Abstract
Metabolic Syndrome (MetS), a clustering of risk factors for type 2 diabetes mellitus and cardiovascular disease, has been associated with cognitive dysfunction and brain abnormalities. This review describes the literature on the impact of MetS on brain and cognition and suggests directions for future research.
A literature search for reports of MetS and cognition and brain imaging was conducted for both non-elderly adults and adolescents. No studies were found describing MetS and brain or cognition among adolescents; therefore we also included studies investigating individual components of MetS in this age group. Most studies found associations between MetS and cognitive dysfunction. Multiple cognitive domains were affected by MetS in adults. In adolescents, the majority of findings were in executive functioning. Brain imaging literature in adults implicated MetS in ischemic stroke, white matter alterations and altered brain metabolism. For adolescents, individual MetS factors were linked to volume losses in the hippocampus and frontal lobes.
MetS negatively impacts cognitive performance and brain structure. Potential explanatory models include impaired vascular reactivity, neuroinflammation, oxidative stress, and abnormal brain lipid metabolism. We posit that insulin resistance-associated impairment in cerebrovascular reactivity is an important mechanism underlying brain deficits seen in MetS.
Keywords: Metabolic Syndrome, Cognitive Performance, Adults, Adolescents, Brain Imaging
Introduction
The Metabolic Syndrome (MetS) has been called a global epidemic by the WHO1 and is considered a major public health problem,2 with 34% of Americans over the age of 20 estimated to be affected.3–4 Among adolescents, 9.4% are estimated to have MetS and the prevalence rises to 44.2% among those that are obese.5 Therefore, the MetS is one of the few clinical syndromes that affects a large portion of the general population that is potentially reversible by established interventions.6
MetS is known to affect cognition and raise the risk for dementia.7–8 Interest in understanding the pathophysiologic mechanisms underlying MetS and its impact on brain function, will inform possible interventions. Positive cognitive changes have been seen with some interventions targeting MetS components.9–10
MetS, first described as Syndrome X was proposed by Reaven in 1988 11 in an attempt to provide a unifying pathophysiologic explanation for the tendency of impaired fasting glucose, dyslipidemia, and hypertension to cluster in some individuals, who were at increased risk for CVD and T2DM 12. Because insulin resistance (IR) is thought to be the key underlying condition in Syndrome X, others then coined the term ‘The Insulin Resistance Syndrome’.13 This focus on the associations between IR and other cardiovascular risk factors led to the creation of clinical MetS definitions by the World Heart Federation (WHO)14, the International Diabetes Federation (IDF)15, and the National Cholesterol Education Program Third Adult Treatment Panel (NCEP ATP III)16, in an attempt to identify patients at increased risk for CVD and T2DM.
The most commonly used definition for MetS in the U.S. is the one described by the NCEP ATP III, which is the presence of three or more of the following criteria: (1) abdominal obesity: waist >102 cm (>40 in) for men or >88 cm (>35 in) for women; (2) triglycerides ≥150 mg/dL; (3) high-density lipoprotein <40 mg/dL for men or <50 mg/dL for women; (4) blood pressure ≥130/≥85 mmHg or current use of anti-hypertensive medications; and (5) fasting glucose level ≥110 mg/dL. The International Diabetes Federation uses a slightly modified definition where one of the three criteria must be abdominal obesity in addition to two of the other four criteria, and the abnormal threshold for fasting glucose is set at ≥ 100 mg/dL) (or previously diagnosed type 2 diabetes).
Goals of the review
Three reviews have been published recently regarding MetS and cognitive decline in older adults with a focus on individuals at high risk for dementia or with dementia.17–19 This review concentrates on the impact of MetS on cognitive functioning and brain integrity in functionally normal non-elderly adults and adolescents. Although our focus is predominantly in brain associations to MetS proper, our review for young populations also highlights associations of the individual MetS factors with cognition and brain due to the paucity of research for this population. We focus particular attention to insulin resistance, as in our opinion, it is central to the impact of the syndrome on brain. At the end of the review we provide a brief overview of one potential explanatory model for the impact of MetS on brain.
Literature Selection
Cognition
Only articles that examined cognitive functioning as an outcome associated with a diagnosis of MetS were selected for the adult review. Use of multiple neuropsychological tests for at least one cognitive domain was required. Reports that relied on self-report or that utilized only global/screening measures of functioning such as the Mini-Mental State Examination were excluded. Electronic databases were searched utilizing terms ‘“Metabolic Syndrome” paired “cognition”, “cognitive function”, “cognitive performance”, or “neuropsychological function.”
For the children and adolescent search, inclusion criteria and search terms mirrored that for the adult studies with the exception that papers addressing Prader-Willi Syndrome and/or focusing on children younger than 10 years of age were excluded. An expanded search was conducted including terms such as “obesity”, “overweight”, “body mass index”, “hypertension”, “lipids”, “HDL”, “triglycerides”, “blood pressure”, “insulin resistance”, and “hyperglycemia”. A total of 20 studies were included, 10 for adults and 10 for adolescents.
Brain Imaging
For the brain imaging literature, MEDLINE searches were performed for keywords and terms such as “metabolic syndrome”, “brain”, “cerebral”, “infarct”, “lesion”, “MRI”, “diffusion tensor imaging”, and “spectroscopy”. The literature reporting the brain involvement in MetS in adults was quite limited and we found no publications among children or adolescents describing the associations between MetS and brain. Therefore, among children or adolescents we expanded our search to also include the terms, “insulin resistance”, “pre-diabetes”, and “type 2 diabetes mellitus” (the extreme of the MetS spectrum).
Neuropsychological Assessment of Cognition
Neuropsychological tests assess functioning in cognitive domains such as intelligence, memory and learning, language, executive functioning, processing speed and sensory-perceptual abilities. However, the literature offers little consistency in individual tests used to measure particular cognitive domains.
Impact of MetS on Cognition in Adults
A summary of the studies included in this review can be found in Table 1. Most studies report that MetS and its components have a negative impact on cognition (i.e.,20–26). However, findings may vary by sex, with men being more affected in some reports23, 27; women in others26, and some reporting no sex differences.24
Table 1.
Ten studies of the association between cognition and Metabolic Syndrome in non-elderly adults (mean age <65 years)
| Reference | Clinical population (MetS) |
Control Group (No MetS) |
Cognitive tests | Covariates/Exclusions | Results |
|---|---|---|---|---|---|
| Bokura, et.al. (2010)* | 186 Japanese mean age 61.2 | 1357 mean age 62.2 | Koh's Test, FAB, (sig) Okabe's Test (non-sig) | Age, gender, education, smoking, alcohol use, subclinical ischemic brain lesions Exclusions: Neurological & Psychiatric Diseases |
MetS is associated with impaired executive function independent of silent brain lesions |
| Cavalieri, et.al. (2010) * | 232 mean age 65.1 | 587 (W: 149 ± 25.3) mean age 64.8 | BLG, WCST, TMT-B, DSB (sig), PPT (not Sig) | Model 1: Age, education, gender, depressive mood, coronary heart disease, physical activity Model 2: Model 1 plus WML volume, presence of lacunes, silent infarcts, brain parenchymal fraction Exclusions: None |
MetS is related to memory and executive function in men but not women; further compromise with high hs-CRP and increasing MetS components |
| Gatto, et.al. (2008) † | 112 mean age 61.8 | 741 (BMI: 26.7 ± 4.7) mean age 60.8 | SDMT, TMT-B, JLO, Block Design, Letter-Number Sequencing, Category Fluency, BNT, Shipley, CVLT-II, Logical Memory I and II, Faces I and II. (None significant) | Age, gender, race, education, income, smoking, CVD risk factors, statins, anti-hypertensives, depression Exclusions: CVD; diabetes; uncontrolled lipid abnormalities, hypertension, other endocrine or significant kidney disease; alcohol/substance abuse; hormone therapy |
Correlation between hypertension and lower cognition; Significant cognitive impairment with increasing MetS factors |
| Haley, et.al. (2010)+ | 13 mean age 47.6 | 25 (BMI:26.8 ± 4.8) mean age 51.3 | MMSE, WASI-IQ, Animal Fluency, CVLT-II, RCF, DSS, COWAT, TMT, GPT, BDI (none were significant) | Age, gender, education, depression Exclusions: Neurological disease, major psychiatric illness, substance abuse, MRI contraindications, age: <40, >60 |
No significant cognitive differences |
| Hassenstab, et.al. (2010)* | 73 mean age 60.4 | 70 (BMI: 25.0 ±3.4) mean age 60.1 | Shipley, Phonemic & Category Fluency, WMS-R & WAIS-R (selected subtests), CVLT, Stroop, Category Fluency (mixed findings) | Age, gender, education, T2DM Exclusions: Significant psychiatric, neurological or other medical diseases; T2DM; < 12 years education |
Significant reductions in recall, lower overall IQ; increasing MetS factors associated with lower performance |
| Komulainen, et.al. (2007)* | 13 Women mean age 63.6 | 88 (BMI 26.9 ±3.9) mean age 63.8 | WRT (sig), Stroop, LDST, MMSE (non sig) | Age, education, depression, hormone
replacement therapy, BMI, prevalent cardiovascular disease Exclusions: None |
MetS at baseline = greater risk of memory impairment at follow-up; memory declines with increasing MetS factors |
| Muller, et.al. (2009)* | 295 mean age 59 | 528 (BMI: 26 ± 3) mean age 58 | 15WLT, RCF, VET, BSAT, Letter Fluency, DART (all sig) | Model 1: Age, sex, education, intellectual functioning, smoking, alcohol use Model 2: Model 1 plus extent of vascular disease, atherosclerosis, inflammation Exclusions: None |
MetS is related to memory and visuospatial dysfunction but not executive dysfunction |
| Schuur, et.al. (2010)* | 434 mean age 61.4 | 1464 mean age 46.2 | Stroop (sig), DART, AVLT, TMT, verbal fluency, WAIS-III block design (non sig) | Age, gender, smoking, alcohol use, education, depression, APOE Exclusions: Dementia or inability to perform a neuropsychological tests |
MetS and high HOMA-IR associated with executive dysfunction in women but not men |
| Segura, et.al. (2010)* | 19 Spanish mean age 61.26 | SDMT associated with FA (sig); Vocabulary (WAIS-III); GPT, CPT-III | Age, education, IQ, gender Exclusions: Hx of psychiatric or neurological disease; <8 years of education, left handed; For controls: any MetS-vascular risk factor |
No significant cognitive differences between groups | |
| Tournoy, et.al. (2010)* | 1007 European men mean age 61.0 | 2145 (BMI: 26.3 ± 3.3) mean age 59.3 | RCF, CTRM, DSST (sig when applied to individual MetS components) | Age, age leaving education, smoking, alcohol consumption, physical activity, depression, hs-CRP, and center location Exclusions: None |
MetS not associated with cognitive impairment; Diabetes linked to poorer memory, executive functions and processing speed |
used NCEP-APT III MetS criteria
used IDF MetS criteria
used a modified NCEP-APT III MetS criteria
Multiple cognitive domains are affected, even after controlling for medical factors such as CVD and T2DM 28, silent brain lesions22, education and socioeconomic status22–23, depressive mood, coronary heart disease, and magnetic resonance imaging (MRI) findings.23 MetS has been linked to deficits in memory, visuospatial abilities, executive functioning, processing speed, and overall intellectual functioning.22–26, 28–29
A few studies report no significant associations between MetS and cognition.30–31 Lack of significant findings could be due to the low sensitivity of the test battery chosen as well as the health status of the “control” group, which often includes subjects with one or two risk factors for MetS. For example, Gatto et al., (2008), showed no group differences; however, regression analysis of their whole population using the actual number of MetS criteria met (0 to 5) showed significant reductions in cognitive performance with each additional MetS criterion met.32
Impact of MetS on cognition in Children and Adolescents
There is currently no literature on MetS and cognition in children and adolescents, but there is some on individual MetS components. In 2011, Smith et al33 published a review exploring the links between obesity and cognition across the lifespan. In children, the majority of findings on cognition in obesity have been predominantly in executive functioning,34–37 a cognitive domain known to depend on an intact frontal lobe. Frontal lobes are still developing during adolescence,38 which may render this brain region more vulnerable to metabolic dysregulation. Impaired executive function may also play a role in the development of obesity, particularly if it leads to impaired response inhibition and overeating.39 Reductions in attention and global functioning or IQ have also been reported in childhood obesity.34, 37, 40–41 Impairments in attention can contribute to poor performance in other cognitive domains and may help explain the deficits reported in executive functioning and IQ.
Lande and colleagues (2003)42 found that 50% of the children with elevated blood pressure were overweight or obese and that those with systolic blood pressure > 90th percentile for age, sex and height scored significantly worse on attention/concentration, visual-spatial, and math tasks.
Impaired fasting glucose (IFG), an important MetS component, is often a precursor for T2DM, which has been strongly linked to cognitive dysfunction in adults.43 Our lab reported that obese adolescents with T2DM perform consistently worse than well-matched, also obese, peers on global functioning, executive function, memory and attention.44 Given that it is rare to find a young individual with T2DM, who does not also fulfill criteria for MetS, it is likely that similar findings will be present among obese adolescents with MetS.
There is only one report failing to find statistically significant cognitive impairments associated with obesity and excess weight.45 However, in this report the heaviest group of children consistently scored lower on all but one of the cognitive measures assessed.
Impact of MetS on Brain in Adults - Imaging
Individual MetS components are known to have independent negative brain consequences,46–49 but evidence of brain involvement in MetS as a whole remains rather limited. MetS is a known risk factor for ischemic stroke.50 There have been a handful of reports of subclinical ischemic brain damage in adults with MetS. Increased silent brain infarction has been observed in both elderly51 and middle-aged individuals with MetS.22, 52 Others have reported increased prevalence of intracranial arteriosclerosis,53 periventricular white matter hyperintensivities (PWMH), and subcortical white matter (WM) lesions.54 Using diffusion tensor imaging (DTI), Segura et al. (2009) characterized reductions of WM micro-structural integrity involving primarily the frontal and temporal lobes.55 More WM abnormalities have been associated with increasing number of MetS components present51, 54 and these associations may also be driven by individual vascular risk factors.51–52, 54
Haley et al. (2010) demonstrated changes in brain metabolism characterized by increased myoinositol/creatine and glutamate/creatine ratios in occipito-parietal gray matter in cognitively intact middle-aged adults with MetS.56 Increased myoinositol/creatine ratios, suggestive of increased microglia or neuroinflammation, have been reported in T2DM.57 Using functional MRI, Hoth et al. (2011) observed blunted brain activation in the absence of cognitive compromise.58 Taken together, these subclinical alterations in cerebral metabolism and cerebrovascular reactivity may represent early brain compromise associated with peripheral metabolic disturbances.
Impact of MetS on Brain in Children and in Adolescents - Imaging
No data currently exists on the impact of MetS on the pediatric brain. Most individuals with MetS have IR,59 which is likely the driving force behind the brain complications reported in MetS.60–61 Bruehl et al. (2011) reported that relative to those without IR, obese adolescents with T2DM had smaller hippocampal volumes and more frontal lobe atrophy.62 In addition, we have described among adolescents with IR, a blunted cortisol awakening response (CAR), a good indicator of the hypothalamic-pituitary-adrenal (HPA) axis integrity.63 More importantly, the finding of an inverse relationship between CAR and fasting insulin levels as well as between CAR and hippocampal volumes supports the role of metabolic disturbances in brain structural abnormalities, which lead to the HPA axis dysregulation.64 Further, we have described specific gray matter volume reductions in the orbitofrontal cortex, associated with disinhibition of feeding behavior among obese adolescents (with and without IR).37
Discussion
There is a lack of consensus on the relationships between MetS and its components and cognitive health, which is partly explained by a lack of consistency in the cognitive domains selected for assessment, differences in quality of tests selected, demographics of populations studied (i.e., differences in age, race, sex, and educational level), lack of a standard definition of MetS, cross-sectional versus longitudinal study designs, and difficulty in uncoupling the impact of individual or combinations of MetS factors from that of the syndrome itself. Cognitive and brain abnormalities associated with MetS may result from synergy of the different component risk factors. In addition, few studies utilized a control group free of any MetS risk factor. Furthermore, sex differences have not been extensively addressed and findings to date have been inconsistent. MetS has been associated with poorer executive performance in women, but not in men.26 Moreover, impairments have been found in varying cognitive domains across the lifespan with memory preserved until the 6th decade when impairments are found25 indicating that MetS may be an important contributory factor in worsening memory for women. Although some of the studies that we cited exclude individuals with major psychiatric or neurological illness, studies either do not specify other medications or if they do, they do not account for them. Given that we excluded studies with subjects with a mean age over 65 years, it is less likely that the reported studies will have the confounding effects of the poly pharmacy that often occur in the elderly. However, we have no way of identifying whether some cognitive findings reported in the literature are due to pharmacological side effects.
Potential Explanatory Model for Brain Deficits Associated with MetS
A number of potential explanatory models have been proposed for the ill effect of MetS on brain and cognition including neuroinflammation, oxidative stress, abnormal brain lipid metabolism, and impaired vascular reactivity among others. Since a discussion of all of these models would be beyond the scope of this brief review, we will utilize an explanatory model based on IR-associated vascular reactivity problems as an example.
Impaired cerebrovascular reactivity65, increased carotid stiffness, and intima-media thickness (IMT)66–67 have been reported in adults with MetS. Given that the carotid artery is the main blood supply to the central nervous system and that carotid atherosclerosis has been linked to cognitive impairment68 and increased brain atrophy69, such findings suggest that the WM damage seen in adults with MetS are likely vascular in nature.
Similarly, endothelial dysfunction, carotid stiffness, and intima-media thickness also have been reported in children with MetS,70–72 obesity,73–76 hypertension,77–79 and T2DM.80–81 Those with uncontrolled T2DM have more severe carotid alterations.80 Vascular involvement likely plays a role in cognitive and brain impairment in adults.82–83 Given the increasing vascular abnormalities with increasing metabolic alterations along the MetS spectrum in children, MetS also likely adversely impacts brain structure and function in adolescents.
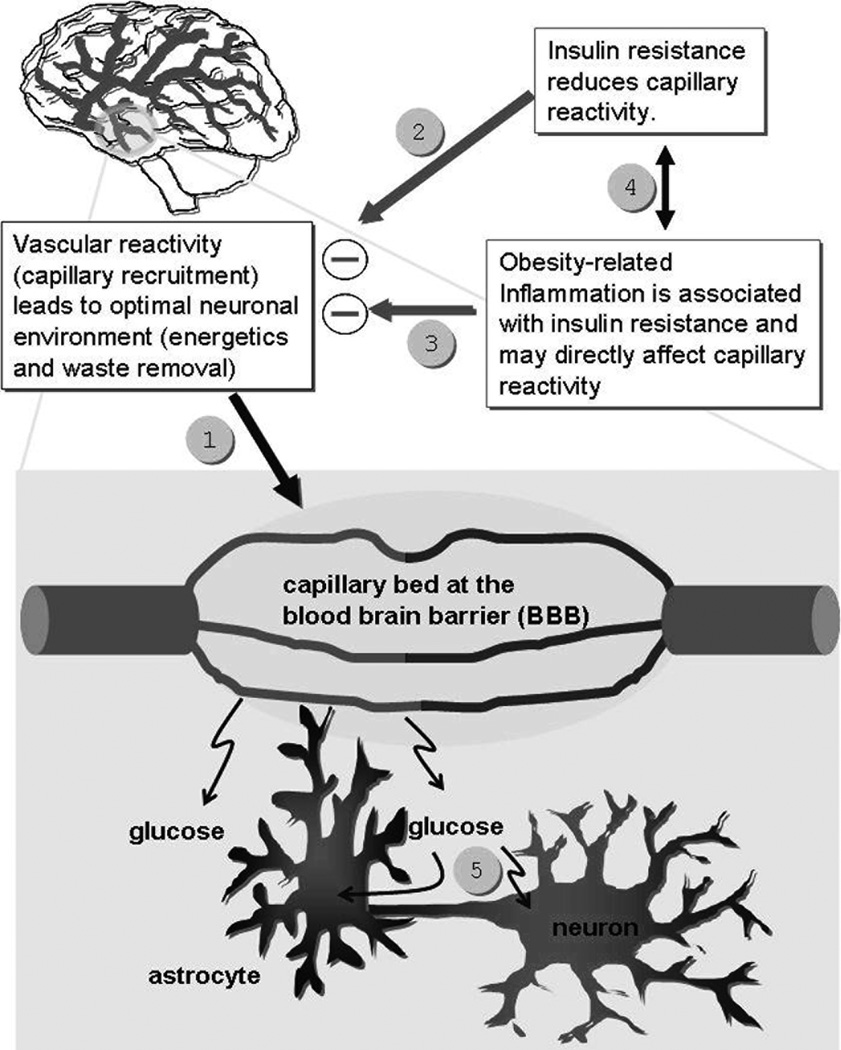
We propose the damaging effects of MetS and IR on brain integrity are partly dependent on the vascular reactivity abnormalities associated with those conditions.84 We suggest a conceptual model that posits that when a region of the brain is activated (as when performing a cognitive task), there is increased synaptic activity in that region, which normally results in regional vasodilatation.85 Vascular reactivity is key to maintaining energy-dependent processes such as regional brain activation by clearing the metabolic “waste” produced by neuronal activity (CO2, excess lactate, other metabolites, heat, etc).85 We know that in T2DM and MetS there are impairments in endothelial-dependent vasodilatation.86–87 Consequently, individuals with MetS may not be able to maintain an optimal neuronal environment, particularly during periods of high demand. We propose that among individuals with insulin resistance and MetS vascular reactivity (capillary recruitment, #1 in Figure 1), a mechanism that maintains an optimal neuronal environment during brain activation, is dysfunctional. We propose that these reductions in vascular reactivity are due to the direct or indirect deleterious effects of insulin resistance (#2) and/or obesity-associated inflammation (#3) on the micro-vasculature. There is new evidence to support both that insulin resistance leads to inflammation and that inflammation leads to IR (#4). The resultant effect of both insulin resistance and inflammation is to reduce cerebral vascular reactivity. The impaired vascular reactivity may, in turn, lead to an inability to maintain energy-dependent processes and clear metabolic “waste” under conditions of increased demand. We propose that this endothelial dysfunction, when coupled with other potentially damaging influences such as inflammation, HPA axis dysregulation, or increased oxidative stress, may damage the brain, particularly those regions more vulnerable to damage. The model does not address possible problems that may occur after vascular reactivity (#5 in diagram above).
Figure 1.
Model describing hypothesized brain vascular reactivity abnormalities resulting in brain impairments.
Future studies should also explore other explanatory models including the impact of inflammation as a potential mediator for the damaging effects of MetS on brain structure and function. Assessment of inflammation and oxidative stress directly in brain by using MRI-based spectroscopy could be a logical next direction to understand the associations between MetS and cognitive impairments. Furthermore, future studies should use prospective longitudinal designs, which will allow stronger conclusions about possible mechanisms and better inform follow-up animal models. Intervention studies as well as those that incorporate protective factors, such as a well balanced healthy diet and exercise, will also assist in better elucidating candidate mechanisms and iteratively improve interventions intending to protect the brain.
Table 2.
Ten cross-sectional studies of the association between cognition and factors of the Metabolic Syndrome in children and adolescents
| Reference | MetS Factor | Clinical Population |
Control Group |
Cognitive tests | Covariates | Results |
|---|---|---|---|---|---|---|
| Cserjesi, et.al. (2007) | Elevated Waist Circumference | 12 obese boys mean age 12.1 | 12 Age-matched non-obese boys | D2 Attention Endurance Test, WCST (sig), Digit Span Memory Task, Raven Matrices, Semantic Verbal Fluency (non-sig) | None | Obese performed worse on WCST & D2 attention endurance task despite similar memory & intelligence |
| Gunstad, et.al. (2008) | Elevated Waist Circumference | 45 BMI≥ 95th % 6–19 y.o.; mean age of entire sample = 12.45 | 433 BMI <95th % divided into 3 weight groups | Digit Span Backward, TMT-B, Verbal Recall, Animal Fluency, Finger Tapping (all non-significant) | Age, estimated IQ | No associations between BMI & cognition |
| Lande et.al., (2003) | Elevated Waist Circumference, Hypertension | 5,077 6–16 y.o. (NHANES) | None | WISC-R Block Design & Digit Span, WRAT Arithmetic (sig), WRAT Reading (non-sig) | Race, sex, parent ed., poverty, meds/antihistamines, general health, lead level, BMI, heart rate | Those with systolic BP >90th % performed worse on block design, digit span and math; 55.6% of these were overweight or obese. |
| Li, et.al. (2008) | Elevated Waist Circumference | 360 BMI ≥ 95th % 8–16 y.o. mean age 12.03 | 2,159 BMI < 95th % divided into 2 weight groups | WISC-R Block Design & Digit Span, global functioning (sig), WRAT Reading & Arithmetic (non-sig) | Age, gender, ethnicity, education, marital status of family head, family income, dwelling, hours watching TV, exercise, health status, blood pressure, heart rate, iron deficiency, psych & social variables | Those with BMI ≥ 95th % performed significantly worse on digit span, block design & global functioning |
| Lokken, et.al. (2009) | Elevated Waist Circumference | 25 12–19 y.o., mean age 16.2, mean BMI=54 | Compared performance across existing normative test data | Digit Span, CPT, Verbal Inference, Switching Attn, Maze Task (sig), Go-No-Go (non-sig) | None | Obesity associated with worse performance, esp. attention and executive functioning |
| Maayan, et.al. (2011) | Elevated Waist Circumference | 54 obese 14–21 y.o. Mean age 17.32 | 37 lean 14–21 y.o. Mean age 17.50 | COWAT, TMT, Stroop, Attn/Concentration & Memory Indexes from WRAML-2 (all sig) | IQ | Obese performed worse on all cognitive measures |
| Parisi, et.al. (2010) | Elevated Waist Circumference | 71 Overweight and 51 obese 6–13 y.o. | 188 6–13 y.o. | WISC-R, SDAG (parents) (varying results) | None | Sig. weight group differences on PIQ; BMI group predicts PIQ; Gender & parental ed. predicts VIQ; Parent ed. predicts TIQ |
| Pauli-Pott, et.al. (2012) | Elevated Waist Circumference | 177 overweight and obese 8–15 y.o. | None | Go-No-Go & Incompatibility Tasks of the Attention Assessment Battery (all significant) | Age, gender, education, SES, general mental ability | Obese showed more inattention; at younger ages, high impulsivity is associated with higher body weight |
| Verdejo-Garcia, et.al. (2010) | Elevated Waist Circumference | 8 overweight & 19 obese 13–16 y.o. | 34 Normal weight 13–16 y.o. | 5 digit test, TMT, Iowa Gambling (sig), Stroop, Letter Number Seq., Similarities, Zoo Map, Rev. Strategy Application, (non-sig) | Age | Excess weight perform worse on inhibition, flexibility & decision making |
| Yau, et.al. (2010) | Elevated Waist Circumference, Hyperglycemia | 18 obese T2DM, mean age 16.46 y.o. | 18 Obese non-T2DM mean age 17.16 y.o. | DSST, IQ, WRAML Verbal (sig), WASI Subtests, WRAT, WRAML Visual & Working Memory, Dig Vig, WCST, ToL, COWAT(non-sig) | Matched on age, sex, grade, SES, BMI, Waist, WHR & Sleep apnea | T2DM performed significantly worse performance on all cognitive domains |
ACKNOWLEDGEMENTS
The study was supported by grants from the National Institutes of Health DK064087 and DK083537.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Potenza MV, Mechanick JI. The metabolic syndrome: definition, global impact, and pathophysiology. Nutr Clin Pract. 2009;24:560–577. doi: 10.1177/0884533609342436. [DOI] [PubMed] [Google Scholar]
- 2.Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 3.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009:1–7. [PubMed] [Google Scholar]
- 4.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 5.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Case CC, Jones PH, Nelson K, O'Brian Smith E, Ballantyne CM. Impact of weight loss on the metabolic syndrome. Diabetes Obes Metab. 2002;4:407–414. doi: 10.1046/j.1463-1326.2002.00236.x. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 8.Case CC, Jones PH, Nelson K, O'Brian Smith E, Ballantyne CM. Impact of weight loss on the metabolic syndrome. Diabetes Obes Metab. 2002;4:407–414. doi: 10.1046/j.1463-1326.2002.00236.x. [DOI] [PubMed] [Google Scholar]
- 9.Bourdel-Marchasson I, Lapre E, Laksir H, Puget E. Insulin resistance, diabetes and cognitive function: consequences for preventative strategies. Diabetes Metab. 2010;36:173–181. doi: 10.1016/j.diabet.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29:345–351. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
- 11.Reaven GM. Role of Insulin Resistance in Human-Disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 12.Zavaroni I, Bonora E, Pagliara M, Dall'Aglio E, Luchetti L, Buonanno G, Bonati PA, Bergonzani M, Gnudi L, Passeri M, et al. Risk factors for coronary artery disease in healthy persons with hyperinsulinemia and normal glucose tolerance. N Engl J Med. 1989;320:702–706. doi: 10.1056/NEJM198903163201105. [DOI] [PubMed] [Google Scholar]
- 13.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 14.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Preventionl; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 16.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Crichton GE, Elias MF, Buckley J, Murphy KJ, Bryan J, Frisardi V. Metabolic Syndrome, Cognitive Performance, and Dementia. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-111022. [DOI] [PubMed] [Google Scholar]
- 18.Frisardi V, Solfrizzi V, Capurso C, Imbimbo BP, Vendemiale G, Seripa D, Pilotto A, Panza F. Is insulin resistant brain state a central feature of the metabolic-cognitive syndrome? J Alzheimers Dis. 2010;21:57–63. doi: 10.3233/JAD-2010-100015. [DOI] [PubMed] [Google Scholar]
- 19.Hao ZL, Wu B, Wang DR, Liu M. Association between metabolic syndrome and cognitive decline: a systematic review of prospective population-based studies. Acta Neuropsychiatr. 2011;23:69–74. doi: 10.1111/j.1601-5215.2011.00527.x. [DOI] [PubMed] [Google Scholar]
- 20.Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Journal of the Neurological Sciences. 2009;283:307–308. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segura B, Jurado MA. [Metabolic syndrome and ageing: cognitive impairment and structural alterations of the central nervous system] Rev Neurol. 2009;49:417–424. [PubMed] [Google Scholar]
- 22.Bokura H, Nagai A, Oguro H, Kobayashi S, Yamaguchi S. The association of metabolic syndrome with executive dysfunction independent of subclinical ischemic brain lesions in Japanese adults. Dement Geriatr Cogn Disord. 2010;30:479–485. doi: 10.1159/000322057. [DOI] [PubMed] [Google Scholar]
- 23.Cavalieri M, Ropele S, Petrovic K, Pluta-Fuerst A, Homayoon N, Enzinger C, Grazer A, Katschnig P, Schwingenschuh P, Berghold A, Schmidt R. Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care. 2010;33:2489–2495. doi: 10.2337/dc10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassenstab JJ, Sweat V, Bruehl H, Convit A. Metabolic syndrome is associated with learning and recall impairment in middle age. Dement Geriatr Cogn Disord. 2010;29:356–362. doi: 10.1159/000296071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Helkala EL, Haapala I, Nissinen A, Rauramaa R. Metabolic syndrome and cognitive function: a population-based follow-up study in elderly women. Dement Geriatr Cogn Disord. 2007;23:29–34. doi: 10.1159/000096636. [DOI] [PubMed] [Google Scholar]
- 26.Schuur M, Henneman P, van Swieten JC, Zillikens MC, de Koning I, Janssens AC, Witteman JC, Aulchenko YS, Frants RR, Oostra BA, van Dijk KW, van Duijn CM. Insulin-resistance and metabolic syndrome are related to executive function in women in a large family-based study. Eur J Epidemiol. 2010;25:561–568. doi: 10.1007/s10654-010-9476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haring R, Volzke H, Felix SB, Schipf S, Dorr M, Rosskopf D, Nauck M, Schofl C, Wallaschofski H. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes. 2009;58:2027–2031. doi: 10.2337/db09-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller M, van Raamt F, Visseren FL, Kalmijn S, Geerlings MI, Mali WP, van der Graaf Y. Metabolic syndrome and cognition in patients with manifest atherosclerotic disease: the SMART study. Neuroepidemiology. 2010;34:83–89. doi: 10.1159/000264825. [DOI] [PubMed] [Google Scholar]
- 29.Segura B, Jurado MA, Freixenet N, Albuin C, Muniesa J, Junque C. Mental slowness and executive dysfunctions in patients with metabolic syndrome. Neurosci Lett. 2009;462:49–53. doi: 10.1016/j.neulet.2009.06.071. [DOI] [PubMed] [Google Scholar]
- 30.Tournoy J, Lee DM, Pendleton N, O'Neill TW, O'Connor DB, Bartfai G, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean ME, Moseley CM, Punab M, Silman AJ, Vanderschueren D, Wu FC, Boonen S. Association of cognitive performance with the metabolic syndrome and with glycaemia in middle-aged and older European men: the European Male Ageing Study. Diabetes Metab Res Rev. 2010;26:668–676. doi: 10.1002/dmrr.1144. [DOI] [PubMed] [Google Scholar]
- 31.Haley AP, Gonzales MM, Tarumi T, Miles SC, Goudarzi K, Tanaka H. Elevated cerebral glutamate and myo-inositol levels in cognitively normal middle-aged adults with metabolic syndrome. Metab Brain Dis. 2010;25:397–405. doi: 10.1007/s11011-010-9221-y. [DOI] [PubMed] [Google Scholar]
- 32.Gatto NM, Henderson VW, St John JA, McCleary C, Hodis HN, Mack WJ. Metabolic syndrome and cognitive function in healthy middle-aged and older adults without diabetes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:627–641. doi: 10.1080/13825580802036936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes Rev. 2011;12:740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 34.Cserjesi R, Molnar D, Luminet O, Lenard L. Is there any relationship between obesity and mental flexibility in children? Appetite. 2007;49:675–678. doi: 10.1016/j.appet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Lokken KL, Boeka AG, Austin HM, Gunstad J, Harmon CM. Evidence of executive dysfunction in extremely obese adolescents: a pilot study. Surg Obes Relat Dis. 2009;5:547–552. doi: 10.1016/j.soard.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Verdejo-Garcia A, Perez-Exposito M, Schmidt-Rio-Valle J, Fernandez-Serrano MJ, Cruz F, Perez-Garcia M, Lopez-Belmonte G, Martin-Matillas M, Martin-Lagos JA, Marcos A, Campoy C. Selective alterations within executive functions in adolescents with excess weight. Obesity (Silver Spring) 2010;18:1572–1578. doi: 10.1038/oby.2009.475. [DOI] [PubMed] [Google Scholar]
- 37.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring) 2011;19:1382–1387. doi: 10.1038/oby.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: A structural MRI study. J Int Neuropsych Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- 39.Pauli-Pott U, Albayrak O, Hebebrand J, Pott W. Association between inhibitory control capacity and body weight in overweight and obese children and adolescents: dependence on age and inhibitory control component. Child Neuropsychol. 2010;16:592–603. doi: 10.1080/09297049.2010.485980. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Dai Q, Jackson JC, Zhang J. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity (Silver Spring) 2008;16:1809–1815. doi: 10.1038/oby.2008.296. [DOI] [PubMed] [Google Scholar]
- 41.Parisi P, Verrotti A, Paolino MC, Miano S, Urbano A, Bernabucci M, Villa MP. Cognitive profile, parental education and BMI in children: reflections on common neuroendrocrinobiological roots. J Pediatr Endocrinol Metab. 2010;23:1133–1141. doi: 10.1515/jpem.2010.178. [DOI] [PubMed] [Google Scholar]
- 42.Lande MB, Kaczorowski JM, Auinger P, Schwartz GJ, Weitzman M. Elevated blood pressure and decreased cognitive function among school-age children and adolescents in the United States. J Pediatr. 2003;143:720–724. doi: 10.1067/S0022-3476(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 43.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70–81 years. Brit Med J. 2004;328:548–551. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yau PL, Javier DC, Ryan CM, Tsui WH, Ardekani BA, Ten S, Convit A. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53:2298–2306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunstad J, Spitznagel MB, Paul RH, Cohen RA, Kohn M, Luyster FS, Clark R, Williams LM, Gordon E. Body mass index and neuropsychological function in healthy children and adolescents. Appetite. 2008;50:246–251. doi: 10.1016/j.appet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Gold SM, Dziobek I, Rogers K, Bayoumy A, McHugh PF, Convit A. Hypertension and hypothalamo-pituitary-adrenal axis hyperactivity affect frontal lobe integrity. J Clin Endocrinol Metab. 2005;90:3262–3267. doi: 10.1210/jc.2004-2181. [DOI] [PubMed] [Google Scholar]
- 47.Manschot S, Biessels G, de Valk H, Algra A, Rutten G, van der Grond J, Kappelle L on behalf of the Utrecht Diabetic Encephalopathy Study G. Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia. 2007;50:2388–2397. doi: 10.1007/s00125-007-0792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward MA, Bendlin BB, McLaren DG, Hess TM, Gallagher CL, Kastman EK, Rowley HA, Asthana S, Carlsson CM, Sager MA, Johnson SC. Low HDL Cholesterol is Associated with Lower Gray Matter Volume in Cognitively Healthy Adults. Front Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurl S, Laukkanen JA, Niskanen L, Laaksonen D, Sivenius J, Nyyssonen K, Salonen JT. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke; a journal of cerebral circulation. 2006;37:806–811. doi: 10.1161/01.STR.0000204354.06965.44. [DOI] [PubMed] [Google Scholar]
- 51.Kwon HM, Kim BJ, Park JH, Ryu WS, Kim CK, Lee SH, Ko SB, Nam H, Lee YS, Yoon BW. Significant association of metabolic syndrome with silent brain infarction in elderly people. J Neurol. 2009;256:1825–1831. doi: 10.1007/s00415-009-5201-8. [DOI] [PubMed] [Google Scholar]
- 52.Park K, Yasuda N, Toyonaga S, Tsubosaki E, Nakabayashi H, Shimizu K. Significant associations of metabolic syndrome and its components with silent lacunar infarction in middle aged subjects. J Neurol Neurosurg Psychiatry. 2008;79:719–721. doi: 10.1136/jnnp.2007.134809. [DOI] [PubMed] [Google Scholar]
- 53.Park JH, Kwon HM. Association between metabolic syndrome and previous ischemic lesions in patients with intracranial atherosclerotic stroke. Clinical Neurology and Neurosurgery. 2008;110:215–221. doi: 10.1016/j.clineuro.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Bokura H, Yamaguchi S, Iijima K, Nagai A, Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke. 2008;39:1607–1609. doi: 10.1161/STROKEAHA.107.508630. [DOI] [PubMed] [Google Scholar]
- 55.Segura B, Jurado MA, Freixenet N, Falcon C, Junque C, Arboix A. Microstructural white matter changes in metabolic syndrome: A diffusion tensor imaging study. Neurology. 2009;73:438–444. doi: 10.1212/WNL.0b013e3181b163cd. [DOI] [PubMed] [Google Scholar]
- 56.Haley AP, Gonzales MM, Tarumi T, Miles SC, Goudarzi K, Tanaka H. Elevated cerebral glutamate and myo-inositol levels in cognitively normal middle-aged adults with metabolic syndrome. Metabolic brain disease. 2010;25:397–405. doi: 10.1007/s11011-010-9221-y. [DOI] [PubMed] [Google Scholar]
- 57.Ajilore O, Haroon E, Kumaran S, Darwin C, Binesh N, Mintz J, Miller J, Thomas MA, Kumar A. Measurement of Brain Metabolites in Patients with type 2 Diabetes and Major Depression Using Proton Magnetic Resonance Spectroscopy. Neuropsychopharmacology. 2006;32:1224–1231. doi: 10.1038/sj.npp.1301248. [DOI] [PubMed] [Google Scholar]
- 58.Hoth KF, Gonzales MM, Tarumi T, Miles SC, Tanaka H, Haley AP. Functional MR imaging evidence of altered functional activation in metabolic syndrome. AJNR. American journal of neuroradiology. 2011;32:541–547. doi: 10.3174/ajnr.A2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. 2007;49:2112–2119. doi: 10.1016/j.jacc.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 60.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 61.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 62.Bruehl H, Sweat V, Tirsi A, Shah B, Convit A. Obese adolescents with type 2 diabetes mellitus have hippocampal and frontal lobe volume reductions. Neuroscience and Medicine. 2011;2:34–42. doi: 10.4236/nm.2011.21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ursache A, Wedin W, Tirsi A, Convit A. Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- 65.Giannopoulos S, Boden-Albala B, Choi JH, Carrera E, Doyle M, Perez T, Marshall RS. Metabolic syndrome and cerebral vasomotor reactivity. Eur J Neurol. 2010;17:1457–1462. doi: 10.1111/j.1468-1331.2010.03087.x. [DOI] [PubMed] [Google Scholar]
- 66.Sipila K, Moilanen L, Nieminen T, Reunanen A, Jula A, Salomaa V, Kaaja R, Kukkonen-Harjula K, Lehtimaki T, Kesaniemi YA, Koivistoinen T, Nieminen MS, Tuomilehto J, Kahonen M. Metabolic syndrome and carotid intima media thickness in the Health 2000 Survey. Atherosclerosis. 2009;204:276–281. doi: 10.1016/j.atherosclerosis.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 67.Koivistoinen T, Hutri-Kahonen N, Juonala M, Aatola H, Koobi T, Lehtimaki T, Viikari JS, Raitakari OT, Kahonen M. Metabolic syndrome in childhood and increased arterial stiffness in adulthood: the Cardiovascular Risk In Young Finns Study. Annals of Medicine. 2011;43:312–319. doi: 10.3109/07853890.2010.549145. [DOI] [PubMed] [Google Scholar]
- 68.Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, Au R, DeCarli C, Wolf PA. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke; a journal of cerebral circulation. 2009;40:1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muller M, van der Graaf Y, Algra A, Hendrikse J, Mali WP, Geerlings MI. Carotid atherosclerosis and progression of brain atrophy: the SMART-MR study. Annals of Neurology. 2011;70:237–244. doi: 10.1002/ana.22392. [DOI] [PubMed] [Google Scholar]
- 70.Komulainen P, Kivipelto M, Lakka TA, Hassinen M, Helkala EL, Patja K, Nissinen A, Rauramaa R. Carotid intima-media thickness and cognitive function in elderly women: a population-based study. Neuroepidemiology. 2007;28:207–213. doi: 10.1159/000108112. [DOI] [PubMed] [Google Scholar]
- 71.Huang K, Zou CC, Yang XZ, Chen XQ, Liang L. Carotid intima-media thickness and serum endothelial marker levels in obese children with metabolic syndrome. Archives Of Pediatrics & Adolescent Medicine. 2010;164:846–851. doi: 10.1001/archpediatrics.2010.160. [DOI] [PubMed] [Google Scholar]
- 72.Iannuzzi A, Licenziati MR, Acampora C, Renis M, Agrusta M, Romano L, Valerio G, Panico S, Trevisan M. Carotid artery stiffness in obese children with the metabolic syndrome. The American Journal of Cardiology. 2006;97:528–531. doi: 10.1016/j.amjcard.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 73.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, Struijker-Boudier HA, Safar ME, Staessen JA. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23:1839–1846. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 74.Zhu W, Huang X, He J, Li M, Neubauer H. Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. Eur J Pediatr. 2005;164:337–344. doi: 10.1007/s00431-005-1642-y. [DOI] [PubMed] [Google Scholar]
- 75.Pandit D, Kinare A, Chiplonkar S, Khadilkar A, Khadilkar V. Carotid arterial stiffness in overweight and obese Indian children. J Pediatr Endocrinol Metab. 2011;24:97–102. doi: 10.1515/jpem.2011.086. [DOI] [PubMed] [Google Scholar]
- 76.Stabouli S, Kotsis V, Karagianni C, Zakopoulos N, Konstantopoulos A. Blood pressure and carotid artery intima-media thickness in children and adolescents: the role of obesity. Hellenic journal of cardiology : HJC = Hellenike kardiologike epitheorese. 2012;53:41–47. [PubMed] [Google Scholar]
- 77.Wong LJ, Kupferman JC, Prohovnik I, Kirkham FJ, Goodman S, Paterno K, Sharma M, Brosgol Y, Pavlakis SG. Hypertension impairs vascular reactivity in the pediatric brain. Stroke. 2011;42:1834–1838. doi: 10.1161/STROKEAHA.110.607606. [DOI] [PubMed] [Google Scholar]
- 78.Pall D, Kiss I, Katona E. Importance of ambulatory blood pressure monitoring in adolescent hypertension. Kidney Blood Press Res. 2012;35:129–134. doi: 10.1159/000331057. [DOI] [PubMed] [Google Scholar]
- 79.Settakis G, Pall D, Molnar C, Katona E, Bereczki D, Fulesdi B. Hyperventilation-induced cerebrovascular reactivity among hypertensive and healthy adolescents. Kidney Blood Press Res. 2006;29:306–311. doi: 10.1159/000097018. [DOI] [PubMed] [Google Scholar]
- 80.Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth With Obesity and Obesity-Related Type 2 Diabetes Mellitus Demonstrate Abnormalities in Carotid Structure and Function. Circulation. 2009;119:2913–2919. doi: 10.1161/CIRCULATIONAHA.108.830380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kotb NA, Gaber R, Salama M, Nagy HM, Elhendy A. Clinical and biochemical predictors of increased carotid intima-media thickness in overweight and obese adolescents with type 2 diabetes. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2012;9:35–41. doi: 10.1177/1479164111421804. [DOI] [PubMed] [Google Scholar]
- 82.Breteler MM. Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Annals of the New York Academy of Sciences. 2000;903:457–465. doi: 10.1111/j.1749-6632.2000.tb06399.x. [DOI] [PubMed] [Google Scholar]
- 83.Breteler MMB, van Swieten JC, Bots G, Grobbee DE, Claus JJ, van den Hout JHW, van Harskamp F, Tanghe HLJ, de Jong PTVM, van Gijn J, Hofman A. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 84.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26(Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 85.Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Tooke JE, Goh KL. Endotheliopathy precedes type 2 diabetes. Diabetes Care. 1998;21:2047–2049. doi: 10.2337/diacare.21.12.2047. [DOI] [PubMed] [Google Scholar]
- 87.Tooke JE, Hannemann MM. Adverse endothelial function and the insulin resistance syndrome. J Intern Med. 2000;247:425–431. doi: 10.1046/j.1365-2796.2000.00671.x. [DOI] [PubMed] [Google Scholar]