Abstract
The Bordetella pertussis heme utilization gene cluster hurIR bhuRSTUV encodes regulatory and transport functions required for assimilation of iron from heme and hemoproteins. Expression of the bhu genes is iron regulated and heme inducible. The putative extracytoplasmic function (ECF) σ factor, HurI, is required for heme-responsive bhu gene expression. In this study, transcriptional activation of B. pertussis bhu genes in response to heme compounds was shown to be dose dependent and specific for heme; protoporphyrin IX and other heme structural analogs did not activate bhu gene expression. Two promoters controlling expression of the heme utilization genes were mapped by primer extension analysis. The hurI promoter showed similarity to σ70-like promoters, and its transcriptional activity was iron regulated and heme independent. A second promoter identified upstream of bhuR exhibited little similarity to previously characterized ECF σ factor-dependent promoters. Expression of bhuR was iron regulated, heme responsive, and hurI dependent in B. pertussis, as shown in a previous study with Bordetella bronchiseptica. Further analyses showed that transcription originating at a distal upstream site and reading through the hurR-bhuR intergenic region contributes to bhuR expression under iron starvation conditions in the absence of heme inducer. The pattern of regulation of the readthrough transcript was consistent with transcription from the hurI promoter. The positions and regulation of the two promoters within the hur-bhu gene cluster influence the production of heme transport machinery so that maximal expression of the bhu genes occurs under iron starvation conditions only in the presence of heme iron sources.
The innate immune system of the human host defends against invading microorganisms in part by sequestering iron, a nutrient essential for virtually all living cells. The majority of host iron is maintained intracellularly in the form of hemoproteins, while extracellular iron is bound by the host glycoproteins transferrin and lactoferrin (44, 49). Successful microbial pathogens have evolved mechanisms to overcome host iron restriction (21, 32, 46), including production and utilization of low-molecular-weight iron chelators termed siderophores (40), utilization of siderophores produced by other organisms, and direct removal of iron from host proteins via specific bacterial cell surface receptors (11, 59).
In gram-negative and some gram-positive bacterial species, genes encoding iron transport systems are repressed when intracellular iron levels are high by the Fur protein with ferrous iron as the corepressor (17, 22). When bacterial cells encounter an iron-limiting environment such as the human host, their intracellular iron stores are depleted, resulting in derepression of iron acquisition genes. Fur derepression is sufficient for full expression of the genes in certain iron uptake systems, while in other systems, positive transcriptional regulation requiring the presence of the cognate iron source is also necessary for maximal gene expression (12). Positive regulators of iron acquisition systems are of three main classes: AraC-like proteins (4, 9, 18, 24, 43), two-component signal transduction systems (14, 50), and extracytoplasmic function (ECF) σ factors (1, 13, 30, 31, 57).
ECF σ factors are members of the σ70 superfamily of bacterial sigma factors and are utilized by diverse species to regulate genes in response to extracytoplasmic stimuli (37, 45). ECF σ factors involved in regulating iron stress responses have been termed members of the iron starvation subfamily of ECF regulators (58). These ECF σ factors and their specific anti-σ factors are produced under iron-limiting conditions, but the σ factors remain inactive until the cognate iron source is sensed in the environment. In the presence of the appropriate iron source, a signaling cascade is initiated at the cell surface by the cognate outer membrane receptor. The signal is transduced to the anti-σ factor, which then either releases or activates the σ factor, allowing it to associate with core RNA polymerase and initiate transcription of genes encoding iron acquisition functions (6, 58). Members of the iron starvation family of ECF σ factors include FecI (1), PupI (31), and PvdS (13), which regulate a subset of iron uptake genes in Escherichia coli, Pseudomonas putida, and Pseudomonas aeruginosa, respectively. Recently, the putative ECF σ factors HurI of Bordetella pertussis and Bordetella bronchiseptica (57) and RhuI of Bordetella avium (30) were shown to regulate expression of heme iron transport genes.
Since greater than 90% of the iron within the human body is associated with heme and hemoproteins (42), bacteria that can access these compounds in vivo and utilize host heme iron have a significant nutritional advantage. Vibrio cholerae (25, 26), pathogenic E. coli (55), Shigella species (36), Yersinia species (53, 54), and P. aeruginosa (41) produce TonB-dependent cell surface receptors and ATP-binding cassette transporters that allow utilization of heme, hemoglobin, and other hemoproteins. A second type of heme uptake system, employed by species such as Serratia marcescens (33), Yersinia pestis (47), and P. aeruginosa (41), involves production and secretion of small heme-binding proteins termed hemophores that obtain and ferry host heme to specific bacterial cell surface receptors.
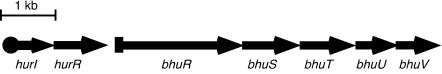
B. pertussis, the causative agent of the human disease whooping cough, and B. bronchiseptica, a closely related mammalian respiratory pathogen, possess multiple systems for iron retrieval under iron-limiting environmental conditions. They produce the siderophore alcaligin (8, 20, 28, 38) and are capable of using siderophores produced by other organisms (3). Both species possess the heme utilization gene cluster bhuRSTUV, which encodes transport functions required for assimilation of iron from heme and hemoproteins (Fig. 1) (56). B. avium, a more distantly related pathogen of turkeys and chickens, has an orthologous gene cluster encoding a functional heme utilization system (39). Expression of B. pertussis and B. bronchiseptica bhu genes is regulated by iron and the presence of heme via Fur and the ECF regulators encoded by the hurIR genes, located immediately upstream of the bhu gene cluster (56, 57).
FIG. 1.
B. pertussis and B. bronchiseptica heme iron utilization genetic locus. The hurI and hurR genes encode a σ factor and cytoplasmic membrane protein (anti-σ factor), respectively. The bhuRSTUV genes encode components of the heme iron transport machinery. BhuR is the outer membrane receptor, and BhuS is a predicted heme binding protein; BhuT, BhuU, and BhuV are components of the periplasmic binding protein-dependent ATP-binding cassette transporter system. The solid circle upstream of hurI represents a predicted σ70-like promoter, while the solid rectangle upstream of bhuR denotes a putative HurI-dependent, heme-responsive promoter. The arrows indicate the direction of transcription.
We showed in a previous study that HurI, a putative ECF σ factor, is required for heme-activated bhuR transcription and for maximal levels of heme utilization (57). In the present study, the kinetics of the transcriptional response to heme inducer and the structural characteristics of the inducer were examined. We have identified transcriptional start sites for the iron-regulated hurI and heme-inducible bhuR genes and have demonstrated the hurI dependence of heme-responsive bhuR expression in B. pertussis. Furthermore, iron-regulated bhuR transcription in the absence of heme was assessed, and it was found that transcription from an upstream promoter, reading through the hurR-bhuR intergenic region, contributes to bhuR expression. These data support a model for transcriptional regulation of heme utilization genes that allows Bordetella cells to sense heme and respond by maximally producing the heme transport machinery.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bordetella strains and recombinant plasmids used in this study are listed in Table 1. E. coli DH5α (Invitrogen, Gaithersburg, Md.) was used as the host strain in routine cloning procedures. Plasmid vectors pGEM3Z (Promega, Madison, Wis.) and pRK415 (29) were used in the construction of recombinant plasmids. A pRK415 derivative, plasmid pRK40 (57), carries a promoterless trp′-′lacZ gene and was used to construct all bhuR-lacZ transcriptional fusions (Table 1).
TABLE 1.
Bordetella strains and reporter plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| UT25Sm1 | B. pertussis; spontaneous streptomycin-resistant derivative of wild-type strain UT25 | 19 |
| PM8 | B. pertussis UT25Sm1 ΔhurI | 57 |
| B013N | B. bronchiseptica; spontaneous nalidixic acid-resistant derivative of wild-type B013 | 2 |
| Plasmids | ||
| pRK40 | pRK415 with 3.3-kb EcoRI-HindIII trp′-′lacZ insert fragment; Tetr | 57 |
| pRK41 | pRK40 with 0.5-kb B. pertussis UT25 ′hurR-bhuR′ insert fragment; bhuR-lacZ transcriptional fusion; Tetr | 57 |
| pRK42 | pRK40 with 2.1-kb B. pertussis UT25 hurIR-bhuR′ insert fragment; hurIR bhuR-lacZ transcriptional fusion; Tetr | 57 |
| pRK45 | pRK40 with 0.3-kb B. pertussis UT25 bhuR′ insert fragment; bhuR-lacZ transcriptional fusion; Tetr | This study |
| pRK47 | pRK42 with 12-nt block substitution in bhuR promoter region; hurIR bhuR-lacZ transcriptional fusion; Tetr | This study |
| pRK48 | pRK42 with unique BglII site in hurR-bhuR intergenic region; hurIR bhuR-lacZ transcriptional fusion; Tetr | This study |
| pRK49 | pRK48 with Ω Cm cassette inserted into BglII site, hurIR Ω Cm bhuR-lacZ transcriptional fusion; Tetr | This study |
| pRK50 | pRK40 with B. pertussis UT25 0.38-kb bhuR′ insert fragment; bhuR-lacZ transcriptional fusion; Tetr | This study |
| pRK51 | pRK40 with B. pertussis UT25 0.44-kb ′hurR-bhuR′ insert fragment; bhuR-lacZ transcriptional fusion; Tetr | This study |
Growth media and chemical solutions.
Luria-Bertani (LB) (48) broth or agar plates were used to culture E. coli strains. B. pertussis strains were cultured on Bordet-Gengou (BG) agar (5); B. bronchiseptica strains were cultured on LB agar. All Bordetella liquid cultures were grown in Stainer-Scholte (SS) minimal medium (51, 52). SS medium was deferrated by Chelex100 (Bio-Rad, Richmond, Calif.) as described previously (2). Iron-depleted SS medium contained no iron supplements, while iron-replete SS medium was supplemented with FeSO4 to a final concentration of 36 μM. Bovine hemin chloride (Sigma, St. Louis, Mo.) was maintained as a 1 mM stock solution as described previously (56) and added to iron-depleted cultures at a final concentration of 5 μM unless otherwise indicated. Ethanolic stock solutions of chlorophyll a (Sigma) were prepared at a concentration of 1 mM; aqueous solutions of protoporphyrin IX (PPIX) and cytochrome c (both from Sigma) were maintained at concentrations of 400 μM and 500 μM, respectively, and zinc-PPIX (Sigma) was dissolved in N,N-dimethyl formamide at a concentration of 800 μM. Each porphyrin compound was added to liquid cultures at a final concentration of 5 μM unless otherwise indicated. Tetracycline and ampicillin were used at final concentrations of 15 μg/ml and 100 μg/ml, respectively.
Bacterial culture conditions.
B. pertussis and B. bronchiseptica strains were grown on agar plates and subcultured to iron-replete SS medium. B. bronchiseptica cells were grown with shaking at 37°C for 24 h, washed, and inoculated at a dilution of 1:200 to iron-replete and iron-depleted SS medium. After 18 h of growth, hemin was added as appropriate to iron-depleted cultures. All cultures were harvested for β-galactosidase assays or RNA isolation 4 h after the addition of hemin (after a total of 22 h of growth). A similar procedure was used to culture B. pertussis strains except that iron-replete SS cultures were grown for 36 h; subcultures were inoculated at an initial optical density (600 nm) of 0.08 and grown for 24 h prior to hemin addition.
RNA isolation and primer extension analysis.
Total RNA was harvested from cultures by a modification (27) of the acid-guanidinium thiocyanate-phenol-chloroform extraction method of Chomczynski and Sacchi (10). Primer extension reactions contained 25 μg of RNA, 1 pmol of 32P-end-labeled primer, 1X Superscript II buffer (Stratagene, La Jolla, Calif.), 1 mM deoxynucleoside triphosphate mixture, 10 μM dithiothreitol, 1 mg of bovine serum albumin per ml, in a total reaction volume of 20 μl. This mixture was heated to 70°C for 5 min to denature the RNA, hybridized at 45°C for 30 min, and cooled to 37°C for 10 min. Superscript II RNase H− reverse transcriptase (10 units) (Stratagene) was added to each reaction, which was incubated for an additional 30 min at 37°C. The primer extension reaction was stopped, and primer-extended cDNA was isolated by standard methods (48). Prior to loading on an 8% polyacrylamide gel, the products were denatured by boiling for 5 min. One-half of the final volume was loaded on the gel next to a nucleotide sequencing ladder generated by appropriate primers with plasmid DNA templates.
Reverse transcription-PCR analysis.
Reverse transcription reactions with Bordetella RNA as templates were performed as described for primer extension, except that nonradiolabeled primer was used. After reverse transcription, the mixture was diluted by addition of an equal volume of distilled water, and 2 μl was used as the template for PCR. The following components were used in the PCR: water to a total volume of 50 μl, 1X Pfu Turbo buffer (Stratagene), 800 μM deoxynucleoside triphosphate mixture, 2 μl of diluted reverse transcription reaction, 8 pmol of each primer, 5% dimethyl sulfoxide, 1 unit of Pfu Turbo DNA polymerase (Stratagene). The thermal cycler was programmed for one cycle of denaturation at 96°C for 5 min, 30 cycles of denaturation at 96°C for 1 min, primer annealing at 62°C for 1 min, and extension at 72°C for 30 s, and one cycle at 72°C for 10 min.
Genetic methods.
Bordetella pertussis nucleotide sequence data were produced by the Bordetella Sequencing Group at the Sanger Centre (http://www.sanger.ac.uk/Projects/B_pertussis/). Other nucleotide sequences were obtained from GenBank at the National Center for Biotechnology Information at the National Library of Medicine.
Reporter plasmid pRK40 and bhuR-lacZ plasmids pRK41 and pRK42 were described previously (57). β-Galactosidase assays of cells carrying reporter plasmids were performed by a modification (7) of the method of Miller (35). The results reported are representative of at least two experimental trials. Deletion derivatives of the bhuR promoter fragment were generated by PCR with B. pertussis cosmid pCPbhu1 (carrying hurIR bhuRSTUV) (56) as the template. The source of the Ω chloramphenicol (Cm) cassette used to construct the terminator insertion in plasmid pRK49 was mini-Tn5 Cm (15).
The block substitution and BglII site insertion in plasmids pRK47 and pRK48, respectively, were constructed by whole-plasmid PCR mutagenesis by a method described previously (60). Briefly, primers that were antisense to one another were designed to be complementary to the hurR-bhuR intergenic region except for the bases to be substituted. Primers mECF1 and mECF2 contained a 12-nucleotide block substitution in the center of each primer, with 16 nucleotides of complementarity to the template DNA flanking both sides of the mutation. Primers Bgl1 and Bgl2 contained three single-nucleotide substitutions to create a BglII restriction site. These primer sequences were as follows: mECF1, 5′-CGTGCCTGCTCTCGATCCCTTTCCTTCTTCATGGTTTACGCTTGC-3′; mECF2, 5′-AAGCGTAAACCATGAAGAAGGAAAGGGATCGAGAGCAGGCACGAG-3′; Bgl1, 5′-CGGCAAAAAAAATTCCAGATCTCTGTCCGGTTTCGACG-3′; and Bgl2, 5′-CGTCGAAACCGGACAGAGATCTGGAATTTTTTTTGCCG-3′.
For whole-plasmid PCR mutagenesis, the following components were mixed in order: water to a total volume of 50 μl, 100 ng of p3Z102 plasmid DNA, 50 pmol of each primer, 1 mM deoxynucleoside triphosphate mixture, 1X Pfu Turbo buffer (Stratagene), 5% dimethyl sulfoxide, and 2.5 U of Pfu Turbo DNA polymerase (Stratagene). The thermal cycler was programmed for one cycle of denaturation at 96°C for 5 min, 16 cycles of denaturation at 96°C for 1 min, primer annealing at 55°C for 1 min, and extension at 68°C for 10 min. Following the PCR, DpnI was added to digest the methylated parental template DNA. E. coli DH5α was transformed with 10 μl of the reaction, and plasmids from several independent transformants were sequenced to identify plasmids containing the desired mutations.
RESULTS
Temporal analysis of bhuR induction.
The transcriptional response of iron-starved Bordetella cells to various heme concentrations was monitored to determine the sensitivity and time course of bhu gene activation. Wild-type B. bronchiseptica B013N cells carrying the hurIR bhuR-lacZ plasmid pRK42 were cultured in parallel in iron-replete SS medium and iron-depleted SS medium with or without hemin. Cells grown in iron-replete medium showed low levels of β-galactosidase activity (≈400 Miller units) that remained constant for the duration of the experiment (data not shown). Cells grown in iron-depleted medium without hemin showed ≈2-fold-higher levels of reporter gene activity compared with iron-replete cells, demonstrating iron-regulated bhu gene expression.
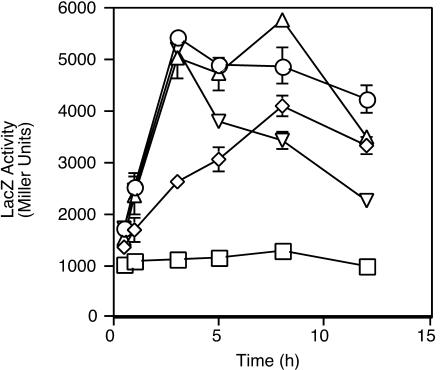
Replicate cultures of iron-starved cells were exposed to concentrations of hemin ranging from 0.32 μM to 20 μM (Fig. 2), and transcription of bhuR was activated in response to all concentrations of hemin tested. Interestingly, the lowest concentration of hemin (0.32 μM) did not measurably stimulate the growth of iron-starved cells (data not shown) but did induce bhuR transcription (Fig. 2), indicating that heme responsiveness and bhuR activation are highly sensitive. The induction kinetics of bhuR transcriptional activation varied with the concentration of hemin provided. Cells exposed to a low concentration of hemin (0.32 μM) showed a modest induction that slowly increased to a maximum at ≈8 h after hemin addition. Cells induced by intermediate heme concentrations (1.25 or 5 μM) showed higher peak levels of transcription that increased rapidly within 2 h and declined from maximum levels after 8 h of heme exposure. Cells given the highest dose of heme (20 μM) showed a pattern of rapid but transient induction followed by a sharper decrease in transcriptional activity, consistent with uptake of heme iron resulting in Fur-mediated repression of the fusion gene. The cultures exhibiting the highest sustained levels of bhuR transcription were those exposed to intermediate concentrations of hemin.
FIG. 2.
Analysis of induction kinetics of bhuR heme-responsive transcription. B. bronchiseptica cells carrying hurIR bhuR-lacZ reporter fusion plasmid pRK42 were cultured in iron-depleted SS medium with or without (squares) hemin. Parallel cultures were assayed for β-galactosidase activity at the indicated times after addition of hemin to the following concentrations: 20 μM (inverted triangles); 5 μM (triangles); 1.25 μM (circles); and 0.32 μM (diamonds).
Analysis of inducer specificity.
Initiation of the signaling cascade that results in bhu gene transcriptional activation involves recognition of heme by the BhuR receptor protein (57). As a means to elucidate the structural requirements for BhuR inducer recognition, molecules structurally similar to heme were tested for their ability to induce bhuR transcription. Cytochrome c is a hemoprotein in which the heme moiety is covalently linked to the cytochrome protein. Although intact cytochrome c cannot supply nutritional iron to Bordetella cells (data not shown), it was hypothesized that recognition of heme at the cell surface, independent of transport, could lead to signaling and transcriptional activation of bhuR. PPIX, the heme biosynthetic precursor lacking a coordinated iron atom, zinc-PPIX, and chlorophyll a, a porphyrin with a coordinated magnesium atom, all bear significant structural similarity to heme.
To test bhuR transcriptional responsiveness to these heme analogs, B. bronchiseptica B013N(pRK42) was grown in iron-depleted SS medium and exposed to chlorophyll a, PPIX, zinc-PPIX, cytochrome c, or hemin. In multiple experiments, cells exposed to hemin showed at least a fourfold induction of bhuR transcription over levels exhibited by iron-starved cells. The highest level of induction in response to any other compound tested was a 1.5-fold induction in response to PPIX (data not shown). To further assess whether PPIX was a weak inducer of bhuR transcription, iron-starved B013N(pRK42) cells were exposed to PPIX concentrations of 5, 10, 25, and 50 μM and bhuR transcriptional activity was monitored. In contrast to the response to hemin (Fig. 2), there was no dose-dependent transcriptional activation in response to PPIX (data not shown), indicating that BhuR recognition of inducer is highly specific for the porphyrin ring with bound iron.
Mapping of the transcriptional initiation site for hurI.
To elucidate the genetic mechanisms mediating inducible expression of heme utilization genes, the positions and features of promoters within the heme utilization gene cluster were defined. The hurI and hurR genes encode a putative ECF σ factor and cytoplasmic membrane regulator, respectively. In previous studies (56, 57), potential σ70-like promoter elements and Fur binding sites were identified upstream of hurI (shown in Fig. 3A), and functional Fur binding activity in this region was demonstrated, suggesting that hurI transcription was iron repressible.
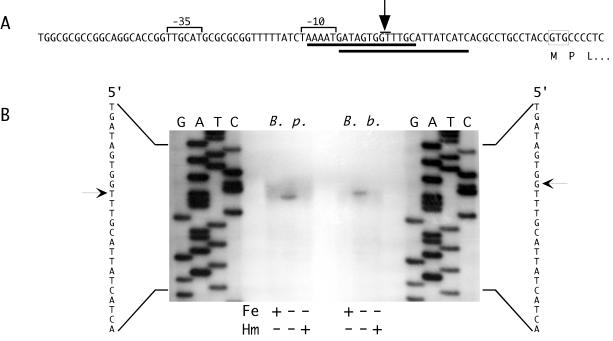
FIG. 3.
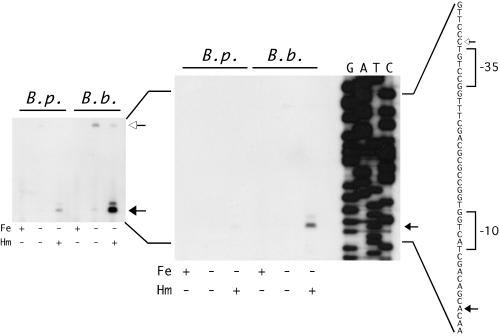
Mapping of the hurI promoter by primer extension. (A) The nucleotide sequence and features of the B. pertussis hurI promoter region are shown. The predicted hurI GTG start codon is boxed. Solid lines below the sequence indicate predicted Fur binding sites. The arrow points to a bar above the +1 positions for the B. pertussis and B. bronchiseptica hurI genes. The −10 and −35 promoter elements are designated by brackets. (B) The autoradiogram shows the results of primer extension analysis of total RNA isolated from B. pertussis (B. p.) and B. bronchiseptica (B. b.) cells cultured under iron-replete (Fe+, Hm−), iron-depleted (Fe−, Hm−), and iron-depleted with hemin supplementation (Fe−, Hm+) conditions. Arrows designate the hurI transcriptional initiation sites in B. pertussis (left) and B. bronchiseptica (right).
To directly examine hurI expression and identify the hurI transcription initiation site, total RNA isolated from wild-type B. pertussis UT25Sm1 and wild-type B. bronchiseptica B013N cells was analyzed in primer extension experiments. A hurI transcript was undetectable in cells grown under iron-replete conditions but was present in RNA isolated from iron-starved cells (Fig. 3B), demonstrating iron regulation at the hurI promoter. Addition of heme to iron-starved cultures resulted in a significant reduction in hurI transcript levels, suggesting that the iron requirements of the cells were satisfied by the added heme and that Fur repression of hurI was resumed.
A single major hurI transcription initiation site was observed in both B. pertussis and B. bronchiseptica. In B. pertussis, the site corresponded to a T residue that was 27 nucleotides upstream of the predicted hurI start codon (Fig. 3A), while in B. bronchiseptica, the major site was the upstream adjacent G residue. Consistent with previous predictions (57), the transcription initiation sites were optimally spaced from σ70-like −10 and −35 elements: 5′-TAAAAT-3′ and 5′-TTGCAT-3′, respectively. The initiation sites and promoter elements overlap predicted Fur binding sites, consistent with a promoter occlusion mechanism of Fur repression. The lack of canonical Shine-Dalgarno sequences suggests that the translational efficiency of the hurI mRNA may be low.
Genetic and biochemical characterization of bhuR promoter determinants.
Other ECF σ factors regulating a variety of functions in response to extracytoplasmic signals have been described (34, 37, 45), and these sigma factors recognize promoter sequences distinct from those typical of σ70 promoters (16, 37). Nucleotide sequence alignments comparing the bhuR upstream region with promoter sequences of other ECF σ factor-regulated genes identified potential −10 and −35 elements that we previously hypothesized to comprise the bhuR promoter (Fig. 4) (56). Based on these predictions, an oligonucleotide primer (PE1, Fig. 4) was designed for mapping of the transcription initiation site by primer extension analysis. However, in multiple experiments, a bhuR-specific extension product was not produced by PE1 (data not shown). At that time, it was hypothesized that the extremely high G+C content of the predicted bhuR initial transcribed region (93% from positions 370 to 410, Fig. 4) may be causing premature termination of reverse transcription.
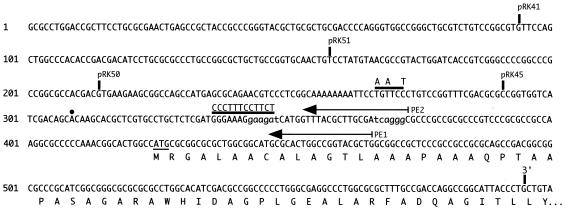
FIG. 4.
Features of the bhuR promoter region. The nucleotide sequence of the bhuR upstream region and 5′ bhuR coding sequences (GenBank accession number AY032627) are shown. Solid vertical lines labeled pRK41, pRK51, pRK50, and pRK45 denote the 5′ limits of the bhuR promoter region used to construct the corresponding bhuR-lacZ plasmid-borne fusions (Table 1). The vertical line labeled 3′ indicates the lacZ fusion junction for all bhuR-lacZ constructs. Nucleotides 343 to 348 and 367 to 372 shown in lowercase letters represent ECF σ-like −35 and −10 elements that were predicted based on similarity to other promoters. The horizontal bar over nucleotides 336 to 347 shows the position of the block substitution mutation constructed in plasmid pRK47; the bar over nucleotides 266 to 271 shows the position of the BglII site engineered in plasmid pRK48. Nucleotide changes are indicated above the bars. Arrows labeled PE1 and PE2 indicate the positions of antisense bhuR primers used in primer extension analyses. The dot denotes the transcription initiation site determined with primer PE2. Amino acids of the N-terminal region of the BhuR protein are designated below the nucleotide sequence.
To genetically test the prediction that sequences located at positions 335 to 346 constituted a critical part of the HurI-dependent, heme-responsive bhuR promoter, a block substitution mutation in the predicted −35 region was constructed (Fig. 4) and analyzed in the context of a transcriptional hurIR bhuR-lacZ fusion (plasmid pRK47). B013N(pRK47) showed the same pattern of iron-regulated, heme-responsive bhuR transcription as cells carrying the wild-type fusion gene (pRK42) (data not shown), indicating that the residues mutated in pRK47 did not constitute part of the heme-responsive bhuR promoter.
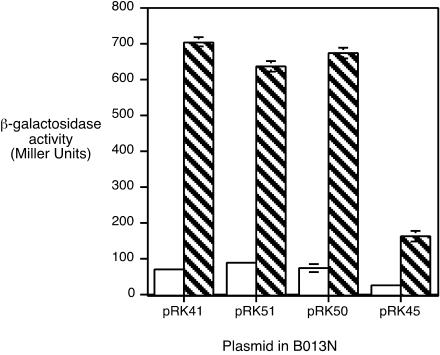
Since predictions based on nucleotide sequence alignments with other ECF σ factor promoters did not allow identification of the bhuR promoter, a series of deletions in the bhuR upstream DNA region was constructed to spatially define the minimal region required for maximal heme-responsive bhuR promoter activity. It was previously shown that a 0.5-kb region encompassing the 3′ region of hurR, the 0.2-kb hurR-bhuR intergenic region, and 5′ bhuR sequences carried all the regulatory determinants necessary to direct hurI-dependent, heme-responsive transcription of a bhuR-lacZ fusion (pRK41) in B. bronchiseptica (57). Successive 5′ deletions of this region were obtained by PCR, yielding 0.44-kb, 0.38-kb, and 0.3-kb fragments, which were used to construct bhuR-lacZ fusion plasmids pRK51, pRK50, and pRK45, respectively (Fig. 4).
B. bronchiseptica B013N carrying the bhuR-lacZ fusion plasmids were grown in iron-depleted medium with or without hemin and assayed for β-galactosidase activity (Fig. 5). B013N(pRK41) showed a ninefold induction of bhuR transcription when iron-starved cells were exposed to hemin. Cells carrying fusion plasmid pRK51 or pRK50 exhibited essentially equivalent levels of transcriptional activity and induction, indicating that bhuR promoter determinants mediating heme responsiveness were contained within the 0.21-kb region upstream of the bhuR start codon carried on plasmid pRK50 (Fig. 4). However, B013N(pRK45) showed markedly reduced transcriptional activity under both growth conditions, and induction in response to hemin was reduced to only ≈3-fold (Fig. 5). This result indicated that nucleotide sequences between the pRK50 and pRK45 endpoints (positions 214 and 291, Fig. 4) were required for wild-type levels of bhuR promoter activity. The residual activity and partial heme responsiveness of the fusion borne on pRK45 suggested that part of the bhuR promoter may be contained on this cloned DNA fragment.
FIG. 5.
Determination of the minimal heme-responsive bhuR promoter region. B. bronchiseptica B013N carrying bhuR-lacZ reporter plasmid pRK41, pRK51, pRK50, or pRK45 was cultured in parallel in iron-depleted SS medium with (hatched bars) or without (open bars) hemin supplementation. Bars represent Miller units of LacZ activity ± 1 standard deviation (n = 3).
Based on these genetic analyses of the bhuR promoter, it was hypothesized that the bhuR +1 position was located further upstream of the bhuR open reading frame than originally predicted and that failure to obtain extension products in previous experiments was perhaps due to the distance between primer PE1 and the transcription initiation site, as well as the G+C composition of sequences upstream of the bhuR coding sequences. Additional primer extension analyses performed with primer PE2 (complementary to a region upstream of the high-G+C tract) (Fig. 4) demonstrated the presence of a bhuR transcript in RNA samples from iron-starved B. pertussis and B. bronchiseptica cells that were exposed to hemin (Fig. 6). The transcript was undetectable in RNA samples from iron-replete cultures and was present in very low abundance in iron-starved B. bronchiseptica cells (detectable only after extended exposures of the autoradiogram).
FIG. 6.
Mapping the bhuR promoter region by primer extension analysis. The autoradiogram at the right shows the results of primer extension with bhuR-specific primer PE2 on B. pertussis (B. p.) and B. bronchiseptica (B. b.) total RNA from iron-replete cultures (Fe+, Hm−) and iron-depleted cultures with (Fe−, Hm+) or without (Fe−, Hm−) hemin. The inset is an overexposure of the same autoradiogram to show less abundant products. The sequence of the bhuR promoter region is shown to the right. The major bhuR transcriptional start site is indicated by a solid arrow; the deduced −10 and −35 promoter elements are indicated by brackets. The open arrow upstream of the −35 element indicates the position of the larger iron-regulated product (likely derived from an upstream promoter) in both B. pertussis and B. bronchiseptica samples.
The major bhuR transcription initiation site corresponded to an A residue 116 nucleotides upstream of the predicted bhuR start codon (Fig. 4 and Fig. 6) in both Bordetella species. A minor initiation site mapped to a G residue 2 nucleotides further upstream. A larger primer extension product (open arrow, Fig. 6) was detected in RNA samples from iron-starved B. bronchiseptica cells (Fig. 6, inset), and was present in very low abundance in RNA from iron-starved B. bronchiseptica cells induced with hemin. This primer extension product was also detectable in RNA from iron-starved B. pertussis when the autoradiogram was significantly overexposed. This larger product maps to a site 36 nucleotides upstream of the major bhuR transcription initiation site and is likely to be derived from a longer iron-regulated transcript initiating upstream of bhuR, possibly at the hurI promoter. The greater abundance of this larger product in RNA from iron-starved cells in the absence of hemin is similar to the pattern of expression of the hurI transcript (Fig. 3), suggesting that this larger primer extension product may be derived from a transcript initiating at the hurI promoter. It is possible that termination of reverse transcription may occur at this point on the transcript due to the presence of a secondary structure in the mRNA.
We previously reported that in B. bronchiseptica, heme-inducible transcription of a bhuR-lacZ fusion was dependent on hurI (57). To determine whether bhuR transcription was also initiated in a hurI-dependent manner in B. pertussis, primer extension experiments with RNA obtained from wild-type (UT25Sm1) and ΔhurI mutant (PM8) B. pertussis strains were performed (data not shown). Similar to the results shown in Fig. 6, in wild-type B. pertussis the bhuR transcript was most abundant in iron-starved cells exposed to hemin. In contrast, the B. pertussis ΔhurI mutant showed no detectable bhuR transcript under any of the conditions tested (data not shown), indicating that production of the heme-inducible bhuR transcript is dependent on the HurI σ factor in B. pertussis. The larger iron-regulated product seen in primer extension experiments (such as that shown in Fig. 6) was also observed in other experiments with both wild-type and hurI mutant strains (data not shown), indicating that this transcript is not hurI dependent.
A nucleotide sequence alignment of the bhuR promoter region with other ECF σ factor- dependent promoters is shown in Fig. 7. Consistent with previous observations of other investigators (16, 37), certain features of the ECF σ factor promoters of other organisms, including the −35 elements and spacing between −35 and −10 elements, are fairly well conserved, while the −10 elements are poorly conserved. It has been proposed that the −10 element may provide specificity for promoter recognition by a particular ECF σ factor, since many bacterial genomes appear to encode multiple ECF sigma factors (37, 58). The bhuR promoter shows little sequence similarity to other ECF σ factor promoters, even that of fecA, which is regulated by another member of the iron starvation subfamily of ECF σ factors (1). Interestingly, although determination of the P. putida pupB promoter (regulated by the ECF σ factor PupI) has not been reported, alignment of the pupB upstream region with the bhuR promoter region revealed striking similarities in what are predicted to be the −35 and −10 elements in each of these promoters. In addition, a tract of A residues upstream from the predicted −35 element is present in the promoter regions of both bhuR and pupB. The functional significance of this sequence feature, if any, is unknown.
FIG. 7.
Alignment of ECF σ factor-dependent promoter regions. The promoters of known ECF σ factor-dependent genes are aligned. The transcriptional start site of each gene is underlined. The −10 and −35 promoter elements are indicated by underlined lowercase letters. (A) Genes (GenBank accession numbers): bhuR, B. pertussis heme receptor (AY032627); fecA, E. coli ferric citrate receptor (S79758); dagAP2, Streptomyces coelicolor agarase promoter 2 (X05811); rpoHP3, E. coli heat shock σ factor promoter 3 (AF127104); algD, P. aeruginosa alginate (M28683); pvdF, P. aeruginosa pyoverdin biosynthesis (U07359); carQ, Myxococcus xanthus transcriptional regulator (X71062). (B) Alignment of B. pertussis bhuR and P. putida pupB pseudobactin receptor gene (X73598) promoter regions. Putative −10 and −35 elements are indicated by underlined lowercase letters. The start codons and the B. pertussis +1 position are underlined.
Analysis of transcription through the hurR-bhuR intergenic region.
The requirement for BhuR in heme-responsive transcriptional activation of the bhu genes was demonstrated previously (57), suggesting a role for the receptor as an environmental heme sensor and signal-transducing protein in addition to its function as a heme transporter. Results from the present study indicate that the bhuR promoter is active almost exclusively under iron starvation conditions in the presence of heme; however, in order to have BhuR displayed on the cell surface to act as a heme sensor, some transcription of bhuR likely occurs under iron starvation conditions in the absence of heme.
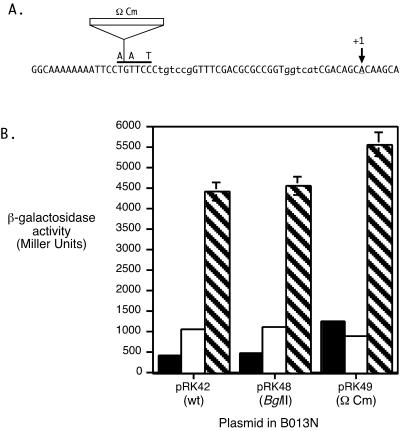
To determine if iron-regulated transcription originating at a distal upstream promoter reads through the hurR-bhuR intergenic region to contribute to iron-regulated bhuR expression, a polar Ω Cm element insertion was constructed in the hurR-bhuR intergenic region. Plasmid-borne transcriptional lacZ fusions with the wild-type parental hurIR bhuR′ fragment (pRK42), the fragment containing an engineered BglII site (pRK48), and the fragment containing the Ω Cm insertion (pRK49) (Table 1, Fig. 8A) were analyzed in wild-type B. bronchiseptica.
FIG. 8.
Analysis of transcriptional readthrough in the hurR-bhuR intergenic region. (A) The nucleotide sequence of a portion of the hurR-bhuR intergenic region is shown from 5′ to 3′. The first nucleotide corresponds to position 251 in Fig. 4. The solid horizontal bar over the nucleotide sequence indicates the position of the BglII site; the nucleotide substitutions that created this restriction site are indicated above the bar. The insertion site of the chloramphenicol resistance cassette containing transcriptional terminators on both ends (Ω Cm) is indicated. The −35 and −10 elements of the bhuR promoter are indicated in lowercase letters. The major bhuR transcriptional initiation site is indicated with an arrow labeled +1. (B) B. bronchiseptica B013N cells carrying the designated plasmids were cultured in iron-replete (solid bars), iron-depleted (open bars), or iron-depleted medium with hemin (hatched bars) and assayed for β-galactosidase activity. Bars represent LacZ activity ± 1 standard deviation (n = 3). Parental plasmid pRK42 (wt) contains the wild-type hurIR bhuR-lacZ transcriptional fusion. Plasmid pRK48 (BglII) is identical to pRK42 except for three substituted nucleotides (indicated in A) that create a BglII site. The 3.2-kb Ω Cm cassette was cloned into the BglII site of pRK48 to construct plasmid pRK49 (Ω Cm).
B013N(pRK42) and B013N(pRK48) exhibited equivalent levels of iron-regulated, heme-inducible bhuR expression (Fig. 8B). Levels of β-galactosidase activity were increased by ≈2-fold in response to iron starvation compared with levels in iron-replete cells, and further activated by approximately 4.5-fold by the addition of heme. In contrast, in B013N(pRK49), the β-galactosidase activities of iron-replete and iron-depleted cultures were nearly equivalent, indicating that the insertion abolished iron-regulated bhuR expression. However, transcription of bhuR was heme activated ≈5.5-fold over iron-depleted levels in B013N(pRK49), demonstrating that the Ω Cm insertion did not disrupt heme-responsive bhuR promoter function. These results suggest that transcription resulting in iron-regulated, heme-independent bhu gene expression originates upstream of the site of the Ω cassette insertion. Transcription from the hurI promoter was shown to be iron regulated, and thus it is likely that transcription from the hurI promoter reads through the hurR-bhuR intergenic region and into bhuR to allow low levels of bhuR transcription under iron-limiting conditions in the absence of heme induction.
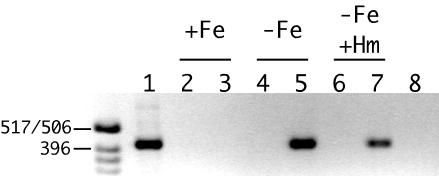
Additional evidence indicating that iron-regulated transcription through the hurR-bhuR intergenic region contributes to bhuR expression in the absence of inducer was obtained by reverse transcription-PCR analysis. Total RNA from B. pertussis cells grown under iron-replete conditions and iron-depleted conditions with and without hemin supplementation was reverse transcribed, and the products were used as the template in PCR. The predicted 0.44-kb product, encompassing the ′hurR-bhuR′ region, was obtained when cosmid DNA carrying the entire hur-bhu genetic system was used as a control template (Fig. 9, lane 1). A ′hurR-bhuR′ transcript was not detected in RNA from cells grown in iron-replete medium (Fig. 9, lane 3), consistent with Fur repression at the hurI promoter. In contrast, transcripts spanning the hurR-bhuR intergenic region were detected in RNA samples from iron-starved cells cultured with and without hemin (Fig. 9, lanes 5 and 7). These results confirm that RNA transcripts initiating upstream of the heme-inducible bhuR promoter (likely at the hurI promoter) proceed through the hurR-bhuR intergenic region and into bhuR under iron-limiting conditions in the absence of inducer, thus allowing BhuR to be produced at a low level for heme sensing and transport.
FIG. 9.
Reverse transcription-PCR analysis of transcription in the hurR-bhuR intergenic region. Total RNA from wild-type B. pertussis cells was isolated, reverse transcribed, and used as a template in PCR analysis. The 0.44-kb product encompasses 3′ hurR sequences, the hurR-bhuR intergenic region, and 5′ bhuR sequences. The positive control DNA template was cosmid pCPbhu1 (lane 1); negative control reactions contained no reverse transcriptase (lanes 2, 4, and 6) or RNA template treated with RNase prior to reverse transcription (lane 8). Cells were grown in iron-replete (lanes 2 and 3), iron-depleted (lanes 4, 5, and 8), and iron-depleted with hemin (lanes 6 and 7) medium. The sizes of DNA markers (in base pairs) are indicated at the left. The image is inverted from the ethidium bromide-stained agarose gel photographed under UV transillumination.
DISCUSSION
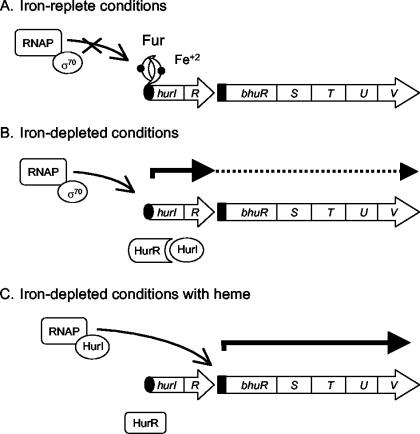
Studies on the B. pertussis heme utilization system to date (56, 57) support the model proposed in Fig. 10 for iron-repressible and heme-responsive transcriptional regulation of bhu genes. Under iron-replete conditions, Fur and iron repress hurI promoter activity (Fig. 10A). Under iron-depleted conditions, Fur derepression of the hurI promoter allows transcription initiation at hurI, resulting in HurI and HurR protein production. However, in the absence of heme, HurI remains inactive through its association with HurR. Some transcription initiated at the hurI promoter reads through the hurR-bhuR intergenic region and the bhu genes (Fig. 10B), allowing low levels of BhuR to be produced and displayed on the cell surface. When BhuR binds heme, a signal is transduced through HurR, and HurI is released and can associate with core RNA polymerase to direct high levels of transcription at the bhuR promoter (Fig. 10C). Transcription at the hurI promoter may continue until the cell's intracellular iron stores are replenished, at which time Fur repression will resume. HurI-dependent transcription of the bhu genes may diminish and eventually cease as the HurI protein turns over and no new protein is produced.
FIG. 10.
Model for molecular mechanisms of transcriptional regulation of the Bordetella heme iron utilization system. The genetic regulation of Bordetella heme utilization genes under three different environmental conditions is depicted as described in the Discussion. The hur and bhu genes are identified by open arrows, which indicate the direction of transcription. Solid arrows represent transcripts originating at the hurI and bhuR promoters; the dashed line with a solid arrowhead indicates a putative low-abundance readthrough transcript. Curved arrows point to positions of transcription initiation. The solid oval and solid rectangle indicate the iron-regulated hurI promoter and heme-responsive bhuR promoter, respectively. RNAP, RNA polymerase core enzyme.
The hurI and bhuR promoters were mapped by mutational and primer extension analyses. The transcription initiation site for the hurI gene was consistent with previous predictions of σ70-like promoter elements. Iron regulation of hurI was observed, consistent with predicted Fur binding sites and previous determination of functional Fur binding activity in the hurI promoter region (56). Several lines of evidence suggest that an iron-regulated polycistronic transcript initiating at the hurI promoter and reading through bhuR provides a low level of bhuR expression in the absence of heme inducer. First, the hurI and hurR open reading frames overlap, and no other obvious promoter elements exist within these coding regions, suggesting that they are cotranscribed. Additionally, reverse transcription-PCR experiments identified iron-regulated transcripts encompassing the hurR-bhuR intergenic region, indicating that readthrough transcription occurs. Concordantly, insertion of a terminator downstream of hurR abolished the wild-type pattern of iron-regulated bhuR expression but did not affect heme-activated expression (Fig. 8), indicating that the transcript encompassing the hurR-bhuR intergenic region was iron regulated and hurI independent, which is a pattern of expression identical to that of the hurI transcript (Fig. 3). Iron-regulated, heme-independent bhuR expression is predicted to be crucial for BhuR production in the absence of inducer, which would allow B. pertussis cells to sense the presence of heme in the environment.
The bhuR transcription initiation site was identified in both B. pertussis and B. bronchiseptica. Consistent with our previous studies examining the activity of bhuR-lacZ reporter fusions (57), the bhuR transcript was found to be iron regulated, heme inducible, and hurI dependent. A second, larger product was also identified with a bhuR-specific primer in primer extension analyses. This product was iron regulated but not heme responsive or hurI dependent; thus, this pattern of expression is very similar to that of the hurI transcript. The bhuR promoter shares little similarity with characterized ECF σ factor promoters from other organisms, including the fecA promoter of E. coli, which is regulated by the iron starvation ECF sigma factor FecI. Interestingly, the bhuR promoter region shares several features with the predicted P. putida pupB promoter region, including the presence of an adenine-rich region upstream of the predicted −35 elements, suggesting that the regulation of these promoters may also be similar.
Though the concentrations of heme to which Bordetella cells are exposed in vivo are unknown, the success of another obligate human respiratory pathogen, Haemophilus influenzae, implies that heme may be accessed in this niche by capable organisms. Similar to B. pertussis, nontypeable H. influenzae is a noninvasive organism that colonizes the human nasopharynx. Haemophilus species are incapable of synthesizing protoporphyrin IX, the precursor of heme, and require exogenously supplied heme or porphyrin in order to grow aerobically (23). Thus, their ability to successfully colonize the nasopharynx and cause upper respiratory disease in humans indicates that their heme requirements are satisfied in the host environment. Unlike Haemophilus species, B. pertussis and B. bronchiseptica can synthesize heme precursors and thus do not require heme as a growth factor. However, heme internalized via the Bhu system may be used both as an iron source and as a prosthetic group for direct incorporation into cytochromes and other metabolic enzymes.
The bhu system is the second example of a positively regulated Bordetella iron acquisition system for which the substrate is known. The native alcaligin siderophore system is positively regulated by an AraC-like protein, AlcR, in response to iron starvation and the presence of alcaligin. We hypothesize that positive regulation of iron acquisition systems in Bordetella species allows the organisms to prioritize expression of genes based on iron source availability. During the course of infection, it is likely that cells may sense multiple iron sources, for example, heme and ferric alcaligin, simultaneously. Under those circumstances, priority might be assigned to expression of genes that encode utilization functions for the most abundant or most easily assimilated iron source in the environment. The ability to integrate signals received from multiple iron sources and respond appropriately may be critical for B. pertussis in the complex host environment, which changes over the course of infection due to the actions of B. pertussis virulence factors and the host immune responses.
Acknowledgments
We are grateful to Timothy Brickman for critical reading of the manuscript, many useful discussions, and advice and assistance with transcriptional analyses and graphics. We thank Mladen Tomich for technical advice related to primer extension methods. We acknowledge Jenny Walder for technical assistance.
Support for this study was provided by Public Health Service grants R01 AI-31088 (S.K.A.) and T32 AI-07421 (C.K.V.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Angerer, A., S. Enz, M. Ochs, and V. Braun. 1995. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. Fecl belongs to a new subfamily of sigma 70-type factors that respond to extracytoplasmic stimuli. Mol. Microbiol. 18:163-174. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, S. K., and M. O. Clements. 1993. Isolation and characterization of Bordetella bronchiseptica mutants deficient in siderophore activity. J. Bacteriol. 175:1144-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beall, B. 1998. Two iron-regulated putative ferric siderophore receptor genes in Bordetella bronchiseptica and Bordetella pertussis. Res. Microbiol. 149:189-201. [DOI] [PubMed] [Google Scholar]
- 4.Beaumont, F. C., H. Y. Kang, T. J. Brickman, and S. K. Armstrong. 1998. Identification and characterization of alcR, a gene encoding an AraC-like regulator of alcaligin siderophore biosynthesis and transport in Bordetella pertussis and Bordetella bronchiseptica. J. Bacteriol. 180:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordet, J., and O. Gengou. 1906. Le microbe de la coqueluche. Ann. Inst. Pasteur (Paris) 20:731-741. [Google Scholar]
- 6.Braun, V. 1997. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch. Microbiol. 167:325-331. [DOI] [PubMed] [Google Scholar]
- 7.Brickman, T. J., and S. K. Armstrong. 2002. Bordetella interspecies allelic variation in AlcR inducer requirements: identification of a critical determinant of AlcR inducer responsiveness and construction of an alcR(Con) mutant allele. J. Bacteriol. 184</?VOLUMN-NR>:1530-1539. [DOI] [PMC free article] [PubMed]
- 8.Brickman, T. J., J. G. Hansel, M. J. Miller, and S. K. Armstrong. 1996. Purification, spectroscopic analysis and biological activity of the macrocyclic dihydroxamate siderophore alcaligin produced by Bordetella pertussis and Bordetella bronchiseptica. Biometals 9:191-203. [DOI] [PubMed] [Google Scholar]
- 9.Brickman, T. J., H. Y. Kang, and S. K. Armstrong. 2001. Transcriptional activation of Bordetella alcaligin siderophore genes requires the AlcR regulator with alcaligin as inducer. J. Bacteriol. 183:483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen, C. N. 2003. Transferrin-iron uptake by gram-negative bacteria. Front. Biosci. 8:D836-847. [DOI] [PubMed] [Google Scholar]
- 12.Crosa, J. H. 1997. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol. Mol. Biol. Rev. 61:319-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunliffe, H. E., T. R. Merriman, and I. L. Lamont. 1995. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J. Bacteriol. 177:2744-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean, C. R., and K. Poole. 1993. Expression of the ferric enterobactin receptor (PfeA) of Pseudomonas aeruginosa: involvement of a two-component regulatory system. Mol. Microbiol. 8:1095-1103. [DOI] [PubMed] [Google Scholar]
- 15.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enz, S., V. Braun, and J. H. Crosa. 1995. Transcription of the region encoding the ferric dicitrate-transport system in Escherichia coli: similarity between promoters for fecA and for extracytoplasmic function sigma factors. Gene 163:13-18. [DOI] [PubMed] [Google Scholar]
- 17.Escolar, L., J. Perez-Martin, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fetherston, J. D., S. W. Bearden, and R. D. Perry. 1996. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol. Microbiol. 22:315-325. [DOI] [PubMed] [Google Scholar]
- 19.Field, L. H., and C. D. Parker. 1978. Differences observed between fresh isolates of Bordetella pertussis and their laboratory passaged derivatives, p. 124-132. In C. R. Manclark and J. C. Hill (ed.), International symposium on pertussis. U.S. Department of Health, Education, and Welfare, Washington, D.C.
- 20.Giardina, P. C., L. A. Foster, S. I. Toth, B. A. Roe, and D. W. Dyer. 1995. Identification of alcA, a Bordetella bronchiseptica gene necessary for alcaligin production. Gene 167:133-136. [DOI] [PubMed] [Google Scholar]
- 21.Guerinot, M. L. 1994. Microbial iron transport. Annu. Rev. Microbiol. 48:743-772. [DOI] [PubMed] [Google Scholar]
- 22.Hantke, K. 1981. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol. Gen. Genet. 182:288-292. [DOI] [PubMed] [Google Scholar]
- 23.Hardy, G. G., S. M. Tudor, and St. J. W. Geme, 3rd. 2003. The pathogenesis of disease due to nontypeable Haemophilus influenzae. Methods Mol. Med. 71:1-28. [DOI] [PubMed] [Google Scholar]
- 24.Heinrichs, D. E., and K. Poole. 1993. Cloning and sequence analysis of a gene (pchR) encoding an AraC family activator of pyochelin and ferripyochelin receptor synthesis in Pseudomonas aeruginosa. J. Bacteriol. 175:5882-5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henderson, D. P., and S. M. Payne. 1994. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB- dependent proteins. J. Bacteriol. 176:3269-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson, D. P., and S. M. Payne. 1994. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect. Immun. 62:5120-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang, H. Y., and S. K. Armstrong. 1998. Transcriptional analysis of the Bordetella alcaligin siderophore biosynthesis operon. J. Bacteriol. 180:855-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang, H. Y., T. J. Brickman, F. C. Beaumont, and S. K. Armstrong. 1996. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J. Bacteriol. 178:4877-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 30.Kirby, A. E., D. J. Metzger, E. R. Murphy, and T. D. Connell. 2001. Heme utilization in Bordetella avium is regulated by RhuI, a heme-responsive extracytoplasmic function sigma factor. Infect. Immun. 69:6951-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koster, M., W. van Klompenburg, W. Bitter, J. Leong, and P. Weisbeek. 1994. Role for the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J. 13:2805-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lankford, C. E. 1973. Bacterial assimilation of iron. Crit. Rev. Microbiol. 2:273-331. [Google Scholar]
- 33.Letoffe, S., J. M. Ghigo, and C. Wandersman. 1994. Iron acquisition from heme and hemoglobin by a Serratia marcescens extracellular protein. Proc. Natl. Acad. Sci. 91:9876-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lonetto, M. A., K. L. Brown, K. E. Rudd, and M. J. Buttner. 1994. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. 91:7573-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Mills, M., and S. M. Payne. 1995. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J. Bacteriol. 177:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Missiakas, D., and S. Raina. 1998. The extracytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 28:1059-1066. [DOI] [PubMed] [Google Scholar]
- 38.Moore, C. H., L. A. Foster, D. G. Gerbig, D. W. Dyer, and B. W. Gibson. 1995. Identification of alcaligin as the siderophore produced by Bordetella pertussis and B. bronchiseptica. J. Bacteriol. 177:1116-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy, E. R., R. E. Sacco, A. Dickenson, D. J. Metzger, Y. Hu, P. E. Orndorff, and T. D. Connell. 2002. BhuR, a virulence-associated outer membrane protein of Bordetella avium, is required for the acquisition of iron from heme and hemoproteins. Infect. Immun. 70:5390-5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neilands, J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed] [Google Scholar]
- 41.Ochsner, U. A., Z. Johnson, and M. L. Vasil. 2000. Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiology 146:185-198. [DOI] [PubMed] [Google Scholar]
- 42.Panter, S. S. 1994. Release of iron from hemoglobin. Methods Enzymol. 231:502-514. [DOI] [PubMed] [Google Scholar]
- 43.Pradel, E., N. Guiso, and C. Locht. 1998. Identification of AlcR, an AraC-type regulator of alcaligin siderophore synthesis in Bordetella bronchiseptica and Bordetella pertussis. J. Bacteriol. 180:871-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Querinjean, P., P. L. Masson, and J. F. Heremans. 1971. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur. J. Biochem. 20:420-425. [DOI] [PubMed] [Google Scholar]
- 45.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591-624. [DOI] [PubMed] [Google Scholar]
- 46.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 47.Rossi, M. S., J. D. Fetherston, S. Letoffe, E. Carniel, R. D. Perry, and J. M. Ghigo. 2001. Identification and characterization of the hemophore- dependent heme acquisition system of Yersinia pestis. Infect. Immun. 69:6707-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook, J. E., F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 49.Schade, A. L., and L. Caroline. 1946. An iron binding component of human blood plasma. Science 104:340-341. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt, M. P. 1999. Identification of a two-component signal transduction system from Corynebacterium diphtheriae that activates gene expression in response to the presence of heme and hemoglobin. J. Bacteriol. 181:5330-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider, D. R., and C. D. Parker. 1982. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect. Immun. 38:548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 53.Stojiljkovic, I., and K. Hantke. 1992. Hemin uptake system of Yersinia enterocolitica: similarities with other TonB- dependent systems in gram-negative bacteria. EMBO J. 11:4359-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson, J. M., H. A. Jones, and R. D. Perry. 1999. Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect. Immun. 67:3879-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres, A. G., and S. M. Payne. 1997. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 23:825-833. [DOI] [PubMed] [Google Scholar]
- 56.Vanderpool, C. K., and S. K. Armstrong. 2001. The Bordetella bhu locus is required for heme iron utilization. J. Bacteriol. 183:4278-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanderpool, C. K., and S. K. Armstrong. 2003. Heme-responsive transcriptional activation of Bordetella bhu genes. J. Bacteriol. 185:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 59.Wandersman, C., and I. Stojiljkovic. 2000. Bacterial heme sources: the role of heme, hemoprotein receptors and hemophores. Curr. Opin. Microbiol. 3:215-220. [DOI] [PubMed] [Google Scholar]
- 60.Wang, J., and M. F. Wilkinson. 2000. Site-directed mutagenesis of large (13-kb) plasmids in a single-PCR procedure. BioTechniques 29:976-978. [DOI] [PubMed] [Google Scholar]