Abstract
Context
Leucovorin, fluorouracil, and oxaliplatin (FOLFOX) is the standard adjuvant therapy for resected stage III colon cancer. Adding cetuximab to FOLFOX benefits patients with metastatic wild-type KRAS but not mutated KRAS colon cancer.
Objective
To assess the potential benefit of cetuximab added to the modified sixth version of the FOLFOX regimen (mFOLFOX6) in patients with resected stage III wild-type KRAS colon cancer.
Design, Setting, and Participants
A randomized trial of 2686 patients aged 18 years or older at multiple institutions across North America enrolled following resection and informed consent between February 10, 2004, and November 25, 2009. The primary randomized comparison was 12 biweekly cycles of mFOLFOX6 with and without cetuximab. KRAS mutation status was centrally determined. The trial was halted after a planned interim analysis of 48% of predicted events (246/515) occurring in 1863 (of 2070 planned) patients with tumors having wild-type KRAS. A total of 717 patients with mutated KRAS and 106 with indeterminate KRAS were accrued. The 2070 patients with wild-type KRAS provided 90% power to detect a hazard ratio (HR) of 1.33 (2-sided α =.05), with planned interim efficacy analyses after 25%, 50%, and 75% of expected relapses.
Main Outcome Measures
Disease-free survival in patients with wild-type KRAS mutations. Secondary end points included overall survival and toxicity.
Results
Median (range) follow-up was 28 (0–68) months. The trial demonstrated no benefit when adding cetuximab. Three-year disease-free survival for mFOLFOX6 alone was 74.6% vs 71.5% with the addition of cetuximab (HR, 1.21; 95% CI, 0.98–1.49; P=.08) in patients with wild-type KRAS, and 67.1% vs 65.0% (HR, 1.12; 95% CI, 0.86–1.46; P=.38) in patients with mutated KRAS, with no significant benefit in any subgroups assessed. Among all patients, grade 3 or higher adverse events (72.5% vs 52.3%; odds ratio [OR], 2.4; 95% CI, 2.1–2.8; P < .001) and failure to complete 12 cycles (33% vs 23%; OR, 1.6; 95% CI, 1.4–1.9; P < .001) were significantly higher with cetuximab. Increased toxicity and greater detrimental differences in all outcomes were observed in patients aged 70 years or older.
Conclusion
Among patients with stage III resected colon cancer, the use of cetuximab with adjuvant mFOLFOX6 compared with mFOLFOX6 alone did not result in improved disease-free survival.
Patients with resected stage III colon cancer have a 50% chance of cure with surgery.1 Multiple trials have established the benefit of adjuvant chemotherapy in reducing the recurrence risk. Specifically, leucovorin, fluorouracil, and oxaliplatin (FOLFOX or slightly different method, FLOX) provides significant benefit in both disease-free and overall survival compared with the prior standard of fluorouracil and leucovorin.2–4
In the setting of metastatic colorectal cancer, cetuximab and panitumumab are US Food and Drug Administration approved for targeting the epidermal growth factor receptor. Both antibodies alone and in combination with chemotherapy have provided additional benefit to that obtained with chemotherapy alone.5,6 This benefit, however, is limited to patients with tumors expressing the wild-type form of the gene KRAS (NCBI Entrez Gene 3845) as opposed to those with the mutated form of KRAS.7
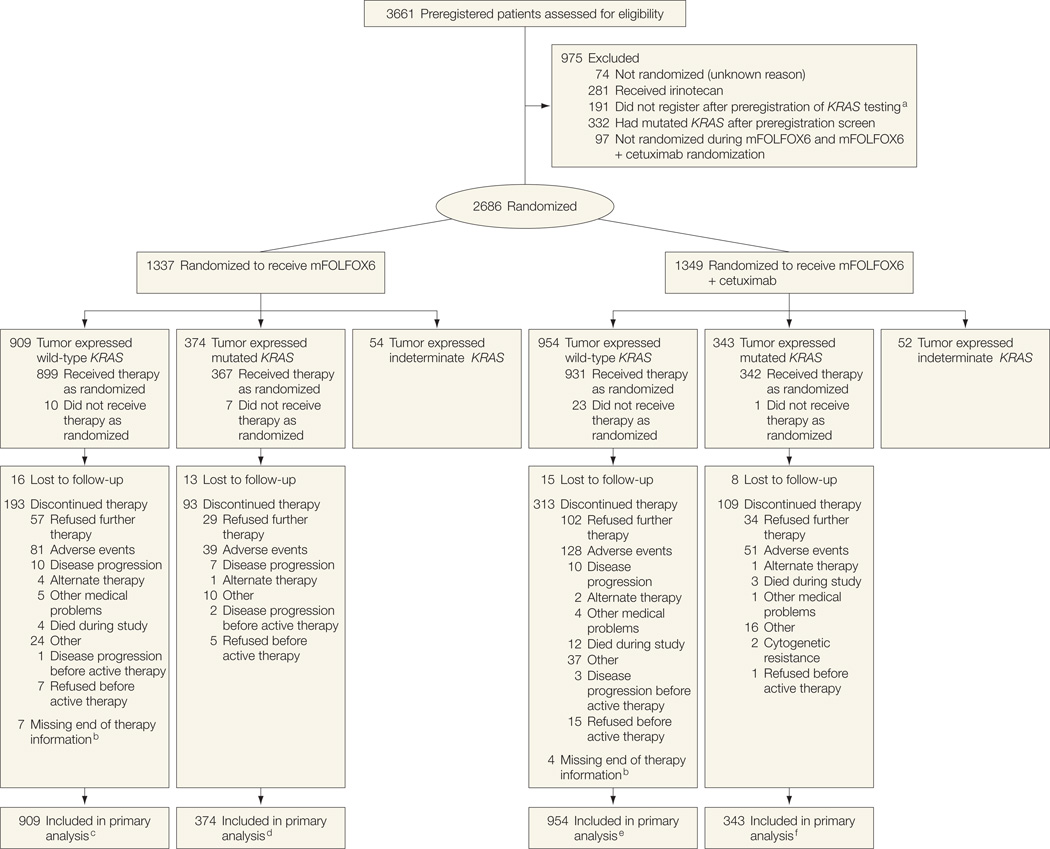
The initial design of this trial (North Central Cancer Treatment Group [NCCTG] N0147) included randomization to either the modified sixth version of the FOLFOX regimen (mFOLFOX6), fluorouracil, leucovorin, and irinotecan (FOLFIRI), or a hybrid regimen consisting of mFOLFOX6 followed up by FOLFIRI. In September 2004, the results from other trials prompted the addition of cetuximab, resulting in 6 groups. The FOLFIRI groups were discontinued mid-2005 based on clinical trial comparisons with fluorouracil and leucovorin.8–10 Patients who received irinotecan are not included in this study. In August 2008, randomization was restricted to patients with tumors expressing centrally confirmed wild-type KRAS (Figure 1). Accrual periods for each treatment group with key dates associated with treatment modifications are shown in eFigure 1 (http://www.jama.com).
Figure 1.
Flow of Patients Through the Trial
mFOLFOX6 indicates the modified sixth version of the leucovorin, fluorouracil, and oxaliplatin regimen.
aThese patients were enrolled after the prospective preregistration for KRAS testing and added but did not go on to the subsequent step of registration due to rumor tissue could not be evaluated for KRAS, investigator or participant decision, eligibility criteria were not met, or other unspecified reasons.
bEither did not have an end-of-treatment form or did not have an end-of-treatment reason on the form.
cIncludes 17 ineligible patients (6 improper histology, 1 pretreatment lab values >14 days from randomization, 2 positive margins, 2 resection not en bloc, and 6 surgery >56 days before randomization).
dIncludes 6 ineligible patients (4 improper histology and 2 positive margins).
eIncludes 16 ineligible patients (9 improper histology, 1 positive margin, 2 resection not en bloc, and 4 surgery >56 days before randomization).
fIncludes 10 ineligible patients (5 improper histology, 2 positive margins, 2 resection not en bloc, and 1 surgery >56 days before randomization).
METHODS
This trial was designed by the NCCTG in collaborationwith the National Cancer Institute (NCI) and the NCI-sponsored cooperative groups. The NCCTG maintains full unrestricted rights to publication of the trial data and performed all analyses. The NCCTG data monitoring committee reviewed this trial semiannually for toxicity and scheduled interim efficacy analyses.
Patients
Patients with completely resected, histologically proven stage III (any TN1-2M0 tumors) colon adenocarcinoma and at least 12 cm from the anal verge were eligible to participate. For patients with locally advanced tumors, an en bloc resection was required. Other eligibility criteria included aged 18 years or older, at least 1 pathologically confirmed–involved lymph node, Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate blood counts of liver and kidney function. No prior chemotherapy, immunotherapy, or radiotherapy for colon cancer was allowed. Investigational review board approval was required at all of the participating centers and all participants provided written informed consent. Mandatory blood and tumor tissue were collected before randomization. The trial was amended in August 2008 to only randomize patients having wild-type KRAS to mFOLFOX6 with or without cetuximab. Patients with tumors expressing undeterminable or mutated KRAS were treated per physician discretion and followed for recurrence and survival.
Treatment
Before starting treatment, patients were randomly assigned in a 1:1 ratio to receive mFOLFOX6 with or without cetuximab. Randomization was stratified by number of involved lymph nodes (1–3 vs ≥4), high histology (poorly differentiated [grade 3], undifferentiated [grade 4]) vs low histology (well differentiated [grade 1], moderately differentiated [grade 2]), and T stage (T1–2 vs T3 vs T4). Both treatment groups received mFOLFOX6, consisting of 12 biweekly courses of oxaliplatin (85 mg/m2) over 2 hours on day 1 with leucovorin (400 mg/m2) and fluorouracil (400 mg/m2) bolus, then 46-hour intravenous fluorouracil (2400 mg/m2) on days 1 to 2 starting within 10 weeks of surgery. Patients enrolled in the cetuximab group received 400 mg/m2 over 2 hours on day 1 of cycle 1, then 250 mg/m2 over 1 hour on day 8 (cycle 1) and day 1 and 8 each of cycles 2 through 12. Standard supportive care included antihistamine before cetuximab and antiemetic therapy, as needed, before receiving mFOLFOX6. All patients received written instructions on diarrhea management.
Patients were assessed biweekly for adverse events using the NCI, Common Toxicity Criteria, version 3.0.11 Guidelines were provided for dose modifications. Mandatory dose modifications were introduced for patients aged 70 years or older following evidence of increased toxicity.
KRAS and BRAF Mutation Status
Assessment of KRAS and BRAF (NCBI Entrez Gene 673) mutational status was performed centrally at the Mayo Clinic in a Clinical Laboratory Improvement Amendments compliant laboratory, using appropriate quality control procedures. Both KRAS and BRAF mutation status was determined using DNA extracted from macrodissected formalin-fixed, paraffin-embedded tumor tissue. For KRAS, testing was performed with the DxS mutation test kit KR-03/04 (DxS), together with the Light-Cycler 480 (Roche Applied Sciences), which assesses for 7 different potential mutations in codons 12 and 13.12 The level of detection was set at 5%. Assessment for the BRAF V600E mutation was performed using a Mayo developed multiplex allele specific polymerase chain reaction–based assay. The polymerase chain reaction primers used for this assay were fluorescently labeled and included the following (wild-type forward [NEDTGATTTTGGTCATGCTACAGT]; mutant forward [6-Fam-CAGTGATTTTGGTCTAGCTTCAGA]; and reverse [GTTTCTTTCTAGTAACTCAGCAGC]). Following amplification, polymerase chain reaction products were analyzed on an ABI 3130xl instrument (Life Technologies, Applied Biosystems) and scored for the presence or absence of the V600E variant only.
Disease Assessments and Follow-up
After completing treatment, disease recurrence was assessed every 6 months until 5 years postrandomization with a physical examination, computed tomographic scan, and laboratory assessment. A follow-up colonoscopy was recommended at years 1 and 4 postresection. Follow-up for all patients was censored at 5 years from randomization for time-to-event analyses.
Statistical Methods
The primary outcome measure was disease-free survival, defined as the time from the date of randomization to the first of documented recurrence of colon cancer (excluding second colon primary cancer) or death from any cause. Based on an assumed 3-year disease-free survival of 70% in the mFOLFOX6 group, 2070 patients with tumors expressing wild-type KRAS were required to achieve 515 events, which provided 90% power to detect a hazard ratio (HR) of 0.75 in the cetuximab-containing group, using a 2-sided log-rank test at P < .05. Interim analyses for efficacy were required after reaching 25%, 50%, and 75% of the planned number of 515 events using an O’Brien-Fleming stopping boundary,12 truncated at ±3.5. The cutoff values for the log-rank statistics for these analyses (3 interim, 1 final) were ±3.5, ±2.996, ±2.361, and ±2.015.
The decision to terminate enrollment was made by the study team following a recommendation from an external data and safety monitoring committee (DSMC) review of a nonprespecified conditional power analysis. Specific stopping rules were built into the trial. At the time of the second planned interim analysis, the results indicated that the experimental group would not be better than the control group. Based on this information, the authors informed the DSMC for the trial through a confidential presentation of the analysis. The DSMC then voted to recommend closure of accrual to the trial, with the study team then being informed of this recommendation. With this recommendation, the study team was obligated to either halt accrual or to provide additional information to the DSMC to alter their decision. Given the results of the analysis, the study team closed the trial to further accrual. The DSMC approved all trial modifications for the primary end point, trial closure, and the release of trial results in a scientific forum.
Patients were randomized using a dynamic allocation procedure.13 All randomized patients are included for efficacy analyses according to intention-to-treat principles, unless otherwise specified. Patients enrolled before August 2008 with retrospectively determined wild-type KRAS were included. Based on data suggesting epidermal growth factor receptor inhibitors may have limited benefit in patients with BRAF mutations,14 secondary analyses are presented excluding such patients.
Secondary end points included time-to-recurrence, overall survival, toxicity, and dose intensity. Time-to-recurrence was defined as time from randomization to recurrence, where patients dying without recurrence were censored for time-to-recurrence at the time of death. Patients lost to follow-up were censored for recurrence (or survival) at the date of their most recent disease assessment (or contact). The Kaplan-Meier method was used to describe the distribution of time-to-recurrence, overall survival, and time-to-treatment discontinuation.15 A Cox proportional hazards regression model was used to explore the associations of patient characteristics with outcome adjusted for the stratification factors.16 P < .05 was considered statistically significant. Analyses were performed by using SAS version 9.2 (SAS Institute Inc) and R version 2.14.17
RESULTS
Study Population
Patient enrollment began February 10, 2004, and was permanently halted on November 25, 2009, after the second planned interim analysis demonstrated a low probability that disease-free survival of the cetuximab group would surpass that of the mFOLFOX6-only group. This conditional power analysis occurred when 2678 patients (1760 patients with wild-type KRAS) had been concurrently randomized to the mFOLFOX6 with or without cetuximab groups, and 246 of 515 (48%) of the planned events had occurred. At that time, the HR for disease-free survival comparing mFOLFOX6 alone with mFOLFOX6 with cetuximab was 1.18 (95% CI, 0.92–1.52; log-rank P=.33, adjusted for stratification factors using a multivariate Cox proportional hazards regression model) in favor of mFOLFOX6. The conditional power analysis showed that if the trial completed enrollment, the probability of having a positive trial was 2.6% if the true HR of adding cetuximab was 0.80. Given the high likelihood of a negative finding, consistent results within secondary end points and subgroup analyses, and increased toxicity observed with cetuximab, the data and safety monitoring committee recommended trial closure.
Table 1 shows patient characteristics and Figure 1 shows the CONSORT flow diagram of participants. A total of 2686 patients comprised our analysis cohort (1863 patients with wild-type KRAS, 717 patients with mutated KRAS, and 106 patients with indeterminate KRAS). A total of 909 patients with wild-type KRAS were randomized to mFOLFOX6 and 954 patients with wild-type KRAS were randomized to mFOLFOX6 with cetuximab. A total of 374 patients with mutated KRAS were randomized to mFOLFOX6 and 343 patients with mutated KRAS were randomized to mFOLFOX6 with cetuximab. A total of 2410 of 2686 patients (89.7%) remained alive with a median (range) follow-up of 28 (0–68) months.
Table 1.
Patient Characteristics at Study Entrya
| No. (%) of Patients |
||||
|---|---|---|---|---|
| Wild-Type KRAS |
Mutated KRAS |
|||
| Characteristics | mFOLFOX6 (n = 909) |
mFOLFOX6 + Cetuximab (n = 954) |
mFOLFOX6 (n = 374) |
mFOLFOX6 + Cetuximab (n = 343) |
| Age, median (range), y | 58 (19–84) | 58 (25–86) | 59 (23–85) | 59 (22–85) |
| Sex | ||||
| Female | 415 (46) | 455 (48) | 190 (51) | 161 (47) |
| Male | 494 (54) | 499 (52) | 184 (49) | 182 (53) |
| Race/ethnicityb | ||||
| White | 788 (87) | 818 (86) | 309 (83) | 297 (87) |
| Black | 50 (6) | 62 (6) | 39 (10) | 27 (8) |
| Other | 71 (7) | 74 (8) | 26 (7) | 19 (5) |
| Adherencec | ||||
| Yes | 139 (15) | 136 (14) | 51 (14) | 42 (12) |
| No | 770 (85) | 818 (86) | 323 (86) | 301 (88) |
| Bowel obstruction | ||||
| Yes | 137 (15) | 157 (16) | 70 (19) | 53 (15) |
| No | 772 (85) | 797 (84) | 304 (81) | 290 (85) |
| Bowel perforation | ||||
| Yes | 42 (5) | 51 (5) | 18 (5) | 20 (6) |
| No | 867 (95) | 903 (95) | 356 (95) | 23 (94) |
| Histologic grade | ||||
| High (grade 3–4) | 247 (27) | 259 (27) | 83 (22) | 70 (20) |
| Low (grade 1–2) | 662 (73) | 695 (73) | 291 (78) | 273 (80) |
| Lymph node involvement | ||||
| 1–3 | 511 (56) | 548 (57) | 236 (63) | 208 (61) |
| >3 | 398 (44) | 406 (43) | 138 (37) | 135 (39) |
| Tumor stage | ||||
| Missing | 1 (0) | 1 (0) | 0 (0) | 1 (0) |
| T1 or T2 | 119 (13) | 158 (17) | 62 (17) | 45 (13) |
| T3 | 686 (76) | 689 (72) | 268 (72) | 257 (75) |
| T4 | 103 (11) | 106 (11) | 44 (12) | 40 (12) |
| BRAF V600E | ||||
| Wild-type | 736 (81) | 743 (78) | 355 (95) | 332 (97) |
| Mutation | 154 (17) | 190 (20) | 0 (0) | 1 (0) |
| Missing | 19 (2) | 21 (2) | 19 (5) | 10 (3) |
Abbreviation: mFOLFOX6, the modified sixth version of the leucovorin, fluorouracil, and oxaliplatin regimen.
Included all patients enrolled in North Central Cancer Treatment Group N0147 from the entire North American cooperative group system.
Race categorized using National Cancer Institute definitions.18 Other race included all other race/ethnicity not classified as white or black.
Any indication of adherence of the tumor or surrounding structures related to inflammation.
Disease-Free Survival
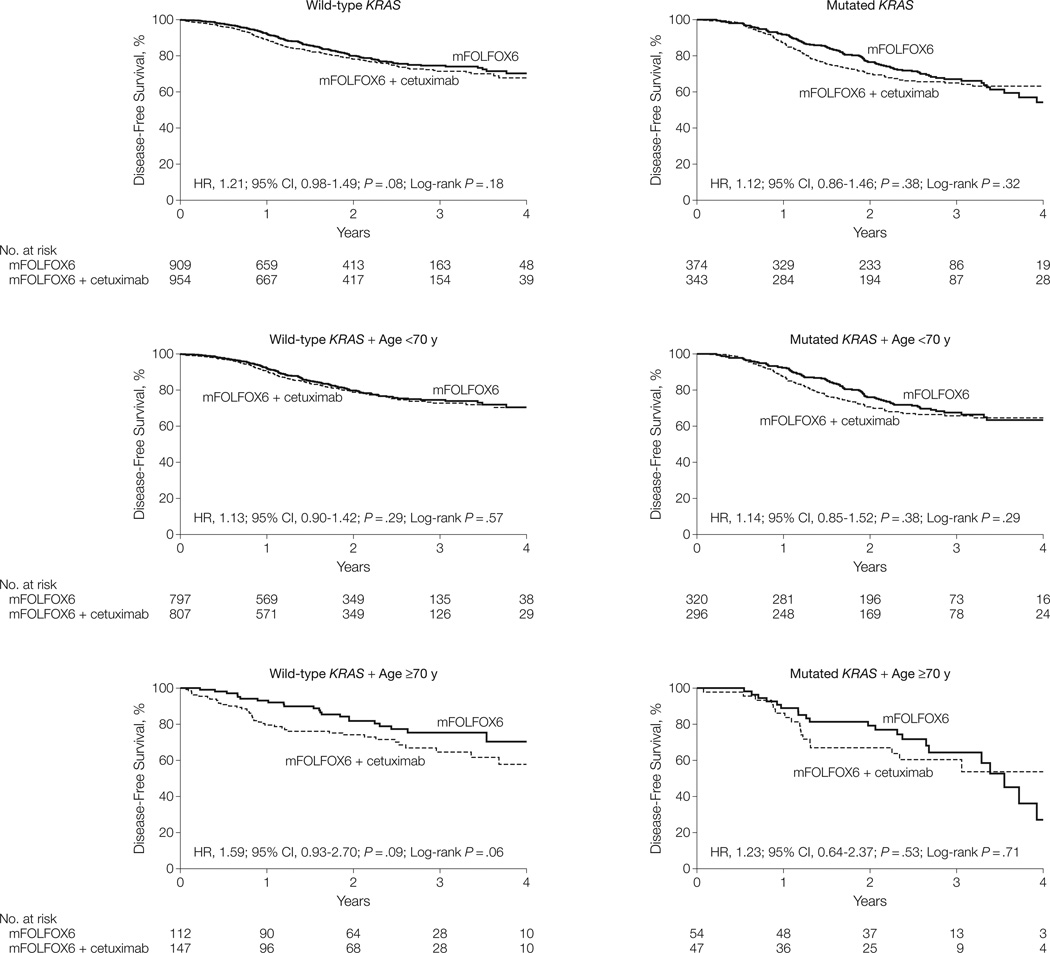
Three-year disease-free survival (Table 2) for mFOLFOX6 alone was 74.6% vs 71.5% with the addition of cetuximab (HR, 1.21; 95% CI, 0.98–1.49; P=.08) in patients with wild-type KRAS, and 67.1% vs 65.0% (HR, 1.12; 95% CI, 0.86–1.46; P=.38) in patients with mutated KRAS, with no evidence of benefit in any individual subgroup (Figure 2). Results are reported from multivariate Cox proportional hazards regression models, adjusted for the stratification factors of histologic grade, N stage, and T stage. Tests for proportional hazards showed no substantial departure (all P > .01), with a single exception of nonproportionality of tumor grade in patients with wild-type KRAS aged 70 years or older (Cox proportional hazards regression test, P=.003).
Table 2.
Patient Outcomes Within the Wild-Type KRAS and Mutated KRAS Patient Groups by Treatment and Age Groupa
|
mFOLFOX6 |
mFOLFOX6 + Cetuximab |
||||||
|---|---|---|---|---|---|---|---|
| Event | Age Group, y |
No. of Events/ Total No. |
3-Year % Event-Free (95% CI)b |
No. of Events/ Total No. |
3-Year % Event-Free (95% CI)b |
Hazard Ratio (95% CI)c |
P Valuec,d |
| Wild-type KRAS | |||||||
| Disease-free survival | All ages | 163/909 | 74.6 (71.1–78.3) | 192/954 | 71.5 (67.8–75.4) | 1.21 (0.98–1.49) | .08 |
| <70 | 141/797 | 74.5 (70.7–78.5) | 152/807 | 72.7 (68.8–76.9) | 1.13 (0.90–1.42) | .29 | |
| ≥70 | 22/112 | 75.3 (66.4–85.5) | 40/147 | 64.5 (55.3–75.4) | 1.59 (0.93–2.70) | .09 | |
| Overall survival | All ages | 78/909 | 87.3 (84.3–90.3) | 94/954 | 85.6 (82.7–88.7) | 1.25 (0.92–1.68) | .15 |
| <70 | 64/797 | 87.4 (84.2–90.7) | 62/807 | 88.4 (85.5–91.5) | 1.02 (0.72–1.44) | .92 | |
| ≥70 | 14/112 | 86.2 (78.9–94.1) | 32/147 | 72.5 (64.0–82.0) | 2.00 (1.05–3.78) | .03 | |
| Time-to-recurrence | All ages | 146/909 | 76.9 (73.4–80.4) | 165/954 | 74.4 (70.8–78.3) | 1.17 (0.93–1.46) | .18 |
| <70 | 128/797 | 76.6 (72.9–80.4) | 137/807 | 75.1 (71.2–79.2) | 1.13 (0.89–1.44) | .31 | |
| ≥70 | 18/112 | 79.0 (70.3–88.8) | 28/147 | 70.8 (61.3–81.6) | 1.36 (0.74–2.50) | .32 | |
| Mutated KRAS | |||||||
| Disease-free survival | All ages | 112/374 | 67.1 (61.8–72.8) | 111/343 | 65.0 (59.8–70.7) | 1.12 (0.86–1.46) | .38 |
| <70 | 91/320 | 67.5 (61.9–73.6) | 94/296 | 65.7 (60.1–71.8) | 1.14 (0.85–1.52) | .38 | |
| ≥70 | 21/54 | 64.3 (50.9–81.3) | 17/47 | 60.4 (46.8–78.0) | 1.23 (0.64–2.37) | .53 | |
| Overall survival | All ages | 44/374 | 87.9 (84.0–91.9) | 49/343 | 82.7 (78.0–87.6) | 1.27 (0.85–1.92) | .25 |
| <70 | 34/320 | 88.5 (84.3–92.8) | 37/296 | 84.7 (79.9–89.8) | 1.24 (0.77–1.98) | .38 | |
| ≥70 | 10/54 | 84.9 (75.1–96.0) | 12/47 | 68.9 (54.5–87.2) | 1.73 (0.74–4.07) | .21 | |
| Time-to-recurrence | All ages | 107/374 | 67.9 (62.6–73.6) | 101/343 | 67.0 (61.7–72.7) | 1.07 (0.81–1.40) | .63 |
| <70 | 87/320 | 68.5 (62.9–74.6) | 87/296 | 67.2 (61.6–73.3) | 1.10 (0.81–1.48) | .55 | |
| ≥70 | 20/54 | 64.3 (50.9–81.3) | 14/47 | 65.5 (51.6–83.1) | 1.05 (0.52–2.11) | .90 | |
| Mutated BRAF | |||||||
| Disease-free survival | All ages | 41/155 | 67.3 (59.3–76.3) | 51/192 | 68.9 (61.7–76.9) | 1.15 (0.76–1.75) | .50 |
| Overall survival | All ages | 30/155 | 74.8 (67.2–83.2) | 38/192 | 73.7 (66.4–81.9) | 1.21 (0.75–1.97) | .43 |
| Time-to-recurrence | All ages | 36/155 | 71.2 (63.4–79.9) | 44/192 | 71.9 (64.8–79.8) | 1.13 (0.72–1.76) | .59 |
| Wild-type BRAF and wild-type KRAS | |||||||
| Disease-free survival | All ages | 119/736 | 76.0 (72.0–80.2) | 138/743 | 72.0 (67.7–76.6) | 1.22 (0.96–1.56) | .11 |
| Overall survival | All ages | 47/736 | 90.1 (86.9–93.4) | 54/743 | 89.3 (86.3–92.4) | 1.22 (0.82–1.81) | .32 |
| Time-to-recurrence | All ages | 108/736 | 77.8 (73.9–81.8) | 119/743 | 74.9 (70.7–79.4) | 1.17 (0.90–1.52) | .23 |
Abbreviation: mFOLFOX6, the modified sixth version of the leucovorin, fluorouracil, and oxaliplatin regimen.
Within all patients having wild-type KRAS, 74.6% (95% CI, 71.1%–78.3%) and 71.5% (95% CI, 67.8%–75.4%) of patients were event free for disease-free survival following treatment with mFOLFOX6 and mFOLFOX6 with cetuximab, respectively. HR of more than 1 indicates the increase in risk associated with the addition of cetuximab and for the event listed for each row. The addition of cetuximab failed to reach statistical significance (disease-free survival: hazard ratio, 1.21; 95% CI, 0.98–1.49; P=.08).
Kaplan-Meier method estimate of the percentage of patients event-free (eg, disease-free, alive) at 3 years, with corresponding 95% CI.
Hazard ratio and P value reported from a multivariate Cox proportional hazards regression model, adjusted for number of nodes, histologic grade, and T stage.
By Wald statistic.
Figure 2.
Disease-Free Survival After Treatment With mFOLFOX6 Alone and mFOLFOX6 With Cetuximab in the Wild-Type KRAS, Mutated KRAS, and Wild-Type KRAS and Mutated KRAS With Age <70 and ≥70 Years Patient Groups
mFOLFOX6 indicates the modified sixth version of the leucovorin, fluorouracil, and oxaliplatin regimen; HR, hazard ratio.
Secondary Outcome Measures
Both time-to-recurrence and overall survival were not significantly different between treatment groups (Table 2). Patients aged 70 years or older with wild-type KRAS who were treated with mFOLFOX6 alone demonstrated significantly better overall survival (HR, 2.00; 95% CI, 1.05–3.78; P=.03), with a 3-year estimate of 86.2% (95% CI, 78.9%–94.1%) for the mFOLFOX6 alone group vs 72.5% (95% CI, 64.0%–82.0%) for the mFOLFOX6 with cetuximab group. The 3-year estimates for disease-free survival for patients with mutated KRAS aged 70 years or older were 64.3% (95% CI, 50.9%–81.3%) vs 60.4% (95% CI, 46.8%–78.0%) for the mFOLFOX6 with cetuximab group (HR, 1.23; 95% CI, 0.64–2.37; P=.53). Subgroup analyses were performed based on age, sex, performance status, and BRAF; no subgroup benefited from the addition of cetuximab (eFigure 2).
Disease-free survival of patients classified as wild-type BRAF (83% in mFOLFOX6 and 80% in mFOLFOX6 with cetuximab) who received mFOLFOX6 with cetuximab did not significantly differ from that of patients who received mFOLFOX6 alone (HR, 1.18; 95% CI, 0.98–1.41; P=.08), in a manner similar to that observed in the patients with wild-type KRAS. There was also no significant difference by treatment for disease-free survival in the subset of patients having both wild-type BRAF and wild-type KRAS (HR, 1.22;95%CI, 0.96–1.56; P=.11) (Table 2).
Chemotherapy
Among patients with wild-type KRAS who continued therapy, dose intensity was similar between the mFOLFOX6 alone and mFOLFOX6 with cetuximab groups (eTable 1). However, the ability to complete at least 6 cycles of therapy differed significantly by treatment (80% in mFOLFOX6 with cetuximab vs 89% in mFOLFOX6 alone, P < .001). In addition, 79% of patients treated with mFOLFOX6 alone received all 12 cycles vs 67% of patients treated with mFOLFOX6 and cetuximab (odds ratio [OR], 1.8; 95% CI, 1.4–2.2; P < .001) (eTable 2).
Similar results were observed in patients with mutated KRAS, although the difference was not significant at cycles 10 (P = .09) and cycles 12 (P = .10) (eTable 2). The proportion of elderly patients with wild-type KRAS completing cycles 6 to 12 of oxaliplatin and fluorouracil was approximately 10% to 20% less than those patients younger than 70 years (all P < .001). Elderly patients (aged ≥70 years) with wild-type KRAS had more frequent dose reductions, receiving a median of 57.5% of planned dose at cycle 12 vs 80% in younger patients with wild-type KRAS. In general, similar results were observed in the patients with mutated KRAS, with smaller differences by age group (eTable 2).
Adverse Events
In patients with wild-type KRAS, grade 3 or higher adverse events occurred in 51.1% of patients receiving mFOLFOX6 alone and 73.3% of patients receiving mFOLFOX6 with cetuximab (OR, 2.6; 95% CI, 2.2–3.2; P < .001) (TABLE 3). In these same patients, grade 3 or higher diarrhea and acne/rash were both significantly more frequent in the mFOLFOX6 with cetuximab group (9.3% vs 15.9% and 0.3% vs 20.0%, respectively; both P < .001). The grade 3 or higher adverse event rates by group within patients with mutated KRAS were similar to those of patients with wild-type KRAS (Table 4).
Table 3.
Grade ≥3 Adverse Events by Treatment Within the Wild-Type KRAS Patient Groupa
| No. (%) of Patients |
|||||||
|---|---|---|---|---|---|---|---|
| mFOLFOX6 (n = 894) |
mFOLFOX6 + Cetuximab (n = 931) |
||||||
| Adverse Eventsb | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | P Valuec |
| Overall | 321 (35.9) | 132 (14.8) | 4 (0.4) | 504 (54.1) | 168 (18.0) | 10 (1.1) | <.001 |
| Hypersensitivity allergy | 21 (2.3) | 2 (0.2) | 0 | 47 (5.0) | 9 (1.0) | 0 | <.001 |
| Cardiovascular | |||||||
| Thrombosis | 18 (2.0) | 15 (1.7) | 1 (0.1) | 19 (2.0) | 14 (1.5) | 1 (0.1) | .86 |
| Infarction | 2 (0.2) | 2 (0.2) | 1 (0.1) | 5 (0.5) | 4 (0.4) | 0 | .32 |
| Constitutional symptoms | |||||||
| Fatigue | 38 (4.3) | 0 | 0 | 58 (6.2) | 2 (0.2) | 0 | .04 |
| Weight loss | 1 (0.1) | 0 | 0 | 7 (0.8) | 0 | 0 | .07d |
| Acne/rash | 3 (0.3) | 0 | 0 | 184 (19.8) | 2 (0.2) | 0 | <.001d |
| Gastrointestinal | |||||||
| Diarrhea | 75 (8.4) | 8 (0.9) | 0 | 145 (15.6) | 3 (0.3) | 0 | <.001 |
| Stomatitis/mucositis | 16 (1.8) | 0 | 0 | 59 (6.3) | 0 | 0 | <.001 |
| Nausea | 32 (3.6) | 0 | 0 | 40 (4.3) | 1 (0.1) | 0 | .37 |
| Vomiting | 25 (2.8) | 2 (0.2) | 0 | 29 (3.1) | 0 | 0 | .91 |
| Anorexia | 9 (1.0) | 0 | 0 | 25 (2.7) | 1 (0.1) | 0 | .005 |
| Neutropenia | 2 (0.2) | 87 (9.7) | 0 | 5 (0.5) | 105 (11.3) | 0 | .20 |
| Infection | 23 (2.6) | 5 (0.6) | 0 | 60 (6.4) | 4 (0.4) | 4 (0.4) | <.001 |
| Febrile neutropenia | 11 (1.2) | 0 | 0 | 18 (1.9) | 5 (0.5) | 0 | .05 |
| Pneumonia | 6 (0.7) | 0 | 0 | 3 (0.3) | 1 (0.1) | 1 (0.1) | .71 |
| Hypomagnesemia | 2 (0.2) | 1 (0.1) | 0 | 16 (1.7) | 4 (0.4) | 0 | <.001d |
| Paresthesias | 135 (15.1) | 3 (0.3) | 0 | 130 (14.0) | 3 (0.3) | 0 | .49 |
| Pulmonary | |||||||
| Dyspnea | 5 (0.6) | 1 (0.1) | 0 | 24 (2.6) | 3 (0.3) | 0 | <.001 |
| Pneumonitis | 3 (0.3) | 2 (0.2) | 0 | 3 (0.3) | 2 (0.2) | 1 (0.1) | .81 |
Abbreviation: mFOLFOX6, the modified sixth version of the leucovorin, fluorouracil, and oxaliplatin regimen.
Patients with wild-type KRAS treated with mFOLFOX6 having grade 3, grade 4, and grade 5 of any classification was 35.9%, 14.8%, and 0.4%, respectively. Addition of cetuximab for these patients resulted in grade 3, grade 4, and grade 5 toxicity rates of 54.1%, 18%, and 1.1%, respectively. During all adverse events experienced, the rate of toxicity of grade 3 or higher was significantly higher for patients with wild-type KRAS treated with cetuximab (2-sided χ2 test, P<.01). Fisher exact test used with at least 1 of the cells in a 2×2 table was less than 5%.
Based on National Cancer Institute, Common Toxicity Criteria, version 3.0.11 Calculated as the maximum severity over all cycles of treatment. Acne/rash includes acne not otherwise specified, rash/desquamation, rash, skin irritation, and rash acneiform. Stomatitis/mucositis includes oral cavity, small bowel, and pharynx. Infection is all infections except pneunomia and febrile neutropenia. Peripheral neuropathy is included in paresthesias.
Two-sided χ2 test comparing the rate of grade 3 or higher by treatment.
By Fisher exact test.
Table 4.
Grade ≥3 Adverse Events by Treatment Within the Mutated KRAS Patient Groupa
| No. (%) of Patients |
|||||||
|---|---|---|---|---|---|---|---|
| mFOLFOX6 (n = 367) |
mFOLFOX6 + Cetuximab (n = 342) |
||||||
| Adverse Eventsb | Grade 3 | Grade 4 | Grade 5 | Grade 3 | Grade 4 | Grade 5 | P Valuec |
| Overall | 139 (37.9) | 64 (17.4) | 1 (0.3) | 164 (48.0) | 78 (22.8) | 5 (1.5) | <.001 |
| Hypersensitivity allergy | 8 (2.2) | 1 (0.3) | 0 | 16 (4.7) | 4 (1.2) | 0 | .02 |
| Cardiovascular | |||||||
| Thrombosis | 9 (2.5) | 3 (0.8) | 0 | 13 (3.8) | 7 (2.0) | 0 | .10 |
| Infarction | 2 (0.5) | 1 (0.3) | 0 | 1 (0.3) | 2 (0.6) | 0 | >.99d |
| Constitutional symptoms | |||||||
| Fatigue | 12 (3.3) | 1 (0.3) | 0 | 14 (4.1) | 3 (0.9) | 0 | .35 |
| Weight loss | 1 (0.3) | 0 | 0 | 3 (0.9) | 0 | 0 | .36d |
| Acne/rash | 0 | 0 | 0 | 69 (20.2) | 4 (1.2) | 0 | <.001d |
| Gastrointestinal | |||||||
| Diarrhea | 27 (7.4) | 2 (0.5) | 0 | 51 (14.9) | 1 (0.3) | 0 | .002 |
| Stomatitis/mucositis | 10 (2.7) | 0 | 0 | 19 (5.6) | 0 | 0 | .06 |
| Nausea | 7 (1.9) | 0 | 0 | 17 (5.0) | 1 (0.3) | 0 | .02 |
| Vomiting | 13 (3.5) | 0 | 0 | 16 (4.7) | 1 (0.3) | 0 | .35 |
| Anorexia | 2 (0.5) | 0 | 0 | 6 (1.8) | 0 | 0 | .16d |
| Neutropenia | 0 | 43 (11.7) | 0 | 1 (0.3) | 45 (13.2) | 0 | .49 |
| Infection | 7 (1.9) | 2 (0.5) | 0 | 14 (4.1) | 3 (0.9) | 1 (0.3) | .05 |
| Febrile neutropenia | 0 | 1 (0.3) | 0 | 6 (1.8) | 1 (0.3) | 0 | .03d |
| Pneumonia | 5 (1.4) | 0 | 0 | 1 (0.3) | 0 (0.0) | 0 | .22d |
| Hypomagnesemia | 0 | 0 | 0 | 4 (1.2) | 2 (0.6) | 0 | .01d |
| Paresthesias | 68 (18.5) | 0 | 0 | 46 (13.5) | 1 (0.3) | 0 | .08 |
| Pulmonary | |||||||
| Dyspnea | 7 (1.9) | 0 | 0 | 7 (2.0) | 0 | 0 | .89 |
| Pneumonitis | 2 (0.5) | 0 | 1 (0.3) | 1 (0.3) | 1 (0.3) | 0 | >.99d |
Abbreviation: mFOLFOX6, the modified sixth version of the leucovorin, fluorouracil, and oxaliplatin regimen.
Patients with mutated KRAS treated with mFOLFOX6 having grade 3, grade 4, and grade 5 of any classification was 37.8%, 17.4%, and 0.3%, respectively. Addition of cetuximab for these patients resulted in grade 3, grade 4, and grade 5 toxicity rates of 47.8%, 22.7%, and 1.5%, respectively. During all adverse events experienced, the rate of toxicity of grade 3 or higher was significantly higher for patients with mutated KRAS treated with cetuximab (2-sided χ2 test, P<.01). Fisher exact test used with at least 1 of the cells in a 2×2 table was less than 5%.
Based on National Cancer Institute, Common Toxicity Criteria, version 3.0.11 Calculated as the maximum severity over all cycles of treatment. Acne/rash includes acne not otherwise specified, rash/desquamation, rash, skin irritation, and rash acneiform. Stomatitis/mucositis includes oral cavity, small bowel, and pharynx. Infection is all infections except pneunomia and febrile neutropenia. Peripheral neuropathy is included in paresthesias.
Two-sided χ2 test comparing the rate of grade 3 or higher by treatment.
By Fisher exact test.
Within the mFOLFOX6 with cetuximab group, any grade 3 or higher toxicity for patients with wild-type KRAS aged younger than 70 years was 72% vs 81% for patients with wild-type KRAS aged 70 years or older (P=.02), primarily due to increased rates of diarrhea (13.5% vs 29.2%, P < .001), dyspnea (2.5% vs 4.9%, P=.13), nausea (3.4% vs 9.7%, P < .001), fatigue (5.0% vs 14.6%, P < .001), infection (6.7% vs 10.4%, P=.12), neutropenia (10.7% vs 18.1%, P = .01), and stomatitis/mucositis (5.6% vs 10.4%, P=.03). Younger patients with wild-type KRAS reported significantly higher rates of acne/rash (21.7% vs 10.4%, P=.002). Significant differences in grade 3 or higher toxicity in older vs younger patients with wild-type KRAS receiving mFOLFOX6 alone were also observed (OR, 1.5; 95% CI, 1.0–2.3; P=.047) (eTable 3).
Four deaths while receiving treatment (3 considered related) occurred in patients with wild-type KRAS receiving mFOLFOX6 alone and 10 deaths (8 considered related) occurred in patients receiving mFOLFOX6 with cetuximab (P=.18). The rate of death during treatment with mFOLFOX6 with cetuximab varied by age (0.5% in patients younger than 70 years vs 4.2% in patients aged 70 years or older, P=.002). In comparison, the rate by age of patients receiving mFOLFOX6 alone was 0.4% in patients younger than 70 years vs 0.9% in patients aged 70 years or older (P=.41). No difference in the rate of death was observed by treatment or by age within treatment within the patients with mutated KRAS.
One patient (0.1%) in the wild-type KRAS group receiving mFOLFOX6 alone and 9 patients (7 with wild-type KRAS and 2 with mutated KRAS) (0.7%) receiving mFOLFOX6 with cetuximab died within 60 days of randomization (P=.02).
COMMENT
In this randomized phase 3 trial for patients with resected stage III colon cancer expressing wild-type KRAS mutations, the addition of cetuximab to mFOLFOX6 did not improve disease-free or overall survival in contradistinction to the original study of cetuximab combined with FOLFOX in metastatic colorectal cancer.5 Multiple trials combining cetuximab with chemotherapy in the metastatic setting have been reported. The first-line trials Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer (CRYSTAL)5 and Oxaliplatin and Cetuximab in the First-Line Treatment of Metastatic Colorectal Cancer (OPUS)19 both demonstrated significant improvements in outcomes with cetuximab in patients with wild-typeKRAS. However, the first-line trials Continuous Chemotherapy plus Cetuximab or Intermittent Chemotherapy (COIN)20 and NORDIC for the trial VII of the Nordic Colorectal Cancer Biomodulation Group (NORDIC VII)21 did not show benefit with the addition of cetuximab.
The reasons for the lack of benefit of mFOLFOX6 with cetuximab in the adjuvant setting remain unclear. The observed 3-year disease-free survival in the mFOLFOX6 alone group, pooled over patients with mutated KRAS and wild-type KRAS, is 72% (95% CI, 69%–75%), identical to that observed in the Multicenter International Study of Oxaliplatin/Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) trial2 that established FOLFOX as the standard of care. The overall rates of death within 60 days of treatment initiation were 0.1% for the mFOLFOX6 alone group and 0.6% for the mFOLFOX6 with cetuximab group, consistent with the 0.3% rate reported for the FOLFOX group of the MOSAIC trial.2 The patients enrolled in NCCTG N0147 were from the entire North American cooperative group system. Thus, it is unlikely that the results could be explained by a problem with patient selection. Other potential explanations include toxicity limiting administration of planned therapy, differential effect related to age, adverse effect of cetuximab on the activity of chemotherapy, effect of a specific subgroup on overall results, and an alteration in the mechanism of action of cetuximab in the setting of micrometastatic disease.
In the COIN trial,22 the addition of cetuximab resulted in dose reductions of chemotherapy at twice the rate observed in patients receiving chemotherapy alone, regardless of age. The ability of patients with wild-type KRAS in NCCTG N0147 to complete the planned course of therapy was reduced in those receiving cetuximab, most notably in patients aged 70 years or older. The inability to give either the planned duration of therapy or dose intensity was primarily related to gastrointestinal symptoms and fatigue. For older patients, the addition of neutropenia further limited therapy with the mFOLFOX6 with cetuximab protocol.
Only 51% of patients with wild-type KRAS aged 70 years or older completed 12 cycles of therapy when receiving cetuximab vs 78% of those receiving mFOLFOX6 alone. Toxicity therefore may have had a direct effect in reducing the potential benefit derived from cetuximab added to mFOLFOX6. Smaller differences were observed by treatment for patients with mutated KRAS than were observed for the patients with wild-type KRAS, suggesting that the ability to complete cetuximab-containing therapy is more challenging in patients with wild-type KRAS vs with mutated KRAS.
The ability of cetuximab to enhance the benefit of chemotherapy appears to be complex. Phase 3 trials assessing the activity of cetuximab in combination with a fluoropyrimidine for metastatic disease have only shown a benefit when an infusional fluoropyrimidine regimen was used. In the COIN20 and NORDIC VII21 clinical trials, the lack of benefit from cetuximab was primarily restricted to patients receiving either capecitabine or bolus fluorouracil as opposed to infusional fluorouracil. A subgroup analysis in the COIN trial demonstrated a benefit to the addition of cetuximab to infusional fluorouracil and oxaliplatin. However, the mFOLFOX6 regimen used in our trial should have overcome this negative interaction, if it exists.
Specific subgroups of patients were evaluated in a posthoc analysis. When disease-free survival outcomes were assessed using other age categories, sex, performance status, grade of the tumor, and T and N status, no subgroup showed benefit (eFigure 2). In addition, with the recognition that patients with a mutated BRAF tumor have a potential worse outcome,23 the disease-free survival for the subgroup of patients with tumors expressing both wild-type KRAS and wild-type BRAF was assessed. This subgroup also showed no benefit and disease-free survival rates were fairly consistent in patients with wild-type KRAS. Additional subgroup analyses are under way using other molecular markers.
The role of adjuvant therapy is to eradicate micrometastatic disease. Molecular characteristics of micrometastases appear to differ from established metastases.24,25 The evolution from the appearance of a tumor with malignant potential to the development of metastatic disease and ultimately the establishment of distant metastatic foci is a dynamic and complex process, exemplified by the epithelial-mesenchymal transition and its reverse mesenchymal-epithelial transition. As the tumor evolves, cell-cell adherence is reduced along the invasion front allowing the migration and spread of tumor cells by epithelial-mesenchymal transition, through repression of E-cadherin.26 Once the metastasizing cells reach its final site, the cells undergo mesenchymal-epithelial transition as they regain the ability to form cell-cell adherence.27 Assessment of signaling pathways suggest that epidermal growth factor has a significant role during epithelial-mesenchymal transition in the change that occurs in Ecadherin.28 Cross talk that develops during this phase between the receptor for epidermal growth factor and other signaling pathways may provide an escape mechanism from anti–epidermal growth factor receptor therapy.29,30 Additional preclinical research is required to establish the importance of these observations in colon cancer.
In addition, the activity of cetuximab on metastatic disease occurs through a variety of mechanisms.31 Cetuximab leads to cell-cycle arrest in G1 phase, as well as induces apoptosis, inhibits tumor angiogenesis, and activates antibody-dependent cellular toxicity. Although these mechanisms of activity have been defined, little is known about acquired or intrinsic resistance to cetuximab beyond mutations in KRAS and possibly BRAF.32,33 BRAF was mutated in 18% of the wild-type KRAS tumors from patients enrolled in our trial. When analyzed by BRAF status, no effect on cetuximab benefit was observed.
In conclusion, in this randomized phase 3 trial for patients with resected stage III colon cancer, no benefit was observed from the addition of cetuximab to mFOLFOX6 therapy, even when restricted to patients with tumors expressing wild-type KRAS and wild-type BRAF. New approaches are needed to identify drugs that may be of benefit in adjuvant therapy, because as shown in our trial promising activity in the metastatic setting did not translate into adjuvant therapy benefit and underscores the importance of performing clinical trials.
Acknowledgments
Funding/Support: This trial was conducted as a collaborative trial of the North Central Cancer Treatment Group (NCCTG), Mayo Clinic, and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35103, CA-35113, CA-35272, CA-114740, CA-32102, CA-14028, CA49957, CA21115, CA31946, CA12027, CA37377 from the National Cancer Institute, Department of Health and Human Services. Bristol-Myers Squibb, ImClone, sanofi-aventis, and Pfizer provided unrestricted support to NCCTG for conduct of trial. Bristol-Myers Squibb provided cetuximab to NCCTG.
Role of the Sponsors: The National Cancer Institute participated in the design and conduct of the trial, but not in the data collection, analysis and interpretation of the data, or in the preparation, review, or approval of the manuscript. Bristol-Myers Squibb, ImClone, sanofi-aventis, and Pfizer had no role in the design and conduct of the trial, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Alberts had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Alberts, Sargent, Nair, Mahoney, Mooney, Chan, Grothey, Goldberg.
Acquisition of data: Alberts, Nair, Mahoney, Thibodeau, Smyrk, Sinicrope, Chan, Gill, Kahlenberg, Shields, Quesenberry, Webb, Goldberg.
Analysis and interpretation of data: Alberts, Sargent, Nair, Mahoney, Mooney, Sinicrope, Chan, Farr, Pockaj, Grothey, Goldberg.
Drafting of the manuscript: Alberts, Sargent, Mahoney, Grothey, Goldberg.
Critical revision of the manuscript for important intellectual content: Alberts, Sargent, Nair, Mahoney, Mooney, Thibodeau, Smyrk, Sinicrope, Chan, Gill, Kahlenberg, Shields, Quesenberry, Webb, Farr, Pockaj, Grothey, Goldberg.
Statistical analysis: Sargent, Mahoney.
Obtained funding: Alberts, Sargent, Goldberg.
Administrative, technical, or material support: Alberts, Sargent, Mahoney, Mooney, Thibodeau, Smyrk, Chan, Goldberg.
Study supervision: Alberts, Sargent, Nair, Mahoney, Sinicrope, Grothey, Goldberg.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Alberts reported receiving grants from Pfizer, Bristol-Myers Squibb, and sanofi-aventis by the North Central Cancer Treatment Group (NCCTG) for support of the conduct of this trial. Dr Sargent reported receiving grants from Pfizer, Bristol-Myers Squibb, and sanofi-aventis for support to NCCTG for conduct of this trial. Dr Nair reported receiving support for travel to meetings from National Cancer Institute Cooperative Group. Dr Sinicrope reported receiving consultancy fees from Merck Serono, receiving grants from the National Institutes of Health, payment for lectures including service on service bureaus from the University of Kansas, and travel meeting expenses from the American Society of Clinical Oncology. Dr Chan reported receiving a grant from the Eastern Cooperative Oncology Group; being a board member of Colorectal Cancer Index and Reviews and HCPLive.com Oncology Advisory Board; being a consultant on advisory boards of Amgen, ImClone, Bristol-Myers Squibb, Genentech, Pfizer, and Celgene; receiving grants and travel meeting expenses from National Comprehensive Cancer Network; receiving travel meeting expenses from Chemotherapy Foundation; and being an institutional investigator in numerous clinical trials involving multiple pharmaceutical companies. Dr Gill reported receiving payment for lectures including service on speakers bureaus from Bristol-Myers Squibb. Dr Kahlenberg reported receiving payment for lectures including service on speakers bureaus from Genentech. Dr Shields reported receiving grant and support for travel to meetings for the study from Southwest Oncology Group. Dr Grothey reported receiving grants and consultancy fees from sanofi-aventis. No other authors provided any financial disclosures.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institutes of Health.
Previous Presentation: Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology; June 4–8, 2010; Chicago, Illinois.
Online-Only Material: The 2 eFigures and 3 eTables are available at http://www.jama.com.
Additional Contributions: We thank all of the patients who elected to participate in this trial. The successful completion of this trial was the result of the collaborative effort between the North Central Cancer Treatment Group and the North American Intergroup (Cancer and Leukemia Group B; Eastern Cooperative Oncology Group; National Cancer Institute of Canada, Clinical Trials Group; National Surgical Adjuvant Breast and Bowel Project; Southwest Oncology Group). We thank all of the data coordinators and research staff for their roles in the implementation and oversight of the trial. We also thank all of the physicians, nurses, and other health care professionals that cared for the patients who participated in this trial.
REFERENCES
- 1.Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122(5):321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 2.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 3.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 4.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 5.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 6.Douillard J-Y, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 7.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26(35):5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 8.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25(23):3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27(19):3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 10.Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802) Ann Oncol. 2009;20(4):674–680. doi: 10.1093/annonc/mdn680. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. [Accessed March 12, 2012];Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. 2003 Mar 31; http://ctep.cancer.gov.
- 12.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 13.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 14.Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22(7):1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 16.Cox DR. Regression models and life tables. J R Stat Soc [Ser A] 1972;B34(2):187–200. [Google Scholar]
- 17.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Accessed March 1, 2012]. 2011. http://www.R-project.org/ [Google Scholar]
- 18.National Cancer Institute. [Accessed March 5, 2012];A Manual for Participants in Clinical Trials of Investigational Agents Sponsored by DCTD, NCI. http://ctep.cancer.gov/investigatorResources/docs/hndbk.pdf.
- 19.Van Cutsem E, Köhne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 20.Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer. Lancet Oncol. 2011;12(7):642–653. doi: 10.1016/S1470-2045(11)70102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tveit K, Guren T, Glimelius B, et al. Randomized phase III study of 5-fluorouracil/floinate/oxaliplatin given continuously or intermittently with or without cetuximab, as first-line treatment of metastatic colorectal cancer [abstract] Ann Oncol. 2010;21(suppl 8):viii9. [Google Scholar]
- 22.Adams RA, Meade AM, Madi A, et al. Toxicity associated with combination oxaliplatin plus fluoropyrimidine with or without cetuximab in the MRC COIN trial experience. Br J Cancer. 2009;100(2):251–258. doi: 10.1038/sj.bjc.6604877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer. Lancet Oncol. 2010;11(8):753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 24.Iiizumi M, Liu W, Pai SK, Furuta E, Watabe K. Drug development against metastasis-related genes and their pathways: a rationale for cancer therapy. Biochim Biophys Acta. 2008;1786(2):87–104. doi: 10.1016/j.bbcan.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riethdorf S, Wikman H, Pantel K. Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123(9):1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- 26.Natalwala A, Spychal R, Tselepis C. Epithelial-mesenchymal transition mediated tumourigenesis in the gastrointestinal tract. World J Gastroenterol. 2008;14(24):3792–3797. doi: 10.3748/wjg.14.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hugo H, Ackland ML, Blick T, et al. Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 28.Shin S-Y, Rath O, Zebisch A, et al. Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer Res. 2010;70(17):6715–6724. doi: 10.1158/0008-5472.CAN-10-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karamouzis MV, Konstantinopoulos PA, Papavassiliou AG. Targeting MET as a strategy to overcome crosstalk-related resistance to EGFR inhibitors. Lancet Oncol. 2009;10(7):709–717. doi: 10.1016/S1470-2045(09)70137-8. [DOI] [PubMed] [Google Scholar]
- 30.Buck E, Eyzaguirre A, Barr S, et al. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6(2):532–541. doi: 10.1158/1535-7163.MCT-06-0462. [DOI] [PubMed] [Google Scholar]
- 31.Vincenzi B, Schiavon G, Silletta M, Santini D, Tonini G. The biological properties of cetuximab. Crit Rev Oncol Hematol. 2008;68(2):93–106. doi: 10.1016/j.critrevonc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28(7):1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 33.Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp Cell Res. 2010;316(7):1083–1100. doi: 10.1016/j.yexcr.2010.01.009. [DOI] [PubMed] [Google Scholar]