Abstract
Phylogeny shows that CD4 T cell memory and lymph nodes (LNs) co-evolved in placental mammals. In ontogeny, retinoic acid orphan receptor (ROR) γ-dependent lymphoid tissue inducer (LTi) cells program the development of mammalian LNs. Here, we show that while primary CD4 T cell expansion is normal in RORγ-deficient mice, the persistence of memory CD4 T cells is RORγ-dependent. Furthermore, using bone marrow chimeric mice we demonstrate that LTi cells are the key RORγ-expressing cell type sufficient for memory CD4 T cell survival in the absence of persistent antigen. This effect was specific for CD4 T cells, since memory CD8 T cells survived equally well in the presence or absence of LTi cells. These data demonstrate a novel role for LTi cells, archetypal members of the innate lymphoid cell family, in supporting memory CD4 T cell survival in vivo.
INTRODUCTION
The hallmark of mammalian immunity is the capacity to make CD4 T cell-dependent memory immune responses, and this underpins the success of vaccination strategies. Phylogeny shows that both LNs and CD4 T cell memory antibody responses evolved in placental animals, as marsupials have evidence of memory (1) and LNs (2, 3), whereas monotremes have neither (4, 5). The formation of LNs is dependent upon RORγ-expressing LTi cells, key members of the recently described innate lymphoid cell family (6). While the function of LTi cells in the developing embryo is clear, their potential roles within mature secondary lymphoid tissue are currently being elucidated.
Recent studies have found them to be important in the repair of lymphoid tissues after pathogen related injury (7), the production of IL-22 (8), and T cell independent production of IgA in the gut (9). We previously found that in mature, but not in embryonic mice, LTi cells express high levels of the TNF family members, OX40-ligand(L) and CD30L (10, 11) and we have linked signalling through the receptors for these molecules with the capacity to mount CD4 memory antibody responses (12, 13). Unlike antigen-presenting cells such as dendritic cells and B cells that can also express OX40L and CD30L, LTi cells completely lack expression of CD80 and CD86, and do not present antigen (13). Since LNs and CD4 memory antibody responses arose in the same evolutionary window, we speculated that LTi cells might provide survival signals required for the maintenance of memory CD4 T cells in the absence of antigenic stimulation. To test this we have now analyzed CD4 memory responses in mice lacking LTi cells. Here we provide direct evidence that LTi cells maintain memory CD4 T cells in vivo, demonstrating a further crucial role for these innate lymphoid cells in supporting adaptive immune responses.
MATERIALS AND METHODS
Mice
Animals were bred in accordance with home office guidelines at the University of Birmingham, Biomedical Services Unit. Mice used were BoyJ, CD3εtg26, CD3εtg26RORγ−/−, RORγ−/−, Rag−/−, Rag−/− × OTII, Rag−/− × SM1. The following mouse was obtained through the NIAID Exchange Program, NIH: C57BL/6-Tg(OT-I)-RAG1<tm1Mom> (14) (15). Experimental and control CD3εtg26RORγ−/− mice were sub-lethally-irradiated (1 × 450 Rad), given BM from CD3εtg26 or CD3εtg26 RORγ−/− mice i.v. and used 4-5wks after reconstitution.
Immunisation and cell transfer
To track antigen specific CD4 T cells, mice were infected i.v. with 107 ActA mutant Listeria monocytogenes (Lm), as described (16). To generate memory T cells, ~5 ×104 SM1, OTII or OTI T cells were transferred into Rag−/− mice, which were then immunised and memory cells harvested 3-4 wks later. To stimulate SM1 cells, recipient mice were immunised i.v. with 107 attenuated Lm expressing FliC peptide (target antigen of SM1 T cells), a kind gift from Dr. Sing Sing Way. To stimulate OTII and OTI cells, recipient mice were immunised i.p. with 100μg alum-precipitated OVA.
Flow cytometry
For tetramer staining, cells from secondary lymphoid tissue were pooled and stained for 1hr at RT with 2W1S:I-Ab. All cell surface staining was done at +4°C for 30mins. Samples were run using a Fortessa (BD) and analysed using Flow-Jo software (Treestar).
Immunofluorescence and image analysis
Frozen tissue sections were cut and stained with as described previously (17).
Statistics
Statistical significance was tested using the Mann-Whitney U Test and a two-tailed p value calculated.
RESULTS AND DISCUSSION
Memory CD4 T cells fail to survive in RORγ−/− mice
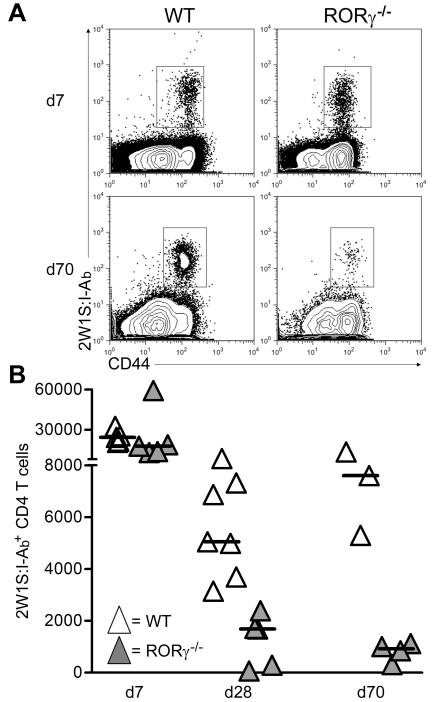
To investigate whether CD4 T cell survival was dependent upon LTi cells, we first immunized RORγ−/− and RORγ−/+ littermate mice with nitrophenylated chicken γ globulin, (NP-CγG). We assessed the primary anti-NP IgM and IgG responses in the serum 7 days (7d) post immunization and found no significant difference (Supplementary Figure 1). To assess a memory response, we analysed splenic NP-specific IgG plasma cells at 4 days post challenge (Supplementary Figure 1). Although RORγ−/+ mounted a characteristic memory response, this was absent in RORγ−/− littermates (Supplementary Figure 1, p=0.006). While consistent with defective memory CD4 T cell survival, these experiments did not discriminate between B or T cell defects in the antibody response. To specifically test memory CD4 T cell survival in RORγ−/− mice, we analysed endogenous 2W1S+ CD4 T cells(16). Mice were infected with an Lm-2W1S peptide (16) and the pMHCII tetramer 2W1S:I-Ab was used to identify responding cells in secondary lymphoid tissue (spleen and LNs from WT mice, only spleen from RORγ−/− mice). Comparable numbers of 2W1S:I-Ab+ CD4 T cells were recovered from WT and RORγ−/− mice 7 days post infection (dpi) (Figures 1A and 1B). No significant differences were seen in the expression of CXCR5, PD-1, Bcl-6 or T-bet by 2W1S+ CD4 T cells at 7 dpi, indicating a normal primary response with formation of follicular T helper cells and other T effector cell subsets. (data not shown) (18). Strikingly, by 28 dpi, numbers of 2W1S:I-Ab+ cells were significantly reduced (p=0.003) in RORγ−/− mice compared with WT controls and this difference was even greater at 70 dpi (Figure 1B).
Figure 1. Memory CD4 T cells fail to survive in RORγ−/− mice.
A, Detection of CD44hi LM-2W1S:I-Ab+ CD4 T cells in secondary lymphoid tissue from WT and RORγ−/− mice at 7 and 70 dpi with Lm-2W1S. Plots representative of 5 mice per time point.
B, Quantitation of total numbers of CD44hi Lm-2W1S:I-Ab+ CD4 T cells isolated from WT (white) and RORγ−/− (grey) at 7, 28 and 70 dpi. Each triangle represents an individual mouse. Results representative of 2 independent experiments. Bars show medians.
RORγ-expressing LTi cells mediate memory CD4 T cell survival
Although these data demonstrated that polyclonal antigen specific CD4 memory T cells were not maintained in RORγ−/− mice, they did not show directly that LTi cells were responsible. Differences between WT and RORγ−/− mice could have been attributed to the absence of LNs in the latter and other cell populations are RORγ-dependent (19-21). Therefore to test directly the requirement for LTi cells we performed the following reductionist experiment, exploiting the CD3εtg26 mouse (22) that lacks T and NK cells but is LTi cell-sufficient (10). These mice were crossed with RORγ−/− mice to make mice deficient in T, NK and LTi cells (CD3εRORγ−/−) (Supplementary Figure 2). To reconstitute LTi cells in the spleen, CD3εRORγ−/− mice were irradiated and reconstituted with bone marrow (BM) from CD3εtg26 mice (Supplementary Figure 2, mice designated LTi+). CD3εRORγ−/− mice irradiated and reconstituted with BM from CD3εRORγ−/− (mice designated LTi−) were used as controls. Since LNs only develop during an embryonic window, they were absent in both LTi− and LTi+ mice. LTi-like cells expressing NK1.1 and NKp46 (23) were absent in the spleens of CD3εtg26 mice compared with the spleens of Rag−/− mice (Supplementary Figure 2). The LTi cells in CD3εtg26 mice expressed high levels of OX40L, whilst expression of OX40L by dendritic cells was comparable in CD3εtg26 and CD3εRORγ−/− mice (Supplementary Figure 2). Therefore we had generated mice with the same secondary lymphoid tissue but sufficient or deficient in LTi cells, to test the role of LTi cells in memory T cell survival.
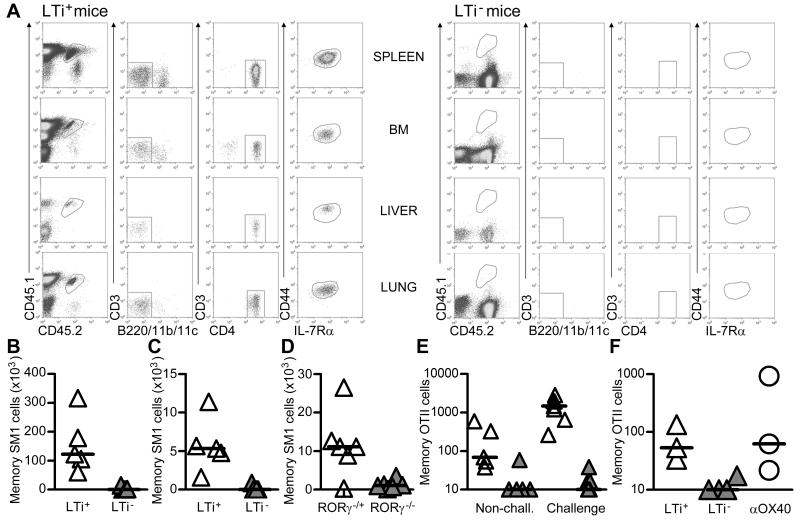
Transfer of pre-made memory cells into LTi+ or LTi− mice would exclude possible effects of LTi cell deficiency on the generation of these cells. A pure cohort of memory CD4 SM1 TCR transgenic T cells was generated (see Materials and methods) and then transferred (~2.5×105) into LTi+ and LTi− hosts. Four wks after transfer of memory SM1 T cells and without further exposure to antigen, a clear population persisted principally in the spleen of LTi+ mice, and to a lesser extent in the BM and other non-lymphoid tissues such as the lung and liver (Figures 2A, B and C). In contrast, this population was almost entirely absent in LTi− mice. To control for complexities arising from chimeric mice, a similar experiment was performed using Rag−/−RORγ−/+ and Rag−/−RORγ−/− mice, with similar results (Figure 2D). We also confirmed these findings with a second TCR transgenic T cell, OTII cells, which again persisted only in LTi+ mice (Figure 2E).
Figure 2. RORγ-dependent splenic LTi cells are sufficient for CD4 memory survival.
A, Spleen, BM, liver and lung tissue from LTi+ and LTi− mice was analysed by flow cytometry for the presence of memory SM1 T cells following their transfer 4 wks previously. Memory SM1 cells express low levels of CD3 and were identified as CD45.1+CD45.2+(CD3lo)CD4+ CD44hiIL-7Rα+ (lacking expression of B220,CD11b and CD11c); data representative of 5 LTi+ and LTi− mice.
Enumeration of memory SM1 cells in the spleen (B) and BM (C) 4 wks after transfer into LTi+ and LTi− mice. Results representative of 2 independent experiments. Bars show medians.
D, Enumeration of memory SM1 cells in secondary lymphoid tissue 4 wks after transfer into Rag−/−RORγ−/+ and Rag−/−RORγ−/− mice respectively. Data pooled from two independent experiments. Bars show medians.
E, Enumeration of memory OTII cells in the spleen after transfer into LTi+ (white) and LTi− (grey) mice, analysed 3 wks after transfer and 4 days post challenge. Data pooled from two independent experiments. Bars show medians.
F, Number of memory OTII cells 14 days after transfer into LTi+ and LTi− mice given either anti-OX40 Abs or control rat IgG, bars show medians. Data representative of two independent experiments.
Finally, we have found that numbers of LTi are dependent on RORγ expression as Rag−/− RORγ−/+ have fewer LTi cells than Rag−/− RORγ+/+ mice (Supplementary Figure 2). In these mice, survival of CD4 T cells is highly correlated with LTi cell number, consistent with CD4 T cell survival influenced by LTi cells. It is currently unclear how LTi cells maintain memory CD4 T cells. Agonistic anti OX40 Abs did maintain memory OTII cells in LTi− mice (Figure 2F), indicating that provision of this signal could keep memory CD4 T cells alive in vivo. Our previous observations that LTi cells express high levels of OX40L and CD30L, and signals by these molecules are essential for the survival of memory CD4 T cells (12, 13), would suggest this may be how LTi cells provide survival signals. We have previously identified that memory OTII cells associate with LTi cells much more frequently than naive OTII cells within the spleen, (24) supporting a model where cellular interactions mediate survival.
LTi cells are not required for memory CD8 T cell survival
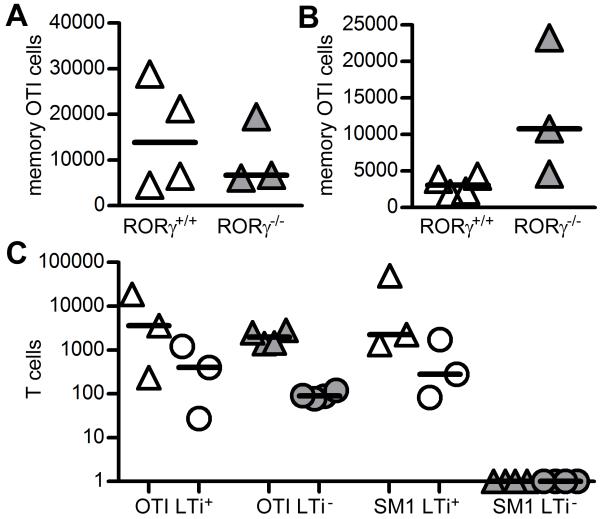
To investigate whether memory CD8 T cell survival was also LTi cell-dependent, memory OTI cells were generated in Rag−/− mice and transferred into Rag−/− RORγ+/+ and Rag−/−RORγ−/−, which were then immunised with OVA. Three wks post immunisation the survival of OTI cells in either the spleen or BM was comparable between LTi cell-sufficient and LTi cell-deficient hosts (Figures 3A and B). When memory OTI and SM1 cells were cotransferred into LTi+ and LTi− chimeric mice, memory OTI cells could be recovered from both LTi+ and LTi− mice, but memory SM1 cells were absent specifically in LTi− mice (Figure 3C).
Figure 3. Memory CD8 T cells do not require LTi cells for survival.
Enumeration of memory OTI cells recovered from secondary lymphoid tissue (A) and BM (B) of Rag−/−RORγ+/+ and Rag−/−RORγ−/− mice 21 days post transfer. Results representative of 2 independent experiments. Bars show medians.
C, Enumeration of memory OTI and SM1 TCR transgenic T cells after co-transfer into LTi+ (white) and LTi− (grey) chimeric mice. Mice analysed 4 wks post transfer, memory cell numbers recovered from the spleen (triangles) and BM (circles) shown. No SM1 cells were detected in LTi− spleen or BM and were given an arbitrary value of 1. Bars show medians.
LTi cells reside at sites of memory cell recirculation
To investigate where LTi cells and memory CD4 T cells might interact in vivo, we analysed RORγ expression in secondary lymphoid tissue to determine the location of these cells. Within the spleen, LTi cells (defined as RORγ+IL-7Rα+CD3−) were located at the marginal sinus and within the bridging channels (Figure 4A), coinciding closely with the sites of lymphocyte entry and trafficking into the white pulp (25). Similarly, within the LN, LTi cells were found clustered at the marginal sinus and interfollicular spaces (Figure 4B), the site where re-circulating T cells enter from the afferent lymph (26). Thus, LTi cells are ideally placed to encounter re-circulating memory CD4 T cells. In addition, these locations are known to be rich in IL-7 expression (27), and the IL-7 signal is recognized as an essential part of CD4 memory survival (28). We have previously shown that in vitro, culture with IL-7 will increase OX40 expression on memory but not naive CD4 T cells (12). Culture with IL-7 also increased CD30L expression by LTi cells in vitro (10). Based on these data and our previous findings, we propose a simple model whereby memory CD4 T cells upregulate OX40 in response to IL-7 signals received as they re-enter lymphoid tissues, enabling them to engage OX40L expressed by LTi cells in the immediate vicinity. Therefore, LTi cells can function as regulators of memory CD4 T cells, distinct from OX40 and CD30 controlled effector function driven by antigen presenting cells (29). In summary, we show that a further function of LTi cells is the support of memory CD4 T cell survival. These data link the evolution of LNs and CD4 memory in placental mammals with LTi cell functions.
Figure 4. LTi cells reside at sites of memory cell recirculation.
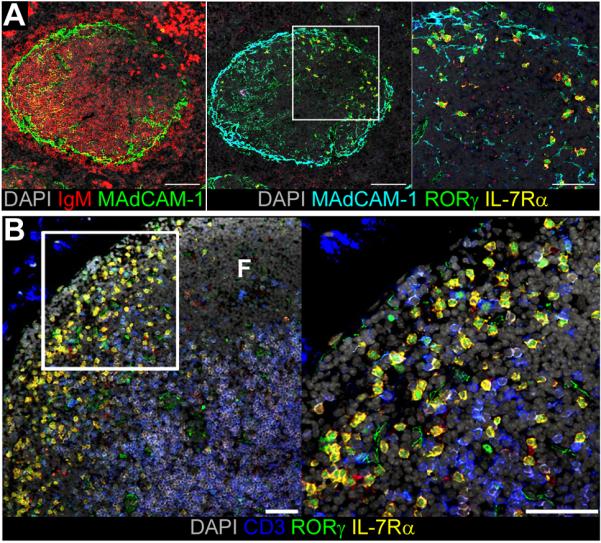
A, Serial sections of CD3ε spleen stained for expression of MAdCAM-1 (green) and IgM (red), or RORγ (green), MAdCAM-1 (turquoise), IL-7Rα (yellow), counterstained with DAPI (grey). Scale bar represents 100μm or 50μm in enlarged panel. Data representative of 6 mice analysed.
B, Sections of mesenteric LN from WT mice stained for expression of RORγ (green), IL-7Rα (yellow), CD3 (blue), counterstained with DAPI (grey). Scale bar represents 50μm, ‘F’ marks follicle.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Dan Littman for permission to use RORγ−/− mice. We are grateful to Dr. Marc Jenkins for provision of 2W1S:I-Ab tetramers and Antonio Pagan for technical advice. The following tetramer was obtained through the NIH Tetramer Facility: 2W1S:I-Ab.
This work was supported by a Programme Grant from the Wellcome Trust to P.L. and G.A.
Abbreviations
- BM
bone marrow
- Lm
Listeria monocytogenes
- LN
lymph node
- LTi
lymphoid tissue inducer
- ROR
retinoic acid orphan receptor.
REFERENCES
- 1.Kreiss A, Wells B, Woods GM. The humoral immune response of the Tasmanian devil (Sarcophilus harrisii) against horse red blood cells. Vet. Immunol. Immunopathol. 2009;130:135–137. doi: 10.1016/j.vetimm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Kreiss A, Obendorf DL, Hemsley S, Canfield PJ, Woods GM. A histological and immunohistochemical analysis of lymphoid tissues of the Tasmanian devil. Anat Rec (Hoboken) 2009;292:611–620. doi: 10.1002/ar.20896. [DOI] [PubMed] [Google Scholar]
- 3.Canfield PJ, Hemsley S. The roles of histology and immunohistology in the investigation of marsupial disease and normal lymphoid tissue. Dev. Comp. Immunol. 2000;24:455–471. doi: 10.1016/s0145-305x(00)00009-4. [DOI] [PubMed] [Google Scholar]
- 4.Diener E, Ealey EH. Immune system in a monotreme: studies on the Australian echidna (Tachyglossus aculeatus) Nature. 1965;208:950–953. doi: 10.1038/208950a0. [DOI] [PubMed] [Google Scholar]
- 5.Wronski EV, Woods GM, Munday BL. Antibody response to sheep red blood cells in platypus and echidna. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003;136:957–963. doi: 10.1016/s1095-6433(03)00325-8. [DOI] [PubMed] [Google Scholar]
- 6.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 7.Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat. Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 8.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Kim M-Y, Anderson G, Martensson I-L, Erlandsson L, Arlt W, White A, Lane PJL. OX40-ligand and CD30-ligand are expressed on adult but not neonatal CD4+CD3-inducer cells: evidence that IL7 signals regulate CD30-ligand but not OX40-ligand expression. J. Immunol. 2005;174:6686–6691. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Han S, Withers DR, Gaspal F, Bae J, Baik S, Shin HC, Kim KS, Bekiaris V, Anderson G, Lane P, Kim MY. CD117 CD3 CD56 OX40Lhigh cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur. J. Immunol. 2011;41:1563–1572. doi: 10.1002/eji.201040915. [DOI] [PubMed] [Google Scholar]
- 12.Gaspal FM, Kim MY, McConnell FM, Raykundalia C, Bekiaris V, Lane PJ. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J. Immunol. 2005;174:3891–3896. doi: 10.4049/jimmunol.174.7.3891. [DOI] [PubMed] [Google Scholar]
- 13.Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/s1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 14.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 15.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 16.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat. Immunol. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, Han S, Withers DR, Gaspal F, Bae J, Baik S, Shin HC, Kim KS, Bekiaris V, Anderson G, Lane P, Kim MY. CD117(+) CD3(−) CD56(−) OX40L(high) cells express IL-22 and display an LTi phenotype in human secondary lymphoid tissues. Eur. J. Immunol. 2011;41:1563–1572. doi: 10.1002/eji.201040915. [DOI] [PubMed] [Google Scholar]
- 18.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Biron C, She J, Higgins K, Sunshine MJ, Lacy E, Lonberg N, Terhorst C. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9402–9406. doi: 10.1073/pnas.91.20.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Withers DR, Gaspal FM, Bekiaris V, McConnell FM, Kim M, Anderson G, Lane PJ. OX40 and CD30 signals in CD4(+) T-cell effector and memory function: a distinct role for lymphoid tissue inducer cells in maintaining CD4(+) T-cell memory but not effector function. Immunol. Rev. 2011;244:134–148. doi: 10.1111/j.1600-065X.2011.01057.x. [DOI] [PubMed] [Google Scholar]
- 25.Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J. Immunol. 2008;181:3947–3954. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, Forster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 2011;12:879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- 27.Repass JF, Laurent MN, Carter C, Reizis B, Bedford MT, Cardenas K, Narang P, Coles M, Richie ER. IL7-hCD25 and IL7-Cre BAC transgenic mouse lines: new tools for analysis of IL-7 expressing cells. Genesis. 2009;47:281–287. doi: 10.1002/dvg.20497. [DOI] [PubMed] [Google Scholar]
- 28.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Gaspal F, Withers D, Saini M, Bekiaris V, McConnell FM, White A, Khan M, Yagita H, Walker LS, Anderson G, Lane PJ. Abrogation of CD30 and OX40 signals prevents autoimmune disease in FoxP3-deficient mice. J. Exp. Med. 2011;208:1579–1584. doi: 10.1084/jem.20101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.