Abstract
Delivery of tumour-associated antigens (TAA) in a way that induces effective, specific immunity is a challenge in anti-cancer vaccine design. Circumventing tumour-induced tolerogenic mechanisms in vivo is also critical for effective immunotherapy. Effective immune responses are induced by professional antigen presenting cells, in particular dendritic cells (DC). This requires presentation of the antigen to both CD4+ and CD8+ T cells in the context of strong co-stimulatory signals. Lentiviral vectors have been tested as vehicles, for both ex vivo and in vivo delivery of TAA and/or activation signals to DC, and have been demonstrated to induce potent T cell mediated immune responses that can control tumour growth. This review will focus on the use of lentiviral vectors for in vivo gene delivery to DC, introducing strategies to target DC, either targeting cell entry or gene expression to improve safety of the lentiviral vaccine or targeting dendritic cell activation pathways to enhance performance of the lentiviral vaccine. In conclusion, this review highlights the potential of lentiviral vectors as a generally applicable ‘off-the-shelf’ anti-cancer immunotherapeutic.
Keywords: dendritic cell, lentiviral vector, cancer, immunotherapy
1. General introduction
The identification of tumour-associated antigens (TAA) (Boon 1996), which are selectively or preferentially expressed by tumour cells, has led to the design of various TAA-based anti-tumour vaccines (Mocellin, Mandruzzato et al. 2004). These vaccines are designed to initiate or re-stimulate anti-tumour immune responses. Although the precise immune effector mechanisms involved in tumour eradication are not well understood yet, it is known that cytotoxic CD8+ T cells (CTL) play a major role (Boon and van der Bruggen 1996). In addition, the activation of CD4+ T helper type 1 cells (TH1) is recognized to be important to initiate the correct immune response and to sustain immune effector mechanisms in vivo, contributing to tumour rejection (Bonehill, Heirman et al. 2004; Bonehill, Heirman et al. 2005). In contrast, other immunological mechanisms can inhibit anti-tumour immunity. These mechanisms are mediated by regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC), and tumour cells themselves (Emens 2003; Whiteside 2008). Thus, anti-cancer vaccines should ideally elicit strong TH1 cellular immune responses which are refractory to inhibitory mechanisms.
In the immune system, antigen presenting cells, particularly dendritic cells (DC), regulate immune responses against pathogens and aberrant cells. DC are a heterogeneous cell population, which exhibits extremely efficient antigen uptake, processing and presenting properties. Upon encounter with pathogens, they undergo a complex maturation programme characterised by they up-regulation of co-stimulatory molecules, pro-inflammatory cytokines and importantly the chemokine receptor CCR7. DC then migrate to lymphoid organs where they stimulate naive and antigen experienced T cells through the presentation of MHC-peptide complexes to the T cell receptor (TCR) (signal 1) and through strong co-stimulation. This stimulation (signal 2) is provided by co-stimulatory molecules such as CD80/CD86 and inflammatory cytokines such as interleukin (IL) -6, tumour necrosis factor-α (TNF-α) and IL-12 (Steinman 2007). Therefore, DC have been extensively studied as a cellular anti-cancer vaccine.
Different strategies for the generation of large numbers of clinical grade DC have been developed, reviewed by Tuyaerts et al (Tuyaerts, Aerts et al. 2007). Furthermore, several viral and non-viral systems have been successfully developed for the genetic modification of DC (Breckpot, Heirman et al. 2004; Mossoba and Medin 2006; Van Tendeloo, Ponsaerts et al. 2007). One of these strategies is the use of HIV-1 (human immunodeficiency virus 1)-based lentiviral vectors (Breckpot, Aerts et al. 2007; Breckpot, Emeagi et al. 2008). HIV-1 is a member of the family Retroviridae, differing from oncoretroviruses, their retroviral cousins, by their ability to integrate their cargo into the host genome independently of its replication status (Lewis and Emerman 1994). This is an important asset in view of DC modification, since human DC are generally differentiated from blood-derived quiescent CD14+ monocytes or from mitotically hypoactive CD34+ cells. The biology of lentiviruses and the development of lentivirus-based gene transfer systems has been reviewed in depth elsewhere (Breckpot, Aerts et al. 2007; He, Munn et al. 2007; Breckpot, Emeagi et al. 2008; Spirin, Vil’gelm et al. 2008).
The first successful transduction of human monocyte-derived DC was described by Unutmaz et al in 1999 (Unutmaz, KewalRamani et al. 1999). Since then, several research groups have reported on the successful transduction of monocyte-(Schroers, Sinha et al. 2000; Dyall, Latouche et al. 2001; Firat, Zennou et al. 2002; Breckpot, Dullaers et al. 2003; Breckpot, Corthals et al. 2004; Lizee, Gonzales et al. 2004) and CD34+-derived human DC (Salmon, Kindler et al. 2000; Oki, Ando et al. 2001; Cui, Golob et al. 2002; Sumimoto, Tsuji et al. 2002), and bone marrow-derived mouse DC (Metharom, Ellem et al. 2001; Zarei, Leuba et al. 2002; Esslinger, Chapatte et al. 2003) with varying efficiencies. Subsequently, it was shown that TAA delivered to DC by lentiviral transduction were processed and presented to established T cell lines, demonstrating the presentation of epitopes derived from various TAA (MAGE-3, Melan-A, tyrosinase and ovalbumin, TRP-2, in the human and mouse system, respectively) (Metharom, Ellem et al. 2001; Firat, Zennou et al. 2002; Breckpot, Dullaers et al. 2003; Lizee, Gonzales et al. 2004). More importantly, in vitro priming of naive CD4+ and CD8+ T cells against weak immunogenic TAA such as MAGE-3, was reported (Breckpot, Dullaers et al. 2003), resulting in the generation of T cell clones and the description of a novel HLA-Cw7-restricted MAGE-3 peptide (Breckpot, Heirman et al. 2004). Similarly, Firat et al primed bulk CTL following in vitro stimulation with DC transduced with lentiviral vectors encoding multiple melanoma antigen-derived epitopes (Metharom, Ellem et al. 2001). Furthermore, lentivirally transduced DC were evaluated in several mouse models as a therapeutic against cancer. Immunization with bone marrow-derived DC transduced with OVA-encoding lentivectors (Breckpot, Dullaers et al. 2003) or tumour antigens such as TRP-2 (Metharom, Ellem et al. 2001) or erbB2 (mouse analogue of human Her-2/neu) (Mossoba, Walia et al. 2008), induced strong CTL responses, decreased tumour growth and tumour protection. Moreover, Wang et al (Wang, He et al. 2006) extended these data in a mouse hepatoma model, immunizing with lentivector transduced DC expressing three hepatoma-associated antigens, self-antigens highly over-expressed in tumour cells. CD4+ and CD8+ T cell responses against all three TAA were demonstrated, resulting in regression of established tumours. Delivery of multiple TAA might overcome the problem of tumour escape due to antigen loss (Dullaers, Van Meirvenne et al. 2006). Importantly, several groups demonstrated that lentivector-modified DC elicited stronger, longer-lasting anti-tumour T cell responses compared with peptide-pulsed or mRNA electroporated DC, both clinically approved DC-based vaccines (Dullaers, Breckpot et al. 2004; He, Zhang et al. 2005; Metharom, Ellem et al. 2005). These studies suggest that ex vivo lentivirally transduced DC are effective in therapeutic treatment of melanoma and other tumours. However, this strategy has important drawbacks. Because the vaccine is patient-specific it requires specialized personnel and facilities for vaccine production. As a consequence, there is the high cost and considerable time required for vaccine production and quality control. It is for that reason that direct lentivector administration in vivo has gained substantial interest. Selective in vivo lentivector targeting to DC or restricting transgene expression in DC will further improve selectivity, safety and efficacy. These topics will be further discussed in the following sections.
2. Lentiviral vectors as an off-the-shelf therapeutic
Encouraged by the first successes of recombinant retro- and lentivirus-mediated gene therapy, lentiviral vectors have been explored to deliver TAA in vivo to induce strong anti-tumour immune responses. This requires that lentiviral vectors transduce antigen presenting cells, preferentially DC, delivering both the tumour antigen and signals for DC activation.
2.1. Broad tropism lentiviral vectors transduce dendritic cells in situ
Broad tropism lentiviral vectors transduce DC in vivo. Dullaers et al (Dullaers, Van Meirvenne et al. 2006) used a PCR-based method to demonstrate the presence of transgene+ cells in the draining lymph node, at day 2 and 10, but not day 25 post administration of lentiviral vectors in the footpad. These data were confirmed in flow cytometry, demonstrating that the PCR signal correlated with a small percentage (less than 1%) of transduced CD11c+ cells (unpublished data Dullaers et al). Esslinger et al (Esslinger, Chapatte et al. 2003) performed immunohistochemical analysis of frozen lymph node sections and found that the majority of the lentivirally transduced cells were CD11c+. More recently, He et al (He and Falo 2006) demonstrated that the green fluorescent protein+ (GFP+) DC found in the lymph node after footpad injection, originated from locally transduced migratory skin DC. Intravenous administration of lentiviral vectors leads to transduction of antigen-presenting cells in the spleen (VandenDriessche, Thorrez et al. 2002; Palmowski, Lopes et al. 2004).
These studies indicate that the first pre-requisite for the success of TAA-encoding lentiviral vectors as a therapeutic, i.e. the in situ transduction of DC and their localization in lymphoid organs has been met.
2.2. Immune responses and inhibition of tumour growth after direct administration of tumour antigen-encoding broad tropism lentiviral vectors
A second pre-requisite for immunisation is that the transduced DC process the lentivirally delivered TAA and subsequently present TAA-derived epitopes in the context of MHC molecules and strong co-stimulation in order to induce strong effector T cell responses. In this case, the degree of TAA-specific CTL induction can be considered as a reliable measure for the value of direct administration of TAA-encoding lentiviral vectors in tumour immunology. Antigen-specific CTL responses could be generated upon direct administration of lentiviral vectors using HLA-Cw3 as a model antigen (Esslinger, Chapatte et al. 2003). Comparison of the immune response generated upon direct administration of lentiviral vectors with that generated upon vaccination with ex vivo lentivirally transduced DC, demonstrated that superior immune responses were generated by the former both in terms of strength and longevity. Similar results were obtained in HLA-A*0201 transgenic mice using a lentivirus encoding a minigene containing the dominant MART-1/Melan-A HLA-A*0201 epitope (Chapatte, Colombetti et al. 2006). Using ovalbumin as an antigen, it was confirmed that direct administration of lentiviral vectors is superior to vaccination with ex vivo transduced DC, both in terms of the number of IFN-γ producing CTL as determined in vitro by ELISPOT and the lytic capacity of CTL as determined by an in vivo CTL assay (Dullaers, Van Meirvenne et al. 2006). Moreover, memory CTL responses were significantly stronger with direct lentiviral vector administration. Other studies performed with relevant tumour antigens such as NY-ESO (Palmowski, Lopes et al. 2004), TRP-2 (Kim, Majumder et al. 2005), TRP-1 (Liu, Peng et al. 2009) and carcinoembryonic antigen (CEA) (Loisel-Meyer, Felizardo et al. 2009), have also shown potent immune responses upon in vivo administration of lentiviral vectors. Chapatte et al (Chapatte, Colombetti et al. 2006) compared direct administration of lentiviral vectors encoding the human MART-1/Melan-A antigen with the clinically approved peptide-adjuvant vaccination strategy in a model of HLA-A*0201 transgenic mice. They demonstrated that the anti-MART-1/Melan-A immune response was higher when immunization was performed with lentiviral vectors when compared to peptide-adjuvant vaccination. Although the generation of a specific CTL response is a convenient read-out for the success of a vaccination strategy, there are many examples of discrepancies between immune responses and anti-tumour responses (Rosenberg, Sherry et al. 2005). Therefore, it is of paramount importance to evaluate the induction of TAA-specific T cells and its influence on tumour growth. Rowe et al (Rowe, Lopes et al. 2006) showed significantly improved protection of direct administration of an ovalbumin-encoding lentiviral vector against a subsequent tumour challenge. More significantly, Dullaers et al (Dullaers, Van Meirvenne et al. 2006) showed that direct administration of lentiviral vectors offers increased protection to a subsequent tumour challenge compared to DC vaccination and a significantly improved survival of tumour bearing mice. Other studies using TRP-2 (Kim, Majumder et al. 2005), TRP-1 (Liu, Peng et al. 2009) or CEA (Loisel-Meyer, Felizardo et al. 2009) as TAA, demonstrated improved survival of tumour bearing mice receiving lentivirus-encoding the TAA. Moreover, Liu et al demonstrated that this type of immunization was able to result in complete regression of small subcutaneous tumours, which correlated with enhanced numbers of CD4+ and functional CD8+ T cells in the tumour environment (Liu, Peng et al. 2009). Therefore, there is substantial evidence for the induction of specific immune responses by TAA-encoding lentiviral vector administration in vivo, which can induce protective and therapeutic immunity.
2.3. Immunogenicity of lentiviral vectors
Although lentiviral vectors do not express viral proteins, administration of lentivectors elicits significant immune responses against transgene-encoded proteins. This suggests immunogenicity of lentiviral vector particles or components present in lentiviral preparations, leading to activation of innate viral-sensing pathways and strong adaptive immune responses.
It has been shown that wild-type HIV-1, from which most recombinant lentiviruses are derived, induces cell- and antibody-mediated responses in humans (Liu, Roberts et al. 1997; McMichael and Phillips 1997). Moreover, HIV-1 activates plasmacytoid DC, a subset of DC specialized in virus recognition, through engagement of toll like receptor (TLR) 7, leading to type I IFN production (Fonteneau, Larsson et al. 2004; Beignon, McKenna et al. 2005). Type I IFN are potent anti-viral cytokines that induce the maturation of plasmacytoid DC, as well as bystander maturation of other DC subsets (Fonteneau, Larsson et al. 2004), which than can initiate adaptive immune responses. As recombinant lentiviral vectors are HIV-derived and contain single stranded RNA (a ligand for TLR7), they may also trigger a similar innate immune response. Interaction of lentiviral vector particles with TLR7 has been demonstrated in a cell line reporter assay (Breckpot, Emeagi et al. 2007). Furthermore, Brown et al. (Brown, Sitia et al. 2006) demonstrated that administration of lentiviral vectors to mice triggers a rapid and transient type I IFN response. The observed effect was independent of the pseudotype, but dependent on functional vector particles, suggesting the necessity of cell entry. Furthermore, as reverse transcription of the viral genome generates double stranded DNA, it has been suggested that TLR9 may be triggered by these lentiviral vectors. Recently, Pichlmair et al (Pichlmair, Diebold et al. 2007) demonstrated that vesicular stomatitis virus glycoprotein (VSV.G) pseudotyped lentivirus preparations are contaminated with tubulovesicular structures of cellular origin, which carry nucleic acids, including plasmid DNA. These structures triggered TLR9 in plasmacytoid DC, hence inducing type I IFN production. However, lentiviral vectors with a gammaretroviral envelope do not trigger TLR9 in plasmacytoid DC but still immunise effectively (Lopes, Dewannieux et al. 2008), suggesting that this particular mechanism is not necessary for potent immune stimulation. Thus all of these studies report activation of plasmacytoid DC by lentiviral vectors, although different mechanisms have been proposed.
In contrast, the effect of lentiviral vectors on conventional DC remains controversial. Some studies with human monocyte-derived DC have been performed. Gruber et al (Gruber, Kan-Mitchell et al. 2000) reported that transduction of immature conventional DC at low multiplicity of infection (MOI) did not result in phenotypical or functional maturation, whereas Tan et al (Tan, Beutelspacher et al. 2005) described that transduction of these DC with a MOI of 500 results in up-regulation of adhesion, co-stimulatory and human leukocyte antigen (HLA) molecules. Furthermore, these DC displayed enhanced allo-stimulatory capacities and an altered cytokine secretion pattern. Breckpot et al (Breckpot, Emeagi et al. 2007) demonstrated that transduction of DC at low MOI results in considerable transgene delivery, without activation, whereas transduction at higher MOI (15–150) indeed leads to phenotypical and functional maturation. It was demonstrated in the latter studies that protein kinase R (PKR), a cytosolic receptor, which interacts with double stranded RNA, an intermediate in the lentiviral replication, is phosporylated upon transduction at high MOI (Tan, Beutelspacher et al. 2005; Breckpot, Emeagi et al. 2007). This PKR phosphorylation leads to the degradation of IκB and subsequent activation of nuclear factor-κB (NF-κB), a transcription factor associated with DC maturation (Taylor, Haste et al. 2005). However, the activation of conventional DC, the DC subset believed to orchestrate the immune response, in vivo has - to our knowledge - not been studied.
It is important to note that induction of a strong anti-tumour response is not only dependent on antigen-recognition by T cells and co-stimulation provided by the DC, but is crucially dependent on an inflammatory environment, in order to overcome tolerance and active inhibitory mechanisms. Such an inflammatory environment can be achieved by strong activation of the innate arm of the immune system, in particular through the engagement of TLR. Two studies, one by Yang et al (Yang, Huang et al. 2004) and the other by Lang et al (Lang, Recher et al. 2005), demonstrate that tolerance of antigen-specific CTL could be broken by persistent TLR ligation. Furthermore, it has been recently described that signalling through certain combinations of TLR on DC not only provided a synergy with respect to the production of cytokines such as IL-12, which is essential for skewing CD4+ T cells toward a TH1 phenotype (Gautier, Humbert et al. 2005; Napolitani, Rinaldi et al. 2005), but also offered protection from inhibitory Treg that quench the anti-tumour immune response (Warger, Osterloh et al. 2006).
In addition to breaking tolerance, a productive CD4+ T cell response is required for the induction of a strong and sustained CTL response. In this regard, it has been recently demonstrated with ex vivo lentivirally transduced DC, cultured in foetal calf serum (FCS) free medium, that transduction with high titer lentiviral vectors, generated in the presence of FCS, results in the transfer of FCS components into MHC class II molecules, as such stimulating FCS-specific CD4+ T cells and providing antigen a-specific CD4+ T cell help (Bao, Guo et al. 2009). Whether the presence of FCS results in the induction of FCS-mediated CD4+ T cell help in vivo remains to be seen. With regard to direct administration of lentiviral vectors, several groups have shown that both a CTL response and an antigen-specific CD4+ T cell response can be induced (Esslinger, Chapatte et al. 2003; Dullaers, Van Meirvenne et al. 2006; Rowe, Lopes et al. 2006). However, not much data is available on the role of CD4+ T cell help in the induction of CTL upon lentiviral immunization. Esslinger et al (Esslinger, Chapatte et al. 2003) showed that CD4 depletion reduces the primary CTL response upon direct administration of lentiviral vectors. Similarly, Dullaers et al (Dullaers, Van Meirvenne et al. 2006) showed that although there was a larger requirement for CD4+ T cell help during the primary response in case of immunization with ex vivo transduced DC compared to direct administration of lentiviral vectors, CD4+ T cell depletion strongly reduced the capacity to mount a recall CTL response in both cases. Interestingly, Marzo et al (Marzo, Vezys et al. 2004) showed that in the case of a VSV infection, a functional CD8+ T cell memory response can be generated in the absence of CD4+ T cells, this in contrast to an infection with Listeria monocytogenes. These authors suggest that the difference might be due to the fact that VSV can directly infect DC whereas Listeria monocytogenes needs to be cross-presented. Since, the currently applied lentiviral vectors are pseudotyped with the envelope of VSV, it needs to be further examined to what extent the CTL response is CD4+ T cell dependent. Overall, these studies indicate that lentiviral vectors induce DC activation through TLR signalling and other mechanisms, explaining their potency as an anti-tumour vaccine.
3. Towards in vivo targeting of dendritic cells by lentiviral vectors
Although direct in vivo administration of lentivectors leads to transduction of DC, the broad tropism of the vectors used in most of the studies so far also implies that other cell types are transduced. The antigen expressed from cells other than DC could be cross-presented by neighboring DC and might further improve the processing and presentation of the antigen (Schulz, Diebold et al. 2005). However, specific transduction of DC could improve the safety and efficacy of the vaccination. Several strategies have been used for this which can be divided in two categories: transductional and transcriptional targeting.
3.1. Transductional targeting
The lentivector tropism is determined by the glycoproteins incorporated in the viral envelope, which interact with specific receptors on the membrane of the target cells. Since lentivectors are usually pseudotyped with heterologous glycoproteins, the natural tropism of the envelope used for pseudotyping can restrict the vector entry to specific cells or tissues.
There is an ever-growing list of glycoproteins that have been successfully used for pseudotyping of lentivectors. Examples are glycoproteins from retroviridae, rhabdoviridae, arenaviridae, flaviviridae, paramyxoviridae, baculoviridae, and filoviridae (Bouard, Alazard-Dany et al. 2009). Although each of these glycoproteins preferentially interacts with specific cell types, finding a natural envelope for DC-specific targeting has been unsuccessful. Therefore, envelopes have been engineered in several ways to re-direct their tropism towards specific cell types.
One way of doing this is by genetically modifying the envelope glycoprotein to ligands that bind to receptors present in the cells to be transduced. However, ligand-fused glycoproteins often results in poor infectivity due to inability of the retargeted envelope to induce membrane fusion and sequestration of the viral particles to some cell surface molecules (Frecha, Szecsi et al. 2008). Another alternative is the modification of the existing envelopes to re-direct receptor attachment without hampering membrane fusion. Yang et al (Yang, Bailey et al. 2006) showed that by introducing a mutation to the envelope of Sindbis virus, its affinity for an ubiquitous receptor (heparan sulphate) was ablated while preserving the capacity to bind to DC-SIGN, a lectin-type receptor present on some DC subsets. OVA-encoding lentivectors pseudotyped with this modified envelope specifically transduced DC in vivo, inducing efficient immune responses against OVA-expressing tumors. However, engineering of re-targeted envelope proteins by fusion to natural ligands has proven to be difficult, and these strategies were at first applied with limited success (Waehler, Russell et al. 2007).
The viral-packaging system can also be exploited to modify the viral envelope. As the virus is generated from a producer cell line, on budding it incorporates part of the cell membrane in its envelope. When a specific receptor is overexpressed on the cell membrane of a producer cell line, it can also be displayed on the viral envelope. When overexpressing stem cell factor (SCF) on the cell membrane of an ecotropic producer cell line, Chandrashekran et al. (Chandrashekran, Blood, 2004 and J Gene Med, 2004) showed that retrovirus thus produced was able to preferentially transduce c-kit expressing human stem cells. Recently, Yang et al. (Yang, PNAS, 2006) demonstrated that this approach can also be applied for lentiviruses. This method could thus be modified to generate lentiviral vectors that can specifically target DC (or other cell types) and still yield high expression of the transgene. Receptors that could be used include CD40 ligand, which interacts with CD40, and CTLA-4, which interacts with CD80 and CD86. As many of these receptors are expressed on other cell types than DC, specificity will still be limited.
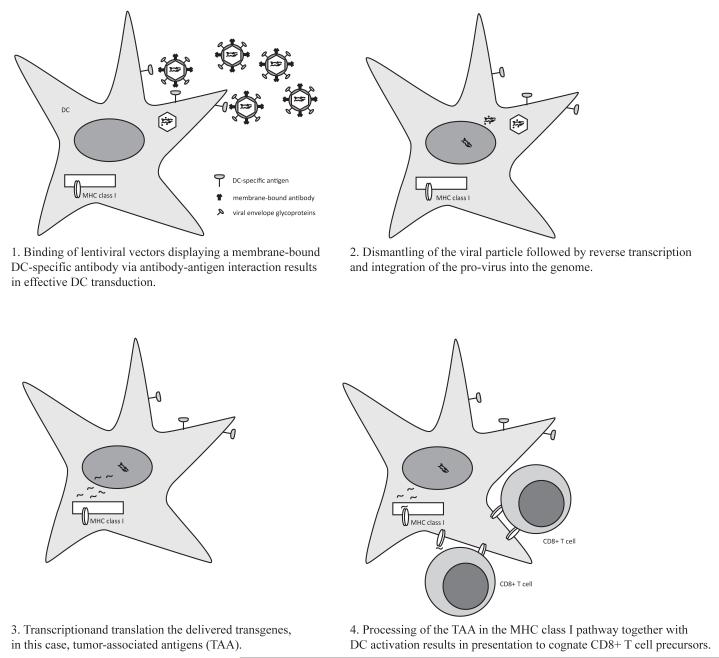
Single-chain antibodies (scFv) specific against surface proteins present on the target cell have been used for this purpose (Fig. 1). Specific targeting of DC has been achieved with antibodies directed against C-type lectins, such as DEC-205 (Bonifaz, J Exp Med, 2002 and 2004) and DC-SIGN (Dzionek, J Exp Med, 2001). Display of scFv in the context of envelope glycoproteins of measles virus seems to be a promising approach since measles virus enters cells through direct fusion at the cell membranes. Target versus non-target cell discrimination has been shown in vitro using this system (Funke, Maisner et al. 2008).
Fig. (1). Transductional targeting of DCs with lentiviral vector.
In this scheme, DC transduction by specific surface targeting is shown as an example. Lentiviral particles are pseudotyped with a fusion protein together with an antibody that is specific for a particular surface protein present only in DC. These lentivectors can then specifically bind to the DC surface when administered in vivo (1). After binding, lentivectors fuse with the DC membrane and the core is incorporated and dismantled in the DC cytoplasm. The genome RNA is reversed-transcribed into a cDNA copy that integrates into the DC genome (2). From the integrated lentivector, transcription takes place leading to synthesis of TAAs, which are subsequently degraded (2). TAAs are processed into peptides and loaded in the MHC I compartment, together with DC activation by the transduction process itself. MHC I-peptide complexes are displayed on the DC surface and presented to TAA-specific CD8 T cells that differentiate in effector anti-tumour CTLs.
As molecular cloning of classic antibodies or fragments thereof offers serious challenges, alternatives have been explored. One of them is the use of antibodies of members of the family of camilidae (i.e., dromedaries, camels, llamas), which produce a unique class of antibodies composed of two identical heavy chains as opposed to the conventional (four-chain) antibody repertoire (Hamers, Nature, 1993). The antigen-binding part of the molecule is composed of only one single variable region (termed VHH, or nanobody). These antigen-specific antibody fragments offer many advantages: (1) they are highly soluble, (2) they can refold after denaturation whilst retaining their binding capacity, (3) cloning and selection of antigen-specific nanobodies obviate the need for construction and screening of large libraries, (4) as nanobodies can be fused to other proteins, it should be possible to present them on the cell membrane of a producer cell line, such as HEK 293T, thus, generating lentiviral particles that incorporated a DC-specific nanobody in their envelope during budding as described above.
3. 2. Transcriptional targeting
Transgene expression can be targeted to specific cell types by the use of cell or tissue-specific promoters. Several promoters have been studied with this purpose (reviewed in (Frecha, Szecsi et al. 2008). In the specific case of DC, different promoters can offer the opportunity to target expression to specific DC subtypes. For example, the CD11c can limit antigen expression to myeloid DC (Noti, Reinemann et al. 1996). BDCA-2 and Langerin promoter results in exclusive expression in pDC (Dzionek, Sohma et al. 2001; Takahara, Omatsu et al. 2002) and Langerhans cells (Takahara, Omatsu et al. 2002) (Takahara, Omatsu et al. 2002), respectively.
Using lentivectors, Gorski et al. (Gorski, Shin et al. 2003) identified the promoters of B7-DC and CCL17 as active in bone marrow-derived DC but not in macrophages. More recently, Lopes et al. (Lopes, Dewannieux et al. 2008) evaluated the use of the mouse dectin-2 promoter to drive GFP expression in mouse BMDC cultures and in human skin-derived Langerhans and dermal DC. When these lentiviral vectors were injected intravenously, GFP expression was detected in splenic dectin-2+ cells, whereas subcutaneous injection resulted in transduced CD11c+ DC in the draining lymph node. Immunization with dectin-2 lentiviruses encoding NY-ESO-1 resulted in an antigen-specific CD8+ T cell response in HLA-A2 transgenic mice and further stimulated a CD4+ T cell response to a newly identified NY-ESO-1 epitope presented by H2 I-Ab. Importantly, it was demonstrated that immunization with dectin-2 lentiviruses was similar to that with lentiviruses containing a strong constitutive viral promoter, demonstrating that targeting antigen expression to DC results in an effective vaccine. These studies demonstrate that the transgene expression limited to DC is a promising and safer strategy in lentivector immunization.
4. TARGETING DENDRITIC CELL DIFFERENTIATION AND MATURATION
Another interesting approach to enhance a lentivector-based vaccine is to increase its intrinsic immunogenicity. Untargeted and DC-targeted lentivector vaccines can elicit potent CD4 and CD8 T cell responses in several infection and tumour models. In addition, the immunogenic potential of lentivectors can be manipulated by expression of molecules that enhance DC maturation or prolong antigen presentation. Tumour cells arise endogenously and most TAA are self-antigens. Therefore, T cells recognizing many of the potential tumour-specific T cells have already been eliminated by central or peripheral tolerance (Walker and Abbas 2002). Additionally, tumour cells have acquired several T cells suppressive mechanisms including antigen presentation in the absence of costimulation, expression of inhibitory/death inducing signals (e.g. PD-L1, FasL), expression of immunomodulatory enzymes (e.g. indoleamine-2,3-deoxygenase) and secretion of suppressive cytokines and chemokines such as IL-10 TGF-β (Zitvogel, Tesniere et al. 2006). Moreover, these immunosuppressive mechanisms promote the differentiation of immune cells suppressive cells such as regulatory DC and T cells, and myeloid-derived suppressor cells (Emens 2003) (Lizee, Radvanyi et al. 2006).
A productive T cell immune response requires specific recognition of an MHC/peptide complex by the TCR (signal 1) together with signaling through co-stimulatory molecules (signal 2). In addition, to establish strong antitumour responses, an inflammatory environment (signal 3) is required. All these requirements can be achieved by strong activation of the innate arm of the immune system, in particular through TLR signalling (van Duin, Medzhitov et al. 2006) (Iwasaki and Medzhitov 2004) (Pasare and Medzhitov 2005) (Rakoff-Nahoum and Medzhitov 2009). In fact, TLR signaling controls DC maturation, including their capacity to migrate, up-regulate co-stimulatory molecules such as CD40, CD80, CD86 and CD70 and produce pro-inflammatory cytokines such as IL-1, IL-6, TNF-α and IL-12 (Iwasaki and Medzhitov 2004; Pasare and Medzhitov 2004; van Duin, Medzhitov et al. 2006). Accordingly, persistent TLR ligation may be necessary to break tumour-induced tolerance in the context of antigen-loaded mature DC (Yang, Huang et al. 2004).
Ideally, a lentivector vaccine could be engineered so that it would include DC activators of differentiation and maturation, and providing at the same time expression of TAAs. This approach would directly target DC activation and tumour antigen presentation, and at the same time prevent the actions of tolerogenic mechanisms over transduced DCs.
4.1. Mechanisms of NF-kB and MAPK activation in dendritic cells
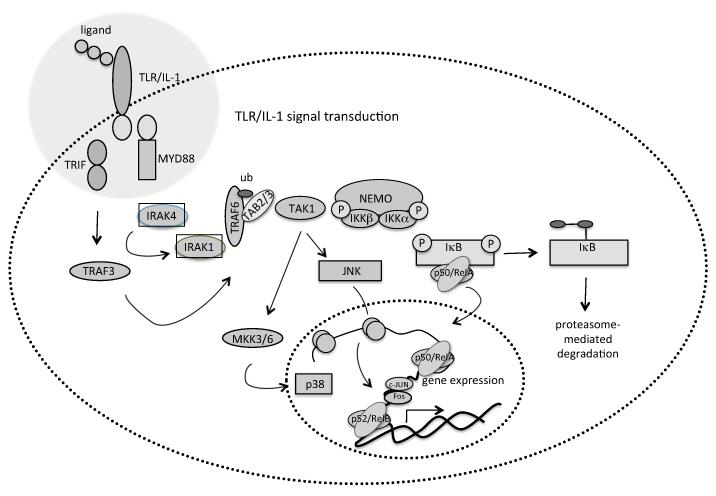
Understanding the mechanisms of TLR signalling and their exploitation is key to enhance lentivector-based vaccines. Binding of TLR to their ligands trigger a complex network of signalling molecules and adaptor proteins that will modulate DC responses in innate and adaptive immunity (Caparros, Munoz et al. 2006). All these networks integrate and converge into a few pathways, such as that of nuclear factor-kB (NF-kB) and mitogen-activated protein kinases (MAPK) (Luft, Rodionova et al. 2006) (Fig. 2). NF-κB is a transcription factor that binds to a 10 base pair consensus DNA element (5′-GGGAATTTCC-3′) and its many variations (Sen and Baltimore 1986). The NF-κB family of proteins is central to immunity and inflammation (Ghosh and Karin 2002). Their members exist as homo- or heterodimeric complexes formed by combinations of five subunits, p65/RelA, RelB, c-Rel, p50 and p52. Of these, RelA, RelB and c-Rel are synthesized in their mature forms and contain a transactivation domain. NF-kB p50 and p52 are synthesized as precursors containing carboxy-terminal ankyrin repeats, which are processed by the proteasome leading to mature proteins. Both p50 and p52 contain a DNA binding domain, but lack a transactivation domain. In particular, RelB is implicated in DC differentiation and maturation (Weih, Carrasco et al. 1995) (Burkly, Hession et al. 1995). All NF-κB proteins present a conserved amino-terminal 300 amino acid Rel homology domain, responsible for dimerization and DNA binding. Furthermore, this domain binds to inhibitory regulatory factors, the inhibitors of kB (IkB) proteins. The binding of IkB keeps cytosolic NF-kB dimers in an inactive state. NF-κB is activated by pro-inflammatory cytokines (such as TNF-alpha, IL-1), T cell delivered signalling (CD40L), bacteria, viruses and cellular stress leading to DC maturation (Rothwarf and Karin 1999). NF-kB pathways share adaptor molecules with MAPK pathways, and can be broadly divided into a classical (canonical) and non-classical pathway (Edwards, Bartlett et al. 2009) (Lee, Jeon et al. 2007) (Akira, Uematsu et al. 2006) (Wang, Miyahara et al. 2008). Both pathways result in activation of IkB kinases (IKK) which will phosphorylate IkB proteins leading to their ubiquitination and degradation. This releases active NF-kB dimers. IkB kinase (IKK) is a multi-subunit protein complex consisting of two catalytic subunits, IKKalpha and IKKbeta and a regulatory subunit, IKKgamma (also called NEMO, NF-κB essential modulator). Phosphorylation of IκB at two critical serine residues (Ser32/Ser36 in IκBalpha and Ser19/Ser23 in IκBbeta) by the IKK complex targets them for proteasomal degradation (Dawson, Hastings et al. 1997). In the classical pathway, it has been shown that IKKbeta, but not IKKalpha, is important in NF-kB activation. Furthermore, it has been demonstrated that these two kinases have distinct rather than overlapping functions (Hu, Wang et al. 1999) (Li, Van Antwerp et al. 1999) (Takeda, Takeuchi et al. 1999) (Li, Chu et al. 1999). The classical pathway includes signalling from TLR/IL-1R family members, intracellular pattern recognition receptors including retinoic acid inducible gene (RIG-I), melanoma differentiation associated factor-5 (MDA-5) and protein kinase R (PKR), as well as TNFR signalling (Hacker and Karin 2006). Mediators such as lymphotoxin-beta, CD40L and receptor activator of NF-kB ligand (RANKL) activate the non-classical pathway, which involves IKKalpha phosphorylation, processing of the p52 precursor p100 and nuclear translocation of the heterodimer p52/RelB (Senftleben, Cao et al. 2001) (Lawrence and Bebien 2007).
Fig. (2). Representation of MAPK and NF-kB pathways involved in DC maturation.
MAPK and NF-kB pathways are involved in DC maturation and can be triggered by engagement of TLRs or other stimuli (not shown). This results in a cascade of enzymatic reactions involving various adaptor and intermediate proteins and finishes with downstream activation of molecules that regulate transcription. MAPK p38 aids the binding of NF-kB to its consensus sequence. Both MAPK JNK and NF-kB control the expression of factors involved in antigen presentation, costimulatory and adhesion molecules, and pro-inflammatory cytokines.
NF-kB targets genes associated with DC maturation, such as cytokines (e.g. IL-6, IL-12, TNF-alpha), chemokines (e.g. MIP-1alpha, MCP1), adhesion molecules (e.g. ICAM-1), inducible effector enzymes (e.g. COX-2) and apoptosis regulators (e.g. c-IAP, XIAP, Bcl-xL). Consequently, the NF-kB pathway is tightly regulated by negative feedback mechanisms that prevent excess inflammation and autoimmunity. Several feedback molecules have been described, including IkB, A20 (or TNF-α inducible protein 3, TNFAIP3), tripartite-motif protein (TRIM) 30alpha and phosphatase PP2A, to name a few (Breckpot, Aerts-Toegaert et al. 2009) (Kawai and Akira 2007) (Coornaert, Carpentier et al. 2009) (Barisic, Strozyk et al. 2008; Bowie 2008; Shi, Deng et al. 2008). The A20 negative regulator has been recently studied and targeted to enhance tumour antigen-specific immune responses. A20 is under the immediate control of NF-kB and it is induced in many cell types, amongst which mouse and human DC (Dixit, Green et al. 1990) (Breckpot, Aerts-Toegaert et al. 2009) (Song, Evel-Kabler et al. 2008) (Beyaert, Heyninck et al. 2000). A20 is an ubiquitin-editing enzyme with an amino-terminal de-ubiquitinase activity and ubiquitinase activity in the C-terminus zinc finger domain, important for modulating NF-kB signaling by interaction with proteins of the TNF-, IL-1/TLR-signaling pathways (Heyninck and Beyaert 1999; Beyaert, Heyninck et al. 2000; Zhang, Kovalenko et al. 2000; Boone, Turer et al. 2004; Wang, Li et al. 2004; Wertz, O’Rourke et al. 2004; Saitoh, Yamamoto et al. 2005).
MAPK are a diverse group of intracellular serine/threonine kinases which are phylogenetically conserved and regulate a wide range of cellular processes, including immune responses (Ardeshna, Pizzey et al. 2000). Three groups of MAPK have been identified: the extracellular signal-regulated protein kinases (ERK) (Boulton, Nye et al. 1991) (Boulton and Cobb 1991), the c-Jun N-terminal kinases (JNK) (Derijard, Hibi et al. 1994) (Kyriakis, Banerjee et al. 1994) and the p38 stress-activated protein kinases (p38) (Lee, Laydon et al. 1994) (Han, Lee et al. 1994). MAPK pathways consist on a three-modular cascade involving activating phosphorylations of downstream kinases by upstream kinases. In this way, MAPK kinase kinases (MAPKKK) are firstly activated by phosphorylation after their recruitment to TLR cytoplasmic domains. These phosphorylated MAPKKK subsequently phosphorylate MAPK kinases (MAPKK), which in turn will phosphorylate MAPK. The TLR cytoplasmic tail contains the Toll/IL-1 receptor (TIR) domain, essential for signal transduction (Beutler 2009), and mainly two TIR domain adaptor molecules, MyD88 (myeloid differentiation factor 88) and TRIF (Toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta) are recruited to the TIR domain. Then, MAPKKK are activated through recruitment of various protein kinases and scaffold proteins, such as ubiquitin ligases TNFR-associated factor 6 (TRAF6) and TRAF3 (Hacker, Redecke et al. 2006). MAPK activation has been shown to depend on MyD88-TRAF6-mediated recruitment of a complex containing MAPKKK TAK1 (transforming growth factor-beta-activated kinase-1) (Sato, Sanjo et al. 2005), but also in an alternative pathway involving TRIF-TRAF3 (Hacker, Redecke et al. 2006).
In DC, ERK is involved in cell survival (Rescigno, Martino et al. 1998), regulation of inflammation and immune suppression (Agrawal, Dillon et al. 2006) (Escors, Lopes et al. 2008). MAPK p38 comprises at least four splice isoforms from which p38α and p38β are ubiquitously expressed. In the context of TLR activation, p38 phosphorylation is completely dependent on TAK1 (Sato, Sanjo et al. 2005), which phosphorylates MKK3 and MKK6, the two main p38 MAPKK (Ninomiya-Tsuji, Kishimoto et al. 1999) (Moriguchi, Kuroyanagi et al. 1996). Classical p38 targets range from kinases such as MK2 and transcription factors such as ATF, p53, c/EBP and NFAT just to mention a few (Zarubin and Han 2005). MAPK p38 plays an important part in DC maturation and secretion of pro-inflammatory cytokines. MAPK p38 is involved in up-regulation of DC co-stimulatory molecules and maturation markers such as CD40, CD80, CD86, CD83 and MHC class II (Arrighi, Rebsamen et al. 2001), and secretion of IL-1beta, TNF-alpha, IL-6 and IL-12 (Arrighi, Rebsamen et al. 2001) (Yu, Kovacs et al. 2004) (Lu, Yang et al. 1999). MAPK c-Jun N-terminal kinase (JNK) proteins are encoded by 3 genes with 10 or more alternative splice forms (Gupta, Barrett et al. 1996), and they are activated by UV irradiation, environmental and chemical stress, pro-inflammatory cytokines and TLR signalling (Hacker, Redecke et al. 2006) (Wang, Deng et al. 2001) (Ninomiya-Tsuji, Kishimoto et al. 1999) (Gupta, Barrett et al. 1996). JNK is activated by dual phosphorylation by MAPKK, MKK4 and MKK7. The classical JNK targets are c-Jun, from which it receives its name, ATF2, p53, Elk-1 and c-Myc amongst others (Junttila, Li et al. 2008) (Morton, Davis et al. 2004) (Gupta, Barrett et al. 1996) (Morton, Davis et al. 2003). ATF2 and c-Jun are components of AP-1, a transcription factor that transactivates many proinflammatory genes. Consequently, JNK is involved in inflammatory responses and inhibitors are being evaluated in clinical trials for the treatment of autoimmune diseases such as rheumatoid arthritis as well as asthma, inflammatory diseases and some types of leukaemia (Roberts and Der 2007). In the context of TLR signalling, TAK1 is involved in JNK activation through MKK3 and MKK7 phosphorylation (Sato, Sanjo et al. 2005) (Wang, Deng et al. 2001) (Ninomiya-Tsuji, Kishimoto et al. 1999) (Hammaker, Boyle et al. 2007). In general, JNK activity enhances DC maturation and proinflammatory cytokine secretion, although at lower levels compared to p38 (Escors, Lopes et al. 2008) (Nakahara, Uchi et al. 2004). Therefore, the specific delivery of TAAs with modulators of MAPK pathways to DC could enhance tumour-specific immune responses.
4.2. Lentivector-modification of DC to mimic persistent TLR activation
In recent years, strategies to deliver activation signals to DCs simultaneously to expressing TAAs have been developed. One of these strategies aims to mimic persistent TLR activation to drive DC maturation. DC express TLR4 which binds LPS resulting in DC maturation (da Silva Correia, Soldau et al. 2001) (Ardeshna, Pizzey et al. 2000) (Arrighi, Rebsamen et al. 2001). LPS-mediated activation remarkably enhances stimulation of DC-mediated immune responses in vitro, and could overcome suppression by regulatory T cells, a critical factor in anti-tumour immunology (Pasare and Medzhitov 2003). However, its clinical use is prohibited due to cytotoxicity. Therefore, a constitutive active TLR4 (caTLR4) for DC maturation has been evaluated (Abdel-Wahab, Cisco et al. 2005) (Bonehill, Tuyaerts et al. 2008) (Cisco, Abdel-Wahab et al. 2004) (Xu, Darcy et al. 2007). This was achieved by truncating positions M620 to P621 and linkeage to the nerve growth factor or the LAMP1 leader sequences (Abdel-Wahab, Cisco et al. 2005) (Cisco, Abdel-Wahab et al. 2004) (Bonehill, Tuyaerts et al. 2008) [187, 189][188]. Alternatively, TLR4 cytoplasmic domain was linked to the extracellular single-chain immunoglobulin anti-erbB2 (Xu, Darcy et al. 2007). Delivery of these active TLR4 constructs resulted in NF-kB activation leading to DC maturation, and moreover, these DC were stimulated MELAN-A and Trp2-specific CTL (Abdel-Wahab, Cisco et al. 2005) (Bonehill, Tuyaerts et al. 2008) (Cisco, Abdel-Wahab et al. 2004).
Another strategy consisted on the introduction of the major TLR adaptor molecules MyD88, TRIF or IRAK-1, which were shown to stimulate downstream signals in the absence of TLR stimuli. Akazawa et al. expressed MyD88 and TRIF in mouse DC using lentiviral vectors, resulting in DC with different properties. MyD88-modified DC produced IL-6 and IL-12p40, but no up-regulation of phenotypic markers, whereas TICAM-1 expression stimulated interferon IFN-beta production and increased levels of CD86. Both MyD88 and TRIF increased the allo-stimulatory capacity of modified DC, and tumour outgrowth was delayed after immunization with these modified DC (Akazawa, Shingai et al. 2007). Xu et al. generated retroviral vectors encoding chimeric proteins consisting of the extracellular single-chain immunoglobulin anti-erbB2 linked to either MyD88 or IRAK-1. These experiments were performed in an immortalized DC line, JAWS II, and only the IRAK-1 chimera mediated IL-12 and TNF-alpha secretion. The latter demonstrated enhanced OVA-specific OT-II CD4 T cell responses (Xu, Darcy et al. 2007).
4.3. Lentivector-targeted activation of NF-kB in dendritic cells
NF-κB has been one of the first pathways to be targeted in DC as an adjuvant by overexpressing NF-κB inducing kinase (NIK) using adenovirus vectors. This led to increased DC maturation and increased Th1 GFP-specific immune responses, although the relevance of this strategy in anti-viral or anti-tumour immunity was not assessed (Andreakos, Williams et al. 2006). Sustained NF-κB activation in DC using lentiviral vectors has been achieved by expressing Kaposi’s sarcoma associated human herpes virus (KSHV) vFLIP (Rowe, Lopes et al. 2009). In this case, DC maturation was enhanced by up-regulation of MHC I and II, co-stimulatory molecules CD80, CD86, CD40 and ICAM-I, and increased secretion of TNF-alpha and IL-12. vFLIP-modified DC significantly increased antigen-specific CD8+ T cell responses resulting in enhanced anti-tumour and anti-parasite immunity (Rowe, Lopes et al. 2009) (Karwacz, Mukherjee et al. 2009).
Another effective approach leading to sustained NF-kB activation consists of down-regulation of negative feed-back mechanisms. As mentioned above, A20 is one of the feedback regulators of NF-kB. A20 deactivates several adaptor molecules of the TNFR, IL-1/TLR signaling pathways by ubiquitination/de-ubiquitination activities. In this way, A20 controls IKK and thus the degradation of IkBalpha. Therefore, A20 down-regulation could result in prolonged NF-kB activation, mimicking persistent TLR ligation and resulting in DC with enhanced stimulatory capacity. Lentivirally delivered A20-targeted shRNA and direct introduction of siRNA were applied to downregulate A20 (Breckpot, Aerts-Toegaert et al. 2009) (Song, Evel-Kabler et al. 2008). These approaches showed that A20 controls DC maturation, cytokine production and immunostimulatory potency. Human DC with down-regulated A20 expression increase NF-kB activity and show enhanced and sustained IL-10 and IL-12 secretion. These DC were more potent in stimulating MelanA-specific CD8+ T cells (Breckpot, Aerts-Toegaert et al. 2009). Mouse DC with down-regulated A20 expression showed enhanced expression of co-stimulatory molecules and pro-inflammatory cytokines and they were refractory to regulatory T cell inhibition, leading to activated tumour-infiltrating CTL and T helper cells (Song, Evel-Kabler et al. 2008). Therefore, A20 is an ideal target for anti-tumour immunotherapy, since it enables DC to induce strong effector T cell responses and inhibit regulatory T cells.
4.4. Lentivector-targeted activation of MAPK pathways in dendritic cells
Lentivectors have been used to increase DC immunogenicity by introducing specific genes that modulate intracellular MAPK pathways. ERK and p38 were activated by expressing MEK1 and MKK6 mutants containing glutamate and aspartate residues in their activation loop, mimicking activating phosphorylated serine or threonine residues (Raingeaud, Whitmarsh et al. 1996). A fusion protein between MKK7 and JNK1 was expressed to achieve constitutive JNK1 phosphorylation (Escors, Lopes et al. 2008). In addition, expression of constitutive activators prevents inactivation by phosphatase-dependent negative feedback mechanisms, which may be important to counteract tolerogenic mechanisms in anti-tumour immunity. In the absence of TLR stimulation, p38 activation resulted in a DC maturation phenotype different from full maturation as achieved by LPS treatment (Escors, Lopes et al. 2008). Particularly, there was specific up-regulation of co-stimulatory molecules CD80, CD40 and ICAM-I, and absence of significant secretion of pro-inflammatory cytokines (Escors, Lopes et al. 2008). This is in contrast to studies using p38 inhibitors after TLR stimulation (Arrighi, Rebsamen et al. 2001) (Lu, Yang et al. 1999; Yu, Kovacs et al. 2004). Consequently, p38 activation may not be directly involved in transcriptional up-regulation of pro-inflammatory cytokine genes (Saccani, Pantano et al. 2002). Interestingly, co-expression of OVA with the p38 activator in DC significantly increased antigen-specific CD4+ and CD8+ T cell responses leading to increased anti-tumour immunity in a OVA-expressing lymphoma model (Escors, Lopes et al. 2008) (Karwacz, Mukherjee et al. 2009). Additionally, MAPK p38 constitutive activation also increased CD8+ T cell responses to human tumour antigens NY-ESO in a humanized HLA-A2 mouse model and MelanA/MART-1 in a human DC-T cell culture (Escors, Lopes et al. 2008). Specific activation of JNK1 in DC showed only a moderate up-regulation of CD80 and ICAM-I and no significant secretion of pro-inflammatory cytokines, confirming previous studies which suggested that JNK marginally control DC maturation (Escors, Lopes et al. 2008) (Nakahara, Uchi et al. 2004). On the other hand, increased antigen-specific CD8+ T cell expansion was achieved in mice after subcutaneous vaccination with LV expressing MKK7-JNK1, suggesting that JNK1 may play a subtle but important role in DC in vivo (Escors, Lopes et al. 2008).
5. POTENTIAL CLINICAL USE OF LENTIVIRUS VECTORS FOR CANCER IMMUNOTHERAPY
5.1. Lentivector persistence in DC
The duration of antigen presentation is clearly a relevant issue in the setting of LVs as potential genetic vaccines, since these vectors permanently integrate their genome into the host cell. Consequently, it would be expected that APCs targeted by LVs would produce sustained expression of the antigen for the life time of the cells. In the post-vaccination scenario with lentivectors, there is no clear picture of the factors that could influence the effects of a sustained antigen presentation.
Antigen persistence has been shown in the bone marrow, liver and spleen of mice injected systemically in vivo with lentivectors (Pan, Gunther et al. 2002; Kimura, Koya et al. 2007). We have recently shown that this route of immunization not only results in the transduction of lymphocytes, macrophages and different DC subsets, but also in transduction of a DC precursor within the spleen (Arce, Rowe et al. 2009). As a result, the percentages of GFP+ DC increased over time and GFP+ DC were still detectable two months after immunization. Presentation of OVA decreased over time, however it was still detectable after 2 months.
Although there evidence that suggests that protracted antigen presentation can result in tolerance and impairment of the immune response (Zinkernagel and Hengartner 2004), a recent study showed that prolonged antigen expression promotes immunization even in the absence of a dendritic cell activation signal (Obst, van Santen et al. 2007). In fact prolongation of antigen presentation, achieved by inhibiting apoptosis of DC, has been shown to be more effective in vaccination (Kim, Hung et al. 2003). In some way, lentiviral vector immunization seems to mimic persistent viral infection, which can result in high level CD8+ T cell responses, generated both from memory cells and naïve CD8+ T-cell recruitment (Snyder, Cho et al. 2008). For cancer treatment, this is an important characteristic to help prevent tumour recurrence. Whether persistent memory plays a role in this is not completely clear, but there is no evidence of tolerance to the transgene in the long term.
5.2. Clinical trials
In spite of their extensive pre-clinical use, translation of lentiviral vectors into the clinical scenario is still in its early days. Results from the use of autologous CD4+ T cells modified with a conditionally-replicating lentivector expressing an antisense gene against the HIV envelope showed sustained gene transfer and no evidence of insertional mutagenesis after 21-36 months. There was self-limiting mobilization of the vector and improvement of the immune function in four out of five subjects (Levine, Humeau et al. 2006). These results are promising in the sense that they show efficacy and safety of the vector. To our knowledge, no clinical trials using lentivectors for active immunization have yet been begun. We would suggest that the potential benefits of tumour therapy outweigh the risks of lentiviral vector immunisation in patients with advanced stage cancer. However, the potential for lentiviral vectors, even when targeted, to transduce dividing cells such as DC precursors imparts a theoretical risk of insertional mutagenesis. Therefore the future development of non-integrating lentiviral vectors, which could be sufficiently safe even for prophylactic vaccination, is essential. Such vectors were first used pre-clinically to treat a mouse model of inherited retinopathy (Yanez-Munoz, Balaggan et al. 2006), and have also been used for gene delivery to non-dividing tissues such as muscle (Apolonia, Waddington et al. 2007). Recently we and others have shown immunisation with non-integrating lentiviral vectors encoding OVA, HBV (Karwacz, Mukherjee et al. 2009), HIV (Negri, Michelini et al. 2007), SIV (Michelini, Negri et al. 2009), or West Nile virus (Coutant, Frenkiel et al. 2008) antigens. It will now be of importance to examine the duration and quality of the immune response and the effectiveness of tumour therapy in comparison to integrating lentiviral vectors.
Acknowledgements
Karine Breckpot is funded by the Fund for Scientific Research-Flanders (FWO-Vlaanderen). David Escors is funded by an Arthritis Research Campaign Career Development Fellowship.
Abbreviations
- CEA
carcinoembryonic antigen
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- FCS
foetal calf serum
- GFP
green fluorescent protein
- HIV
human immunodeficiency virus
- HLA
human leukocyte antigen
- IFN
interferon
- IL
interleukin
- MDSC
myeloid-derived suppressor cells
- MHC
major histocompatibility complex
- MOI
multiplicity of infection
- PKR
protein kinase R
- TAA
tumour-associated antigen
- TCR
T cell receptor
- TH
T helper cell
- TLR
toll like receptor
- TNF-α
tumour necrosis factor-α
- Treg
regulatory T cell
REFERENCES
- Abdel-Wahab Z, Cisco R, et al. Cotransfection of DC with TLR4 and MART-1 RNA induces MART-1-specific responses. J Surg Res. 2005;124(2):264–73. doi: 10.1016/j.jss.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Dillon S, et al. ERK−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2006;176(10):5788–96. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- Akazawa T, Shingai M, et al. Tumor immunotherapy using bone marrow-derived dendritic cells overexpressing Toll-like receptor adaptors. FEBS Lett. 2007;581(18):3334–40. doi: 10.1016/j.febslet.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, et al. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Andreakos E, Williams RO, et al. Activation of NF-kappaB by the intracellular expression of NF-kappaB-inducing kinase acts as a powerful vaccine adjuvant. Proc Natl Acad Sci U S A. 2006;103(39):14459–64. doi: 10.1073/pnas.0603493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolonia L, Waddington SN, et al. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol Ther. 2007;15(11):1947–54. doi: 10.1038/sj.mt.6300281. [DOI] [PubMed] [Google Scholar]
- Arce F, Rowe HM, et al. Lentiviral vectors transduce proliferating dendritic cell precursors leading to persistent antigen presentation and immunization. Mol Ther. 2009;17(9):1643–50. doi: 10.1038/mt.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshna KM, Pizzey AR, et al. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96(3):1039–46. [PubMed] [Google Scholar]
- Arrighi JF, Rebsamen M, et al. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166(6):3837–45. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- Bao L, Guo H, et al. High-titer lentiviral vectors stimulate fetal calf serum-specific human CD4 T-cell responses: implications in human gene therapy. Gene Ther. 2009;16(6):788–95. doi: 10.1038/gt.2009.34. [DOI] [PubMed] [Google Scholar]
- Barisic S, Strozyk E, et al. Identification of PP2A as a crucial regulator of the NF-kappaB feedback loop: its inhibition by UVB turns NF-kappaB into a pro-apoptotic factor. Cell Death Differ. 2008;15(11):1681–90. doi: 10.1038/cdd.2008.98. [DOI] [PubMed] [Google Scholar]
- Beignon AS, McKenna K, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115(11):3265–75. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler BA. TLRs and innate immunity. Blood. 2009;113(7):1399–407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaert R, Heyninck K, et al. A20 and A20-binding proteins as cellular inhibitors of nuclear factor-kappa B-dependent gene expression and apoptosis. Biochem Pharmacol. 2000;60(8):1143–51. doi: 10.1016/s0006-2952(00)00404-4. [DOI] [PubMed] [Google Scholar]
- Bonehill A, Heirman C, et al. Genetic approaches for the induction of a CD4+ T cell response in cancer immunotherapy. J Gene Med. 2005;7(6):686–95. doi: 10.1002/jgm.713. [DOI] [PubMed] [Google Scholar]
- Bonehill A, Heirman C, et al. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J Immunol. 2004;172(11):6649–57. doi: 10.4049/jimmunol.172.11.6649. [DOI] [PubMed] [Google Scholar]
- Bonehill A, Tuyaerts S, et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16(6):1170–80. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183(3):725–9. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T. a. P. v. d. B. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183(3):725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DL, Turer EE, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5(10):1052–60. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Bouard D, Alazard-Dany D, et al. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157(2):153–65. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton TG, Cobb MH. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991;2(5):357–71. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton TG, Nye SH, et al. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65(4):663–75. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Bowie AG. TRIM-ing down Tolls. Nat Immunol. 2008;9(4):348–50. doi: 10.1038/ni0408-348. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Aerts-Toegaert C, et al. Attenuated expression of A20 markedly increases the efficacy of double-stranded RNA-activated dendritic cells as an anti-cancer vaccine. J Immunol. 2009;182(2):860–70. doi: 10.4049/jimmunol.182.2.860. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Aerts JL, et al. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene Ther. 2007;14(11):847–62. doi: 10.1038/sj.gt.3302947. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Corthals J, et al. Activation of monocytes via the CD14 receptor leads to the enhanced lentiviral transduction of immature dendritic cells. Hum Gene Ther. 2004;15(6):562–73. doi: 10.1089/104303404323142015. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Dullaers M, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med. 2003;5(8):654–67. doi: 10.1002/jgm.400. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Emeagi P, et al. Activation of immature monocyte-derived dendritic cells after transduction with high doses of lentiviral vectors. Hum Gene Ther. 2007;18(6):536–46. doi: 10.1089/hum.2007.006. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Emeagi PU, et al. Lentiviral vectors for anti-tumor immunotherapy. Curr Gene Ther. 2008;8(6):438–48. doi: 10.2174/156652308786848058. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Heirman C, et al. Identification of new antigenic peptide presented by HLA-Cw7 and encoded by several MAGE genes using dendritic cells transduced with lentiviruses. J Immunol. 2004;172(4):2232–7. doi: 10.4049/jimmunol.172.4.2232. [DOI] [PubMed] [Google Scholar]
- Breckpot K, Heirman C, et al. Exploiting dendritic cells for cancer immunotherapy: genetic modification of dendritic cells. J Gene Med. 2004;6(11):1175–88. doi: 10.1002/jgm.615. [DOI] [PubMed] [Google Scholar]
- Brown BD, Sitia G, et al. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2006 doi: 10.1182/blood-2006-10-049312. [DOI] [PubMed] [Google Scholar]
- Burkly L, Hession C, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373(6514):531–6. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- Caparros E, Munoz P, et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107(10):3950–8. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- Chapatte L, Colombetti S, et al. Efficient induction of tumor antigen-specific CD8+ memory T cells by recombinant lentivectors. Cancer Res. 2006;66(2):1155–60. doi: 10.1158/0008-5472.CAN-05-2597. [DOI] [PubMed] [Google Scholar]
- Cisco RM, Abdel-Wahab Z, et al. Induction of human dendritic cell maturation using transfection with RNA encoding a dominant positive toll-like receptor 4. J Immunol. 2004;172(11):7162–8. doi: 10.4049/jimmunol.172.11.7162. [DOI] [PubMed] [Google Scholar]
- Coornaert B, Carpentier I, et al. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009;284(13):8217–21. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutant F, Frenkiel MP, et al. Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS One. 2008;3(12):e3973. doi: 10.1371/journal.pone.0003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Golob J, et al. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood. 2002;99(2):399–408. doi: 10.1182/blood.v99.2.399. [DOI] [PubMed] [Google Scholar]
- da Silva Correia J, Soldau K, et al. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276(24):21129–35. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- Dawson S, Hastings R, et al. The 26S-proteasome: regulation and substrate recognition. Mol Biol Rep. 1997;24(1-2):39–44. doi: 10.1023/a:1006800522814. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76(6):1025–37. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Dixit VM, Green S, et al. Tumor necrosis factor-alpha induction of novel gene products in human endothelial cells including a macrophage-specific chemotaxin. J Biol Chem. 1990;265(5):2973–8. [PubMed] [Google Scholar]
- Dullaers M, Breckpot K, et al. Side-by-side comparison of lentivirally transduced and mRNA-electroporated dendritic cells: implications for cancer immunotherapy protocols. Mol Ther. 2004;10(4):768–79. doi: 10.1016/j.ymthe.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Dullaers M, Van Meirvenne S, et al. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 2006;13(7):630–40. doi: 10.1038/sj.gt.3302697. [DOI] [PubMed] [Google Scholar]
- Dyall J, Latouche JB, et al. Lentivirus-transduced human monocyte-derived dendritic cells efficiently stimulate antigen-specific cytotoxic T lymphocytes. Blood. 2001;97(1):114–21. doi: 10.1182/blood.v97.1.114. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Sohma Y, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194(12):1823–34. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MR, Bartlett NW, et al. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther. 2009;121(1):1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens LA. A new twist on autologous cancer vaccines. Cancer Biol Ther. 2003;2(2):161–3. doi: 10.4161/cbt.2.2.283. [DOI] [PubMed] [Google Scholar]
- Escors D, Lopes L, et al. Targeting dendritic cell signalling to regulate the response to immunisation. Blood. 2008;111(6):3050–61. doi: 10.1182/blood-2007-11-122408. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Chapatte L, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J Clin Invest. 2003;111(11):1673–81. doi: 10.1172/JCI17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat H, Zennou V, et al. Use of a lentiviral flap vector for induction of CTL immunity against melanoma. Perspectives for immunotherapy. J Gene Med. 2002;4(1):38–45. doi: 10.1002/jgm.243. [DOI] [PubMed] [Google Scholar]
- Fonteneau JF, Larsson M, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78(10):5223–32. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frecha C, Szecsi J, et al. Strategies for targeting lentiviral vectors. Curr Gene Ther. 2008;8(6):449–60. doi: 10.2174/156652308786848003. [DOI] [PubMed] [Google Scholar]
- Funke S, Maisner A, et al. Targeted cell entry of lentiviral vectors. Mol Ther. 2008;16(8):1427–36. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier G, Humbert M, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201(9):1435–46. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gorski KS, Shin T, et al. A set of genes selectively expressed in murine dendritic cells: utility of related cis-acting sequences for lentiviral gene transfer. Mol Immunol. 2003;40(1):35–47. doi: 10.1016/s0161-5890(03)00085-3. [DOI] [PubMed] [Google Scholar]
- Gruber A, Kan-Mitchell J, et al. Dendritic cells transduced by multiply deleted HIV-1 vectors exhibit normal phenotypes and functions and elicit an HIV-specific cytotoxic T-lymphocyte response in vitro. Blood. 2000;96(4):1327–33. [PubMed] [Google Scholar]
- Gupta S, Barrett T, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. Embo J. 1996;15(11):2760–70. [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006(357):re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Hacker H, Redecke V, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439(7073):204–7. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- Hammaker DR, Boyle DL, et al. Regulation of the JNK pathway by TGF-beta activated kinase 1 in rheumatoid arthritis synoviocytes. Arthritis Res Ther. 2007;9(3):R57. doi: 10.1186/ar2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee JD, et al. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265(5173):808–11. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- He Y, Falo LD. Induction of T cell immunity by cutaneous genetic immunization with recombinant lentivector. Immunol Res. 2006;36(1-3):101–17. doi: 10.1385/IR:36:1:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Munn D, et al. Recombinant lentivector as a genetic immunization vehicle for antitumor immunity. Expert Rev Vaccines. 2007;6(6):913–24. doi: 10.1586/14760584.6.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang J, et al. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol. 2005;174(6):3808–17. doi: 10.4049/jimmunol.174.6.3808. [DOI] [PubMed] [Google Scholar]
- Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett. 1999;442(2-3):147–50. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- Hu MC, Wang Y, et al. Hematopoietic progenitor kinase-1 (HPK1) stress response signaling pathway activates IkappaB kinases (IKK-alpha/beta) and IKK-beta is a developmentally regulated protein kinase. Oncogene. 1999;18(40):5514–24. doi: 10.1038/sj.onc.1202740. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, et al. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. Faseb J. 2008;22(4):954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Karwacz K, Mukherjee S, et al. Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy. J Virol. 2009;83(7):3094–103. doi: 10.1128/JVI.02519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13(11):460–9. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kim JH, Majumder N, et al. Induction of therapeutic antitumor immunity by in vivo administration of a lentiviral vaccine. Hum Gene Ther. 2005;16(11):1255–66. doi: 10.1089/hum.2005.16.1255. [DOI] [PubMed] [Google Scholar]
- Kim TW, Hung CF, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112(1):109–17. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Koya RC, et al. Lentiviral vectors with CMV or MHCII promoters administered in vivo: immune reactivity versus persistence of expression. Mol Ther. 2007;15(7):1390–9. doi: 10.1038/sj.mt.6300180. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369(6476):156–60. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lang KS, Recher M, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11(2):138–45. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Bebien M. IKKalpha in the regulation of inflammation and adaptive immunity. Biochem Soc Trans. 2007;35(Pt 2):270–2. doi: 10.1042/BST0350270. [DOI] [PubMed] [Google Scholar]
- Lee CH, Jeon YT, et al. NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 2007;29(1):19–35. doi: 10.1002/biof.5520290103. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372(6508):739–46. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006;103(46):17372–7. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68(1):510–6. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, et al. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284(5412):321–5. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu W, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189(11):1839–45. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Roberts RL, et al. Antibody-directed natural cytotoxicity results in enhanced killing of HIV gp120-coated CEMNKR cells. Clin Immunol Immunopathol. 1997;83(2):139–46. doi: 10.1006/clin.1997.4330. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peng Y, et al. Lentivector immunization stimulates potent CD8 T cell responses against melanoma self-antigen tyrosinase-related protein 1 and generates antitumor immunity in mice. J Immunol. 2009;182(10):5960–9. doi: 10.4049/jimmunol.0900008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizee G, Gonzales MI, et al. Lentivirus vector-mediated expression of tumor-associated epitopes by human antigen presenting cells. Hum Gene Ther. 2004;15(4):393–404. doi: 10.1089/104303404322959542. [DOI] [PubMed] [Google Scholar]
- Lizee G, Radvanyi LG, et al. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res. 2006;12(16):4794–803. doi: 10.1158/1078-0432.CCR-06-0944. [DOI] [PubMed] [Google Scholar]
- Loisel-Meyer S, Felizardo T, et al. Potent induction of B- and T-cell immunity against human carcinoembryonic antigen-expressing tumors in human carcinoembryonic antigen transgenic mice mediated by direct lentivector injection. Mol Cancer Ther. 2009;8(3):692–702. doi: 10.1158/1535-7163.MCT-08-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes L, Dewannieux M, et al. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J Virol. 2008;82(1):86–95. doi: 10.1128/JVI.01289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HT, Yang DD, et al. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. Embo J. 1999;18(7):1845–57. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft T, Rodionova E, et al. Adaptive functional differentiation of dendritic cells: integrating the network of extra- and intracellular signals. Blood. 2006;107(12):4763–9. doi: 10.1182/blood-2005-04-1501. [DOI] [PubMed] [Google Scholar]
- Marzo AL, Vezys V, et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol. 2004;173(2):969–75. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, Phillips RE. Escape of human immunodeficiency virus from immune control. Annu Rev Immunol. 1997;15:271–96. doi: 10.1146/annurev.immunol.15.1.271. [DOI] [PubMed] [Google Scholar]
- Metharom P, Ellem KA, et al. Lentiviral vector-mediated tyrosinase-related protein 2 gene transfer to dendritic cells for the therapy of melanoma. Hum Gene Ther. 2001;12(18):2203–13. doi: 10.1089/10430340152710540. [DOI] [PubMed] [Google Scholar]
- Metharom P, Ellem KA, et al. Gene transfer to dendritic cells induced a protective immunity against melanoma. Cell Mol Immunol. 2005;2(4):281–8. [PubMed] [Google Scholar]
- Michelini Z, Negri DR, et al. Development and use of SIV-based Integrase defective lentiviral vector for immunization. Vaccine. 2009;27(34):4622–9. doi: 10.1016/j.vaccine.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Mandruzzato S, et al. Part I: Vaccines for solid tumours. Lancet Oncol. 2004;5(11):681–9. doi: 10.1016/S1470-2045(04)01610-9. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Kuroyanagi N, et al. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271(23):13675–9. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- Morton S, Davis RJ, et al. Signalling pathways involved in multisite phosphorylation of the transcription factor ATF-2. FEBS Lett. 2004;572(1-3):177–83. doi: 10.1016/j.febslet.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Morton S, Davis RJ, et al. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. Embo J. 2003;22(15):3876–86. doi: 10.1093/emboj/cdg388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossoba ME, Medin JA. Cancer immunotherapy using virally transduced dendritic cells: animal studies and human clinical trials. Expert Rev Vaccines. 2006;5(5):717–32. doi: 10.1586/14760584.5.5.717. [DOI] [PubMed] [Google Scholar]
- Mossoba ME, Walia JS, et al. Tumor protection following vaccination with low doses of lentivirally transduced DCs expressing the self-antigen erbB2. Mol Ther. 2008;16(3):607–17. doi: 10.1038/sj.mt.6300390. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Uchi H, et al. Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int Immunol. 2004;16(12):1701–9. doi: 10.1093/intimm/dxh171. [DOI] [PubMed] [Google Scholar]
- Napolitani G, Rinaldi A, et al. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri DR, Michelini Z, et al. Successful immunization with a single injection of non-integrating lentiviral vector. Mol Ther. 2007;15(9):1716–23. doi: 10.1038/sj.mt.6300241. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, et al. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398(6724):252–6. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Noti JD, Reinemann BC, et al. Sp1 binds two sites in the CD11c promoter in vivo specifically in myeloid cells and cooperates with AP1 to activate transcription. Mol Cell Biol. 1996;16(6):2940–50. doi: 10.1128/mcb.16.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obst R, van Santen HM, et al. Sustained antigen presentation can promote an immunogenic T cell response, like dendritic cell activation. Proc Natl Acad Sci U S A. 2007;104(39):15460–5. doi: 10.1073/pnas.0707331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki M, Ando K, et al. Efficient lentiviral transduction of human cord blood CD34(+) cells followed by their expansion and differentiation into dendritic cells. Exp Hematol. 2001;29(10):1210–7. doi: 10.1016/s0301-472x(01)00695-6. [DOI] [PubMed] [Google Scholar]
- Palmowski MJ, Lopes L, et al. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J Immunol. 2004;172(3):1582–7. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- Pan D, Gunther R, et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther. 2002;6(1):19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21(5):733–41. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–8. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Diebold SS, et al. Tubulovesicular structures within vesicular stomatitis virus G protein-pseudotyped lentiviral vector preparations carry DNA and stimulate antiviral responses via Toll-like receptor 9. J Virol. 2007;81(2):539–47. doi: 10.1128/JVI.01818-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, et al. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16(3):1247–55. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Martino M, et al. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188(11):2175–80. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Sherry RM, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175(9):6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- Rothwarf DM, Karin M. The NF-kappa B activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE. 1999;1999(5):RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- Rowe HM, Lopes L, et al. Expression of vFLIP in a lentiviral vaccine vector activates NF-{kappa}B, matures dendritic cells, and increases CD8+ T-cell responses. J Virol. 2009;83(4):1555–62. doi: 10.1128/JVI.00709-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe HM, Lopes L, et al. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol Ther. 2006;13(2):310–9. doi: 10.1016/j.ymthe.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, et al. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3(1):69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Yamamoto M, et al. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol. 2005;174(3):1507–12. doi: 10.4049/jimmunol.174.3.1507. [DOI] [PubMed] [Google Scholar]
- Salmon P, Kindler V, et al. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood. 2000;96(10):3392–8. [PubMed] [Google Scholar]
- Sato S, Sanjo H, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6(11):1087–95. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- Schroers R, Sinha I, et al. Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1-based lentiviral vector system. Mol Ther. 2000;1(2):171–9. doi: 10.1006/mthe.2000.0027. [DOI] [PubMed] [Google Scholar]
- Schulz O, Diebold SS, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433(7028):887–92. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47(6):921–8. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293(5534):1495–9. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Shi M, Deng W, et al. TRIM30 alpha negatively regulates TLR-mediated NF-kappa B activation by targeting TAB2 and TAB3 for degradation. Nat Immunol. 2008;9(4):369–77. doi: 10.1038/ni1577. [DOI] [PubMed] [Google Scholar]
- Snyder CM, Cho KS, et al. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29(4):650–9. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XT, Evel-Kabler K, et al. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med. 2008;14(3):258–65. doi: 10.1038/nm1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin PV, Vil’gelm AE, et al. Lentiviral vectors. Mol Biol (Mosk) 2008;42(5):913–26. [PubMed] [Google Scholar]
- Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Tsuji T, et al. Rapid and efficient generation of lentivirally gene-modified dendritic cells from DC progenitors with bone marrow stromal cells. J Immunol Methods. 2002;271(1-2):153–65. doi: 10.1016/s0022-1759(02)00342-3. [DOI] [PubMed] [Google Scholar]
- Takahara K, Omatsu Y, et al. Identification and expression of mouse Langerin (CD207) in dendritic cells. Int Immunol. 2002;14(5):433–44. doi: 10.1093/intimm/14.5.433. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, et al. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284(5412):313–6. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Tan PH, Beutelspacher SC, et al. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood. 2005;105(10):3824–32. doi: 10.1182/blood-2004-10-3880. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Haste NM, et al. PKR and eIF2alpha: integration of kinase dimerization, activation, and substrate docking. Cell. 2005;122(6):823–5. doi: 10.1016/j.cell.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Tuyaerts S, Aerts JL, et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother. 2007;56(10):1513–37. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unutmaz D, KewalRamani VN, et al. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J Exp Med. 1999;189(11):1735–46. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin D, Medzhitov R, et al. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27(1):49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Van Tendeloo VF, Ponsaerts P, et al. mRNA-based gene transfer as a tool for gene and cell therapy. Curr Opin Mol Ther. 2007;9(5):423–31. [PubMed] [Google Scholar]
- VandenDriessche T, Thorrez L, et al. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood. 2002;100(3):813–22. doi: 10.1182/blood.v100.3.813. [DOI] [PubMed] [Google Scholar]
- Waehler R, Russell SJ, et al. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8(8):573–87. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2(1):11–9. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- Wang B, He J, et al. An effective cancer vaccine modality: lentiviral modification of dendritic cells expressing multiple cancer-specific antigens. Vaccine. 2006;24(17):3477–89. doi: 10.1016/j.vaccine.2006.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Deng L, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412(6844):346–51. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Wang RF, Miyahara Y, et al. Toll-like receptors and immune regulation: implications for cancer therapy. Oncogene. 2008;27(2):181–9. doi: 10.1038/sj.onc.1210906. [DOI] [PubMed] [Google Scholar]
- Wang YY, Li L, et al. A20 is a potent inhibitor of TLR3- and Sendai virus-induced activation of NF-kappaB and ISRE and IFN-beta promoter. FEBS Lett. 2004;576(1-2):86–90. doi: 10.1016/j.febslet.2004.08.071. [DOI] [PubMed] [Google Scholar]
- Warger T, Osterloh P, et al. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108(2):544–50. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- Weih F, Carrasco D, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80(2):331–40. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Wertz IE, O’Rourke KM, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694–9. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–12. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Darcy PK, et al. Tumor-specific dendritic cells generated by genetic redirection of Toll-like receptor signaling against the tumor-associated antigen, erbB2. Cancer Gene Ther. 2007;14(9):773–80. doi: 10.1038/sj.cgt.7701073. [DOI] [PubMed] [Google Scholar]
- Yanez-Munoz RJ, Balaggan KS, et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med. 2006;12(3):348–53. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- Yang L, Bailey L, et al. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci U S A. 2006;103(31):11479–84. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Huang CT, et al. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5(5):508–15. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- Yu Q, Kovacs C, et al. The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J Immunol. 2004;172(10):6047–56. doi: 10.4049/jimmunol.172.10.6047. [DOI] [PubMed] [Google Scholar]
- Zarei S, Leuba F, et al. Transduction of dendritic cells by antigen-encoding lentiviral vectors permits antigen processing and MHC class I-dependent presentation. J Allergy Clin Immunol. 2002;109(6):988–94. doi: 10.1067/mai.2002.124663. [DOI] [PubMed] [Google Scholar]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15(1):11–8. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- Zhang SQ, Kovalenko A, et al. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000;12(3):301–11. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Hengartner H. On immunity against infections and vaccines: credo 2004. Scand J Immunol. 2004;60(1-2):9–13. doi: 10.1111/j.0300-9475.2004.01460.x. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Tesniere A, et al. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–27. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]