Abstract
Most ATPases, involved in energy-driven processes, act in the cytoplasm. However, external membrane-bound ATPases have also been described in parasites and eukaryotic cells. In Mycoplasma hominis, a bacterium lacking a cell wall, the surface-exposed substrate-binding protein OppA of an oligopeptide permease (Opp) contains an ATP binding P-loop structure in the C-terminal region. With ATP affinity chromatography and tryptic digestion in the presence or absence of ATP, the functionality of the Mg2+-dependent ATP binding site is demonstrated. In addition to ATP, ADP also could bind to OppA. The presence of an ATPase activity on the surface of M. hominis is indicated by the inactivation of ATP hydrolyzing activity of intact mycoplasma cells by the impermeable ATPase inhibitor 4′,4′-diisothiocyanostilbene-2′,2′-disulfonic acid and influenced by the ATP analog 5′-fluorosulfonyl-benzoyladenosine. Comparing equimolar amounts of OppA in intact mycoplasma cells and in the purified form indicated that more than 80% of the surface-localized ATPase activity is derived from OppA, implying that OppA is the main ATPase on the surface of mycoplasma cells. Together, these data present the first evidence that the cytoadhesive substrate binding protein OppA of the oligopeptide permease also functions as an ecto-ATPase in Mycoplasma hominis.
The most important high-energy phosphate compounds in living cells are adenosine and guanosine triphosphate (ATP and GTP), which serve as carriers of energy that keeps the organism alive. The mobilization of energy is accomplished by a diverse variety of ATPases (31). In 1982, Walker and coworkers (44) described a sequence similarity in the alpha- and beta-subunits of ATP synthetase. This consensus sequence is also found in myosin, kinases, and other ATP-requiring enzymes. The best conserved of these motifs is a glycine-rich region, [AG]-X(4)-G-K-[ST], which typically forms a flexible loop between a beta-strand and an alpha-helix and interacts extensively with the β- and γ-phosphates of the nucleotide, whereas only limited interactions engaged with the ATP adenine ring. This sequence motif, which is generally referred to as Walker A sequence or P-loop, is present in numerous ATP- or GTP-binding proteins, including the nucleotide-binding subunits of the well known ATP-binding cassette (ABC) transporter.
Besides the highly conserved Walker A motif there is also a less conserved Walker B motif that is involved in attacking the γ-phosphate bond during ATP hydrolysis. Consequently, substitution in the Walker sequences generally impairs ATP hydrolysis to a greater or lesser extent (38). Besides the two hydrophilic peripheral nucleotide-binding subunits, ABC transporters generally consist of two hydrophobic membrane-spanning subunits and a substrate-binding protein which has been described only in bacteria (1, 12). In gram negative bacteria the substrate-binding protein is located within the periplasmic space, whereas in gram positive bacteria it is surface exposed and tethered to the membrane via a lipid anchor (32).
We have previously characterized an oligopeptide permease of the cell wall-less bacterium Mycoplasma hominis (11). This oligopeptide permease consists of two integral membrane proteins, OppB and OppC, two nucleotide-binding proteins, OppD and OppF, and a substrate-binding protein, OppA. The OppA subunit was shown to be involved in cytoadherence, having a lipoprotein attachment site and no further trans-membrane regions (11). The finding that OppA was proteolytically digested by trypsin treatment of intact mycoplasma cells supports the idea that OppA is located on the cell surface of Mycoplasma hominis (10). Surprisingly, computer analysis revealed an ATP-binding P-loop structure not only in the protein sequences of OppD and OppF but also in the C-terminal region of the OppA protein which has never been described previously for a substrate-binding protein. The results presented in this study provide evidence that OppA of M. hominis acts as an ATP hydrolase on the surface of the cell.
MATERIALS AND METHODS
Mycoplasma culture, osmotic lysis, and separation of membrane and cytoplasmic proteins.
Mycoplasma hominis strain FBG was cultivated in PPLO (pleuropneumonia-like organism) broth base medium containing arginine as described previously (5). Stocks of M. hominis FBG were prepared from mid-logarithmic-phase broth culture and stored at −70°C. To prepare lysates of M. hominis, the mycoplasma cells were harvested by centrifugation (15,000 × g, 30 min, 4°C), washed and resuspended in buffer A (120 mM NaCl, 5 mM KCl, 20 mM Tris-HCl, pH 7.5). The cells were disrupted by three freeze-thaw cycles and the addition of 50 volumes of distilled water followed by 30 min of incubation at 37°C and centrifugation (23,500 × g, 45 min, 4°C).
Bacterial strains and plasmids.
Plasmids pXB and pBX (Roche Applied Science, Mannheim, Germany) were used as expression vectors for the heterologous expression of OppA and OppD. The plasmids were propagated in Escherichia coli SG13009 (Qiagen, Hilden, Germany).
DNA manipulations.
All routine DNA manipulation techniques, including plasmid preparation, restriction endonuclease analysis, ligation and transformation of E. coli, were performed as described by Sambrook et al. (36) or according to the manufacturer′s instructions (Qiagen, Hilden, Germany).
PCR, cloning, and sequencing of the DNA fragments.
To generate the oppA and oppD expression plasmids, oligonucleotides were used as primers in PCR to change the mycoplasma tryptophan encoding TGA to the universal codon TGG. To facilitate cloning of the PCR products, restriction sites were inserted in the primer sequence without changing the amino acid sequence. PCR was performed as described by Kitzerow et al. (18) with the oligonucleotide primers published by Hopfe (13).
Mutations in the P-loop motif of OppA were inserted by use of the following primers: PL1 (5′-AAAGGATCCTAAAACCGGAAAATATG-3′) and PL2 (5′-CAGGAGGCATCAATAGAACCAACC-3′) to generate fragment 1; PL3 (5′-TGATGCTCCTGAACTGTCTTTT-3′) and PL4 (5′-TTTGCGGCCGCCTGCAGTTTTTTAGTATCTTTGA-3′) to generate fragment 2. The two fragments were fused by SOE (splicing by overlap extension)-PCR (14) and cloned into a vector which carries the protein coding sequence of the OppA protein and thus replaced the Lys875 with Arg (OppAK875R mutant).
In the same way, the OppAΔP-loop mutant was created by changing the P-loop motif from GKDSSGKS to THASSSAH with the primers PL1 and PL5 (5′-TTTACACATGCCAGTTCAAGTGCACATATAGAACCAACC-3′) for fragment 3 and PL6 (5′-TATATGTGCACTTGAACTGGCATGTGTAAAATCG-3′) and PL4 for fragment 4, which were fused and cloned as described above.
Expression and purification of recombinant proteins.
The recombinant proteins OppA, OppAK875R mutant, OppAΔP-loop mutant, and OppD were each expressed with an N- or C-terminally fused protein C tag. One liter of LB-broth medium (Gibco BRL, Life Technologies Inc., Gaithersburg, Md.) containing ampicillin (100 μg/ml) and kanamycin (25 μg/ml) was inoculated with 50 ml of overnight culture of the respective E. coli SG13009 clone for 3 h at 37°C with vigorous shaking until an A600 of 0.6 to 0.9 was reached. Protein expression was induced by isopropylthio-β-d-galactoside (1 mM). After 3 to 4 h of cultivation at 37°C the cells were harvested by centrifugation (15,000 × g, 20 min, 4°C) and frozen at −70°C. After thawing on ice the cells were resuspended in 40 ml of buffer A. The cells were disrupted by three repeat freeze-thaw cycles in liquid nitrogen followed by three bursts of sonication on ice (5-min bursts at 95 W with a 1-min cooling period between each burst). The lysate was centrifuged (15,000 × g, 20 min, 4°C) and the OppD-containing supernatant was transferred to an anti-protein C affinity matrix (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instructions. The recombinant OppA variants were purified with a Sepharose-coupled anti-OppA antibody (monoclonal antibody DC10) as described by Henrich et al. (10).
The purified MalK protein was a gift from E. Schneider (Humboldt University of Berlin, Institute of Biology).
SDS-PAGE and immunostaining of proteins.
Proteins were separated on 9.5% and 12% polyacrylamide gels (22), transferred to nitrocellulose (Schleicher and Schüll, Dassel, Germany) with a semidry blot apparatus (Phase, Mölln, Germany), and immunostained by the protocol of Kitzerow et al. (19). Monoclonal antibodies DC10 and BG11 (anti-OppA), BA10 (anti-P50), NB12 (anti-P80), CG4 (anti-P60), and AH10 (anti-P55) were used.
Limited proteolysis by trypsin.
Proteolysis of the purified proteins recombinant OppA, OppAK875R mutant, OppAΔP-loop, recombinant OppD, recombinant MalK, and native P50 (0.1 g/ml each) or intact mycoplasma cells (2.5 mg/ml) was performed as described by Schneider et al. (37) by first incubating the samples at 4°C for 10 min with or without 5 mM ATP and 5 mM MgCl2. Trypsin was then added at a final concentration of 0.18 mg/ml (for purified proteins) or 0.05 mg/ml (for mycoplasma cells), and proteolysis was allowed to proceed for 30 min at 4°C. The reaction was terminated by adjusting the sample to the conditions of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (10% [wt/vol] SDS, 0.4 M Tris-HCl, pH 6.8, 0.1% [wt/vol] bromophenol blue, 25% [vol/vol] glycerol, 5% [vol/vol] 2-mercaptoethanol) and immediately boiling for 5 min. After gel electrophoresis, the digestion patterns were analyzed by Western blotting and immunostaining. Experiments were also carried out with GTP, CTP, or ADP instead of ATP (Sigma Aldrich, Taufkirchen, Germany). The necessity of metal ions for ATP binding was determined by adding EDTA (10 mM) to the trypsin assay.
Enzyme-linked immunosorbent assay.
The enzyme-linked immunosorbent assay was performed as described by Henrich et al. (10) with the modification that different concentrations of M. hominis cell lysate and purified OppA were coated to MaxiSorp microtiter plates (Nunc, Wiesbaden, Germany). The amount of OppA was determined by the addition of the DC10 monoclonal antibody.
ATP hydrolysis assay.
The ATPase assay was conducted at 37°C as described by Henkel et al. (9) with minor modifications. Briefly, the assay was performed in microtiter plates by incubating 500 ng of purified protein or intact mycoplasma cells equivalent to 500 ng of native OppA in 20 μl of buffer A with 5 mM ATP and 5 mM MgCl2 for 1 to 60 min. Hydrolysis of ATP was terminated by adding 200 μl of malachite green reagent (5.72% [wt/vol] ammonium molybdate in 6 N HCl, 2.32% [wt/vol] polyvinyl alcohol, 0.0812% [wt/vol] malachite green, and distilled water at a ratio of 1:1:2:2). The relative absorbance of the samples in relation to a blank was measured at 620 nm (Tecan Rainbow, SLT Labinstruments, Crailsheim, Germany). The three nucleotides ATP, GTP, and CTP also show hydrolysis activity in the absence of an ATPase. This value was subtracted from the measured ATPase activities to achieve the real value. Inorganic phosphate (in concentrations varying from 1 to 20 nmol) was used as a standard.
ATP affinity chromatography.
ATP affinity chromatography was performed with 0.5 ml of swollen ATP-agarose (2.1 μmol of ATP/ml packed gel coupled via a 22-Å spacer [Sigma Aldrich, Taufkirchen, Germany]). The crude bacterial lysate (5 to 10 mg of protein/10 ml) was incubated for 2 to 3 h at 4°C with the ATP-agarose on a rotary shaker; 20 μl (for the detection of OppA) and 2 μl (for the detection of clongation factor Tu [EF-Tu]) of the bacterial lysate were resuspended in 5× SDS-PAGE sample buffer before adding to the ATP agarose. The agarose was transferred to a column and washed three times with 5 ml of wash buffer (20 mM Tris-HCl, pH 7.5, 2 mM EGTA, 2 mM MgCl2, 0.01% [wt/vol] n-dodecyl-β-maltoside). ATP-bound proteins were eluted with wash buffer containing ATP (2 or 20 mM); 25-μl aliquots of each 1-ml fraction were analyzed by SDS-PAGE.
Sequence analysis.
Analysis of the DNA and protein sequences and the design of oligonucleotides were facilitated by the Lasergene software (DNA Star Inc., Madison, Wis.). For protein analysis the database of the Expert Protein Analysis System (ExPASy; www.expasy.org) was used. The two-dimensional densitometry was performed with the Aida Image Analyser version 3.24 (Raytest GmbH, Straubenherdt, Germany).
Statistical analysis.
All experiments were performed in triplicate, with similar results obtained with at least five separate cell suspensions or six recombinant protein preparations. Km and Vmax were calculated with a computerized nonlinear regression analysis (Graph Pad Prism, version 3.02, Graph Pad Software Inc., San Diego, Calif.).
RESULTS
OppA binds to an ATP affinity column.
A P-loop structure motif has been found in a variety of proteins, e.g., the guanylate kinase, thymidine kinase, ATP-binding proteins of the ABC transporters, DNA/RNA helicases, and the GTP-binding elongation factor EF-Tu. While these proteins bind nucleotides, others also carrying a P-loop sequence do not bind ATP or GTP, e.g., chymotrypsin or the human ferritin light chain. As computer analysis of the Mycoplasma hominis OppA protein sequence predicted an ATP-binding site (P-loop) in the C-terminal region (amino acids 869 to 876: G-K-D-S-S-G-K-S) (11, 13), we investigated its ATP-binding character. The less conserved Walker B motif R/K-X-X-X-G-X-X-X-L, followed by several hydrophobic amino acids (44), was present between amino acids 737 and 752 (RFDGVTGENLLAWSAD). This indicated that in OppA of M. hominis the structural elements Walker A and B are in the order BA. A similar order has been described for nematode and rabbit myosin (44).
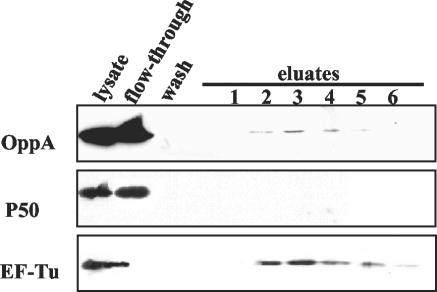
An ATP affinity column was loaded with lysate of the M. hominis isolate FBG. After several washes the bound proteins were eluted with 2 and 20 mM ATP. The proteins were resolved by SDS-PAGE, blotted, and analyzed by immunostaining (Fig. 1). As shown in OppA staining (monoclonal antibody DC10), 2% of the added OppA was retained on the ATP affinity column (determined by two-dimensional densitometry). The elongation factor EF-Tu, which is known to carry an ATP/GTP binding site, was used as a positive control, and the P50 adhesin of M. hominis (10), which does not express an ATP-binding site, was used as a negative control. As was expected, the EF-Tu protein also bound to a GTP affinity column, whereas OppA did not (data not shown).
FIG. 1.
Western blot analysis of ATP-binding proteins. Mycoplasma hominis FBG lysate was applied to an ATP affinity column. The ATP-bound proteins were eluted with 2 mM ATP (lanes 1 to 3) and with 20 mM ATP (lanes 4 to 6), subjected to SDS-PAGE, and immunostained with monoclonal antibodies for the detection of OppA (monoclonal antibody DC10), P50 (monoclonal antibody BG2), and EF-Tu (monoclonal antibody KD2).
The binding of OppA is not a result of its positive charge (4.6) at pH 7.5 because the lipoprotein P60 (18) with a pK of 15.5 at pH 7.5 did not show binding to the ATP affinity column (data not shown). This finding confirms that the adsorption of OppA to the matrix is not due to ion exchange.
It is possible that OppA forms a complex with the other Opp components even in the lysate. To rule out that the retention of OppA on the ATP matrix is mediated through the nucleotide binding proteins OppD and OppF of the Opp complex, binding of purified OppA to the ATP matrix was also proven. These data strongly suggest that OppA belongs to the group of ATP-binding proteins.
Effect of ATP on the tryptic digestion pattern of OppA.
Binding of ATP often induces a conformational change in the protein, demonstrated for MalK, the ATP-binding protein of the maltose transporter of Salmonella enterica serovar Typhimurium (37). The structural alteration in the MalK protein by ATP induced a change in the sensitivity to protease and caused an altered degradation pattern of the ATP-protein complex.
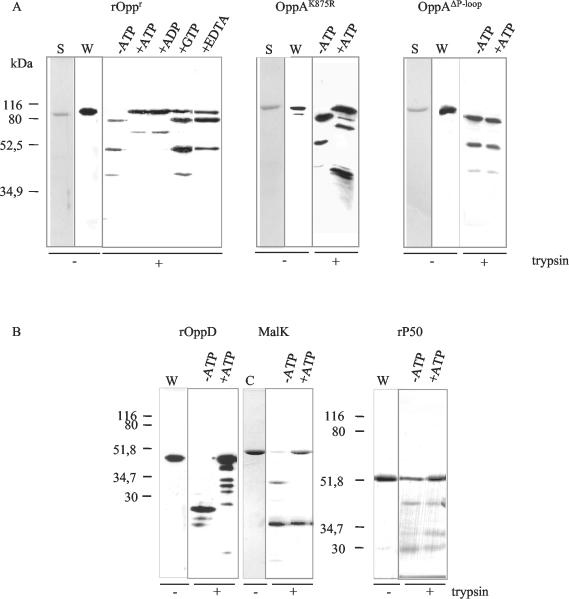
We performed a similar analysis on OppA and OppD of the oligopeptide permease of M. hominis, the P50 adhesin (as a negative control), and MalK (as a positive control). Recombinant OppA, recombinant OppD, and native P50 isolated from M. hominis as well as purified S. enterica serovar Typhimurium MalK were exposed to trypsin in the presence or absence of ATP for 30 min at 4°C. The tryptic peptides were separated by SDS-PAGE and subsequently analyzed by Western blotting and protein staining (Fig. 2). By comparing the Coomassie-stained digestion patterns with the immunostained ones, we ascertained that the antibodies against OppA, P50, and OppD reacted with the major cleavage products (data not shown). As shown in Fig. 2A, OppA was completely digested into three major fragments of 80, 50, and 39 kDa in the absence of ATP. In the presence of ATP, the sensitivity of OppA to proteolysis was markedly reduced, and the degradation pattern was modified. Most of the OppA molecules were resistant to proteolytic degradation when pretreated with ATP, and an additional 65-kDa peptide was produced. As expected, the ATPases OppD and MalK (used as positive controls) showed different tryptic digestion patterns with and without ATP exposure, whereas the proteolytic cleavage pattern of the P50 adhesin (used as a negative control) remained unchanged, indicating the lack of ATP binding (Fig. 2B). These experiments confirm that OppA is an ATP-binding protein.
FIG. 2.
Effect of ATP on the tryptic digestion pattern. After limited tryptic digestion of 0.5 μg of recombinant OppA, OppAK875R mutant, and OppAΔP-loop (A) and recombinant OppD, native MalK, and native P50 (B) in the presence or absence of the depicted nucleotides, the fragments were analyzed by Western blotting with the monoclonal antibodies DC10 (OppA) and BG2 (P50), a polyclonal antiserum directed against OppD, or by Coomassie staining (C) (MalK). The left two lanes of each panel show the purified proteins used in the trypsin assay in silver staining (S) and Western blotting (W).
We used the trypsin assay to analyze the binding characteristics of OppA for ADP, GTP, and CTP. As depicted in Fig. 2A, ADP, like ATP, protected OppA from proteolytic cleavage. Neither GTP (Fig. 2A) nor CTP (data not shown) was able to protect OppA against rapid proteolytic digestion, which is reflected by similar tryptic peptide patterns obtained with and without nucleotide addition. As most ATP-protein interactions require divalent cations, EDTA was used to chelate the Mg2+ ions from the ATP-binding assay. This led to a loss of the protective effect of ATP to proteolysis (Fig. 2A, +EDTA).
Effect of ATP on the tryptic digestion pattern of the OppAK875R and OppAΔP-loop mutants.
In order to confirm that the Walker A motif of OppA is essential for nucleotide binding, we analyzed the effect of ATP on the tryptic digestion pattern of two OppA mutants. In OppAK875R, the lysine residue (Lys875) of the Walker A motif of the nucleotide binding fold, an invariant amino acid important for ATP hydrolysis, was replaced by arginine. This lysine is described in other ATP-binding proteins to be involved in binding of the β- and γ-phosphates of nucleotides (7). Limited trypsin proteolysis of OppAK875R in the presence or absence of ATP revealed a similar protection by ATP as seen with the recombinant OppA protein (Fig. 2A), suggesting binding of ATP. In the OppAΔP-loop mutant, in which the whole P-loop motif GKDSSGKS was changed to THASSSAH, no protective effect of ATP was observed, indicating that ATP binding did not occur. Thus, these data show that the Walker A motif is essential for the binding of ATP.
ATP binding of surface-exposed OppA.
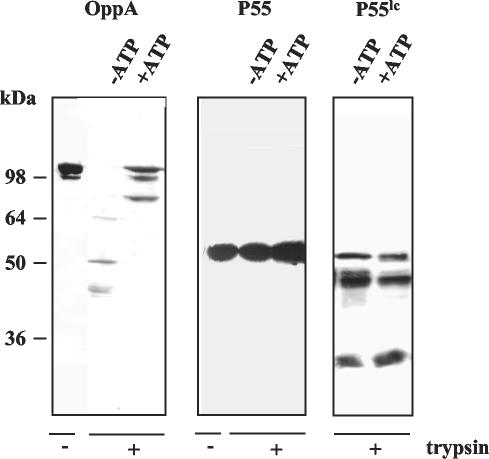
OppA was characterized as a surface-exposed lipoprotein with cytoadhesive qualities (10, 112). To confirm that the ATP-binding site of OppA is accessible from outside the M. hominis cell, intact mycoplasma cells were incubated with and without ATP, followed by trypsin treatment. The membrane-anchored OppA protein was protected by ATP from proteolysis, whereas this protein was rapidly digested in the absence of ATP (Fig. 3). The integrity of the mycoplasma cells was demonstrated by monitoring the proteolysis of cytoplasmic protein P55. Exposure of intact cells to trypsin did not result in digestion of P55, whereas lysis of the mycoplasma cells prior to trypsin exposure led to proteolytic digestion of the cytoplasmic protein P55 (Fig. 3). These data clearly prove that the ATP-binding site of OppA is located outside the mycoplasma cell.
FIG. 3.
Localization of the ATP-binding site. Intact mycoplasma cells (50 μg/20 μl) were incubated with 2 mM trypsin in the presence (+) or absence (−) of 5 mM ATP. The protein patterns were analyzed by Western blotting with the OppA-specific monoclonal antibody DC10 and the P55-specific monoclonal antibody AH10. Since cytoplasmic P55 is susceptible to tryptic digestion, cells were lysed (P55lc) before addition of trypsin.
ATP hydrolysis of OppA and intact mycoplasma cells.
To analyze whether OppA is able not only to bind but also to hydrolyze ATP, we estimated the ATP-hydrolyzing activity of recombinant OppA protein. The purity of the recombinant OppA preparation was demonstrated by silver staining (Fig. 2A).
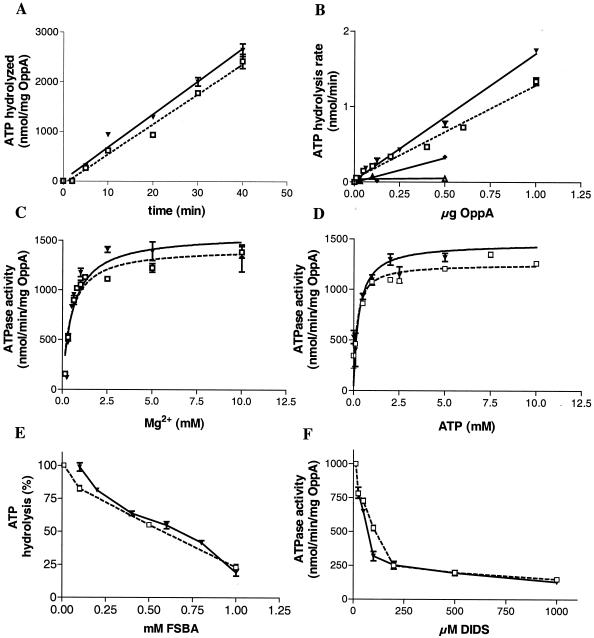
The kinetics of ATP hydrolysis by recombinant OppA measured as the release of free phosphate were linear for at least 40 min (Fig. 4A). In the following experiments, the ATPase assay was therefore terminated after 20 min. ATPase activity was determined in relation to the concentration of OppA. ATP hydrolysis was linear between 0.125 and 1 μg of OppA (Fig. 4B, open square). Therefore, we used 0.5 μg of OppA in all further ATPase assays.
FIG. 4.
Comparison of ATPase activity of intact mycoplasmas, recombinant OppA, OppAK875R mutant, and OppAΔP-loop. ATPase activity of intact cells (▾), recombinant OppA (□), OppAK875R (♦), OppAΔP-loop (▵), and the supernatant (•) was measured in the ammonium molybdate assay as described in Materials and Methods. The amount of OppA present at the surface of intact mycoplasma cells was estimated by an OppA-specific enzyme-linked immunosorbent assay. The ATPase activity was measured as a function of time (A), concentration of OppA (B), concentration of Mg2+ ions (C), and concentration of ATP (D). The effect of increasing concentration of 5′-fluorosulfonyl-benzoyladenosine (FSBA, E) and DIDS (F) on the ecto-ATPase activity of OppA was determined. The values shown are the means (± standard deviations) of the results of three independent experiments.
As demonstrated in the ATP trypsin assay, the binding of ATP to OppA depends on Mg2+ ions. To prove that ATP hydrolysis was also dependent on divalent cations, recombinant OppA was used in the absence of MgCl2 and resulted in a low level of ATP hydrolysis, and addition of MgCl2 to the medium led to an increase in ATPase activity in a dose-dependent manner (Fig. 4C). The data for ATP hydrolysis of OppA were fed into the Michaelis-Menten equation to yield values for Vmax of 1,254 ± 40 nmol/min/mg of OppA protein and Km of 0.18 ± 0.04 mM (Fig. 4D). As expected, mutation of the P-loop structure decreased ATPase activity. In comparison to the wild type, the ATP-hydrolyzing activity of the mutants OppAK875R and OppAΔP-loop decreased to 15% and 6%, respectively (see Fig. 4B).
We also analyzed the specificity of recombinant OppA towards hydrolysis of other nucleoside triphosphates. With GTP and CTP, the release of free phosphate represents only 8% (GTP) and 6% (CTP) of the ATP hydrolysis (data not shown). This result was consistent with the finding described above that GTP and CTP have a lower affinity to OppA than ATP does.
To confirm that the ATP cleavage is accompanied by ATP binding in OppA, a synthetic adenosine analog which is capable of covalent binding to proteins was added to the ATP hydrolysis assay. Pretreatment of OppA with 5′-fluorosulfonyl-benzoyladenosine strongly inhibited ATP hydrolysis in a dose-dependent manner (Fig. 4E). The ATPase inhibitor 4′,4′-diisothiocyanostilbene 2′,2′-disulfonic acid (DIDS) reduced ATPase activity to 25% of that of the control (Fig. 4F).
The effect of ATP on the tryptic digestion pattern of lipid-anchored OppA in the mycoplasma membrane demonstrated that the nucleotide-binding site is located on the surface of the cell. To characterize the contribution of OppA to the ecto-ATPase activity of Mycoplasma hominis, the amount of surface-localized OppA was determined by an OppA-specific enzyme-linked immunosorbent assay analyzing recombinant OppA of known concentration and cells of Mycoplasma hominis in serial dilutions. The ATPase activity of the different samples was determined in parallel. This experiment revealed that regarding the same amount of OppA in both lines (protein versus cells), the purified recombinant OppA represents about 80% to 85% of the ATPase activity of intact mycoplasma cells (Fig. 4B).
The ecto-ATPase activity of intact mycoplasma cells showed the same enzyme kinetic and dependence on the concentrations of enzyme, Mg2+ ions and ATP as the purified OppA protein (Fig. 4A, B, and C, curve with open squares). For mycoplasma cells the Vmax for ATP hydrolysis was 1,460 ± 76 nmol/min/mg, correlating with an OppA concentration on the surface of M. hominis of 0.19 ± 0.03 mM (Fig. 4D).
As observed for the ATPase activity of purified OppA, the ATP hydrolysis activity of intact cells decreased (here about 86%) in the presence of the inhibitor 5′-fluorosulfonyl-benzoyladenosine (Fig. 4E). Further evidence for the surface ecto-ATPase activity of M. hominis cells was provided by inhibition of the hydrolysis with the cell-impermeable reagent DIDS. DIDS inhibited the ATPase activity of mycoplasma cells by 75% (Fig. 4F).
To avoid measurement of ATPase activity from cytoplasmic enzymes, cells of the Mycoplasma hominis strain FBG were collected at the beginning of logarithmic growth and transferred to a phosphate-free ATPase buffer, and the release of inorganic phosphate was measured immediately. During the experiment the mycoplasma cells did not show measurable growth or lysis, as confirmed by determination of the colony-changing units. We also excluded the possibility that the observed ATP hydrolysis was a result of soluble enzymes secreted from the cells by analyzing the supernatant of the mycoplasma cells for ATPase activity, which did not show hydrolyzing activity (Fig. 4B).
These data clearly demonstrate that in M. hominis the substrate-binding protein of the oligopeptide permease has a surface-localized ATP-binding site which functions as the main ecto-ATPase of the organism.
DISCUSSION
Proteins with surface-localized binding sites for nucleotides have been reported for cells such as vascular endothelium (40), smooth muscle cells (23), parasites such as Leishmania tropica (26), the amoeba Dictyostelium discoideum (16), and group C streptococci (28). The data presented here describe the first Mg2+-dependent ATP-binding and -hydrolyzing activity on the external surface of a cell wall-less bacterium. The substrate-binding protein OppA of the oligopeptide permease was shown to be the main ecto-ATPase of M. hominis. Trypsin treatment of protein or cell preparations in the presence or absence of ATP indicated a conformational change of OppA in response to the binding of nucleotides, as seen for other nucleotide-binding proteins (37).
The tryptic digestion pattern of OppA changed dramatically in the presence of ATP and ADP, whereas nucleotides such as GTP and CTP failed to protect OppA against proteolysis. Because of identical tryptic digestion patterns by binding ATP or ADP, it seems likely that the adenosine in both molecules was involved in binding to OppA. This is in accordance with results reported for the hemolysin exporter HlyB of E. coli that also binds ATP and ADP in almost equimolar amounts, inducing a substantial conformational change in HlyB (21). Furthermore, a 95-kDa fragment of the SecA ATPase of E. coli was shown to be highly resistant to protease treatment in the presence of ATP, ATPγS, and ADP but not GTP, also suggesting an adenosine phosphate-mediated change in the protein structure (39).
The experiments shown in this paper revealed that GTP and CTP did not bind to the OppA protein and this protein also exhibited very low GTPase (8%) and CTPase (6%) activity compared with the ATPase activity. There are several types of surface-located enzymes differing in their ability to hydrolyze nucleotides (45). The ecto-ATPases at the surface of parasites (26) and the ATP-binding protein of the histidine permease of S. enterica serovar Typhimurium (29) show reduced hydrolysis activity for the nucleotides GTP, CTP, UTP, and ADP.
Analyses of the crystal structure of different ATP and GTP binding proteins revealed that a distinct lysine residue in the P-loop structure is involved in binding the β-and γ-phosphates of nucleotides (7, 30). We proved that this lysine was important for ATP hydrolysis when analyzing an OppAK875R mutant in which the lysine residue was replaced by arginine. In accordance with the findings of Schneider and coworkers who tested a respective lysine deficient mutant of the MalK protein of S. enterica serovar Typhimurium (37), the OppAK875R mutant displayed a decreased ATPase activity of 85% compared to the wild-type but was still capable of binding ATP. Thus, binding of ATP appears to be sufficient to cause protection against trypsin digestion whereas mutation of the whole Walker A motif is required to decrease ATP binding and hydrolysis.
Several hypotheses for the function of ecto-ATPases in various cell types have been proposed, such as (i) protection from a cytolytic effect of extracellular ATP (6, 34), (ii) regulation of ecto-kinase substrate concentration (33), (iii) involvement in signal transduction (2, 25, 27), and (iv) involvement in cellular adhesion (17, 20, 42). So far the physiological role of OppA in Mycoplasma hominis remains speculative. Indeed, this protein is concomitantly expressed with the four core subunits of the permease, and furthermore, no OppA-deficient mutants were found after analyzing approximately 200 M. hominis isolates, which suggests that OppA probably plays an essential role in M. hominis vitality. A decade ago, when its function as a substrate-binding protein was unknown, OppA was identified as a cytoadherence-mediating lipoprotein of M. hominis (10). With the discovery of the substrate-binding function of the OppA protein, the multifunctionality of this protein has become obvious (11). In other bacteria, transport systems have been shown to take part in processes other than the transport of peptides, e.g., cytoadherence (4), initiation of competence and sporulation (32, 36), or induction of conjugation (24).
Extracellular ATP has been shown to be released from neutrophils, degranulated platelets (8), and epithelial cells colonized by bacteria (3). ATP is known to be an important extracellular signaling molecule which may be involved in cell-mediated cytotoxicity (41). Into and coworkers found that lipoproteins of Mycoplasma fermentans and Mycoplasma salivarius induced an increase in plasma membrane permeability by which ATP is released. This extracellular ATP regulates the cytotoxicity of the lipoproteins, probably by interaction with ATP receptors such as P2X purinergic receptors (15). It has been demonstrated that extracellular ATP has profound effects on cellular functions, causing plasma membrane depolarization, Ca2+ influx, and cell death (6, 41). The ecto-ATPases of parasites might be involved in an escape mechanism allowing a splitting of the ATP released in its vicinity by the colonized cells (43). Recently, in vivo phosphorylation of M. hominis revealed that OppA exists in phosphorylated and nonphosphorylated forms, suggesting a regulatory mechanism (13).
Future work will include the study of the influence of the phosphorylation status of OppA on the ATPase activity and the influence of the ATPase activity on cytoadhesion, peptide transport, cytolysis, and the triggering of specific signal cascades in the host that have been described to alter host cell division and growth (35). Our aim is to achieve an understanding of the multifunctional network in Mycoplasma hominis in which OppA seems to play an important role.
Acknowledgments
We thank Marzena Czarna for excellent technical assistance; Gaby Reichmann, Colin MacKenzie, Klaus Pfeffer, and Heiner Schaal for critically reading the manuscript; Sybille Scheuring for the DNA sequencing work (Center for Biological and Medical Research, Heinrich Heine University); and Erwin Schneider (Humboldt University of Berlin, Institute of Biology) for the gift of purified MalK protein.
REFERENCES
- 1.Ames, G. F.-L. 1990. Energy coupling in periplasmic permeases: the histidine permease as a model system. Res. Microbiol. 141:341-348. [DOI] [PubMed] [Google Scholar]
- 2.Clifford, E. E., K. A. Martin, P. Dalal, R. Thomas, and G. R. Dubyak. 1997. Stage-specific expression of P2Y receptors, ecto-apyrase, and ecto-5′-nucleotidase in myeloid leukocytes. Am. J. Physiol. 42:C973-C987. [DOI] [PubMed] [Google Scholar]
- 3.Crane, J. K., R. A. Olson, H. M. Jones, and M. E. Duffey. 2002. Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G74-G86. [DOI] [PubMed] [Google Scholar]
- 4.Cundell, D. R., B. J. Pearce, J. Sandros, A. M. Naughton, and H. R. Masure. 1995. Peptide permease from Streptococcus pneumoniae affect adherence to eukaryotic cells. Infect. Immun. 63:2493-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmann, R.-C., B. Henrich, V. Kolb-Bachofen, and U. Hadding. 1992. Decreased metabolism and viability of Mycoplasma hominis induced by monoclonal antibody-mediated agglutination. Infect. Immun. 60:166-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filippini, A., R. E. Taffs, T. Agui, and M. V. Sitkovsky. 1990. Ecto-ATPase activity in cytolytic T-lymphocytes. Protection from the cytolytic effects of extracellular ATP. J. Biol. Chem. 265:334-340. [PubMed] [Google Scholar]
- 7.Fry, D. C., S. A. Kuby, and Mildvan. 1987. NMR studies of the AMP-binding site and mechanism of adenylate kinase. Biochemistry 26:1645-1655. [DOI] [PubMed] [Google Scholar]
- 8.Gordon, J. L. 1986. Extracellular ATP: effects, sources and fate. Biochem. J. 233:309-319. [DOI] [PMC free article] [PubMed]
- 9.Henkel, R. D., J. L. van de Berg, and R. A. Walsh. 1988. A microassay for ATPase. Anal. Biochem. 169:312-318. [DOI] [PubMed] [Google Scholar]
- 10.Henrich, B., R.-C. Feldmann, and U. Hadding. 1993. Cytoadhesins of Mycoplasma hominis. Infect. Immun. 61:2945-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henrich, B., M. Hopfe, A. Kiterow, and U. Hadding. 1999. The adherence-associated lipoprotein P100, encoded by an opp operon structure, funtions as the oligopeptide-binding domain OppA of a putative oligopeptide transport system in Mycoplasma hominis. J. Bacteriol. 181:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgens, C. F. 1992. ABC transporters: from microorganismus to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 13.Hopfe, M. 2002. Ph.D. thesis. Characterization of the oligopeptide permease in Mycoplasma hominis (Charakterisierung der Oligopepidpermease von Mycoplasma hominis). www.ulp.uni-duesseldorf.de/diss/Mathematisch-Naturwissenschaftliche/2002/Hopfe.pdf. Heinrich-Heine University, Düsseldorf, Germany.
- 14.Horten, R. H., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:161-166. [DOI] [PubMed] [Google Scholar]
- 15.Into, T., K. Okada, N. Inoue, M. Yasuda, and K.-I. Shibata. 2002. Extracellular ATP regulates cell death of lymphocytes and monocytes induced by membrane-bound lipoproteins of Mycoplasma fermentans and Mycoplasma salivarium. Microbiol. Immunol. 46:667-675. [DOI] [PubMed] [Google Scholar]
- 16.Juliani, M. T., and C. Klein. 1981. A protein kinase of the plasma membrane of Dictyostelium discoideum. Biochim. Biophys. Acta 662:256-264. [DOI] [PubMed] [Google Scholar]
- 17.Kirley, T. L. 1997. Complementary DNA cloning and sequencing of the chicken muscle ecto-ATPase. Homology with the lymphoid cell activation antigen CD39. J. Biol. Chem. 272:1076-1081. [DOI] [PubMed] [Google Scholar]
- 18.Kitzerow, A., and B. Henrich. 2001. The cytosolic HinT protein of Mycoplasma hominis interacts with two membrane proteins. Mol. Microbiol. 41:279-287. [DOI] [PubMed] [Google Scholar]
- 19.Kitzerow, A., U. Hadding, and B. Henrich. 1999. Cyto-adherence studies of the adhesion P50 of Mycoplasma hominis. J. Med. Microbiol. 48:485-493. [DOI] [PubMed] [Google Scholar]
- 20.Knowles, A. F. 1995. The rat liver ecto-ATPase/C-CAM cDNA detects induction of carcinoembryonic antigen but not the mercurial-insensitive ecto-ATPase in human hepatoma Li-7A cells treated by epidermal growth factor and cholera toxin. Biochem. Biophys. Res. Commun. 207:529-535. [DOI] [PubMed] [Google Scholar]
- 21.Koronakis, V., C. Hughes, and E. Koronakis. 1993. ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol. Microbiol. 8:1163-1175. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 23.Lamport-Vrana, S. J., E. Vrana, and J. S. Fedan. 1991. Involvement of ecto-phosphoryl transfer in contractions of the smooth muscle of the guinea pig vas deferens to adenosine 5′-triphosphate. J. Pharmacol. Exp. Ther. 258:339-348. [PubMed] [Google Scholar]
- 24.Leonard, B. A., A. Podbielski, P. J. Hedberg, and G. M. Dunny. 1996. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc. Natl. Acad. Sci. USA 93:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolis, R. N., M. J. Schell, S. L. Taylor, and A. L. Hubbard. 1990. Hepatocyte plasma membrane Ecto-ATPase (pp120/HA4) is a substrate for tyrosine kinase activity of the insulin receptor. Biochem. Biophys. Res. Commun. 166:562-566. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-Fernandes, J. R., P. M. L. Dutra, C. O. Rodrigues, J. Saad-Nehme, and A. H. C. S. Lopes. 1997. Mg-dependent ecto-ATPase activity in Leishmania tropica. Arch. Biochem. Biophys. 341:40-46. [DOI] [PubMed] [Google Scholar]
- 27.Najjar, S. M., D. Accili, N. Philippe, J. Jernberg, R. Margolis, and S. I. Taylor. 1993. pp120/ecto-ATPase, an endogenous substrate of the insulin receptor tyrosine kinase, is expressed as two variably spliced isoforms. J. Biol. Chem. 268:1201-1206. [PubMed] [Google Scholar]
- 28.Nickel, V., S. Prehm, M. Lansing, A. Mausolf, A. Podbielski, J. Deutscher, and P. Prehm. 1998. An ectoprotein kinase of group C streptococci binds hyaluronan and regulates capsule formation. J. Biol. Chem. 273:23668-23673. [DOI] [PubMed] [Google Scholar]
- 29.Nikaido, K., P. Q. Liu, and G. F. Ames. 1997. Purification and characterization of HisP, the ATP-binding subunit of a traffic ATPase (ABC transporter), the histidine permease of Salmonella typhimurium. Solubility, dimerization, and ATPase activity. J. Biol. Chem. 272:27745-27752. [DOI] [PubMed] [Google Scholar]
- 30.Pai, E. F., W. Kabsch, U. Krengel, K. Holmes, J. John, and A. Wittinghofer. 1989. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature 341:209-214. [DOI] [PubMed]
- 31.Pedersen, P. L., and E. Carafoli. 1987. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 12:146-150. [Google Scholar]
- 32.Perego, M., C. F. Higgins, R. Pearce, M. P. Gallagher, and J. A. Joch. 1991. The oligopeptide transport system of Bacillus subtilis plays a role in the initiation of sporulation. Mol. Microbiol. 5:173-185. [DOI] [PubMed] [Google Scholar]
- 33.Plesner, L. 1995. Ecto-ATPases: identities and functions. Int. Rev. Cytol. 158:141-214. [DOI] [PubMed] [Google Scholar]
- 34.Redegeld, F., A. Filippini, and M. Sitkovsky. 1991. Comparative studies of the cytotoxic T lymphocyte-mediated cytotoxicity and of extracellular ATP-induced cell lysis. Different requirements in extracellular Mg2+ and pH. J. Immunol. 147:3638-3645. [PubMed] [Google Scholar]
- 35.Rottem, S., and M. F. Barile. 1993. Beware of mycoplasmas. Trends Biotechnol. 11:143-151. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Schneider, E., S. Wilken, and R. Schmid. 1994. Nucleotide-induced conformational changes of MalK, a bacterial ATP binding cassette transporter protein. J. Bio. Chem. 269:20456-20461. [PubMed] [Google Scholar]
- 38.Schneider, E., and S. Hunke. 1998. ATP-binding-cassette (ABC) transport systems: functional and structural aspects of the ATP hydrolyzing subunits/domains. FEMS Microbiol. Rev. 22:1-20. [DOI] [PubMed] [Google Scholar]
- 39.Shinkai, A., L. H. Mei, H. Tokuda, and S. Mizushima. 1991. The conformation of SecA, as revealed by its protease sensitivity, is altered upon interaction with ATP, presecretory proteins, everted membrane vesicles, and phospholipids. J. Biol. Chem. 266:5827-5833. [PubMed] [Google Scholar]
- 40.Skubitz, K. M., and D. D. Ehresmann. 1992. The angiogenesis inhibitor beta-cyclodextrin tetradecasulfate inhibits ecto-protein kinase activity. Cell Mol. Biol. 38:543-560. [PubMed] [Google Scholar]
- 41.Steinberg, T. H., and F. Di Virgilio. 1991. Cell-mediated cytotoxicity: ATP as an effector and the role of target cells. Curr. Opin. Immunol. 3:71-75. [DOI] [PubMed] [Google Scholar]
- 42.Stout, J. G., R. S. Strobel, and T. L Kirley. 1995. Properties of and proteins associated with the extracellular ATPase of chicken gizzard smooth muscle. A monoclonal antibody study. J. Biol. Chem. 270:11845-11850. [DOI] [PubMed] [Google Scholar]
- 43.Vasconcelos, E. G., S. T. Ferreira, T. M. U. Carvalho, W. De Souza, A. M. Kettlun, M. Mancilla, M. A. Valenzuela, and S. Verjovshi-Almeida. 1996. Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni. Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J. Biol. Chem. 271:22139-22145. [DOI] [PubMed] [Google Scholar]
- 44.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases, and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann, H. 1999. Nucleotides and cd39: principal modulatory players in hemostasis and thrombosis. Nat. Med. 5:987-988. [DOI] [PubMed] [Google Scholar]