Immunol. 2005;35(1):31–41. doi: 10.1002/eji.200425524. [DOI] [PubMed] [Google Scholar]
- 38.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautés-Fridman C, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99(6):2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 42.Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61(1):79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114(2):357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114(3):596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, et al. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 47.Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107(32):14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10(7):778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


