Summary
We describe a case of dural arteriovenous fistulas (DAVFs) involving the superior sagittal sinus (SSS) successfully treated with stent placement for an occluded sinus and transarterial embolization.
A 61-year-old man who had been treated with anticoagulation for a known SSS thrombosis presented with a sudden onset of headache. CT scan revealed an intraventricular hemorrhage and cerebral angiography revealed DAVFs involving the SSS which had severe venous congestion and sinus occlusion. We treated this case with a staged endovascular approach which consisted of stent placement for the occluded sinus and transarterial intravenous embolization resulting in complete eradication of DAVFs.
Recanalization of an occluded sinus by stent placement can reduce venous congestion and transarterial intravenous embolization can obliterate dural arteriovenous shunts. This staged strategy is feasible and should be considered a first option of treatment, especially for DAVFs which presented with intracranial hemorrhage and aggressive venous hypertension.
Key words: superior sagittal sinus, dural arteriovenous fistulas, stent placement, transarterial embolization
Introduction
Dural arteriovenous fistulas (DAVFs) involving the superior sagittal sinus (SSS) are rare but frequently have aggressive features including intracranial hemorrhage and venous hypertension 1. Therapeutic intervention of DAVFs in this region remains a challenge even with a variety of methods. We present a case of ruptured DAVFs involving the SSS that was successfully treated with stent placement for an occluded sinus and transarterial embolization.
Case Report
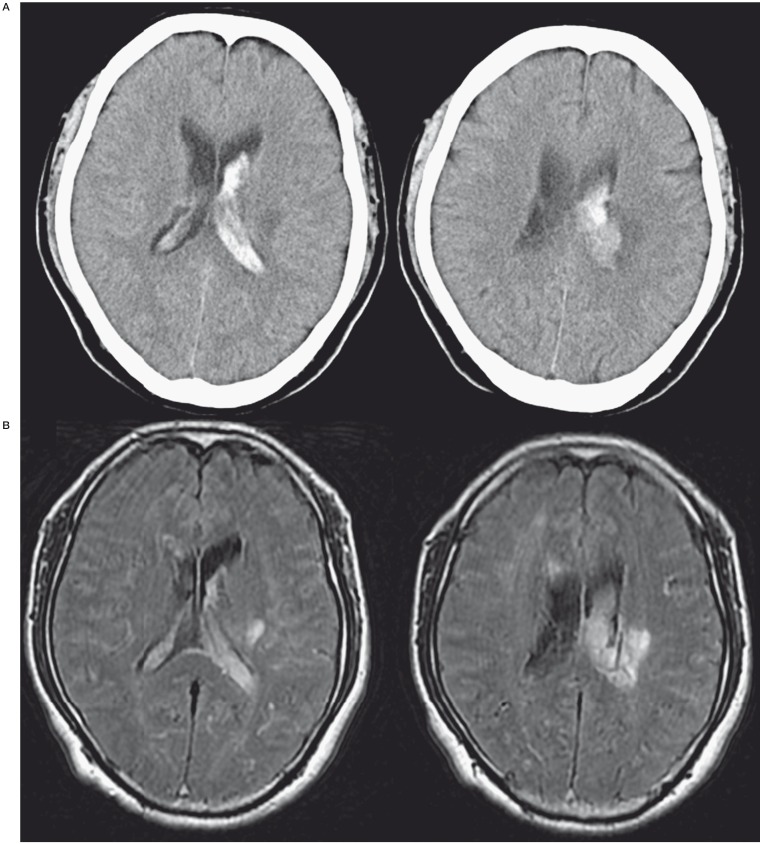
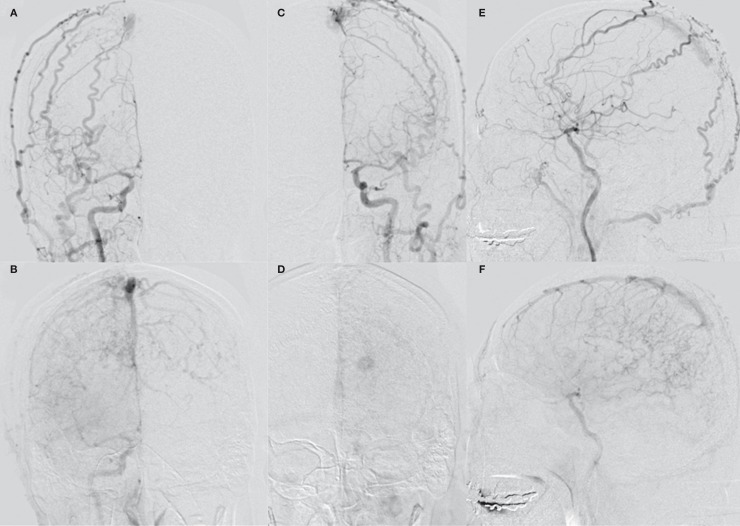
A 61-year-old man suddenly developed headaches. He had no history of head trauma, infection, or neurosurgical intervention, but had a known SSS thrombosis and had been treated with anticoagulation for one year at another hospital. He also suffered from dizziness, especially when going downstairs and his cognitive impairment had been pointed out by his family a few months ago. Computed tomography on admission revealed an intraventricular hemorrhage in the left lateral ventricle (Figure 1A). Fluid-attenuated inversion recovery images on MRI showed hyperintensity along the sulcus which implied cerebral edema (Figure 1B). Magnetic resonance venography revealed enlarged cortical veins and an occlusion of the SSS. Cerebral angiography revealed DAVFs involving the SSS fed by the bilateral MMA, STA, and OA (Figure 2). DAVFs drained into the cortical veins of the bilateral frontoparietal region and a varix in the left midregion of the cortical vein was observed. The posterior third of the SSS was occluded. These DAVFs were classified as a type IIa+b fistulas according to Cognard et al 1. It was unclear whether DAVFs had been present for one year, but they were discovered due to the intracranial hemorrhage and marked venous congestion. Therefore, aggressive treatment was needed.
Figure 1.
A) A CT scan on admission shows intraventricular hemorrhage in the left lateral ventricle. B) An MRI FLAIR image on admission shows hyperintensities along the sulcus which implies cerebral edema.
Figure 2.
A-D) Anterior-posterior view of a common carotid artery angiogram (A, right, arterial phase; B, right, late phase; C; left, arterial phase; D, left, late phase) demonstrating dural arteriovenous fistulas in the superior sagittal sinus. The feeders include the bilateral middle meningeal arteries, superficial temporal arteries, and occipital arteries. Severe venous congestion and a varix are observed; E,F) Lateral view of a right common carotid artery angiogram (E, arterial phase; F, late phase) demonstrating dural arteriovenous fistulas in the superior sagittal sinus. The posterior third of the sinus is occluded.
Intervention
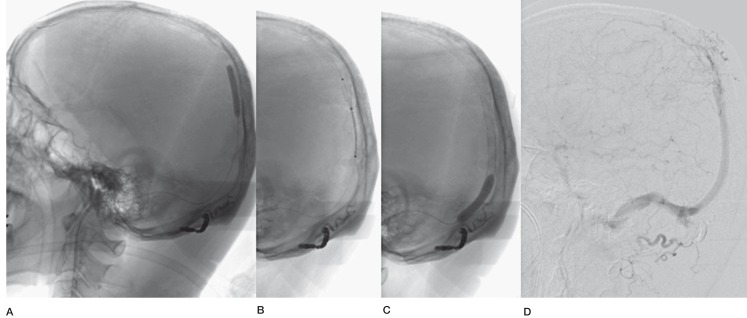
One day after admission, endovascular treatment was performed. Each 7F sheath was placed in the right femoral artery and the left femoral vein. Throughout the procedure, heparin was administered to maintain an activated clotting time of 200 to 250 seconds. After transarterial embolization of the right occipital artery with NBCA and microcoils, we attempted a transvenous approach. A 7F guiding catheter (Roadmaster; Goodman, Nagoya, Japan) was placed in the right jugular bulb and a 4F guiding catheter (Cerulean G40; Medikit, Tokyo, Japan) was advanced to the right transverse sinus. Through the 4F guiding catheter, a microcatheter (Excelsior SL10; Boston Scientific, Natick, MA,USA) was advanced over a microguidewire (GT wire 16; Terumo, Tokyo, Japan) into the thrombosed SSS. The tip of the microguidewire was frequently checked from an anteroposterior view and a lateral view to avoid perforation. Finally, the microguidewire was successfully advanced through the thrombosed sinus, and was followed by the microcatheter into the anterior part of the SSS. The microguidewire was then exchanged with a long wire (Transend 14 300 cm; Boston Scientific), and a PTA balloon catheter (Gateway 3.0 mm*9.0 mm; Boston Scientific) was introduced over the wire. PTA was performed four times from the distal portion of the occluded sinus to the confluence. Post-procedural angiography revealed no recanalization of the sinus. Therefore, the PTA balloon was exchanged for one with a larger balloon size (RX-Genity 5.0 mm*30 mm; Kaneka, Osaka, Japan), and PTA was performed again at the occluded sinus (Figure 3A). The occluded sinus was then recanalized and antegrade flow was observed down the SSS to the jugular vein, and retrograde cortical venous drainage was markedly improved. To maintain this antegrade flow, we planned a stent placement in this recanalized sinus. Aspirin and clopidogrel were administered via a nasogastric tube. A self-expanding stent (CarotidWallstent 8 mm*21 mm; Boston Scientific) was then inserted over the guidewire and an attempt was made to introduce it into the SSS. It was difficult to advance them into the SSS, so we advanced a 7F guiding catheter as far as possible into the transverse sinus over a 4F inner catheter and 0.035 inch wire. Finally, the stent was advanced into the SSS and deployed from the patent sinus (Figure 3B). A post-dilation balloon (Aviator 6.0 mm*40 mm; Cordis, Miami Lakes, FL, USA) was introduced into the stent and dilated at a nominal pressure. Similarly, three stents were deployed from the SSS to the confluence (Figure 3C). Postprocedural angiography revealed good recanalization of the SSS and antegrade venous flow, and venous congestion was much improved (Figure 3D). No complications occurred during or after the procedure.
Figure 3.
A) Percutaneous transluminal angioplasty (PTA) balloon was dilated at the occluded superior sagittal sinus. B) A self-expanding stent was deployed. C) Three stents were deployed and post-dilation with a PTA balloon was performed.D) Postprocedural angiography revealed recanalization of the SSS and antegrade venous flow.
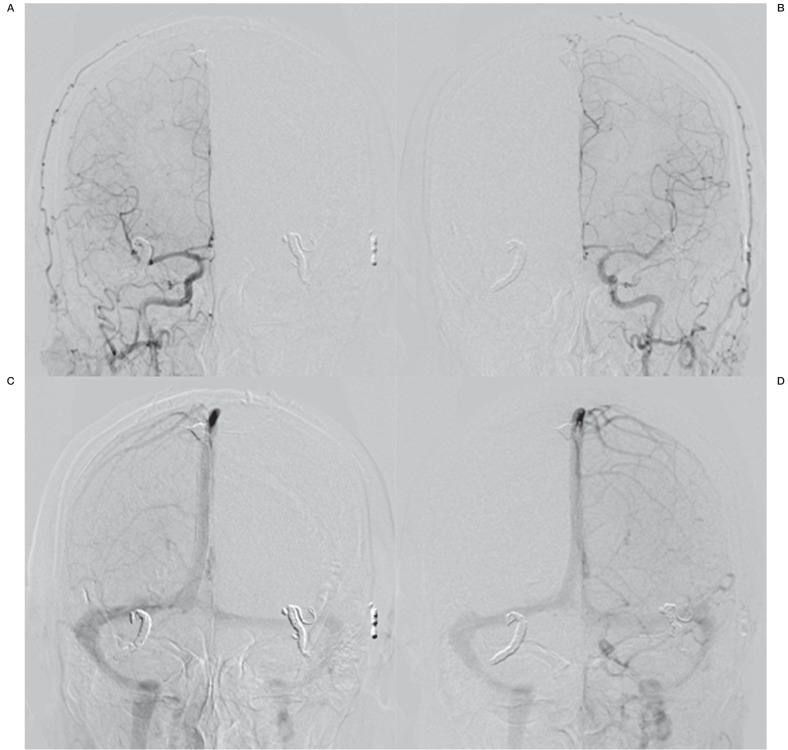
In another session, additional transarterial embolizations were performed in the bilateral STA, the left OA, and the right MMA with NBCA and microcoils. In these procedures, we advanced a flow-directed catheter (Marathon: EV3, CA, USA) across a fistula site via the right MMA, and reached the parasinus venous pouch which was regarded as the shunting point of these DAVFs. We performed a transarterial intravenous embolization with microcoils along the stent wall, and postprocedural angiography revealed no opacification of the fistulous draining vein and total obliteration of the fistula (Figure 4).
The patient was discharged without neurological deficits. Follow-up angiography at three months post-intervention showed complete closure, no recanalization of the fistula, and good patency of the stents.
Figure 4.
A-D) Anterior-posterior view of a common carotid artery angiogram (A, right, arterial phase; B, right, late phase; C, left, arterial phase; D, left, late phase) demonstrating total obliteration of fistulas and normal antegrade venous flow.
Discussion
Dural arteriovenous fistulas (DAVFs) involving the superior sagittal sinus (SSS) are rare but frequently have aggressive features including intracranial hemorrhage and venous hypertension 1. In a nationwide survey of Japan, though DAVFs involving the SSS represented 5.3% of all DAVFs, the incidence of bleeding caused by rupture of the draining veins or by venous hypertension was greater than that of DAVFs in other locations 2. Therefore, DAVFs involving the SSS often require intensive treatment until complete occlusion has been achieved. However, they are difficult to treat because of their unique midline location, multiple feeding arteries, and critical venous drainages 3.
The treatment options for DAVFs involving the SSS include endovascular embolization with transarterial or transvenous approaches, surgery (sinus isolation or resection) combined with endovascular embolization, and radiation therapy 3-10. Transarterial embolization is a comparatively safe and effective method to reduce blood flow to the fistulas, however, it is difficult to extirpate fistulas in this region using this method alone. Some cases were reported with transarterial intravenous catheterization and coil embolization via the middle meningeal artery 6,7. In the present case, we also used this approach in an additional session and it was very effective. It can obliterate DAVFs under limited conditions, but it depends on the tortuosity of the access route and the size of the fistulas.
Transvenous embolization has been reported as sinus packing or target embolization which is used for other DAVFs in the region of a cavernous sinus or transverse sinus 4. However, a transvenous approach is often difficult because DAVFs involving the SSS are strongly associated with sinus occlusion, so a microcatheter needs to be introduced through the occluded sinus. If the transvenous approach is difficult, standard techniques include transvenous embolization with a surgical approach, and surgical isolation or resection of the sinus 11,12. Surgical removal of the fistulous sinus, which has a high obliteration rate, is not always feasible and carries the risks associated with open surgery 12. Furthermore, transvenous sinus embolization or surgical resections have more problems. If the involved sinus does not participate in normal venous drainage, total occlusion or total resection can be performed safely 10. However, sinus occlusion or resection often carries the risk of damaging normal venous drainage and causing disastrous results. Therefore these procedures should be performed carefully after firstly recognizing that the fistulous portion of the SSS does not have a normal sinus function.
There have been few reports on the treatment of DAVFs involving the SSS with irradiation 8, but the efficacy of radiation therapy in treating transverse-sigmoid sinus DAVFs suggests that irradiation may be an effective treatment 4. However, there is a delayed response (about 6-12 months) after irradiation, and patients remain at risk of hemorrhage during this time lag 13. Radiosurgery alone is a safe therapy only in clinically stable patients who can tolerate symptom resolution in a delayed fashion.
Of these options, the treatment selection should be chosen on an individual basis, taking into consideration the angiographic appearance of the lesion and feasibility.
Recently, on the other hand, some authors reported on the use of stent placement for DAVFs in other locations 14-19. In 2000, Murphy et al. described a case of DAVFs involving a transverse and sigmoid sinus treated with recanalization using transluminal angioplasty and stent placement in a partially thrombosed fistulous sinus 18. They emphasized that recanalization of the sinus could restore venous outflow and correct venous hypertension, and that stent deployment at the site of the fistula could achieve complete closure of multiple DAVFs feeders. Other authors subsequently described that stent placement for DAVFs is a promising technique and should be considered a first-line treatment 15,16.
As described above, DAVFs, especially those involving the SSS, are often associated with sinus occlusion or thrombosis. When DAVFs and venous sinus thrombosis coexist, the cause-effect relationship between the two conditions is still debated. In fact, our case had been treated as a sinus thrombosis until intracerebral hemorrhage occurred. It was suspected that DAVFs developed after a sinus thrombosis, but this has yet to be confirmed. Many authors believe that a common mechanism in the development of DAVFs is venous hypertension 20, which may result from obstruction of venous outflow by sinus or venous thrombosis, stenosis, or from alterations in blood flow due to increased viscosity or hypercoagulable states.
Two hypotheses have been proposed for the pathogenesis of DAVFs. The first is based on physiological arteriovenous shunts between meningeal arterial networks and dural venous sinuses. An increase in sinus and venous pressure, for example, by obstruction of the venous outflow by a sinus thrombosis, may open these channels to create DAVFs. The second hypothesis, as shown in a rat model, suggests that venous hypertension induced by an obstruction to venous outflow may reduce cerebral perfusion and lead to ischemia, followed by angiogenesis 15,21. On the basis of this theory, a correction in venous hypertension should reduce cerebral venous edema and switch off this vicious cycle which may create DAVFs. Also, recanalization of the thrombosed or occluded sinus using balloon angioplasty or stent placement may correct venous hypertension.
In the present case, we treated ruptured DAVFs involving the SSS successfully by stent placement and transarterial embolization. We first planned to treat DAVFs by transarterial embolization followed by transvenous embolization if possible. However, we confirmed that shunts collect at the parasinus, so it was difficult to reach this point with a transvenous approach, and we were also uncertain if obliterating the shunts by sinus packing alone was possible. Therefore, when we could advance the microguidewire and microcatheter into the patent SSS through the thrombosed sinus, we decided to try balloon angioplasty for the occluded sinus, which resulted in recanalization of the sinus and restored venous antegrade flow. Hence, we decided on stent deployment at the sinus to maintain venous antegrade flow. As a result, recanalization of the occluded sinus transformed DAVFs from type IIa+b to type IIb.
The risk of intracranial hemorrhage due to venous hypertension is much higher in patients who have experienced a previous hemorrhage 22. Thus, in these circumstances, we suggest that staged therapy is needed: first to ameliorate high venous pressure and restore cerebral perfusion, and then to obliterate DAVFs accordingly. This strategy is very reasonable and physiological, especially for DAVFs which have aggressive features, and should be considered a first option of treatment. To our knowledge, this is the first case that was treated by stent placement for DAVFs involving the SSS.
However, angioplasty and stent placement for the involved sinus of DAVFs need particular conditions, so all cases may not be successfully treated like ours. This technique has some limitations and problems. First, it might be not always feasible to recanalize a totally occluded SSS. Endovascular access and delivery are very important problems that need to be addressed. It is often difficult to advance a microguidewire and a microcatheter through occluded sinus. To prevent perforation or migration to cortical vein, they should be advanced gently. If it is impossible to advance them, other strategies such as surgical or transarterial approaches must be considered. Furthermore, PTA balloon and stent delivery often present difficulties due to many septa inside venous sinus walls and the acute angle of the sinuses. To advance the balloon or stent, it would be important to introduce a guiding catheter as far as possible into the transverse sinus. It is expected that improved devices which have a more flexible and softer shaft may make it easier to complete this method successfully.
Second, the long-term effects of stent placement for sinus occlusion are not yet known. To prevent restenosis or thrombosis, the best medical treatment remains unsettled, but previous reports of transverse sinus stenting for idiopathic intracranial hypertension suggest that dual antiplatelets might prevent restenosis 23,24. In our opinion, the patency of the stents, especially in the SSS, would be maintained as long as the antegrade venous flow to the jugular vein keep draining: in other words, unless the vicious cycle of DAVFs and sinus thrombosis is resumed. So, to maintain patency of the venous stents, additional embolization which can result in shunt obliteration would be more important.
In addition, the choice of stent type remains a problem. Previous reports used self-expanding stents in the recanalization of occluded sinus in DAVFs 15,17,18. We think that self-expandable and closed-cell type stents such as Wallstent are suitable for recanalization of thrombosed sinus in DAVFs because their radial force may also induce compression of the fistulous dural wall of the sinus.
Despite these problems, we propose this staged therapy as the first choice treatment because it is the most physiological. In case of failure, other treatment options may be applied.
In conclusion, we reported the successful treatment of DAVFs involving the SSS by stent placement for an occluded sinus and transarterial embolization. Stent placement could restore venous outflow and correct venous hypertension, and subsequent transarterial embolization obliterated the shunt flow. This staged strategy is feasible and should be considered a first treatment option, especially for DAVFs which presented with intracranial hemorrhage and aggressive venous hypertension.
Abbreviations
DAVFs = dural arteriovenous fistulas;
SSS = superior sagittal sinus;
MRI = magnetic resonance imaging;
MMA = middle meningeal artery;
STA = superficial temporal artery;
OA = occipital artery;
NBCA = n-butyl-2-cyanoacrylate;
PTA = percutaneous transluminal angioplasty
References
- 1.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–680. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 2.Kuwayama N, Kubo M, Endo S. Epidemiological survey on dural arteriovenous fistulas in Japan. Report of Grant-in-Aid for Scientific Research. 2005 [Google Scholar]
- 3.Halbach VV, Higashida RT, Hieshima GB, et al. Treatment of dural arteriovenous malformations involving the superior sagittal sinus. Am J Neuroradiol. 1988;9:337–343. [PMC free article] [PubMed] [Google Scholar]
- 4.Kiyosue H, Hori Y, Okahara M, et al. Treatment of intracranial dural arteriovenous fistulas: current strategies based on location and hemodynamics, and alternative techniques of transcatheter embolization. Radiographics. 2004;24:1637–1653. doi: 10.1148/rg.246045026. [DOI] [PubMed] [Google Scholar]
- 5.Kurl S, Saari T, Vanninen R, et al. Dural arteriovenous fistulas of superior sagittal sinus: case report and review of literature. Surg Neurol. 1996;45:250–255. doi: 10.1016/0090-3019(95)00361-4. [DOI] [PubMed] [Google Scholar]
- 6.Fukai J, Terada T, Kuwata T, et al. Transarterial intravenous coil embolization of dural arteriovenous fistula involving the superior sagittal sinus. Surg Neurol. 2001;55:353–358. doi: 10.1016/s0090-3019(01)00469-4. [DOI] [PubMed] [Google Scholar]
- 7.Kiyosue H, Okahara M, Matsumoto S, et al. Coil embolization of superior sagittal sinus dural arteriovenous fistula by transarterial intrasinus catheterization. Cardiovasc Intervent Radiol. 2004;27:405–407. doi: 10.1007/s00270-003-5028-0. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama K, Shin M, Kurita H, et al. Stereotactic radiosurgery for dural arteriovenous fistula involving the superior sagittal sinus. Case report. J Neurosurg. 2002;97:481–483. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 9.Pierot L, Visot A, Boulin A, et al. Combined neurosurgical and neuroradiological treatment of a complex superior sagittal sinus dural fistula: technical note. Neurosurgery. 1998;42:194–197. doi: 10.1097/00006123-199801000-00044. [DOI] [PubMed] [Google Scholar]
- 10.Toyota S, Fujimoto Y, Wakayama A, et al. Complete cure of superior sagittal sinus dural arteriovenous fistulas by transvenous embolization through the thrombosed sinus in a single therapeutic session. A case report. Interv Neuroradiol. 2008;14:319–324. doi: 10.1177/159101990801400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houdart E, Saint-Maurice JP, Chapot R, et al. Transcranial approach for venous embolization of dural arteriovenous fistulas. J Neurosurg. 2002;97:280–286. doi: 10.3171/jns.2002.97.2.0280. [DOI] [PubMed] [Google Scholar]
- 12.Kakarla UK, Deshmukh VR, Zabramski JM, et al. Surgical treatment of high-risk intracranial dural arteriovenous fistulae: clinical outcomes and avoidance of complications. Neurosurgery. 2007;61:447–449. doi: 10.1227/01.NEU.0000290889.62201.7F. discussion 457-449. [DOI] [PubMed] [Google Scholar]
- 13.Koebbe CJ, Singhal D, Sheehan J, et al. Radiosurgery for dural arteriovenous fistulas. Surg Neurol. 2005;64:392–398. doi: 10.1016/j.surneu.2004.12.026. discussion 398-399. [DOI] [PubMed] [Google Scholar]
- 14.Choi BJ, Lee TH, Kim CW, et al. Reconstructive treatment using a stent graft for a dural arteriovenous fistula of the transverse sinus in the case of hypoplasia of the contralateral venous sinuses: technical case report. Neurosurgery. 2009;65:E994–996. doi: 10.1227/01.NEU.0000351772.45417.92. discussion E996. [DOI] [PubMed] [Google Scholar]
- 15.Levrier O, Metellus P, Fuentes S, et al. Use of a self-expanding stent with balloon angioplasty in the treatment of dural arteriovenous fistulas involving the transverse and/or sigmoid sinus: functional and neuroimaging-based outcome in 10 patients. J Neurosurg. 2006;104:254–263. doi: 10.3171/jns.2006.104.2.254. [DOI] [PubMed] [Google Scholar]
- 16.Liebig T, Henkes H, Brew S, et al. Reconstructive treatment of dural arteriovenous fistulas of the transverse and sigmoid sinus: transvenous angioplasty and stent deployment. Neuroradiology. 2005;47:543–551. doi: 10.1007/s00234-005-1377-5. [DOI] [PubMed] [Google Scholar]
- 17.Malek AM, Higashida RT, Balousek PA, et al. Endovascular recanalization with balloon angioplasty and stenting of an occluded occipital sinus for treatment of intracranial venous hypertension: technical case report. Neurosurgery. 1999;44:896–901. doi: 10.1097/00006123-199904000-00133. [DOI] [PubMed] [Google Scholar]
- 18.Murphy KJ, Gailloud P, Venbrux A, et al. Endovascular treatment of a grade IV transverse sinus dural arteriovenous fistula by sinus recanalization, angioplasty, and stent placement: technical case report. Neurosurgery. 2000;46:497–500. doi: 10.1097/00006123-200002000-00048. discussion 500-491. [DOI] [PubMed] [Google Scholar]
- 19.Yeh PS, Wu TC, Tzeng WS, et al. Endovascular angioplasty and stent placement in venous hypertension related to dural arteriovenous fistulas and venous sinus thrombosis. Clin Neurol Neurosurg. 2010;112:167–171. doi: 10.1016/j.clineuro.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Terada T, Higashida RT, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg. 1994;80:884–889. doi: 10.3171/jns.1994.80.5.0884. [DOI] [PubMed] [Google Scholar]
- 21.Tsai LK, Jeng JS, Liu HM, et al. Intracranial dural arteriovenous fistulas with or without cerebral sinus thrombosis: analysis of 69 patients. J Neurol Neurosurg Psychiatry. 2004;75:1639–1641. doi: 10.1136/jnnp.2003.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soderman M, Pavic L, Edner G, et al. Natural history of dural arteriovenous shunts. Stroke. 2008;;39:1735–1739. doi: 10.1161/STROKEAHA.107.506485. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JN, Owler BK, Cousins C, et al. Venous sinus stenting for refractory benign intracranial hypertension. Lancet. 2002;359:228–230. doi: 10.1016/S0140-6736(02)07440-8. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. Am J Neuroradiol. 2011;32:1408–1414. doi: 10.3174/ajnr.A2575. [DOI] [PMC free article] [PubMed] [Google Scholar]