Summary
Balloon test occlusion (BTO) of the internal carotid artery (ICA) combined with cerebral blood flow (CBF) study is a sensitive test for predicting the outcome of permanent ICA occlusion. However, false negative results sometimes occur using single photon emission tomography (SPECT). We have recently developed a rapid positron emission tomography (PET) protocol that measures not only the CBF but also the cerebral oxygen metabolism before and during BTO in succession. We measured acute changes in regional CBF and OEF/CMRO2 before and during BTO in three cases with large or giant cerebral aneurysms using the rapid PET protocol.
Although no patients showed ischemic symptoms during BTO, PET studies exhibited mildly to moderately decreased CBF (9∼34%) compared to the values obtained before BTO in all cases. The average OEF during BTO was significantly increased (21% and 43%) than that of before BTO in two cases. The two cases were considered to be non-tolerant for permanent ICA occlusion and treated without ICA sacrifice.
Measurement of the CBF and OEF/CMRO2 using a rapid PET protocol before and during BTO is feasible and can be used for accurate assessment of tolerance prediction in ICA occlusion.
Key words: balloon test occlusion, cerebral blood flow, cerebral oxygen metabolism, internal carotid artery, positron emission tomography
Introduction
Occlusion of the ICA is sometimes necessary to treat giant ICA aneurysms and tumors encasing the ICA. The consequences of ICA occlusion range from no symptoms to devastating hemispheric strokes 1. The balloon test occlusion (BTO) of the ICA has been used to predict whether a patient can tolerate temporary or permanent occlusion of the ICA. If the patient develops any sign of hemispheric ischemia during BTO, permanent ICA occlusion should not be performed or an extracranial-intracranial (EC-IC) bypass is considered before ICA occlusion 2. However, BTO with neurological evaluation alone has a high false negative rate 3. To improve the sensitivity, several reports have proposed CBF measurement to predict the likelihood of ischemic complications following ICA occlusion during BTO 4-10. Single photon emission tomography (SPECT) allows semi-quantitative determination of the CBF throughout the entire brain and is reported to be useful for evaluating collateral blood flow after permanent ICA occlusion 4,6,8. However, up to 20% of the patients did not tolerate ICA sacrifice, even though they had symmetrical SPECT scans during BTO 11,12. Besides the CBF, oxygen extraction fraction (OEF) and cerebral metabolic rate of oxygen (CMRO2), which can be obtained only with positron emission tomography (PET) study, provide us important indices for assessing ischemic degree 13-15. However, the complex nature of the PET procedure and its relatively long protocol limit the applicability of PET study to BTO. One of our colleagues developed a new dual tracer autoradiographic (DRAG) method to shorten PET examination time 16,17. This method enabled us to measure the CBF and OEF/CMRO2 values before and during BTO in succession. In this manuscript, we report initial three cases of PET study for evaluating the cerebral blood flow and metabolism before and during BTO, especially describing the feasibility of rapid quantitative measurement of the CBF and OEF/CMRO2.
Materials and Methods
Three women with large or giant cerebral aneurysms involving the ICA underwent BTO to evaluate brain tolerance to sacrifice the ICA. Written informed consent was obtained from all patients after a detailed explanation of the test procedure.
Balloon test occlusion
After diagnostic cerebral angiography, a 5-French double-lumen balloon catheter was inserted into the ICA of the affected side with systemic heparinization (5,000-10,000 IU). One lumen is used to inflate the balloon, and the central lumen is a distal lumen beyond the balloon connecting to a pressure transducer. Cross-filling via the communicating arteries was examined by contralateral carotid angiogram or vertebral angiogram under balloon inflation of the objective carotid artery. A trial BTO was performed for 15 min to examine neurological signs or changes in the stump pressure.
Complete occlusion of the ICA was confirmed by a sudden drop in the stump pressure and contrast injection. After trial BTO, patients were transferred from the angiography suite to the radioisotope suite with the balloon deflated but still in place under frequent monitoring of the activated coagulation time.
An adhesive strip was affixed to the catheter at the location of the catheter entrance into the sheath so that it could later be inflated during PET scanning without the use of fluoroscopy.
PET examination
PET acquisition was performed in 2D mode using an ECAT HR+ scanner (Siemens-CTI, Knoxville, USA), which provided 63 tomographic slice images for an axial field-of-view of approximately 150 mm. A five-minute transmission scan using a 68Ge rod source was conducted to correct tissue attenuation. Intermittent arterial blood sampling and radioactivity concentration measurements were performed throughout PET scanning using a catheter implanted in the brachial artery to obtain the arterial input function using an auto well gamma counter (ARC-400, Aloka, Tokyo, Japan). Arterial blood samples were also used to measure hematocrit and blood gas tensions. In the radioisotope suite, the patients were positioned on a table for the PET camera. First, baseline measurements of CBF and OEF/CMRO2 were performed without balloon inflation. The PET protocol was originally developed as the DRAG method 16,17, in which 15O2 and H215O (or C15O2) are sequentially administered during a single PET scanning to measure the OEF/CMRO2 and CBF. The method was further improved by eliminating the need for CBV data so that the total scan time is shortened to less than 15 min 17. In the present study, we applied the method by administering firstly 15O2 (3000 MBq/min) followed by C15O2 (500 MBq/min) after ten minutes during a single PET scanning (10 s × 6; 20 s × 6; 30 s × 4; 120 s × 3; 5 s × 12; 10 s × 9). The arterial input function was determined by frequent arterial sampling during the PET scanning. After the baseline measurement, the balloon was inflated after sufficient time for eliminating the tracer. Complete occlusion of the ICA was confirmed by a sudden drop in the stump pressure. The ICA was occluded for 15 minutes for the BTO PET study and CBF and OEF/CMRO2 values were measured during BTO. Total examination time in the radioisotope suite was about 50 minutes. Several circular regions of interest (ROI) were placed on CBF images in the middle cerebral artery (MCA) territory. The size of each ROI was 10 mm in diameter. OEF and CMRO2 values were extracted from the same ROIs on CBF images.
Results
None of the three patients exhibited neurologic symptoms during BTO in the angiography suite. Physiological parameters including blood pressure and blood gas tensions were within normal range and were not significantly different between before and during BTO PET examinations.
Case 1
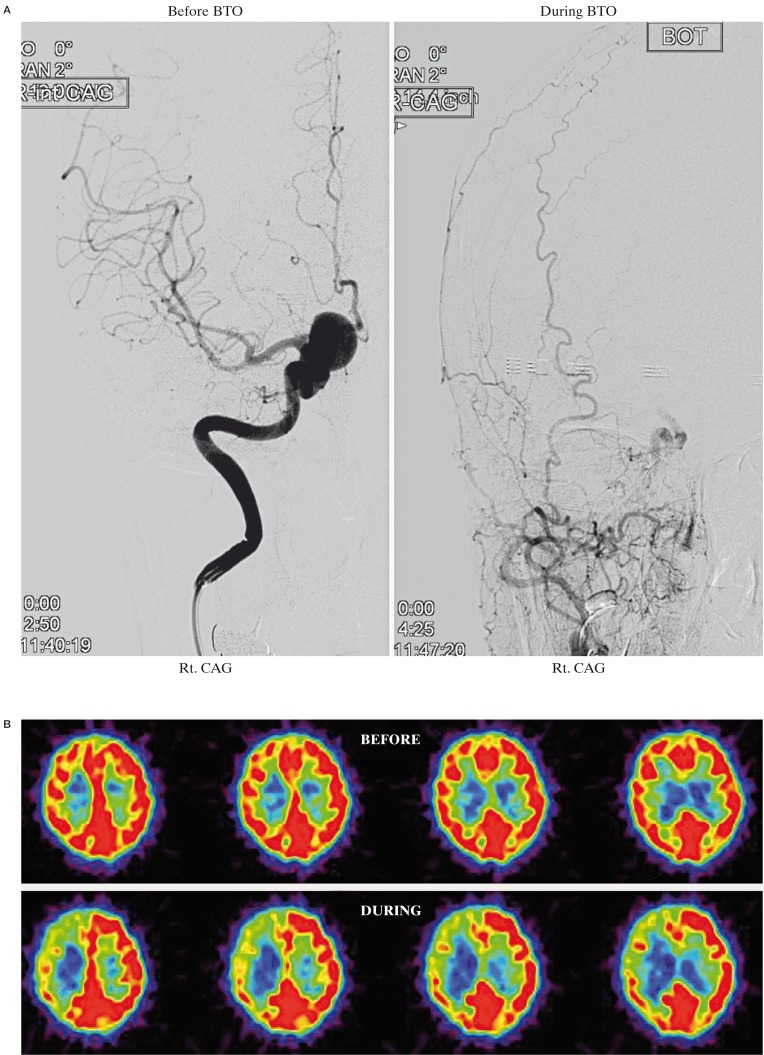
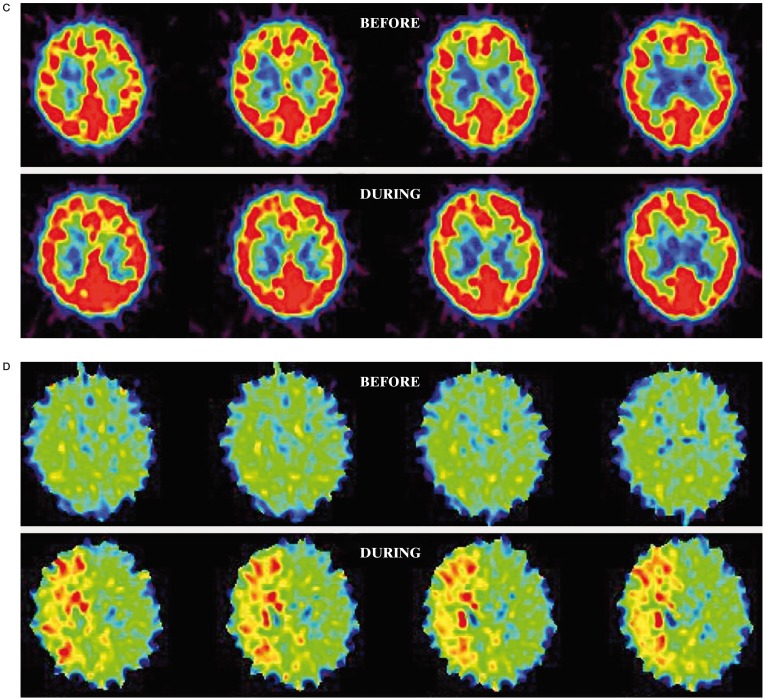
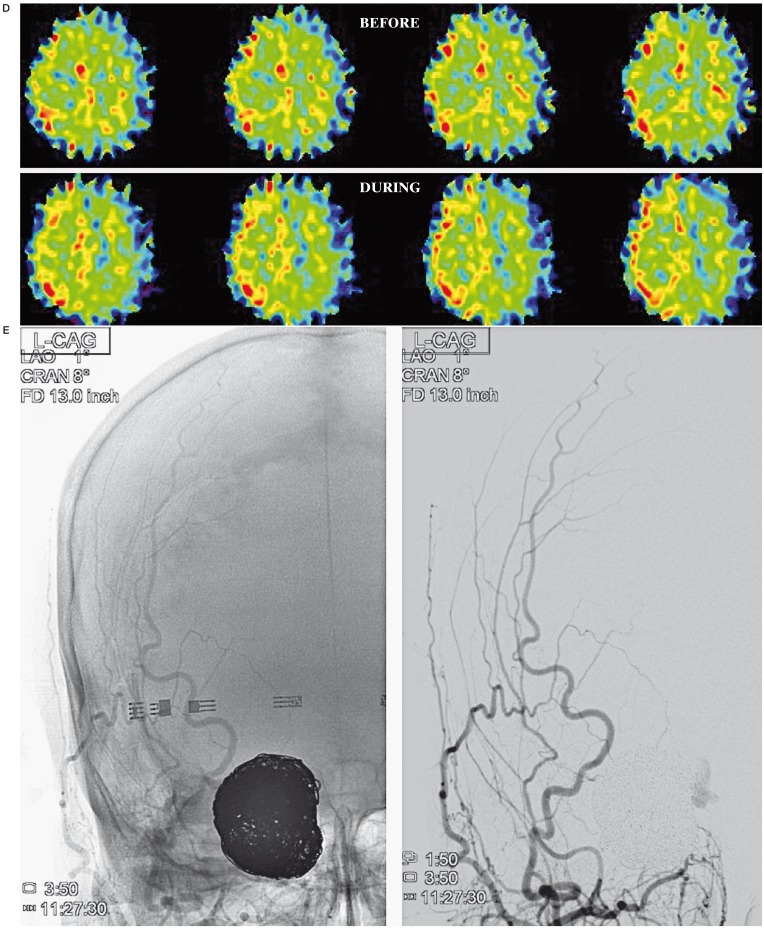
A 66-year-old woman was admitted to our hospital with left upper-quadrant homonymous hemianopsia. Right ICA angiogram demonstrated a large paraclinoid aneurysm (16 × 12 mm) (Figure 1A). The average CBF in the right MCA territory during BTO was 38.4 ml/100 g/min, which was 34% lower than that of before BTO (57.9 ml/100 g/ min) and 35% lower compared to the contralateral value (58.8 ml/100 g/ min) (Figure 1B). The average CMRO2 in the right MCA territory during BTO (4.9 ml/100 g/min) was slightly higher than that of before BTO (4.5 ml/100 g/min) (Figure 1C). The average OEF in the right MCA territory during BTO was 0.66, which was 43% higher than that of before BTO (0.46) (Figure 1D). This patient was considered to be non-tolerant for permanent ICA occlusion and treated with successful neck clipping.
Figure 1.
A) Right ICA angiogram shows a large paraclinoid aneurysm. The right ICA is completely occluded by a balloon during BTO. B) During BTO of the right ICA, a moderate decrease in CBF in the right MCA territory is demonstrated by PET study. The CMRO2 was symmetrical (C) and the OEF in the right MCA territory is markedly increased (D). This patient was considered to be non-tolerant for permanent ICA occlusion.
Case 2
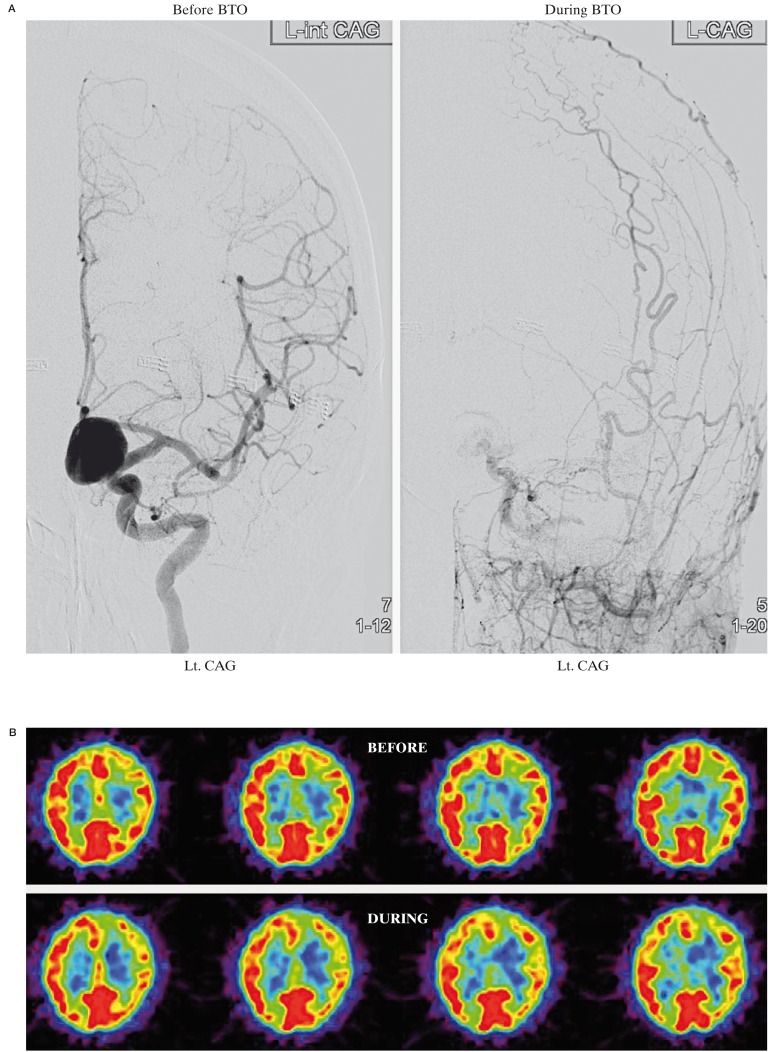
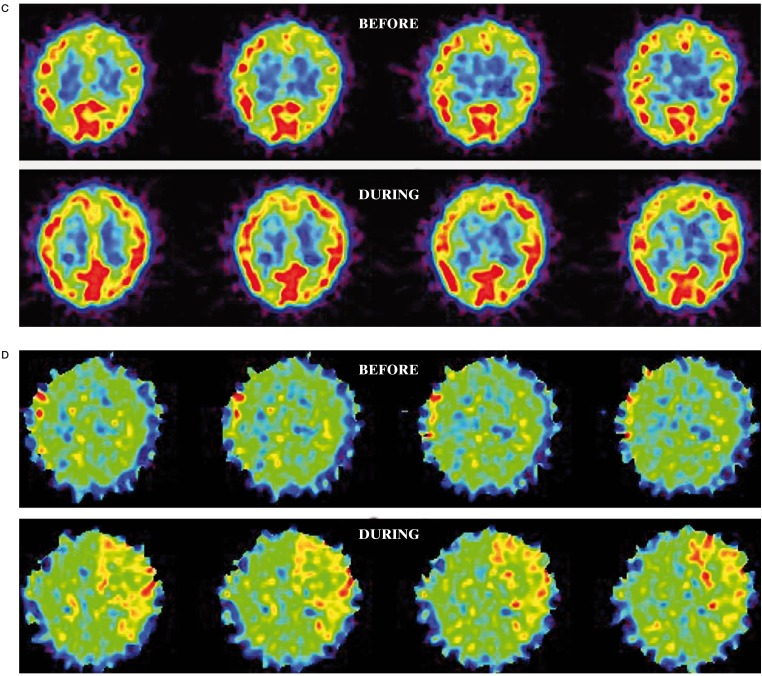
A 50-year-old woman was admitted to our hospital with an incidentally found cerebral aneurysm. left ICA angiogram demonstrated a large paraclinoid aneurysm (16 × 14 mm) (Figure 2A). BTO resulted in mild global reduction of CBF in the bilateral hemispheres.
The average CBF in the left MCA territory during BTO was 41.2 ml/100 g/min, which was 13% lower than that of before BTO (47.0 ml/100 g/ min) and 15% lower compared to the contralateral value (48.6 ml/100 g/ min) (Figure 2B). The average CMRO2 in the left MCA territory during BTO (3.8 ml/100 g/min) was slightly higher than that of before BTO (3.3 ml/100 g/min) (Figure 2C). The average OEF in the left MCA territory during BTO was 0.57, which was 21% higher than that of before BTO (0.47) (Figure 2D). This patient was considered to be non-tolerant for permanent ICA occlusion and treated with successful neck clipping.
Figure 2.
A) left ICA angiogram shows a large paraclinoid aneurysm. The left ICA is completely occluded by a balloon during BTO. B) During BTO of the left ICA, a mild decrease in CBF in the left MCA territory is demonstrated by PET study. The CMRO2 was symmetrical (C) and the OEF in the left MCA territory is mildly increased (D). This patient was considered to be non-tolerant for permanent ICA occlusion.
Case 3
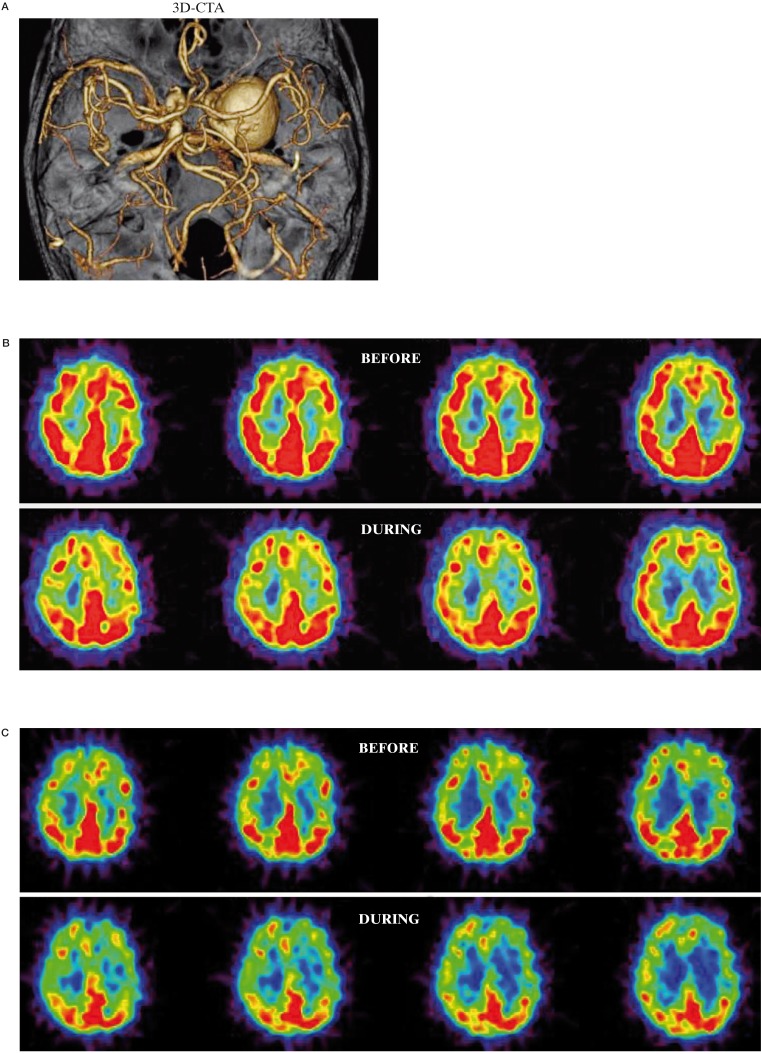
A 60-year-old woman was admitted to our hospital with right facial numbness and blurred vision. Right ICA angiogram demonstrated a giant cavernous aneurysm (30 × 25 mm) (Figure 3A). BTO resulted in mild global reduction of CBF in the bilateral hemispheres. The average CBF in the right MCA territory during BTO was 46.6 ml/100 g/min, which was 9% lower than that of before BTO (51.0 ml/100 g/ min) and 9% lower compared to the contralateral value (51.5 ml/100 g/ min) (Figure 3B). The average CMRO2 in the right MCA territory during BTO (3.0 ml/100 g/min) was slightly lower than that of before BTO (3.3 ml/100 g/min) (Figure 3C). The average OEF in the left MCA territory during BTO was 0.51, which was slightly higher than that of before BTO (0.48) (Figure 3D). This patient was considered to be tolerant for permanent ICA occlusion and treated with ICA occlusion without acute or later neurological deficits at six months after the treatment.
Figure 3.
A) 3D-CT angiogram shows a giant cavernous aneurysm. B) During BTO of the right ICA, a mild global decrease in CBF in the bilateral hemispheres is demonstrated by PET study. The CMRO2 was symmetrical (C) and the OEF in the right MCA territory is slightly increased (D). This patient was considered to be tolerant for permanent ICA occlusion and treated with coil embolization with ICA occlusion (E).
Discussion
Occlusion of the ICA was demonstrated to carry 26% risk of ischemia of the ipsilateral hemisphere, of which 46% were fatal, when performed in non-selective patients 3. Balloon test occlusion (BTO) has been developed as a method to assess the tolerance of permanent ICA occlusion. Linskey et al. reported that the risk of ischemic complications was reduced to 13% by selective carotid occlusion using BTO 3. However, the appearance of neurological symptoms during BTO exhibited low negative and positive predictive values and was not a good predictor of sustained patient outcome 9. To improve the sensitivity, various reports have proposed adjunctive methods to predict the likelihood of ischemic complications following ICA occlusion, such as measurement of the stump pressure 4,18 and the CBF 4-10 during BTO.
Measurement of the blood flow has demonstrated that substantial cerebral hypoperfusion occurs even when the patient does not show any neurological signs during BTO and may evaluate a potential risk after the ICA occlusion 19,20. Among several methods of CBF measurement, SPECT is considered to be the most useful method for evaluating the collateral circulation during BTO 4,6,8. SPECT images acquired after the completion of BTO reflect the tracer distribution during occlusion when the tracer is injected. Although no definite quantitative criteria are now available to define perfusion abnormalities that may cause cerebral infarction, an interhemispheric difference in tracer uptake less than 10% (CBF ratio < 0.9) is generally accepted to be asymmetric and safe 21. Palestro et al. 22 reported that the negative predictive value of the symmetric perfusion (CBF ratio > 0.9) during BTO was 100% in 14 patients who underwent carotid occlusion. In the SPECT study, however, up to 20% of the patients did not tolerate ICA sacrifice, even though they had symmetrical SPECT scans during BTO 11,12. Moreover, a previous study has shown that a considerable reduction in CBF might occur under hypotension after ICA sacrifice, even with negative BTO 23. It has also been suggested that SPECT asymmetry analysis carries a high rate of false positive test results 6,24, because of the semi-quantitative nature of the data analysis. The false negative rate of the BTO was lowered to 3-10% by applying quantitative CBF analysis with xenon-enhanced CT 3,25. They proposed a flow of less than 30 ml/100 g/min as the threshold for occurrence of neurological symptoms after ICA occlusion 3,25. These results indicate that SPECT asymmetry analysis is not accurate to predict ischemic complications after permanent ICA occlusion and quantitative CBF analysis improves the precision of risk evaluation.
PET study is considered to be the most reliable method for measuring the CBF throughout the entire brain. Besides CBF, CBV, oxygen extraction fraction (OEF) and cerebral metabolic rate of oxygen (CMRO2) provide us with important indices that can be used for assessing the ischemic degree in chronic and acute cerebrovascular disease. In a primate study of cerebral ischemia, CMRO2 not CBF measurement may be the best predictor of reversible or irreversible tissue damage 14,15. OEF and CMRO2 values provide a true definition of ischemia in patients with aneurysmal subarachnoid hemorrhage 13. These values can be quantitatively obtained by PET using 15O-labelled compounds. The computational formulae to compute these parametric images are based on a single-tissue compartment model for oxygen kinetics and generally require a data set of C15O scan for cerebral blood volume (CBV), C15O2 (or H215O) scan for CBF and 15O2 scan together with CBF and CBV values for OEF/CMRO2. Several quantitative approaches have been developed and applied in clinical assessment. In the steady-state method, the parametric images are estimated from the data set that is acquired while in the steady state reached during the continuous inhalation of 15O2 and C15O2 as well as bolus inhalation of C15O, requiring waiting time to reach equilibrium. This method can be employed using a simple procedure and mathematical formula, but requires a prolonged data-acquisition time (approximately 50-60 min). Recently, one of our colleagues developed a dual tracer autoradiographic (DRAG) method to shorten the PET examination period 16. This method applies sequential administration of dual tracers of 15O2 and C15O2 during a single PET scan and computes CBF and CMRO2 autoradiographically. Clinical studies demonstrate the possibility of neglecting or fixing the CBV value in the computation of the OEF and CMRO2 without significant bias in normal controls and patients with cerebrovascular disease 17,26. In our institution, we introduced the rapid DRAG method two years ago. This change reduced the burden of patients and clinicians and shortened PET examination time from 50 minutes to 15 minutes and enabled us to measure the CBF and OEF/CMRO2 values before and during BTO in succession. To the best of our knowledge, only a few studies have applied the PET evaluation for BTO. Brunberg et al. 5 applied H215O PET for quantitative measurement of regional CBF during BTO to predict the adequacy of collateral flow after permanent carotid occlusion. They demonstrated that patients having a CBF reduction to 25-35 ml/100 g/min during balloon occlusion may bear a risk of cerebral infarction after permanent ICA occlusion even when there was no clinical symptom 5. This is the first report to measure the blood flow and metabolism in the brain using PET before and during BTO. Although we have not clarified critical values of the OEF/CMRO2 during BTO, which may carry the risk of ischemic complications after permanent ICA occlusion, this approach can improve the sensitivity to predict ischemic complication after permanent ICA occlusion. This study is preliminary and should be performed in a greater number of patients. Our study has several limitations and drawbacks. It should be mentioned that while the findings are suggestive they are still preliminary because of the limited number of cases and this represents a possible limitation to the conclusion drawn. Even though PET study can provide more useful information for the patients, movement to radioisotope suite and longer indwelling time of balloon including inflation and deflation in PET room may require further risk-taking. Moreover, compared to intravenous injection of radioisotope, inhalation of gas tracer used in this study requires additional devices and may limit availability in other institutions.
Conclusion
Measurement of the CBF and OEF/CMRO2 using a new, rapid PET protocol before and during BTO is feasible. With its quantitative character and brief scanning time, our rapid PET can offer a suitable method for predicting tolerance to ICA occlusion. This method may further improve the precision of the test and to reduce the risk of complications following ICA sacrifice with surgery.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (C) (21591845) from the Ministry of Education, Science and Culture of Japan.
References
- 1.Maves MD, Bruns MD, Keenan MJ. Carotid artery resection for head neck cancer. Ann Otol Rhinol Laryngol. 1992;10:778–781. doi: 10.1177/000348949210100912. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu H, Matsumoto Y, Tominaga T. Parent artery occlusion with bypass surgery for the treatment of internal carotid artery aneurysms: clinical and hemodynamic results. Clin Neurol Neurosurg. 2010;112:32–39. doi: 10.1016/j.clineuro.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Linskey ME, Jungreis CA, Yonas H, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. Am J Neuroradiol. 1994;15:829–843. [PMC free article] [PubMed] [Google Scholar]
- 4.Tomura N, Omachi K, Takahashi S, et al. Comparison of technetium Tc 99m hexamethylpropyleneamine oxime single- photon emission tomography with stump pressure during balloon occlusion test of the internal carotid artery. Am J Neuroradiol. 2005;26:1937–1942. [PMC free article] [PubMed] [Google Scholar]
- 5.Brunberg JA, Frey KA, Horton JA, et al. [15O]H2O positron emission tomography determination of cerebral blood flow during balloon test occlusion of the internal carotid artery. Am J Neuroradiol. 1994;15:725–732. [PMC free article] [PubMed] [Google Scholar]
- 6.Field M, Jungreis CA, Chengelis N, et al. Symptomatic cavernous sinus aneurysms: management and outcome after carotid artery occlusion and selective cerebral revascularization. Am J Neuroradiol. 2003;24:1200–1207. [PMC free article] [PubMed] [Google Scholar]
- 7.Katano H, Nagai H, Mase M, et al. Measurement of regional cerebral blood flow with H215O positron emission tomography during Matas Test. Acta Neurochir (Wien) 1995;135:70–77. doi: 10.1007/BF02307417. [DOI] [PubMed] [Google Scholar]
- 8.Kato K, Tomura N, Takahashi S, et al. Balloon occlusion test of the internal carotid artery: correlation with stump pressure and 99mTc-HMPAO SPECT. Acta Radiol. 2006;47:1073–1078. doi: 10.1080/02841850600977745. [DOI] [PubMed] [Google Scholar]
- 9.Marshall RS, Lazar RM, Young WL, et al. Clinical utility of quantitative cerebral blood flow measurements during internal carotid artery test occlusion. Neurosurgery. 2002;50:996–1005. doi: 10.1097/00006123-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Murphy KJ, Deveikisz JP, Brunberg JA, et al. [O-15]H2O positron emission tomography determination of cerebral blood flow reserve after intravenous acetazolamide during balloon test occlusion of the internal carotid artery. Interv Neuroradiol. 1998;30:57–62. doi: 10.1177/159101999800400107. [DOI] [PubMed] [Google Scholar]
- 11.Larson JJ, Tew JM Jr, Tomsick TA, et al. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery. 1995;36:26–30. [PubMed] [Google Scholar]
- 12.Lorberboym M, Pandit N, Machac J, et al. Brain perfusion imaging during preoperative temporary balloon occlusion of the internal carotid artery. J Nucl Med. 1996;37:415–419. [PubMed] [Google Scholar]
- 13.Carpenter DA, Grubb RL Jr, Temple LW, et al. Cerebral oxygen metabolism after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 1991;11:837–844. doi: 10.1038/jcbfm.1991.143. [DOI] [PubMed] [Google Scholar]
- 14.Frykholm P, Andersson JLR, Valtysson J, et al. A metabolic threshold of irreversible ischemia demonstrated by PET in a middle cerebral artery occlusion-reperfusion primate model. Acta Neurol Scand. 2000;102:18–26. doi: 10.1034/j.1600-0404.2000.102001018.x. [DOI] [PubMed] [Google Scholar]
- 15.Frykholm P, Hillered L, Långström B, et al. Relationship between cerebral blood flow and oxygen metabolism, and extracellular glucose and lactate concentrations during middle cerebral artery occlusion and reperfusion: a microdialysis and positron emission tomography study in nonhuman primates. J Neurosurg. 2005;102:1076–1084. doi: 10.3171/jns.2005.102.6.1076. [DOI] [PubMed] [Google Scholar]
- 16.Kudomi N, Hayashi T, Teramoto N, et al. Rapid quantitative measurement of CMRO(2) and CBF by dual administration of (15)O-labeled oxygen and water during a single PET scan-a validation study and error analysis in anesthetized monkeys. J Cereb Blood Flow Metab. 2005;25:1209–1224. doi: 10.1038/sj.jcbfm.9600118. [DOI] [PubMed] [Google Scholar]
- 17.Kudomi N, Watabe H, Hayashi T, et al. Separation of input function for rapid measurement of quantitative CMRO2 and CBF in a single PET scan with a dual tracer administration method. Phys Med Biol. 2007;52:1893–1908. doi: 10.1088/0031-9155/52/7/009. [DOI] [PubMed] [Google Scholar]
- 18.Morishima H, Kurata A, Miyasaka Y, et al. Efficacy of the stump pressure ratio as a guide to the safety of permanent occlusion of the internal carotid artery. Neurol Res. 1998;20:732–736. doi: 10.1080/01616412.1998.11740592. [DOI] [PubMed] [Google Scholar]
- 19.Mathews D, Walker BS, Purdy PD, et al. Brain blood flow SPECT in temporary balloon occlusion of carotid and intracerebral arteries. J Nucl Med. 1993;34:1239–1243. [PubMed] [Google Scholar]
- 20.Simonson TM, Ryals TJ, Yuh WT, et al. MR imaging and HMPAO scintigraphy in conjunction with balloon test occlusion: value in predicting sequelae after permanent carotid occlusion. Am J Roentgenol. 1992;159:1063–1068. doi: 10.2214/ajr.159.5.1414776. [DOI] [PubMed] [Google Scholar]
- 21.Monsein LH, Jeffery PJ, van Heerden BB, et al. Assessing adequacy of collateral circulation during balloon test occlusion of the internal carotid artery with 99mTc-HMPAO SPECT. Am J Neuroradiol. 1991;12:1045–1051. [PMC free article] [PubMed] [Google Scholar]
- 22.Palestro CJ, Sen C, Muzinic M, et al. Assessing collateral cerebral perfusion with technetium-99m-HMPAO SPECT during temporary internal carotid artery occlusion. J Nucl Med. 1993;34:1235–1238. [PubMed] [Google Scholar]
- 23.Tanaka F, Nishizawa S, Yonekura Y, et al. Changes in cerebral blood flow induced by balloon test occlusion of the internal carotid artery under hypotension. Eur J Nucl Med. 1995;22:1268–1273. doi: 10.1007/BF00801611. [DOI] [PubMed] [Google Scholar]
- 24.Yonas H, Linskey M, Johnson DW, et al. Internal carotid balloon test occlusion does require quantitative CBF. Am J Neuroradiol. 1992;12:1147–1152. [PMC free article] [PubMed] [Google Scholar]
- 25.Witt JP, Yonas H, Jungreis C. Cerebral blood flow response pattern during balloon test occlusion of the internal carotid artery. Am J Neuroradiol. 1994;15:847–856. [PMC free article] [PubMed] [Google Scholar]
- 26.Sasakawa Y, Kudomi N, Yamamoto Y, et al. Omission of [15O]CO scan for PET CMRO2 examination using 15O-labelled compounds. Ann Nucl Med. 2011;25:189–196. doi: 10.1007/s12149-010-0438-y. [DOI] [PubMed] [Google Scholar]