Abstract
A large gene downstream of the primary Bacteroides cellulosolvens cellulosomal scaffoldin (cipBc, now renamed scaA) was sequenced. The gene, termed scaB, contained an N-terminal leader peptide followed by 10 type I cohesins, an “X” domain of unknown structure and function, and a C-terminal S-layer homology (SLH) surface-anchoring module. In addition, a previously identified gene in a different part of the genome, encoding for a dockerin-borne family 48 cellulosomal glycoside hydrolase (Cel48), was sequenced completely, and a putative cellulosome-related family 9 glycosyl hydrolase was detected. Recombinant fusion proteins, comprising dockerins derived from either the ScaA scaffoldin or Cel48, were overexpressed. Their interaction with ScaA and ScaB cohesins was examined by immunoassay. The results indicated that the ScaB type I cohesin of the new anchoring protein binds selectively to the ScaA dockerin, whereas the Cel48 dockerin binds specifically to the type II ScaA cohesin 5. Thus, by virtue of the 11 type II ScaA cohesins and the 10 type I ScaB cohesins, the relatively simple two-component cellulosome-integrating complex would potentially incorporate 110 enzyme molecules onto the cell surface via the ScaB SLH module. Compared to previously described cellulosome systems, the apparent roles of the B. cellulosolvens cohesins are reversed, in that the type II cohesins are located on the enzyme-binding primary scaffoldin, whereas the type I cohesins are located on the anchoring scaffoldin. The results underscore the extensive diversity in the supramolecular architecture of cellulosome systems in nature.
Cellulosomes are multienzyme complexes designed for the efficient degradation of plant cell wall polysaccharides in general and cellulose in particular (3, 5-8, 17, 18, 51, 52). The cellulosome, first described for the anaerobic thermophile, Clostridium thermocellum (4, 27, 31, 32), comprises a central scaffoldin subunit that incorporates the various enzymes into the complex and targets the complex to the substrate (19, 20, 53, 54). For this purpose, the scaffoldin molecule carries multiple cohesin modules that selectively bind to a complementary domain, the dockerin, located on the enzymes. The high-affinity cohesin-dockerin interaction is responsible for the cellulosome architecture. Substrate targeting is mediated by a cellulose-binding carbohydrate-binding module (CBM), which is located on the scaffoldin molecule (47).
Two major types of cellulosome have thus far been described. In one, many of the cellulosomal genes are arranged in a cluster on the genome, consisting of the scaffoldin gene followed sequentially downstream by the various dockerin-containing enzyme genes. Examples of this type of cellulosome system can be found in various mesophilc clostridia, such as C. cellulolyticum (1, 45), C. cellulovorans (57, 58), C. josui (24), and C. acetobutylicum (43, 49). In the other type, a more elaborate arrangement is observed wherein the scaffoldin gene is clustered together on the genome with one or more anchoring proteins that serve to selectively incorporate the cellulosome onto the cell surface. Each anchoring protein bears one or more cohesins and an S-layer homology (SLH) module for this purpose, such that the anchoring cohesin(s) bind strongly to the resident dockerin of the primary (enzyme-recognizing) scaffoldin. In this type of cellulosome system, the genes for the various enzymes are distributed elsewhere on the genome either alone or in small clusters. Evidence for the scaffoldin-anchoring gene cluster arrangement was first reported for C. thermocellum (19, 36). Recently, similar scaffoldin gene clusters have also been described for Acetivibrio cellulolyticus (13, 59) and Ruminococcus flavefaciens (15, 48).
We describe here the presence of a pair of scaffoldin genes on the chromosome of Bacteroides cellulosolvens. One of the genes encodes for the previously reported (14) primary scaffoldin, CipBc (herein renamed ScaA). Another scaffoldin-coding gene, scaB, is located immediately downstream of the scaA gene. The modular architecture of the two proteins, the modular functions, and intermodular interactions are discussed.
MATERIALS AND METHODS
Preparation of B. cellulosolvens.
Cellulose-binding extracellular proteins and cell-associated protein fractions were prepared from cellobiose-grown cells of B. cellulosolvens ATCC 35603 as described previously (13). Cellulose-binding proteins were obtained by adsorbing cell-free culture supernatant fluids with a 1% volume of a 10-mg/ml suspension of amorphous cellulose (28).
Isolation of genomic DNA and construction of genomic libraries.
B. cellulosolvens genomic DNA was isolated according to the protocol of Murray and Thompson (41). B. cellulosolvens genomic libraries were constructed by using the Lambda ZAP II undigested vector kit for an SacI library and the Uni-ZAP XR vector kit for an EcoRI-XhoI library. Both kits were obtained from Stratagene Cloning Systems (La Jolla, Calif.). Both libraries included fragments that reflected star activities (aberrant cleavage) of SacI and EcoRI. In some instances, this phenomenon facilitated application of genome walking with overlapping fragments when we used a single library (see Results).
PCR and subcloning.
PCRs were performed by using a Master Personal device (Eppendorf, Hamburg, Germany) at various annealing temperatures, 55 to 60°C, and DNA polymerase of TaKaRa ExTaq was purchased from TaKaRa Bio, Inc. (Shiga, Japan). The resulting PCR fragments were cloned by using the pGEM-T vector system 1 (Promega Corp., Madison, Wis.). Escherichia coli XL-1 strains were used as host cells for transformation. DNA samples were purified by using either the QIAquick PCR purification kit (Qiagen, Inc., Valencia, Calif.), or agarose gel DNA extraction kit (Roche Diagnostics Corp., Indianapolis, Ind.). Plasmids were purified by using the High Puri plasmid isolation kit (Boehringer Mannheim, Germany).
Library screening.
B. cellulosolvens genomic DNA libraries were screened with appropriate probes prepared by PCR with relevant primers. Southern blotting was performed according to the protocol described in the DIG Application Manual for Filter Hybridization (Roche Molecular Biochemicals).
Genomic-walking PCR.
Two B. cellulosolvens genomic DNA libraries were screened by using either one- or two-step PCR, which were applied to amplify the forward and backward sequences of a known region. In the first step, λ phage libraries were used as templates, and PCR was performed with a combination of a specific primer (Table 1), designed from a previously sequenced region of the target gene, and a lambda phage vector primer, i.e., either T3 and M13/pUC(−21) or T7 and M13/pUC(−20). For the second step, a 100-fold dilution of the first PCR product served as a template for PCR with a nested primer, designed from an inner sequence of the known region, and one of the latter primers from the phage vector.
TABLE 1.
Primers used in this study
| Primer | Nucleotide sequencea | Locationb | Comments |
|---|---|---|---|
| F-BC-1 | GGAAGTGTTTTCACAAATGTGTCG | Signal peptide, cipBc (scaA) | Probe for library screening |
| R-BC-1 | CCAATCAAACACCATAGTACCGTC | Coh-1, scaA | Probe for library screening |
| F-BC-AN-1 | GCCTGATTATGGAACATTGTTGCAGGG | Coh-11, scaA | Probe for library screening |
| R-BC-AN-1 | CTGCTGGGAACTCGGAAATCATTCC | Doc, scaA | Probe for library screening |
| Coh-F | AGACCATGGGTTCAGGAGTAGTAGCAAC | Coh5, scaA | Expression, NcoI, His tag |
| Coh-R | AGTCTCGAGTCCGTTTATTGAAGAAGCCTG | Coh5, scaA | Expression, XhoI, His tag |
| PBC-Coh11-N | CTGCCATGGGATCTGTATTGACAGCT | Coh11, scaA | Expression, NcoI, His tag |
| PBC-Coh11-C | TACCTCGAGTGTGCCTTTTGGATAGAT | Coh11, scaA | Expression, XhoI, His tag |
| F-EX-BCcohB3 | GGGCCATGGGGAAAAGTTCACCAGGAAATAAAATG | Coh3, scaB | Expression, NcoI, His tag |
| R-EX-BCcohB3 | CCCCTCGAGATTAGTTACAGTAATGCTTCCATC | Coh3, scaB | Expression, XhoI, His tag |
| F-9dxyn-docGH48 | ATAGGTACCTCCATTTGTTAAATTAAAAGGTGAC | Doc, cel48A | Expression, KpnI, fused to Xyn T6 |
| R-9dxyn-docGH48 | CCCGGATCCAAACATTACGACCTCTGCTGATTT | Doc, cel48A | Expression, BamHI, fused to Xyn T6 |
| F-9dxyn-docCipBc | ATAGGTACCTCCAAAAGGCACAGCTACAGTATTA | Doc, scaA | Expression, KpnI, fused to Xyn T6 |
| R-9dxyn-docCipBc | GGGGGATCCTTTTTGTTCTGCTGGGAACTCGGA | Doc, scaA | Expression, BamHI, fused to Xyn T6 |
Residues shown in italics were added to the 5′ ends to generate the designated restriction enzyme cleavage sites (underlined [see Comments column]).
Abbreviations: Doc, dockerin domain; Coh, cohesin domain.
DNA sequencing.
DNA sequencing was performed either directly on PCR products or on cloned fragments on an ABI Prism 3100 genetic analyzer (Applied Biosystems, Foster City, Calif.) at the Sequencing Lab of Tel Aviv University in Israel. The resulting sequences were compared to known cellulosome-related proteins.
Cloning and overexpression of recombinant proteins.
The appropriate genes were subcloned into expression vectors via PCR (Fig. 1 for details). The PCR products were cloned into either the pET28a or pET9d vectors, and their intact sequences were verified by DNA sequencing. The clones (Table 2) were expressed either in E. coli BL21(DE3) for cohesins or in E. coli BL21(DE3)/pLyS (Stratagene) for xylanase-dockerins at 16°C, grown in the presence of 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). After growth, the cultures were lysed by sonication according to the method of Ding et al. (15). The expressed proteins were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10 to 12%) and staining with Coomassie brilliant blue.
FIG. 1.
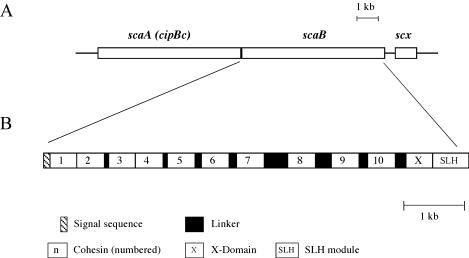
Scheme showing the disposition on the genome and the modular organization of the scaB gene from B. cellulosolvens. The gene is positioned immediately downstream of scaA (cipBc). scaB contains an N-terminal signal sequence, 10 copies of type I cohesin domains (numbered), an “X” domain of unknown function, and a C-terminal SLH module. Cohesins 1 and 2 and cohesins 3 and 4 are closely attached with little or no identifiable linker sequences, whereas Pro/Thr-rich linker segments of varied length separate the other cohesins. An additional ORF (scx) appears downstream of scaB that bears strong resemblance to members of the family of sodium-calcium exchanger integral membrane proteins.
TABLE 2.
Expressed proteins prepared in this study
| Protein | Modular content | Plasmid | Tag |
|---|---|---|---|
| CohA5 | Cohesin 5 of CipBc (ScaA) | pET28a | C-terminal His tag |
| CohA11 | Cohesin 11 of CipBc (ScaA) | pET28a | C-terminal His tag |
| CohB3 | Cohesin 3 of ScaB | pET28a | C-terminal His tag |
| Xyn-DocA | Hybrid construct consisting of G. stearothermophilus Xyn T6 harboring the B. cellulosolvens ScaA dockerin at the C terminus | pET9d | N-terminal His tag |
| Xyn-DocGH48 | Hybrid construct consisting of Xyn T6 and the B. cellulosolvens Cel48A dockerin at the C terminus | pET9d | N-terminal His tag |
Immunoblotting.
Proteins were subjected to SDS-PAGE (10%), and the separated proteins were then transferred onto a nitrocellulose membrane and rinsed with wash buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 25 mM CaCl2). The membrane was then incubated for 2 to 3 h with blocking buffer (3% bovine serum albumin in washing buffer) and rinsed five times with the wash buffer. The membrane was then incubated overnight at 4°C with the recombinant His-tagged protein. The membrane was then treated with peroxidase-conjugated antibody [anti-His(C-terminal)-horseradish peroxidase-labeled mouse antibody] detection system according to the supplier's instructions (Invitrogen Corp., Carlsbad, Calif.). Bands were visualized by using a chemiluminescent substrate (Supersignal Substrate [Western blotting]; Pierce Biotechnology, Rockford, Ill.) according to the manufacturer's instructions.
Peptide sequencing.
Selected protein bands were excised from SDS-PAGE gels and subjected to proteolysis with Lys-C (Promega), and the resultant peptides were resolved on reversed-phase high-pressure liquid chromatography, analyzed, and sequenced by Edman degradation (Protein Center, Technion, Haifa, Israel). Alternatively, the bands were treated with trypsin, and the tryptic peptides were identified by matrix-assisted laser desorption ionization-mass spectrometry in the Maiman Institute for Proteome Research in Tel Aviv University in Israel. The identified peptide sequence data were compared to the sequences of known genes.
Protein sequence analysis.
Potential signal sequences were determined by the SignalP V2.0 program (42). Similarity searches were performed by using the BLAST (basic local alignment search tool) program provided by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/). The parameters for molecular weight, theoretical pI, amino acid composition, and extinction coefficient were computed by using the ProtParam Tool (http://www.expasy.org/tools/protparam.html), which is available at the SWISS-PROT website (2). Multiple sequence alignment and phylogenetic trees were generated by using the CLUSTAL W program (http://www2.ebi.ac.uk/clustalw/). Dockerins, cohesins, and other enzyme sequences were obtained from either GenBank (http://www.ncbi.nlm.nih.gov/), the SWISS-PROT website, or via the Carbohydrate-Active Enzymes server (CAZy [http://afmb.cnrs-mrs.fr/∼pedro/CAZY/db.html]), designed by Coutinho and Henrissat (10, 12).
Nucleotide sequence accession number.
The DNA sequence for the scaB and scx genes have been deposited in the GenBank database under accession number AF224509; the GenBank accession number for cel48A is AY374129.
RESULTS
Identification of the B. cellulosolvens scaffoldin cluster.
The disposition on the genome and modular architecture of scaB are illustrated in Fig. 1. The scaB gene is located immediately downstream of the gene that encodes for the primary scaffoldin (formerly termed cipBc, now renamed scaA). In addition, another open reading frame (ORF), scx, was sequenced downstream of scaB, the sequence of which bears strong resemblance to members of the family of sodium-calcium exchanger integral membrane proteins.
The original cipBc sequence lacked the first few amino acid residues and the entire upstream promoter region. Within the framework of the present study, it was of interest to complete the initial and upstream portion of the “scaA” sequence. An EcoRI/XhoI library was screened with a probe derived from the 0.5-kb PCR product via primers of F-BC-1 and R-BC-1 (Table 1), and a 2-kb insert was obtained. The resultant sequence indicated “MRT” as the missing residues at the onset of the cipBc sequence.
In order to sequence the portion of the genome downstream of scaA, two different Lambda ZAPII genomic DNA libraries were generated by using SacI and EcoRI-XhoI, respectively. Primers F-BC-AN-1 and R-BC-AN-1 (Table 1) were designed from the C-terminal portion of the cipBc (scaA). The resultant PCR product was used as a probe for Southern blotting of the SacI genomic DNA library. The isolated positive phage served as a template for PCR, with primers F-BC-AN-1 and M13/pUC(−20), and the resultant 2.4-kb product was sequenced. It was noted that the SacI cleavage site at the C terminus of this fragment was AAGCTA instead of GAGCTC, indicating star activity of the restriction enzyme. Successive genome-walking protocols, with the EcoRI-XhoI and SacI libraries in alternating fashion and appropriate primers (documented in Table 1), yielded overlapping fragments of 1.1, 2.1, 2.2, and 4 kb, respectively. With the exception of the 4-kb fragment, all of these fragments reflected different types of star activity (at the 3′ terminus) by both EcoRI and SacI. In one case (i.e., the generation of the 2.1 and 2.2 overlapping fragments), the infidelity of the restriction enzyme (SacI) enabled successive application of the same library. The final corrected sequence of the entire 9.5-kb segment was verified by direct PCR with B. cellulosolvens genomic DNA as a template.
Sequence analysis of the intergenic flanking regions of the B. cellulosolvens scaffoldin cluster.
Sequence analysis revealed several regulatory regions flanking the scaA and scaB genes. A deduced ribosome-binding site sequence (5′-AGGGGG-3′) is located several bases upstream of the scaA ATG start codon, and two putative −10 and −35 regions that resemble σA promoters occur farther upstream. In addition, 36 bases downstream of the stop codon of scaA, there is a palindromic sequence corresponding to an mRNA hairpin loop with a ΔG of −19.6 kcal/mol. This loop structure probably represents a rho-independent terminator. Upstream of the scaB gene, there is also a typical ribosome binding site (5′-AGGGGG-′3) and a putative σA promoter (5′-AATAAT-3). However, no potential terminator structures were detected downstream of the gene. Based on the sequence analysis, it is likely that scaA and scaB are transcribed separately.
Description of ScaB.
scaB encodes for a 2,299-residue protein that contains a signal peptide, 10 consecutive cohesin modules, followed by an X domain (of unknown structure and function) and an SLH module at the C terminus. The most likely cleavage site of the signal peptide is between residues 29 and 30 (QFA-AT). The signal peptide is somewhat unusual in that a TTG initiation codon is present rather than the more common ATG codon. This is consistent both with the fact that the TTG codon is preceded 10 bp upstream by a typical Shine-Dalgarno sequence (AGGGGG) and with the AT-rich composition (63.9%) of the B. cellulosolvens genome (as calculated from the accumulated 16.6 kb of genomic DNA that has been sequenced in our lab) (26, 56). In addition, as mentioned above, a putative promoter precedes the TTG codon. Based on the deduced ScaB sequence, the mature protein (following cleavage of the signal peptide) exhibits a theoretical molecular weight of 240,333 and a calculated pI of 5.70 for the unfolded protein.
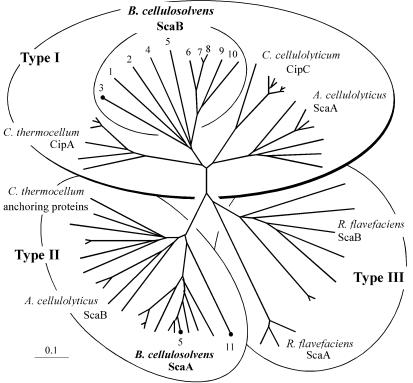
The 10 ScaB cohesins can all be classified as type I cohesins. Phylogenetic analysis reveals that these cohesins form a cluster (Fig. 2, inner circle) in close proximity to the other type I cohesins from the cellulosomes of C. thermocellum, C. cellulolyticum (representative of other related mesophilic clostridia), and A. cellulolyticus. The ScaB cohesins seem to have evolved from a common ancestral point on the tree; cohesins 1 through 5 are the most diversified, whereas cohesins 6 through 10 form a well-conserved branch. Cohesins 7 and 8 are nearly identical. Little if any linker segment separates between the first two cohesins and cohesins 3 and 4 (Fig. 1B). The other cohesins are linked by relatively long Pro/Thr-rich regions, the longest of which are the ∼140-residue stretch between cohesins 7 and 8 and the ∼90-residue stretch between cohesins 8 and 9.
FIG. 2.
Phylogenetic relationships of the B. cellulosolvens ScaB cohesins relative to known cohesins of types I, II, and III. All 10 ScaB cohesins map together on a separate branch of the type I cohesins. Cohesins CohB3, CohA5, and CohA11, which were expressed and used as probes in the present study, are indicated. Scale bars in this and subsequent figures indicate the percentage (0.1) of amino acid substitutions. The GenBank or Swiss-Prot accession numbers for scaffoldin sequences used to construct this tree are as follows: ScaA (AF155197), ScaB (AY221112), and ScaC (AY221113) from A. cellulolyticus; ScaA (AF224509) and ScaB (the present study) from B. cellulosolvens; CipC (U40345) from C. cellulolyticum; CipA (Q06851), OlpA (Q06848), OlpB (Q06852), and Orf2p (Q06853) from C. thermocellum; and ScaA (AJ278969) and ScaB (AJ278969) from R. flavefaciens.
The C terminus of ScaB comprises an X domain and an SLH module. The two modules are closely associated, with little or no detectable linker sequence. The SLH module is closely related to those of the anchoring proteins from C. thermocellum and A. cellulolyticus (Fig. 3). The sequence of the X domain exhibits high similarity with only a few other entries in the protein and nucleotide databases, including two hypothetical proteins (gi|23021211|ref|ZP_00060895.1 and gi|23022175|ref|ZP_00061799.1) from the emerging C. thermocellum genome and another (gi|23138343|ref|ZP_00120007.1) from that of Cytophaga hutchinsonii. As in B. cellulosolvens ScaB, the hypothetical C. thermocellum proteins include a C-terminal SLH module immediately downstream of the X domain, whereas the conserved portion of the Cytophaga hutchinsonii protein is located internally with no SLH module.
FIG. 3.
Multiple sequence alignment of the SLH module of B. cellulosolvens ScaB with those of other anchoring scaffoldins. The sequence of the ScaB module (Bacce-ScaB) is aligned with those of A. cellulolyticus ScaC (Acece-ScaC) and three C. thermocellum anchoring proteins (Clotm-OlpA, -OlpB, and -Orf2p). Consensus symbols shown at the bottom of the four sequences denote the degree of conservation of each position, where the identity of all sequences is denoted by an asterisk, conservation of the residues is denoted by a colon, and “semiconservation” is denoted by a period, as defined by the EBI server (http://www2.ebi.ac.uk/clustalw/).
Sequencing of a gene encoding for a family 48 glycoside hydrolase.
In previous study (14), we identified a 10-residue stretch of amino acids that showed clear homology with a highly conserved region of the family 48 glycoside hydrolases. The latter stretch was extended by genomic-walking PCR, with PstI- and EcoRI-pUC19 genomic libraries and relevant primers (14). A partial sequence (702 bp) of the cel48A gene was initially cloned by using the latter strategy. Another new primer (BC48-R2 [Table 1]), designed from the known sequence, was applied together with primer T7 from the phage vector for PCR, with the amplified genomic EcoRI-XhoI library as a template. This strategy resulted in the amplification of a fragment of 7 kb, which was purified and sequenced, thus enabling the completion of the C-terminal portion of the gene. Using a similar approach, primers BC48-R3 and M13/pUC(−21) were used to identify a relevant 2-kb fragment with the EcoRI-XhoI library as a template. Its sequence completed the N terminus of the gene, and the final corrected sequence of the entire cel48A gene was verified by direct PCR with B. cellulosolvens genomic DNA as a template.
Description of Cel48A.
B. cellulosolvens cel48A encodes for a 752-residue protein that contains a standard 23-residue signal peptide at the N terminus. The deduced sequence indicates that the mature protein exhibits a theoretical molecular weight of 81,698 and a calculated pI of 4.81 for the unfolded protein. Cel48A is characterized by a relatively simple modular architecture, consisting of the family 48 catalytic domain, a short ∼10-residue linker (7 of which are prolines), and a typical C-terminal dockerin domain.
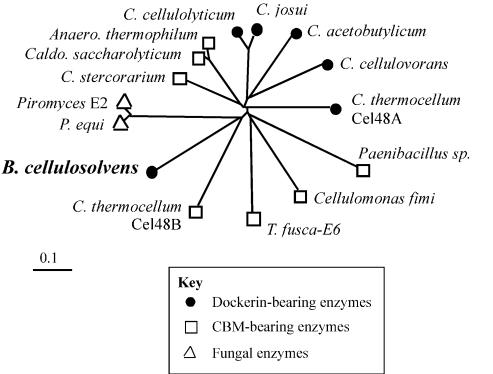
Phylogenetic analysis of the family 48 glycoside hydrolases is illustrated in Fig. 4. In general, the various cellulases of this family appear to have diverged extensively from each other, as evinced from their tendency to radiate at a distance from focal points on or near the short central branch of the tree. The close clustering of certain cellulases (e.g., C. cellulolyticum and C. josui) has been noted earlier (55) and likely reflects the close evolutionary relationship of the parent microorganisms. In contrast, the cellulosomal and noncellulosomal family 48 enzymes from C. thermocellum (Cel48A and Cel48B, respectively) are greatly diverged, indicating that the two enzymes may have been acquired from different sources. The sequence of the B. cellulosolvens family 48 catalytic domain is indeed closest to those of the noncellulosomal Cel48B from C. thermocellum and the fungal enzymes. The latter observation underscores the findings of a previous study (55) indicating that the phylogenetic distribution of the family 48 enzymes appears not to reflect modular architecture.
FIG. 4.
Phylogenetic relationships of the B. cellulosolvens Cel48A catalytic domain relative to known sequences classified as family 48 glycoside hydrolases. The GenBank or Swiss-Prot accession numbers for enzyme sequences used to prepare the phylogenetic tree are as follows: 1,4-β-glucanase from Anaerocellum thermophilum (P96311), Cel48A from B. cellulosolvens (AY374129 [the present study]), cellulase A from Caldicellulosiruptor saccharolyticus (P22534), cellobiohydrolase B from Cellulomonas fimi (P50899), probable processive endoglucanase (CelF ortholog) from C. acetobutylicum (AE007607), processive endocellulase CelF from C. cellulolyticum (P37698), exoglucanase S from C. cellulovorans (O65986), exoglucanase from C. josui (O82831), exocellobiohydrolase II from C. stercorarium (P50900), cellulase S (Cel48A) and hypothetical protein (Cel48B) from C. thermocellum (X80993 and ZP_00059879, respectively), 1,4-β-cellobiosidase from Paenibacillus sp. strain BP-23 (CAD32945), cellulase Cel48A from Piromyces sp. strain E2 (AF449412), cellulase Cel48A from Piromyces equi (AF449413), and β-1,4-exocellulase E6 from T. fusca (AAD39947).
Completion of the B. cellulosolvens Cel48A sequence provided the first insight into the nature of the enzyme-borne dockerin from this species. The Cel48A dockerin contains all of the normal calcium-binding residues and other features consistent with the “F-hand” motif that characterizes the dockerin domain (11, 38, 39, 44). As has been observed in the past for other types of newly described dockerins (13-15, 40, 44, 48, 59), the putative recognition residues of the Cel48A dockerin (i.e., MA/MA) appear to be distinct from those of all of the other known enzyme- or scaffoldin-borne dockerins derived from the different cellulosome-producing species (Table 3).
TABLE 3.
Predicted specificity residues of dockerins derived from the indicated proteins
| Organism | Proteina | Residueb at position
|
|||
|---|---|---|---|---|---|
| 10 | 11 | 10′ | 11′ | ||
| B. cellulosolvens | Cel48A | M | A | M | A |
| ScaA | S | D | S | D | |
| A. cellulolyticus | Cel9B | S | I | S | L |
| ScaA | L | E | L | E | |
| ScaB | I | N | I | N | |
| C. thermocellum | Enzymes* | S | T | S | T |
| CipA | L | L | M | Q | |
| C. cellulolyticus | Enzymes* | A | L/I | A | L/I |
| R. flavefaciens | Enzymes* | L | A | G/N/A | D |
| ScaA | V | A | A | V | |
*, Consensus residues represent the dominant amino acid(s) that appears in the designated position from the indicated group of cellulosomal enzymes.
The four residues indicated represent positions of the calcium-binding motifs in the first (10 and 11) and second (10′ and 11′) duplicated segments, respectively (44).
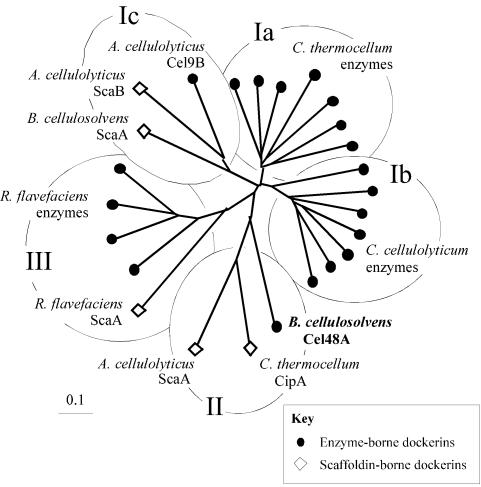
Phylogenetic analysis of the B. cellulosolvens Cel48A dockerin sequence (Fig. 5) revealed a relatively close association with the only two scaffoldin-borne dockerins that are also known to recognize type II cohesins (i.e., A. cellulolyticus ScaA and C. thermocellum CipA). In contrast, the scaffoldin-borne ScaA dockerin of B. cellulosolvens (Fig. 5) maps on a branch of the tree with another scaffoldin-borne dockerin (A. cellulolyticus ScaB) and an enzyme-borne dockerin (Acece-Cel9B), both from the cellulolytic system of A. cellulolyticus. The three latter dockerins recognize type I cohesins. Since the cohesins of the primary (enzyme-integrating) B. cellulosolvens scaffoldin map as type II cohesins, it is not surprising that the enzyme-borne dockerin sequence would map together with dockerins known to bind to type II cohesins, perhaps portending the recognition properties of the Cel48A dockerin described below.
FIG. 5.
Phylogenetic analysis of the dockerins of ScaA and the Cel48 enzyme. The B. cellulosolvens Cel48A-borne dockerin maps on a branch of the tree (designated II), shared by other dockerins that recognize type II cohesins. Type I cohesin-recognizing dockerins are located on branches designated I. Clostridial-enzyme-based dockerins map on species-specific branches (Ia and Ib), whereas the only known A. cellulolyticus enzyme-based dockerin clusters together with scaffoldin-based dockerins from A. cellulolyticus and B. cellulosolvens (Ic). The scaffoldin- and enzyme-based type-III cohesin-recognizing dockerins from R. flavefaciens cluster together on a separate branch of the tree (designated III). Sequences used to construct this tree are among those listed in the legends to Fig. 2 and 4 or as follows: Cel9B from A. cellulolyticus (59); Cel5A (M93096), Cel8C (M87018), Cel9E (M87018), Cel9G (M87018), Cel9H (AF316823), and Cel9 M (AF316823) from C. cellulolyticum; CbhA (X80993), CelB (X03592), CelD (X04584), CelF (X60545), CelH (M31903), and XynV (AF047761) from C. thermocellum; and EndA (Z83304), EndB (AJ298117), XynB (Z35226), and XynD (S61204) from R. flavefaciens.
Cohesin-dockerin interactions.
Within a given organism, the specificity characteristics of the various cohesins and dockerin counterparts have been used to determine the theoretical supramolecular organization of its known cellulosomal components. It was thus of interest to verify the specificities among representative cohesins and dockerins of the B. cellulosolvens system. For this purpose, cohesin 3 (CohB3) was selected to represent the ScaB cohesins, and cohesins 5 and 11 (CohA5 and CohA11) were selected as representative ScaA cohesins (Table 2).
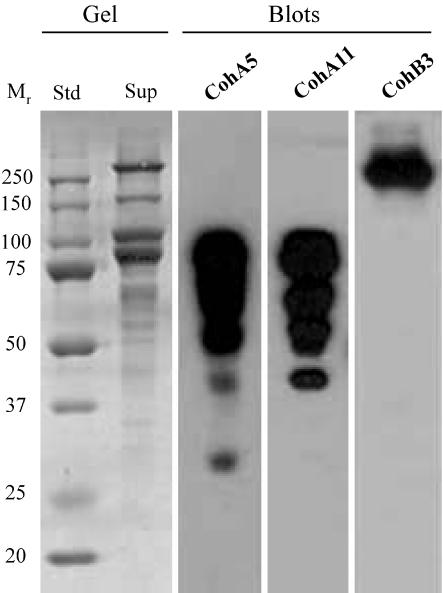
CohA5 was used as a probe in a previous report (14), and the results indeed indicated that the type II ScaA cohesins recognize a series of cell-free supernatant-derived protein bands. The latter bands were presumed to represent cellulosomal enzymes. We also prepared CohA11, since the sequence of this particular cohesin comprises a marked divergence from those of the other 10 ScaA cohesins (see Fig. 2). It was thus worthwhile determining whether the specificity would differ significantly from that of Coh5A. The results of affinity blotting experiments demonstrated that both CohA5 and CohA11 recognize a similar array of labeled bands in the cell-free supernatant (Fig. 6), indicating that, despite the observed sequence divergence, both ScaA cohesins exhibit similar specificities for numerous enzyme-borne dockerins. In contrast to the ScaA cohesins, the CohB3 probe essentially labeled a single high-molecular-weight band that had previously been demonstrated to represent ScaA (14).
FIG. 6.
Affinity blotting of cell-derived B. cellulosolvens proteins, probed by ScaA- and ScaB-based cohesins. Cells were grown on cellobiose and centrifuged, and the cell-free supernatant fluids were subjected to SDS-PAGE (Gel) and then blotted onto nitrocellulose membranes (Blots). Gels were stained with Coomassie brilliant blue. The blots were probed with either His-tagged ScaB cohesin 3 (CohB3) or ScaA cohesins (CohA5 and CohA11), and the labeled bands were detected by chemiluminescence with peroxidase-conjugated, anti-His-tag antibody.
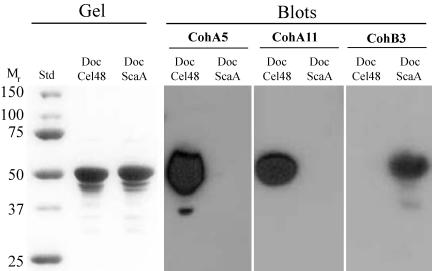
In order to verify further the specificity characteristics of the cohesin-dockerin interaction in the B. cellulosolvens system, cohesins CohA5, CohA11, and CohB3 were used in affinity blotting experiments against selected xylanase-based dockerin fusion proteins (Table 2), whereby the dockerins were derived from either ScaA or the cellulosomal enzyme Cel48A. The xylanase system (xylanase T6 from Geobacillus stearothermophilus) was used as a standard fail-safe carrier vehicle that facilitates expression, folding, and stability of the attached dockerin (59). The results (Fig. 7) again demonstrate that, despite the evolutionary divergence of CohA5 and CohA11, both ScaA-derived cohesins recognize the enzyme-borne Cel48A dockerin and, as expected, fail to recognize its own dockerin. Conversely, the ScaB-derived cohesin probe labeled the ScaA dockerin but not the dockerin of Cel48A.
FIG. 7.
Affinity blotting of Cel48A- and ScaA-based dockerins probed by recombinant cohesins from ScaA and ScaB. Dockerins from B. cellulosolvens cellulosomal Cel48A and ScaA were fused individually to G. stearothermophilus xylanase T6, and the resultant fusion proteins were expressed in an appropriate E. coli host cell system. The isolated fusion proteins were subjected to SDS-PAGE (Gel), transferred to nitrocellulose membranes (Blots), and probed with the recombinant cohesins from ScaA (CohA5 and CohA11) and ScaB (CohB3) as described in the legend to Fig. 6.
Detection of a putative cellulosomal family 9 glycosyl hydrolase.
Western blotting experiments (Fig. 6) revealed 2 major Coomassie blue-stained bands that interacted with the ScaA cohesins, in addition to several less-prominent bands. One of these (82 kDa) was determined to be Cel48 as described above. The second band (∼100 kDa) was subjected to tryptic digestion, and the resultant peptides were subjected to sequencing by MALDI-MS. Two of the peptide sequences showed clear similarity to segments of the family 9 glycoside hydrolases. One of the sequences (QLGFYPNAAK) was homologous to a conserved region of the immunoglobulin-like domain, and the other (WEMEFLK) is typical of the complementary family 9 catalytic domain, such as cellulase E4 from Thermobifida fusca (23, 50). Together, the results imply (albeit inconclusively) that the ∼100-kDa band may represent a theme D family 9 processive endoglucanase that would include a family 4 CBM, an immunoglobulin-like domain, a family 9 catalytic domain, and a dockerin domain (6). Final identification and determination of the modular architecture awaits sequencing of the completed gene for the enzyme.
DISCUSSION
After the establishment of the cellulosome concept in C. thermocellum (4, 27, 31, 32); several lines of evidence indicated that cellulosomes are also produced in other cellulolytic bacteria (9, 16, 25, 30, 46, 49). Initially, the definitive characteristics of cellulase activity, αGal-specific lectin binding, immunochemical cross-reactivity, and cellulosome-related structures were correlated with the bacterial cell surface in numerous cellulolytic strains. In the case of B. cellulosolvens, the accumulating evidence was especially in favor of the production of a surface-bound cellulosome system. Interestingly, 16S rRNA analysis has indicated that this bacterium is closer to the clostridial cluster than to the Cytophaga-Flavobacterium-Bacteroides phylum (37).
In early studies (29), cellulosome-like complexes were indeed identified in both cell-associated and cell extracts of this bacterium. In this context, an ∼230-kDa protein component of the observed complexes interacted both with the lectin and with antibodies specific for the scaffoldin subunit of the C. thermocellum cellulosome. More intriguing, perhaps, was the finding that cellulosome-derived oligosaccharides from both C. thermocellum and B. cellulosolvens shared an unusual αGalf-containing trisaccharide backbone but were distinguished by different substituents (21, 22). Nevertheless, final verification of a true cellulosome system in B. cellulosolvens awaited sequencing of its components and identification of cellulosome-signature sequences (i.e., the cohesins and the dockerins).
Any doubt as to the precise definition of B. cellulosolvens as a cellulosome-producing organism was dispelled upon the sequencing of a bona fide primary scaffoldin from this species, originally termed CipBc and herein renamed ScaA (14). Like the scaffoldins of C. thermocellum and A. cellulolyticus, the scaffoldin of B. cellulosolvens contains multiple cohesins, an internal cellulose-binding CBM, and a C-terminal dockerin domain. The singularity of the B. cellulosolvens ScaA scaffoldin, however, lies in its record number of cohesins that were classified as type II rather than type I on the basis of sequence homology.
Prior to the sequencing of ScaA, all known primary scaffodin-borne cohesins that recognized dockerin-containing cellulosomal enzyme subunits were classified as type I. In contrast, the type II cohesins were hitherto associated exclusively with cell surface-anchoring proteins that carry SLH modules (33-35). It was generally anticipated that the observed distinction between the two types of cohesins would represent a more general theme and that the type II cohesin-dockerin interaction would invariably mediate attachment of cellulosomes to the cell surface. Consequently, the discovery of the type II cohesins in the B. cellulosolvens primary scaffodin was puzzling (14), and the presence of the C-terminal ScaA dockerin domain raised several additional questions. In previous study with the cellulosomal systems of C. thermocellum, A. cellulolyticus, and R. flavefaciens (13, 15, 19, 48, 59), it was determined that genes encoding for scaffoldins that carry a C-terminal dockerin domain are clustered on the genome, together with other scaffoldin-related genes. It was thus intriguing to know whether scaffoldin-related gene(s) would be discovered immediately downstream of B. cellulosolvens ScaA. If so, it was of interest whether such gene(s) would encode for a multiple cohesin-containing protein and whether the protein would serve as an anchoring scaffoldin that bears a C-terminal SLH module. Moreover, the implicit question remained whether the ScaA dockerin domain and the cohesins of the anticipated anchoring proteins would be of type I or type II.
The sequence of B. cellulosolvens ScaB indeed confirmed that this protein plays the role of a cell surface-anchoring scaffoldin by virtue of its C-terminal SLH module. Its 10 resident cohesin domains belong to the type I group of cohesins, and the status of the B. cellulosolvens scaffoldins is thus the reverse of that of the C. thermocellum cellulosome system. Hence, the type II cohesins of the primary scaffoldin, ScaA, bind to the enzyme-borne dockerins, whereas the type I cohesins of the anchoring scaffoldin, ScaB, bind to the dockerin of ScaA. Indeed, biochemical examination of the various cohesin-dockerin specificities in the B. cellulosolvens system corroborated this premise whereby representative ScaA cohesins appear to recognize numerous dockerin-containing enzymes (and the Cel48 dockerin in particular), whereas the ScaB cohesins recognize the ScaA dockerin (Fig. 6 and 7). These results also confirm, by definition, the type of dockerin borne by the different cellulosomal components. In this regard, the phylogenetic relationship of the various dockerins (Fig. 5) may provide a clue as to the dockerin type. Nevertheless, the definitive classification of a given dockerin is inherent in the type of cohesin with which it interacts.
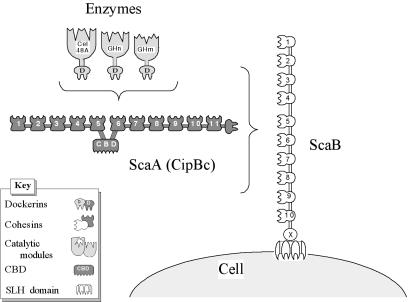
Considering the known modular architecture and specificities of the B. cellulosolvens cellulosomal components as described here, our current view regarding the supramolecular organization of the cellulosome on the surface of this bacterium is presented schematically in Fig. 8. Eleven dockerin-borne enzymes can ostensibly be incorporated into the ScaA polypeptide by virtue of its resident type II cohesin modules. Likewise, the 10 ScaB cohesin modules can conceivably accommodate an equivalent number of ScaA subunits, together with their complement of enzyme molecules, and the entire complex would presumably be attached to the cell surface via the ScaB SLH module. Altogether, the cellulosome apparatus of B. cellulosolvens would comprise a total of 110 enzyme molecules, the highest estimated level of amplification thus far reported for a cellulosomal system.
FIG. 8.
Schematic representation of the proposed cell surface disposition of the known B. cellulosolvens cellulosomal components. Cel48A and other putative dockerin-containing enzymes are incorporated into the ScaA scaffoldin owing to the interaction of their resident dockerin domains with the type II ScaA cohesins. In turn, ScaA, together with its complement of enzymes, is attached in multiple copies to the type I ScaB cohesins, and the cellulosome complex is attached to the cell surface via the ScaB SLH module.
The B. cellulosolvens cellulase system shows all of the features of a powerful and dominant cellulosome assembly, including intimate association with the cell surface, a variety of different plant cell wall-degrading enzymes (cellulases and hemicellulases), substrate targeting, enzyme amplification, and enzyme proximity effects. The variations on the cellulosome theme, as described here for the B. cellulosolvens system, is yet another example of the diversity displayed by cellulosome-producing bacteria in nature. The results of the present study also caution against our tendency toward hasty classification and premature linkage between structural divergence and function.
Acknowledgments
We thank Tali Dror for analysis of the intergenic sequences immediately flanking scaA and scaB. We also thank Shi-You Ding and Adva Mechaly for sequencing initial segments of the cel48A gene.
This research was supported by the Israel Science Foundation (grants 394/03, 771/01, 446/01, and 250/99), by the United States-Israel Binational Agricultural Research and Development Fund (BARD research grant 3106-99C), and by a grant from the United States-Israel Binational Science Foundation (BSF), Jerusalem, Israel. Additional support was provided by the Otto Meyerhof Center for Biotechnology, established by the Minerva Foundation (Munich, Germany).
REFERENCES
- 1.Bagnara-Tardif, C., C. Gaudin, A. Belaich, P. Hoest, T. Citard, and J.-P. Belaich. 1992. Sequence analysis of a gene cluster encoding cellulases from Clostridium cellulolyticum. Gene 119:17-28. [DOI] [PubMed] [Google Scholar]
- 2.Bairoch, A., and R. Apweiler. 2000. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 28:45-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer, E. A., H. Chanzy, R. Lamed, and Y. Shoham. 1998. Cellulose, cellulases and cellulosomes. Curr. Opin. Struct. Biol. 8:548-557. [DOI] [PubMed] [Google Scholar]
- 4.Bayer, E. A., R. Kenig, and R. Lamed. 1983. Adherence of Clostridium thermocellum to cellulose. J. Bacteriol. 156:818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayer, E. A., E. Morag, and R. Lamed. 1994. The cellulosome: a treasure-trove for biotechnology. Trends Biotechnol. 12:378-386. [DOI] [PubMed] [Google Scholar]
- 6.Bayer, E. A., Y. Shoham, and R. Lamed. 2000. Cellulose-decomposing prokaryotes and their enzyme systems. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed. [Online.] Springer-Verlag, New York, N.Y. http://link.springer.de/link/service/books/10125/index.htm.
- 7.Béguin, P., and M. Lemaire. 1996. The cellulosome: an exocellular, multiprotein complex specialized in cellulose degradation. Crit. Rev. Biochem. Mol. Biol. 31:201-236. [DOI] [PubMed] [Google Scholar]
- 8.Belaich, J.-P., A. Belaich, H.-P. Fierobe, L. Gal, C. Gaudin, S. Pagès, C. Reverbel-Leroy, and C. Tardif. 1999. The cellulolytic system of Clostridium cellulolyticum, p. 479-487. In K. Ohmiya, K. Hayashi, K. Sakka, Y. Kobayashi, S. Karita, and T. Kimura (ed.), Genetics, biochemistry, and ecology of cellulose degradation. Uni Publishers Co., Tokyo, Japan.
- 9.Belaich, J.-P., C. Tardif, A. Belaich, and C. Gaudin. 1997. The cellulolytic system of Clostridium cellulolyticum. J. Biotechnol. 57:3-14. [DOI] [PubMed] [Google Scholar]
- 10.Bourne, Y., and B. Henrissat. 2001. Glycoside hydrolases and glycosyltransferases: families and functional modules. Curr. Opin. Struct. Biol. 11:593-600. [DOI] [PubMed] [Google Scholar]
- 11.Chauvaux, S., P. Béguin, J.-P. Aubert, K. M. Bhat, L. A. Gow, T. M. Wood, and A. Bairoch. 1990. Calcium-binding affinity and calcium-enhanced activity of Clostridium thermocellum endoglucanase D. Biochem. J. 265:261-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutinho, P. M., and B. Henrissat. 1999. Carbohydrate-active enzymes: an integrated database approach, p. 3-12. In H. J. Gilbert, G. J. Davies, B. Henrissat, and B. Svensson (ed.), Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 13.Ding, S.-Y., E. A. Bayer, D. Steiner, Y. Shoham, and R. Lamed. 1999. A novel cellulosomal scaffoldin from Acetivibrio cellulolyticus that contains a Family-9 glycosyl hydrolase. J. Bacteriol. 181:6720-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding, S.-Y., E. A. Bayer, D. Steiner, Y. Shoham, and R. Lamed. 2000. A scaffoldin of the Bacteroides cellulosolvens cellulosome that contains 11 type II cohesins. J. Bacteriol. 182:4915-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding, S.-Y., M. T. Rincon, R. Lamed, J. C. Martin, S. I. McCrae, V. Aurilia, Y. Shoham, E. A. Bayer, and H. J. Flint. 2001. Cellulosomal scaffoldin-like proteins from Ruminococcus flavefaciens. J. Bacteriol. 183:1945-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi, R. H., M. Goldstein, S. Hashida, J. S. Park, and M. Takagi. 1994. The Clostridium cellulovorans cellulosome. Crit. Rev. Microbiol. 20:87-93. [DOI] [PubMed] [Google Scholar]
- 17.Doi, R. H., and Y. Tamura. 2001. The Clostridium cellulovorans cellulosome: an enzyme complex with plant cell wall degrading activity. Chem. Rec. 1:24-32. [DOI] [PubMed] [Google Scholar]
- 18.Felix, C. R., and L. G. Ljungdahl. 1993. The cellulosome: the exocellular organelle of Clostridium. Annu. Rev. Microbiol. 47:791-819. [DOI] [PubMed] [Google Scholar]
- 19.Fujino, T., P. Béguin, and J.-P. Aubert. 1993. Organization of a Clostridium thermocellum gene cluster encoding the cellulosomal scaffolding protein CipA and a protein possibly involved in attachment of the cellulosome to the cell surface. J. Bacteriol. 175:1891-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerngross, U. T., M. P. M. Romaniec, T. Kobayashi, N. S. Huskisson, and A. L. Demain. 1993. Sequencing of a Clostridium thermocellum gene (cipA) encoding the cellulosomal SL-protein reveals an unusual degree of internal homology. Mol. Microbiol. 8:325-334. [DOI] [PubMed] [Google Scholar]
- 21.Gerwig, G., J. P. Kamerling, J. F. G. Vliegenthart, E. Morag, R. Lamed, and E. A. Bayer. 1993. The nature of the carbohydrate-peptide linkage region in glycoproteins from the cellulosomes of Clostridium thermocellum and Bacteroides cellulosolvens. J. Biol. Chem. 268:26956-26960. [PubMed] [Google Scholar]
- 22.Gerwig, G., J. P. Kamerling, J. F. G. Vliegenthart, E. Morag, R. Lamed, and E. A. Bayer. 1992. Novel oligosaccharide constituents of the cellulase complex of Bacteroides cellulosolvens. Eur. J. Biochem. 205:799-808. [DOI] [PubMed] [Google Scholar]
- 23.Irwin, D., D.-H. Shin, S. Zhang, B. K. Barr, J. Sakon, P. A. Karplus, and D. B. Wilson. 1998. Roles of the catalytic domain and two cellulose binding domains of Thermomonospora fusca E4 in cellulose hydrolysis. J. Bacteriol. 180:1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakiuchi, M., A. Isui, K. Suzuki, T. Fujino, E. Fujino, T. Kimura, S. Karita, K. Sakka, and K. Ohmiya. 1998. Cloning and DNA sequencing of the genes encoding Clostridium josui scaffolding protein CipA and cellulase CelD and identification of their gene products as major components of the cellulosome. J. Bacteriol. 180:4303-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirby, J., J. C. Martin, A. S. Daniel, and H. J. Flint. 1997. Dockerin-like sequences in cellulases and xylanases from the rumen cellulolytic bacterium Ruminococcus flavefaciens. FEMS Microbiol. Lett. 149:213-219. [DOI] [PubMed] [Google Scholar]
- 26.Kozak, M. 1999. Initiation of translation in prokaryotes and eukaryotes. Gene 234:187-208. [DOI] [PubMed] [Google Scholar]
- 27.Lamed, R., and E. A. Bayer. 1988. The cellulosome of Clostridium thermocellum. Adv. Appl. Microbiol. 33:1-46. [Google Scholar]
- 28.Lamed, R., R. Kenig, E. Setter, and E. A. Bayer. 1985. Major characteristics of the cellulolytic system of Clostridium thermocellum coincide with those of the purified cellulosome. Enzyme Microb. Technol. 7:37-41. [Google Scholar]
- 29.Lamed, R., E. Morag (Morgenstern), O. Mor-Yosef, and E. A. Bayer. 1991. Cellulosome-like entities in Bacteroides cellulosolvens. Curr. Microbiol. 22:27-33. [Google Scholar]
- 30.Lamed, R., J. Naimark, E. Morgenstern, and E. A. Bayer. 1987. Specialized cell surface structures in cellulolytic bacteria. J. Bacteriol. 169:3792-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamed, R., E. Setter, and E. A. Bayer. 1983. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J. Bacteriol. 156:828-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamed, R., E. Setter, R. Kenig, and E. A. Bayer. 1983. The cellulosome: a discrete cell surface organelle of Clostridium thermocellum which exhibits separate antigenic, cellulose-binding and various cellulolytic activities. Biotechnol. Bioeng. Symp. 13:163-181. [Google Scholar]
- 33.Leibovitz, E., and P. Béguin. 1996. A new type of cohesin domain that specifically binds the dockerin domain of the Clostridium thermocellum cellulosome-integrating protein CipA. J. Bacteriol. 178:3077-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leibovitz, E., H. Ohayon, P. Gounon, and P. Béguin. 1997. Characterization and subcellular localization of the Clostridium thermocellum scaffoldin dockerin binding protein SdbA. J. Bacteriol. 179:2519-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemaire, M., I. Miras, P. Gounon, and P. Béguin. 1998. Identification of a region responsible for binding to the cell wall within the S-layer protein of Clostridium thermocellum. Microbiology 144:211-217. [DOI] [PubMed] [Google Scholar]
- 36.Lemaire, M., H. Ohayon, P. Gounon, T. Fujino, and P. Béguin. 1995. OlpB, a new outer layer protein of Clostridium thermocellum, and binding of its S-layer-like domains to components of the cell envelope. J. Bacteriol. 177:2451-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, C., J. W. Urbance, and D. A. Stahl. 1994. Acetivibrio cellulolyticus and Bacteroides cellulosolvens are members of the greater clostridial assemblage. FEMS Microbiol. Lett. 124:151-155. [DOI] [PubMed] [Google Scholar]
- 38.Lytle, B., B. F. Volkman, W. M. Westler, and J. H. D. Wu. 2000. Secondary structure and calcium-induced folding of the Clostridium thermocellum dockerin domain determined by NMR spectroscopy. Arch. Biochem. Biophys. 379:237-244. [DOI] [PubMed] [Google Scholar]
- 39.Lytle, B. L., B. F. Volkman, W. M. Westler, M. P. Heckman, and J. H. D. Wu. 2001. Solution structure of a type I dockerin domain, a novel prokaryotic, extracellular calcium-binding domain. J. Mol. Biol. 307:745-753. [DOI] [PubMed] [Google Scholar]
- 40.Mechaly, A., S. Yaron, R. Lamed, H.-P. Fierobe, A. Belaich, J.-P. Belaich, Y. Shoham, and E. A. Bayer. 2000. Cohesin-dockerin recognition in cellulosome assembly: experiment versus hypothesis. Proteins 39:170-177. [DOI] [PubMed] [Google Scholar]
- 41.Murray, M. G., and W. F. Thompson. 1980. Rapid isolation of high-molecular-weight plant DNA. Nucleic Acids Res. 8:4321-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen, H., S. Brunak, and G. von Heijne. 1999. Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng. 12:3-9. [DOI] [PubMed] [Google Scholar]
- 43.Nolling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagès, S., A. Belaich, J.-P. Belaich, E. Morag, R. Lamed, Y. Shoham, and E. A. Bayer. 1997. Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: prediction of specificity determinants of the dockerin domain. Proteins 29:517-527. [PubMed] [Google Scholar]
- 45.Pagès, S., A. Belaich, H.-P. Fierobe, C. Tardif, C. Gaudin, and J.-P. Belaich. 1999. Sequence analysis of scaffolding protein CipC and ORFXp, a new cohesin-containing protein in Clostridium cellulolyticum: comparison of various cohesin domains and subcellular localization of ORFXp. J. Bacteriol. 181:1801-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponpium, P., K. Ratanakhanokchai, and K. L. Kyu. 2000. Isolation and properties of a cellulosome-type multienzyme complex of the thermophilic Bacteroides sp. strain P-1. Enzyme Microb. Technol. 26:459-465. [DOI] [PubMed] [Google Scholar]
- 47.Poole, D. M., E. Morag, R. Lamed, E. A. Bayer, G. P. Hazlewood, and H. J. Gilbert. 1992. Identification of the cellulose binding domain of the cellulosome subunit S1 from Clostridium thermocellum. FEMS Microbiol. Lett. 99:181-186. [DOI] [PubMed] [Google Scholar]
- 48.Rincon, M. T., S.-Y. Ding, S. I. McCrae, J. C. Martin, V. Aurilia, R. Lamed, Y. Shoham, E. A. Bayer, and H. J. Flint. 2003. Novel organization and divergent dockerin specificities in the cellulosome system of Ruminococcus flavefaciens. J. Bacteriol. 185:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabathe, F., A. Belaich, and P. Soucaille. 2002. Characterization of the cellulolytic complex (cellulosome) of Clostridium acetobutylicum. FEMS Microbiol. Lett. 217:15-22. [DOI] [PubMed] [Google Scholar]
- 50.Sakon, J., D. Irwin, D. B. Wilson, and P. A. Karplus. 1997. Structure and mechanism of endo/exocellulase E4 from Thermomonospora fusca. Nat. Struct. Biol. 4:810-818. [DOI] [PubMed] [Google Scholar]
- 51.Schwarz, W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56:634-649. [DOI] [PubMed] [Google Scholar]
- 52.Shoham, Y., R. Lamed, and E. A. Bayer. 1999. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 7:275-281. [DOI] [PubMed] [Google Scholar]
- 53.Shoseyov, O., and R. H. Doi. 1990. Essential 170-kDa subunit for degradation of crystalline cellulose by Clostridium cellulovorans cellulase. Proc. Natl. Acad. Sci. USA 87:2192-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shoseyov, O., M. Takagi, M. A. Goldstein, and R. H. Doi. 1992. Primary sequence analysis of Clostridium cellulovorans cellulose binding protein A. Proc. Natl. Acad. Sci. USA 89:3483-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steenbakkers, P. J., A. Freelove, B. Van Cranenbroek, B. M. Sweegers, H. R. Harhangi, G. D. Vogels, G. P. Hazlewood, H. J. Gilbert, and H. J. Op den Camp. 2002. The major component of the cellulosomes of anaerobic fungi from the genus Piromyces is a family 48 glycoside hydrolase. DNA Seq 13:313-320. [DOI] [PubMed] [Google Scholar]
- 56.Stenstrom, C. M., E. Holmgren, and L. A. Isaksson. 2001. Cooperative effects by the initiation codon and its flanking regions on translation initiation. Gene 273:259-265. [DOI] [PubMed] [Google Scholar]
- 57.Tamaru, Y., S. Karita, A. Ibrahim, H. Chan, and R. H. Doi. 2000. A large gene cluster for the Clostridium cellulovorans cellulosome. J. Bacteriol. 182:5906-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamaru, Y., C.-C. Liu, A. Ichi-ishi, L. Malburg, and R. H. Doi. 1999. The Clostridium cellulovorans cellulosome and non-cellulosomal cellulases, p. 488-494. In K. Ohmiya, K. Hayashi, K. Sakka, Y. Kobayashi, S. Karita, and T. Kimura (ed.), Genetics, biochemistry, and ecology of cellulose degradation. Uni Publishers Co., Tokyo.
- 59.Xu, Q., W. Gao, S.-Y. Ding, R. Kenig, Y. Shoham, E. A. Bayer, and R. Lamed. 2003. The cellulosome system of Acetivibrio cellulolyticus includes a novel type of adaptor protein and a cell-surface anchoring protein. J. Bacteriol. 185:4548-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]