Abstract
Background
Ketamine has rapid antidepressant effects lasting as long as 1 week in patients with major depressive disorder (MDD) and bipolar depression (BD). Ketamine is extensively metabolized. This study examined the relationship between ketamine metabolites and response, diagnosis, and psychotomimetic symptoms in MDD and BD patients.
Methods
Following a 40-minute ketamine infusion (.5 mg/kg), plasma samples were collected at 40, 80, 110, and 230 minutes and day 1 postinfusion in 67 patients currently experiencing a major depressive episode (MDD, n = 45; BD, n = 22). Concentrations of ketamine, norketamine (NK), dehydronorketamine (DHNK), six hydroxynorketamine metabolites (HNK), and hydroxyketamine (HK) were measured. Plasma concentrations were analyzed by diagnostic group and correlated with patients’ depressive, psychotic, and dissociative symptoms. The relationship between cytochrome P450 gene polymorphisms and metabolites, response, and diagnosis was also examined.
Results
Ketamine, NK, DHNK, four of six HNKs, and HK were present during the first 230 minutes postinfusion. Patients with BD had higher plasma concentrations of DHNK, (2S,6S;2R,6R)-HNK, (2S,6R;2R,6S)-HNK, and (2S,5S;2R,5R)-HNK than patients with MDD, who, in turn, had higher concentrations of (2S,6S;2R,6R)-HK. Higher (2S,5S;2R,5R)-HNK concentrations were associated with nonresponse to ketamine in BD patients. Dehydronorketamine, HNK4c, and HNK4f levels were significantly negatively correlated with psychotic and dissociative symptoms at 40 minutes. No relationship was found between cytochrome P450 genes and any of the parameters examined.
Conclusions
A diagnostic difference was observed in the metabolism and disposition of ketamine. Concentrations of (2S,5S;2R,5R)-HNK were related to nonresponse to ketamine in BD. Some hydroxylated metabolites of ketamine correlated with psychotic and dissociative symptoms.
Keywords: Bipolar disorder, dehydronorketamine, depression, hydroxynorketamine, norketamine, psychosis, response
In the treatment of major depressive disorder (MDD) and bipolar depression (BD), little progress has been made over the last several decades in developing new antidepressants whose mechanism of action is markedly different or whose efficacy is superior to those discovered several decades ago (1). In particular, the considerable lag of onset of antidepressant action has remained essentially unchanged since the first antidepressant was introduced. Current antidepressants largely modulate the serotonergic and noradrenergic systems and usually take several weeks to exert an appreciable response (2). A promising alternative target is the glutamatergic system (3). Notably, studies indicate that antidepressant effects can appear within hours of a single intravenous infusion of the N-methyl-D-aspartate antagonist ketamine (4 –7). Controlled studies from our laboratory observed that, for individuals with MDD, response rates to ketamine are initially robust and fade over the course of a week; response rates at 4 hours, 24 hours, and 72 hours were 56%, 71%, and 35%, respectively (8). Similar studies conducted in patients with BD found that response rates at 4 hours, 24 hours, and 72 hours were 61%, 41%, and 30%, respectively (9); a recent replication study in BD found comparable response rates (10). The most significant side effects associated with ketamine in these studies were its psychotomimetic and dissociative effects, which occurred only at the 40-minute postinfusion time point.
A key step in optimizing the clinical use of ketamine is determining the mechanism by which ketamine exerts its rapid antidepressant effects. Ongoing studies are investigating the molecular, cellular, neurochemical, brain circuit, and pharmacological mechanisms of this agent (11–14). These studies have primarily concentrated on ketamine and its initial N-demethylated metabolite, norketamine (NK). Horacek et al. (15) reported that in healthy volunteers, ketamine and NK blood plasma concentrations decreased prefrontal theta cordance and that this might be key to ketamine’s antidepressant effects. Lahti et al. (16) found no significant correlation between ketamine-induced symptom changes (i.e., psychotomimetic effects) and plasma ketamine concentrations in either normal control subjects or individuals with schizophrenia. Finally, in a controlled study of patients with treatment-resistant BD, no significant relationship was found between ketamine and NK plasma concentrations and antidepressant efficacy (9). In this and in earlier work from our laboratory (8), we noted that the relatively prolonged antidepressant effect associated with a single ketamine infusion (about 1 week) was remarkable given the short half-lives of ketamine and NK (2 hours [17] and 5 hours [18], respectively). These data raise the intriguing question of whether the sustained antidepressant effects observed after a single ketamine infusion might thus be due to active ketamine metabolites whose effects last beyond the timeframe of ketamine and NK.
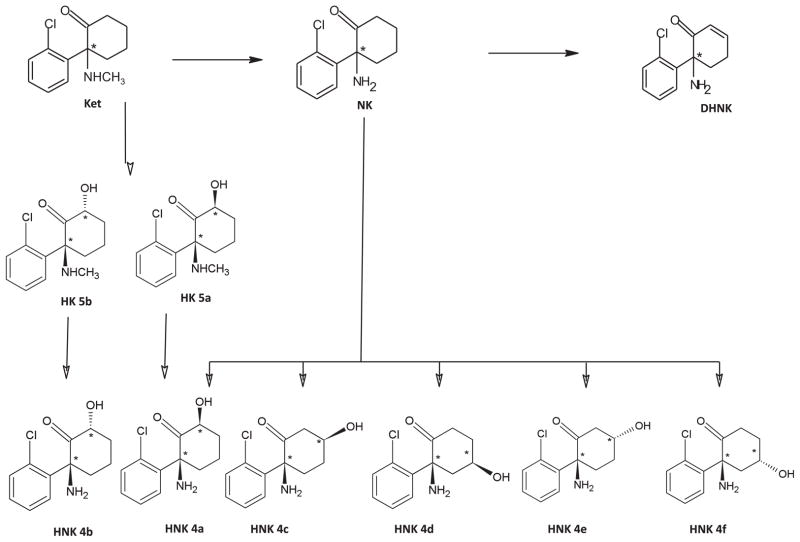
Key to understanding the mechanism of ketamine’s clinical effects is determining whether it does indeed have active metabolites and, if so, characterizing their pharmacologic activity. Similarly, genetic polymorphisms of cytochrome P450 (CYP) enzymes and anti-depressant metabolism have been increasingly studied as an important variable in clinical outcome (19); however, no information is presently available on CYP enzymes and antidepressant response to ketamine. Ketamine was approved by the US Food and Drug Administration for use as an anesthetic agent in the 1970s; since that time, very little systematic research has been conducted into the pharmacologic effects of ketamine metabolites on the central nervous system (i.e., therapeutic effects, adverse events, or neurobiological changes). However, the potential for metabolite-associated effects arises from the observation that ketamine and NK are extensively hydroxylated to a series of six hydroxynorketamine metabolites (HNK4a–4f), as well as two hydroxyketamine metabolites (HK5a,5b); furthermore, NK is also transformed into a dehydronorketamine (DHNK) metabolite (20 –22) (Figure 1; Table S1 in Supplement 1). Initial pharmacologic studies of NK and the (2S,6S;2R,6R)-HNK metabolite (HNK4a in Figure 1, Table S1 in Supplement 1) demonstrated that NK exerted both anesthetic (23) and antinociceptive effects (24,25), while HNK4a had no anesthetic effects in rats (23). No further characterization of the pharmacologic activity of these metabolites or any of the other downstream metabolites of ketamine and NK has been conducted.
Figure 1.
The proposed metabolic pathways of ketamine. DHNK, dehydronorketamine; Ket, ketamine; HK, hydroxyketamine; HNK, hydroxynorketamine; NK, norketamine.
In an initial population pharmacokinetic study of ketamine in BD patients, quantifiable concentrations of ketamine, NK, DHNK, HNK4a, and (2S,6R;2R,6S)-HNK (HNK4b, Table S1 in Supplement 1) were found to be present up to 3 days postketamine infusion (26). Building on these preliminary findings, we designed the present study to examine the relationship between plasma concentrations of ketamine, NK, DHNK, six HNK metabolites (HNK4a–4f), and HK5a,5b and antidepressant efficacy (response), diagnosis, and psychotomimetic effects in patients with treatment-resistant depression (TRD). In addition, we investigated the relationship of CYP gene polymorphisms on pharmacokinetic parameters, response, and diagnosis.
Methods and Materials
Participants
Sixty-seven patients with TRD (MDD = 45; BD = 22) currently experiencing a major depressive episode without psychotic features were enrolled in this study; diagnosis was confirmed by the Structured Clinical Interview for Axis I DSM-IV Disorders–Patient Version (27). The efficacy and side effects of ketamine in a subset of these patients were previously published (9,10,28,29). All subjects were studied as inpatients at the National Institute of Mental Health Clinical Research Center, Mood Disorders Research Unit in Bethesda, Maryland. Briefly, inclusion criteria were a Montgomery–Åsberg Depression Rating Scale (MADRS) (30) score of at least 20 or greater, a current or past history of not responding to specific treatments, and a current major depressive episode lasting at least 4 weeks. Patients with a DSM-IV diagnosis of drug or alcohol dependence or abuse within the past 3 months; serious, unstable illness; or uncorrected hypothyroidism or hyperthyroidism were excluded. Additional study details have been previously published (9,10,28,29).
Over the course of the study, MDD patients were required to be medication-free for at least 2 weeks (5 weeks for fluoxetine) before and during the infusion. Patients with BD were required to take either lithium or valproate within a specified range (serum lithium, .6 –1.2 mEq/L, or valproic acid, 50 –125 μg/mL) and no other psychotropic medications, also for 2 weeks before and during the infusion.
The study was approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health. All subjects provided written informed consent.
Ketamine Administration
Ketamine infusion was conducted as previously described (8). Briefly, patients received a single intravenous infusion of .5 mg/kg of ketamine hydrochloride over the course of 40 minutes. Symptoms were assessed at 40, 80, 110, and 230 minutes following ketamine infusion. Baseline and postketamine scores for depressive and psychotic symptoms were obtained using the MADRS and the Brief Psychiatric Rating Scale (BPRS) positive and total symptoms subscales (31), as well as the Clinician Administered Dissociative States Scale (CADSS) (32); the latter two scales measure psychotic and dissociative symptoms.
Response was considered a 50% or greater improvement from baseline on the MADRS. We did not assess treatment response later than 230 minutes because extensive analyses of previous data (8,9) showed that most patients (~90%) with TRD who will respond to ketamine do so by 230 minutes postinfusion. We also reasoned that if no relationship was present between metabolite concentration and response to ketamine or ketamine-induced psychotomimetic side effects at the 230-minute time point, it would be unlikely to be present at later time points when response rates, sides effects, and quantifiable concentrations of ketamine metabolites begin to diminish (8,9,26).
Bioanalytical Methods
Plasma samples were collected before the ketamine infusion; at 40 minutes (end of the infusion); at 80, 110, and 230 minutes postinfusion; and at day 1 postinfusion. These samples were collected via a separate intravenous line than the one used to administer ketamine. Samples were frozen at −80°C until analysis.
Plasma concentrations of ketamine, NK, DHNK, HNK4a– 4f, and HK5a,5b were determined using a previously described and validated liquid chromatography-mass spectrometry method (33). The chromatographic experiments were carried out on a Shimadzu Prominence high-performance liquid chromatography system (Shimadzu, Columbia, Maryland), and total analyte concentrations were determined using an Eclipse XDB-C18 guard column and a Varian Pursuit XRs 5 C18 analytical column (Varian, Inc., Palo Alto, California). The tandem mass spectrometry analysis was performed using a triple quadrupole mass spectrometer model API 4000 system from Applied Biosystems/MDS Sciex equipped with Turbo Ion Spray (Applied Biosystems, Foster City, California). Data were acquired and analyzed using Analyst version 1.4.2 (Applied Biosystems). Positive electrospray ionization data were acquired using multiple reaction monitoring, and quantification was accomplished using area ratios calculated using D4-ketamine as the internal standard, where the concentration of the internal standard was set at 50 ng/mL.
Genotyping the CYP Variants
A number of cytochrome P450 enzymes have been identified that contribute to the metabolism of ketamine, including CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP3A4, and CYP3A5 (Table S1 in Supplement 1). This study determined the genotypes of CYP2A6, CYP2B6, CYP2C19, and CYP3A5. These CYPs are highly polymorphic, and many alleles and allelic subvariants that affect enzymatic activity have been reported (Human Cytochrome P450 [CYP] Allele Nomenclature Committee; www.cypalleles.ki.se/) (34). The alleles probed in this study are listed in Table S2 in Supplement 1. Because most (93%) of our subjects were Caucasian, these alleles were chosen to cover a high percentage of the known reduced functional and nonfunctional alleles in the Caucasian population (Human Cytochrome P450 [CYP] Allele Nomenclature Committee and the Pharmacogenomics Knowledge Base [PharmGKB] - http://www.pharmgkb.org/) (35). Genotyping was performed for 11 CYPs and their corresponding alleles (except CYP2B6*4) using TaqMan Genotyping Assays (Applied Biosystems); the CYP2B6*4 allele was assayed by modifying a previously described polymerase chain reaction-restriction fragment length polymorphism protocol (36), as described in Table S2 in Supplement 1. As a quality control measure, 10% to 20% of randomly selected samples were re-genotyped for each of the CYPs. In this study, individuals carrying wild-type alleles and alleles that do not affect enzymatic activity were compared with individuals carrying one or more alleles that affect this activity. We specifically examined the influence of these CYPs on ketamine metabolite concentrations, response, and diagnosis.
Data Analysis
Linear mixed models with compound symmetry covariance structure and restricted maximum likelihood estimation were used in a full factorial model to examine the influence of response, diagnosis, and time on various metabolites. Time included all five time points observed from 40 minutes through day 1. Metabolite concentrations became very low or nonexistent after day 1, so they were not included in the analysis. The fixed intercept was included in the model, but random effects were not, given that they did not contribute to the model. Significance was evaluated at p < .05, two-tailed, but following Bonferroni correction for the seven outcomes (NK, DHNK, HNK4a, HNK4b, HNK4c, HNK4f, and HK5a), the cutoff was p < .007. Only values that remained significant after correction were reported, although p values are given before correction. Bonferroni-corrected simple effects tests were used to examine significant omnibus effects; p values for post hoc tests are following multiple comparison correction.
Secondary analyses using Pearson correlations examined the relationship between metabolite levels and psychotomimetic or dissociative side effects at 40 minutes postinfusion.
Results
Subject Characteristics
The subject population in this study included 67 subjects (33 female subjects, 34 male subjects; age range: 21 to 65 years [mean 46.1 ± 12.5 years]; Table 1).
Table 1.
Demographic and Treatment Characteristics of the Patient Sample
| MDD (n = 45) | BD (n = 22) | χ2, p | |
|---|---|---|---|
| n (%) | n (%) | ||
| Gender (Male) | 28 (62) | 6 (27) | 7.22, .007 |
| Response Rate to Ketamine at 230 Minutes | 17 (38) | 15 (68) | 5.48, .02 |
| Mean (SD) | Mean (SD) | t, p | |
|---|---|---|---|
| Age, Years | 46.8 (12.9) | 44.8 (11.7) | .62, .54 |
| Weight | 93.3 (23.5) | 91.5 (19.0) | .33, .75 |
| BMI | 30.2 (6.6) | 31.8 (5.9) | −.96, .34 |
| Clinical Scales | |||
| MADRS | 32.8 (4.7) | 31.7 (3.8) | .98, .33 |
| BPRS total | 36.1 (5.9) | 34.9 (4.6) | .88, .38 |
| BPRS positive | 9.7 (1.4) | 9.9 (1.5) | −.45, .65 |
| CADSS | 4.3 (7.5) | 2.1 (3.4) | 1.36, .18 |
| Percent change in MADRSa | −37.3 (32.1) | −46.5 (33.6) | 1.08, .28 |
BD, bipolar depression; BMI, body mass index; BPRS, Brief Psychiatric Rating Scale; CADSS, Clinician Administered Dissociative States Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; MDD, major depressive disorder; SD, standard deviation.
From baseline to end point.
Differences were observed between diagnostic groups in both the proportion of female subjects and the response rate at 230 minutes.
Plasma Concentrations of Ketamine and Metabolites
Patients with BD
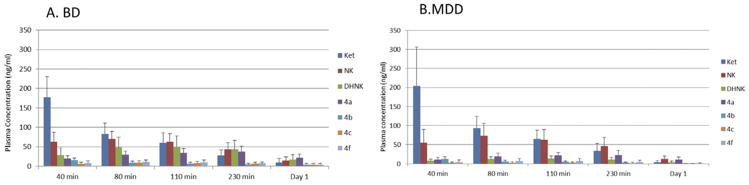
Concentrations of ketamine and its major metabolites were analyzed in the plasma samples obtained from BD patients using the liquid chromatography-tandem mass spectrometry assay; a representative chromatogram is presented in Figure S1 in Supplement 1, and the structure of these compounds is listed in Table S1 in Supplement 1. Significant concentrations of ketamine, NK, DHNK, HNK4a, and HNK4b were present during the first 230 minutes postinfusion, and quantifiable concentrations (≥4 ng/mL) were observed on day 1 in patients with BD (Figure 2A, Table S3 in Supplement 1). At 230 minutes, NK was the major metabolite in 9 of 22 BD patients (41%), DHNK was the major metabolite in another 9 of 22 patients (41%), and HNK4a was the major metabolite in the remaining 4 patients (18%). Measurable concentrations of HNK4c, HNK4d, and HNK4e were present in the 230-minute plasma sample of all the patients; of these, HNK4c was the most predominant. Detectable, but not quantifiable, concentrations (<4 ng/mL) of HK5a were observed in 9 of 22 (41%) patients. Hydroxynorketamine 4d, HNK4e, and HK5b were not detected in any of the samples.
Figure 2.
Plasma concentrations of ketamine and its metabolites over time in patients with treatment-resistant depression (bipolar depression [BD] patients [A] and major depressive disorder [MDD] patients [B]). See Table S1 in Supplement 1 for metabolite identification. DHNK, dehydronorketamine; Ket, ketamine; NK, norketamine.
Patients with MDD
A representative chromatogram of ketamine and its major metabolites in the plasma samples obtained from MDD patients is presented in Figure S2 in Supplement 1. Significant concentrations of ketamine, NK, DHNK, HNK4a, and HNK4b were present during the first 230 minutes postinfusion, and quantifiable concentrations (<4 ng/mL) were observed on day 1 (Figure 2B, Table S3 in Supplement 1). The metabolite pattern of 44 of the 45 MDD patients was determined at 230 minutes, as this sample was missing from 1 patient. At this time point, NK was the major metabolite in 37 of 44 patients (84%), and HNK4a was the major metabolite in the remaining 7 patients (16%). In the MDD patients, measurable concentrations of HNK4c, HNK4d, and HNK4e were present in the 230-minute plasma samples; of these, HNK4c was the most predominant. Measurable concentrations (≥4 ng/mL) of HK5a were observed in 41 of 44 patients (93%). Hydroxynorketamine 4d, HNK4e, and HK5b were not detected in any of the samples.
Response and Diagnosis
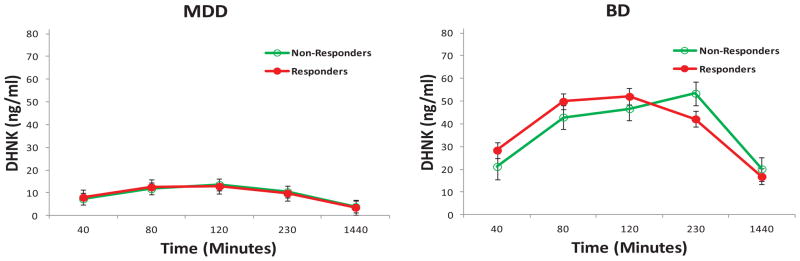
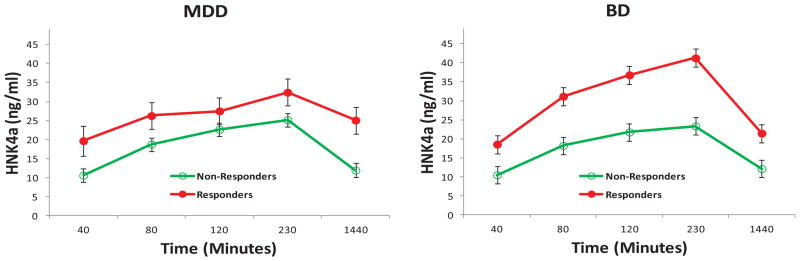
The linear mixed models showed no significant main effects or interactions of response and diagnosis for ketamine (response [R]: F = .15, df = 1,60, p = .70; diagnosis [D]: F = .49, df = 1,60, p = .49; R × D: F = .01, df = 1,60, p = .95), NK (R: F = .04, df = 1,62, p = .85; D: F = .15, df = 1,62, p = .70; R × D: F = .07, df = 1,62, p = .79), or HNK4f levels (R: F = .36, df = 1,62, p = .55; D: F = 4.08, df = 1,62, p = .048; R × D: F = .02, df = 1,62, p = .88). However, significant diagnosis— but not response— effects were present for DHNK (D: F = 27.37, df = 1,63, p < .001; R: F = .56, df = 1,63, p = .46; R × D: F = 1.14, df = 1,63, p = .29), HNK4a (D: F = 79.63, df = 1,64, p < .001; R: F = .02, df = 1,64, p = .88; R × D: F = .03, df = 1,64, p = .87), HNK4b (D: F = 16.07, df = 1,61, p < .001; R: F = .15, df = 1,61, p = .70; R × D: F = .40, df = 1,61, p = .53), and HK5a (D: F = 26.37, df = 1,63, p < .001; R: F = .26, df = 1,63, p = .62; R × D: F = .95, df = 1,63, p = .33). The BD group had higher DHNK (d = 1.32) (Figure 3), HNK4a (d = 2.23) (Figure 4), and HNK4b (d = 1.03) (Figure S3 in Supplement 1) levels than the MDD group, but the MDD group had higher HK5a levels (d = −1.29) (Figure 5).
Figure 3.
Dehydronorketamine (DHNK) concentrations by response and diagnosis in patients with treatment-resistant depression. Dehydronorketamine plasma concentrations were significantly higher in patients with bipolar depression (BD) than in patients with major depressive disorder (MDD).
Figure 4.
Hydroxynorketamine (HNK) 4a plasma concentrations by response and diagnosis in patients with treatment-resistant depression. Hydroxynorketamine 4a plasma concentrations were significantly higher in patients with bipolar depression (BD) than in patients with major depressive disorder (MDD).
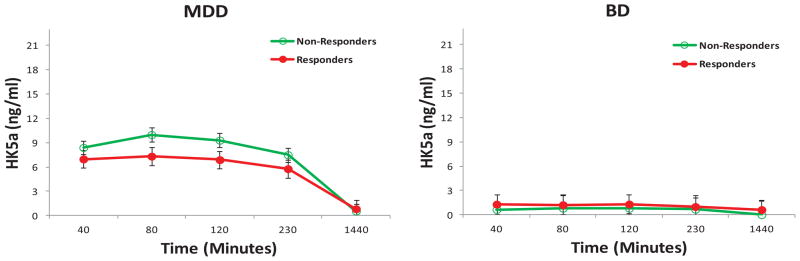
Figure 5.
Hydroxyketamine (HK) 5a plasma concentrations by response and diagnosis in patients with treatment-resistant depression. Hydroxyketamine 5a plasma concentrations were significantly higher in patients with major depressive disorder (MDD) than in patients with bipolar depression (BD).
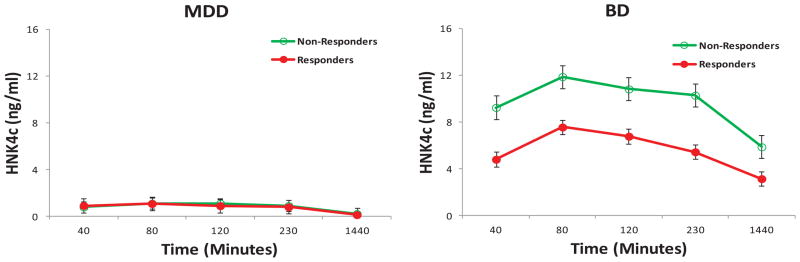
In addition, a significant diagnosis by response interaction was noted for HNK4c (D: F = 122.02, df = 1,62, p < .001; R: F = 11.31, df = 1,62, p = .001; R × D: F = 10.68, df = 1,62, p = .002). BD patients had higher levels of HNK4c than MDD patients in general (df = 2.81) (Figure 6). BD patients who did not respond to ketamine had higher levels of HNK4c than BD patients who did respond to ketamine (p < .001; d = 1.00), but no significant difference was observed between MDD responders and nonresponders (p = .93; d = .02).
Figure 6.
Hydroxynorketamine (HNK) 4c plasma concentrations by response and diagnosis in patients with treatment-resistant depression. HNK 4c plasma concentrations were significantly higher in patients with bipolar depression (BD) than in patients with major depressive disorder (MDD). HNK 4c plasma concentrations were also significantly higher in BD patients who did not respond to ketamine than in those who did respond to ketamine.
Additional linear mixed models were run to determine whether gender might play a role in diagnostic and response differences. Only variables with significant diagnosis or response effects were included. Female subjects had significantly higher DHNK (p = .006), HNK4a (p < .001), and HNK4c levels (p = .008) than male subjects. Male subjects, in turn, had significantly higher HK5a levels than female subjects (p = .006). No difference in HNK4b levels was noted (p = .99). The observed gender difference in the downstream NK metabolites is consistent with data from an earlier pharmacokinetic-pharmacodynamic study in which 10 men and 10 women received a 120-minute infusion of (S)-(+)- ketamine (.95 mg/kg); in that study, female subjects had a 20% higher plasma elimination clearance for (S)-(+)- ketamine and (S)-(+)-norketamine (37). However, the plasma concentrations of DHNK and HNK were not determined, nor were the clearance of (S)-(+)-ketamine to the corresponding HK5a levels.
For metabolites with significant gender effects, models were examined to identify any possible interaction between gender, response, and diagnosis. Given sample size limitations, only diagnosis and response could be included in a single model with gender. When diagnosis and gender were examined together, all the effects previously associated with diagnosis remained significant (DHNK: p < .001; HNK4a: p < .001; HNK4c: p < .001; HK5a: p < .001). When gender was included in the same model with response, the response effect was not significant (p = .46), but the gender effect was significant (p = .009).
Further analyses were performed with the BD group alone to explore the effects of mood stabilizers. Significant interactions were observed between mood stabilizer and time for NK (p = .02) and HK5a (p = .01). Norketamine levels were higher in BD patients receiving lithium, but this was observed only at the 120-minute time point. Hydroxyketamine 5a levels were higher in BD patients receiving valproate; this result was seen at the 40-minute, 80-minute, and 230-minute time points.
Primary Metabolite
The primary metabolite was identified for each patient. NK was the primary metabolite for 84% (37/44) of the MDD group and 41% (9/22) of the BD group (χ2 = 10.50, p = .001). Thus, the odds of having NK as the major metabolite were 6.14 times greater in the MDD group.
Psychotomimetic and Dissociative Side Effects
DHNK, HNK4c, and HNK4f levels were significantly correlated with BPRS total score, BPRS positive symptom subscale score, and/or CADSS score at 40 minutes (DHNK: BPRS total, r = −.27, p = .04; HNK4c: CADSS: r = −.29, p = .03; HNK4f: BPRS total: r = −.38, p = .003, BPRS positive: r = −.28, p = .03, CADSS: r = −.35, p = .006). For all of these correlations, higher levels of the metabolite were associated with fewer symptoms. Correlations with other metabolites were not significant.
Pharmacogenetic Analysis
We examined CYP genotypes in both the BD and MDD groups. The frequencies of the CYP polymorphisms were comparable with those observed in the Caucasian population. Genotypes were not associated with response: CYP2A6 (χ2 = .02, df = 1, p = .88); CYP2B6 (χ2 = .03, df = 1, p = .85); and CYP2C19 (χ2 = 1.84, df = 1, p = .18). The contribution of CYP genotype to diagnosis was also investigated; no difference between diagnostic groups (BD, MDD) was observed: CYP2A6 (χ2 = .18, df = 1, p = .67), CPYP2B6 (χ2 = 1.81, df = 1, p = .18), and CYP2C19 (χ2 = .93, df = 1, p = .33). Finally, we examined the influence of CYP genotype on metabolite levels. Patients with one or more polymorphisms that are consistent with poor or intermediate ability to metabolize CYP2A6 had significantly higher NK levels than patients with the wild-type allele or those whose ability to metabolize CYP2A6 was extensive (F = 4.92, df = 1,49, p = .03); however, this difference was not significant following correction for multiple comparisons. No other comparisons were significant for any metabolite, either before or after correction.
Discussion
To our knowledge, this study is the first to examine the relationship between plasma concentrations of downstream ketamine metabolites and antidepressant efficacy, diagnosis, and psychotic and dissociative symptoms in patients with TRD.
One of the most interesting findings was that levels of several ketamine metabolites—specifically, DHNK, HNK4a, and HNK4c—were consistently higher in patients with BD than in patients with MDD. Only plasma ketamine levels at the 40-minute time point and HK5a concentrations were found to be higher in patients with MDD than those with BD. This suggests that the N-demethylation of ketamine to NK is reduced in patients with MDD relative to BD, while the ring hydroxylation of ketamine to form HK5a is increased in MDD patients relative to those with BD. This is consistent with the observation that the DHNK and HNK metabolites are downstream metabolites of NK (Figure 1). The reason for this difference is unclear. The phenomenon was not related to difference in sex or CYP genotype and did not appear to be due to drug-drug metabolic interactions, although valproic acid is known to inhibit the activity of CYP2C9 and weakly inhibit the activity of CYP2C19 and CYP3A4 (38). One potential reason may be underlying pathophysiological effects on the phenotypic expression of some or all of the CYPs examined in this study. A number of diseases—including diabetes, obesity, inflammation, infection, and liver diseases—affect CYP expression and activity, thereby altering drug metabolism and disposition (39); genotype/phenotype comparisons have also been suggested as possible probes for the effect of disease progression on drug metabolism (40). In particular, inflammation can alter the liver’s ability to metabolize drugs, an effect thought to be predominantly mediated by cytokine production (41,42). The results might also be attributable to differences in past medication history between the diagnostic groups. Thus, future studies of the metabolism and disposition of ketamine in BD and MDD patients should include metabolic genotype-probe drug phenotype studies and assess the impact of past treatment (40).
When plasma concentrations of ketamine, NK, and the downstream ketamine metabolites were studied in conjunction with antidepressant efficacy, diagnosis, and psychotic and dissociative symptoms, higher concentrations of HNK4c were noted in BD patients who did not respond to ketamine than BD patients who did respond to ketamine. This suggests that HNK4c is a pharmacologically active metabolite that may have relevant research and clinical implications. The reason for this difference was not readily discernable, although it is possible that the concomitant use of a mood stabilizer (i.e., lithium or valproate) increased HNK4c plasma concentrations. To fully investigate this possibility, it would be important to study the effects of ketamine and its metabolites in drug-free BD patients. Nevertheless, the results suggest that patients with BD who do not respond to ketamine may be overdosed by having excessively high plasma concentrations of HNK4c. This suggests that we may need to identify new dosing strategies or alternative drug formulations to optimize metabolite plasma concentrations and increase the probability of efficacy.
Finally, an inverse relationship was noted between ketamine metabolites and psychotomimetic or dissociative side effects; higher DHNK, HNK4c, and HNK4f levels were associated with lower BPRS and CADSS scores.
This study had several strengths. Notably, subjects were well characterized and hospitalized for several weeks before and after the infusion, thus reducing the number of variables that could affect drug plasma concentrations (e.g., noncompliance, use of prohibited medications or substances, etc). In addition, blood samples were drawn at the corresponding time points of efficacy and side effect measures. Nevertheless, several limitations also exist. Specifically, BD patients were taking a concomitant mood stabilizer medication and received ketamine in a crossover design, while patients with MDD received ketamine openly. However, we did not think this was likely to affect study results, as there were no differences in demographic and treatment characteristics between the groups. In addition, the manner in which ketamine was administered and the pattern of antidepressant response and side effect profile were similar between patients with BD and MDD.
Taken together, the present results support the hypothesis that patients with BD and MDD may metabolize ketamine differently. In particular, patients with BD appear to be prone to higher concentrations of DHNK, HNK4a, HNK4b, and HNK4c than patients with MDD. In addition, one of these metabolites—HNK4c—was associated with nonresponse to ketamine. In patients with MDD, HK5a levels were found to be consistently higher than in patients with BD. Finally, it is worth mentioning that the durable antidepressant response—lasting up to 1 week or more— observed in some patients in response to ketamine may be due to active metabolites whose effects last beyond the half-life of ketamine and NK. Indeed, in a previous study, we found that quantifiable concentrations of other ketamine metabolites were present up to 3 days postketamine infusion (26). Unfortunately, no data on ketamine metabolite concentrations were collected beyond this time point. Further studies should continue to explore the influence of ketamine metabolites on efficacy, side effects, and neurobiological changes in TRD patients, as well as assess whether the selective delivery of some metabolites to patients could be more advantageous— both in terms of efficacy and adverse event profile—than the primary drug ketamine.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of the National Institute of Aging, National Institutes of Health (NIH), and the National Institute of Mental Health, NIH, as well as NIH Grant MH085098-01 to GL, and the Brain & Behavior Research Foundation Bipolar Disorders Award (CAZ).
Footnotes
Ioline Henter (National Institute of Mental Health) provided invaluable editorial assistance.
Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. CAZ, IWW, and RM have submitted a patent for the use of ketamine metabolites in the treatment of bipolar disorder and major depression. NB, GL, DAL, SLVV, and AR report no biomedical financial interests or other potential conflicts of interest.
ClinicalTrials.gov: Rapid Antidepressant Effects of Ketamine in Major Depression; http://clinicaltrials.gov/ct2/show/NCT00088699; NCT00088699.
Supplementary material cited in this article is available online.
References
- 1.Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: Raising the bar for mental health research. Mol Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: A new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69:946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA., Jr Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–150. doi: 10.1016/j.pharmthera.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 5.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 6.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856– 864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 9.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793– 802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: A randomized controlled add-on trial [published online ahead of print January 30] Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver FW, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: A neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horacek J, Brunovsky M, Novak T, Tislerova B, Palenicek T, Bubenikova-Valesova V, et al. Subanesthetic dose of ketamine decreases prefrontal theta cordance in healthy volunteers: Implications for antidepressant effect. Psychol Med. 2010;40:1443–1451. doi: 10.1017/S0033291709991619. [DOI] [PubMed] [Google Scholar]
- 16.Lahti AC, Warfel D, Michaelidis T, Weiler MA, Frey K, Tamminga CA. Long-term outcome of patients who receive ketamine during research. Biol Psychiatry. 2001;49:869– 875. doi: 10.1016/s0006-3223(00)01037-4. [DOI] [PubMed] [Google Scholar]
- 17.White PF, Schuttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br J Anaesth. 1985;57:197–203. doi: 10.1093/bja/57.2.197. [DOI] [PubMed] [Google Scholar]
- 18.Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 19.Porcelli S, Fabbri C, Spina E, Serretti A, De Ronchi D. Genetic polymorphisms of cytochrome P450 enzymes and antidepressant metabolism. Expert Opin Drug Metab Toxicol. 2011;7:1101–1115. doi: 10.1517/17425255.2011.597740. [DOI] [PubMed] [Google Scholar]
- 20.Hijazi Y, Boulieu R. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2002;30:853– 858. doi: 10.1124/dmd.30.7.853. [DOI] [PubMed] [Google Scholar]
- 21.Yanagihara Y, Kariya S, Ohtani M, Uchino K, Aoyama T, Yamamura Y, Iga T. Involvement of CYP2B6 in n-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2001;29:887– 890. [PubMed] [Google Scholar]
- 22.Portmann S, Kwan HY, Theurillat R, Schmitz A, Mevissen M, Thormann W. Enantioselective capillary electrophoresis for identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J Chromatogr A. 2010;1217:7942–7948. doi: 10.1016/j.chroma.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem. 1986;29:2396–2399. doi: 10.1021/jm00161a043. [DOI] [PubMed] [Google Scholar]
- 24.Herd DW, Anderson BJ, Holford NH. Modeling the norketamine metabolite in children and the implications for analgesia. Paediatr Anaesth. 2007;17:831– 840. doi: 10.1111/j.1460-9592.2007.02257.x. [DOI] [PubMed] [Google Scholar]
- 25.Holtman JR, Jr, Crooks PA, Johnson-Hardy JK, Hojomat M, Kleven M, Wala EP. Effects of norketamine enantiomers in rodent models of persistent pain. Pharmacol Biochem Behav. 2008;90:676– 685. doi: 10.1016/j.pbb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Swarajya LV, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, et al. Simultaneous population pharmacokinetic modeling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression [published online ahead of print February 1] Br J Clin Pharmacol. 2012 doi: 10.1111/j.1365-2125.2012.04198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV-TR Axis I Disorders-Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- 28.Ibrahim L, DiazGranados N, Franco-Chaves J, Brutsche NE, Henter ID, Kronstein P, et al. Course of improvement in depressive symtpoms to a single intravenous infusion of ketamine vs add-on riluzole: Results from a four-week, double-blind, placebo-controlled study [published online ahead of print February 1] Neuropsychopharmacology. 2012 doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 31.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:790– 812. [Google Scholar]
- 32.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 33.Moaddel R, Venkata SL, Tanga MJ, Bupp JE, Green CE, Iyer L, et al. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82:1892–1904. doi: 10.1016/j.talanta.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim SC, Ingelman-Sundberg M. The human cytochrome P450 Allele Nomenclature Committee Web site: submission criteria, procedures, and objectives. Methods Mol Biol. 2006;320:183–191. doi: 10.1385/1-59259-998-2:183. [DOI] [PubMed] [Google Scholar]
- 35.Thorn CF, Klein TE, Altman RB. PharmGKB: the pharmacogenetics and pharmacogenomics knowledge base. Methods Mol Biol. 2005;311:179–191. doi: 10.1385/1-59259-957-5:179. [DOI] [PubMed] [Google Scholar]
- 36.Jacob RM, Johnstone EC, Neville MJ, Walton RT. Identification of CYP2B6 sequence variants by use of multiplex PCR with allele-specific genotyping. Clin Chem. 2004;50:1372–1377. doi: 10.1373/clinchem.2004.031708. [DOI] [PubMed] [Google Scholar]
- 37.Sigtermans M, Dahan A, Mooren R, Bauer M, Kest B, Sarton E, Olofsen E. S(+)-ketamine effect on experimental pain and cardiac output: A population pharmacokinetic-pharmacodynamic modeling study in healthy volunteers. Anesthesiology. 2009;111:892–903. doi: 10.1097/ALN.0b013e3181b437b1. [DOI] [PubMed] [Google Scholar]
- 38.Wen X, Wang JS, Kivisto KT, Neuvonen PJ, Backman JT. In vitro evaluation of valproic acid as an inhibitor of human cytochrome P450 isoforms: Preferential inhibition of cytochrome P450 2C9 (CYP2C9) Br J Clin Pharmacol. 2001;52:547–553. doi: 10.1046/j.0306-5251.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng PY, Morgan ET. Hepatic cytochrome P450 regulation in disease states. Curr Drug Metab. 2001;2:165–183. doi: 10.2174/1389200013338676. [DOI] [PubMed] [Google Scholar]
- 40.Williams ML, Wainer IW. Genotype/phenotype comparisons: A probe for the effect of disease progression on drug metabolism. Curr Opin Drug Discov Devel. 2002;5:144–149. [PubMed] [Google Scholar]
- 41.Shedlofsky SI, Israel BC, McClain CJ, Hill DB, Blouin RA. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J Clin Invest. 1994;94:2209–2214. doi: 10.1172/JCI117582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renton KW. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin Drug Metab Toxicol. 2005;1:629–640. doi: 10.1517/17425255.1.4.629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.