Abstract
Objective
General practitioner (GP) involvement may be instrumental in obtaining successful palliative cancer trajectories. The aim of the study was to examine associations between bereaved relatives’ evaluation of palliative cancer trajectories, place of death, and GP involvement.
Design
Population-based, cross-sectional combined register and questionnaire study.
Setting
The former Aarhus County, Denmark.
Subjects
Questionnaire data on GPs’ palliative efforts and relatives’ evaluations of the palliative trajectories were obtained for 153 cases of deceased cancer patients.
Main outcome measures
A successful palliative trajectory as evaluated retrospectively by the relatives.
Results
Successful palliative trajectories were statistically significantly associated with home death (PR 1.48 (95% CI 1.04; 2.12)). No significant associations were identified between the evaluations of the palliative trajectory at home and GP involvement. “Relative living with patient” (PR 1.75 (95% CI: 0.87; 3.53)) and “GP having contact with relatives” (PR 1.69 (95% CI 0.55; 5.19)) were not significantly associated, but this may be due to the poor number of cases included in the final analysis.
Conclusion
This study indicates that home death is positively associated with a higher likelihood that bereaved relatives will evaluate the palliative trajectory at home as successful. No specific GP services that were statistically significantly associated with higher satisfaction among relatives could be identified, but contact between GPs and relatives seems important and the impact needs further investigation.
Key Words: Denmark, family practice, neoplasm, palliative care, primary health care, terminally ill
GP home visits are associated with death at home in palliative trajectories. However, home death does not necessarily mean a successful palliative trajectory.
Home death is positively associated with a higher likelihood that the bereaved relative will evaluate the palliative trajectory as successful.
Interpersonal relationships, which are difficult to quantify, may be of greater importance than structural services in terms of perceived quality.
Most terminally ill cancer patients and relatives wish for patients to die at home [1–3]. However, home death is not necessarily a proxy for a successful palliative trajectory, and many patients spend most of their palliative period at home even though they die in institutions [4]. Furthermore, the numerous professionals involved, e.g. general practitioners (GPs) and community nurses (CNs), and their services may influence patients’ and relatives’ views [5].
The fact that a large proportion of cancer patients die in hospital has been linked to the presumed poor services of primary health care professionals [3]. However, satisfaction with GPs is high in spite of dissatisfaction with symptom control [6]. Also, interview studies suggest that 24-hour back-up and GP involvement are important elements in bereaved relatives’ evaluation of palliative trajectories [7–9]. Studies have also shown that GP home visits are positively associated with home death [4–10]. However, it remains unclear what constitutes particularly important GP contributions in achieving successful palliative trajectories. This knowledge could be important for guiding GPs to provide the best possible palliative care.
The aim of the study was to examine associations between bereaved relatives’ evaluation of palliative trajectories, place of death, and GP involvement.
Material and methods
Setting
The survey was conducted on 599 deceased cancer patients in the former Aarhus County (approximately 640 000 inhabitants, 12% of the Danish population) from January to July 2007. Questionnaire and health register data were linked using the unique personal identification number allocated to all Danish citizens [11].
Some 98% of Danes are registered with GPs and receive free tax-financed medical care [12]. Danish GPs are responsible for frontline care 24 hours a day and act as gatekeepers for access to specialist treatment. Palliative specialist teams are available during daytime hours and provide support through home visits and telephone advice to other professionals.
Study population and sampling
We included adults in Aarhus County who died of cancer from 1 March to 30 November 2006 and who received palliative home care, and sampled patients by combining official register data.
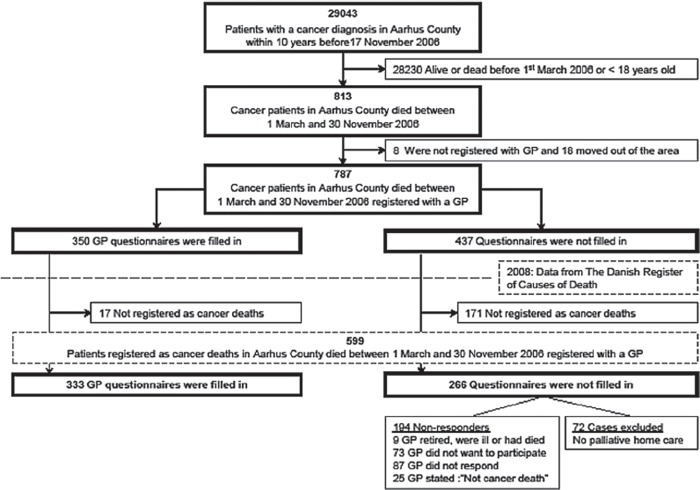
From the county hospital discharge register we identified 29 043 individuals aged 18 years and over who were registered with at least one cancer diagnosis (ICD- 10) (excluding non-melanoma skin cancers) from November 1996 to November 2006. Using The Centralized Civil Register (CRS), we identified 813 individuals among the 29 042 who died between 1 March and 30 November 2006. From the regional health service register we identified their GPs. Eight (1.0%) were not registered with a GP and 18 (2.2%) had moved from the county, leaving 787 patients. Questionnaires were sent to the GPs asking if palliative home care had been provided and whether it was acceptable to contact the bereaved relative. In most cases GPs identified the relative, but if not relatives were identified according to the following priority: spouse, child over 18 years, oldest sibling, parent (CRS).
In late 2008, data on cancer deaths from 2006 were available. After merging with our initial database, 188 patients who did not die of cancer were excluded, reducing our study population to 599 (Figure 1).
Figure 1.
Flow-chart of sampling and GP questionnaire: Responders and non-responders.
Data collection
Questionnaires included themes identified through literature studies, clinical experience, and group interview studies with bereaved relatives [7] and involved professionals [13,14].
The GP questionnaire was pilot-tested among 30 GPs. GPs received small financial compensation, and non-responders were sent two reminders. Relevant GP questionnaire data can be seen in Table I. Furthermore, GPs were asked about duration of the palliative period at home. The palliative period was defined as the patient's last period of life in which all curative treatment was stopped and care and treatment were solely palliative. Data on duration of the palliative period are given in a separate paper [4].
Table I.
Characteristics of 153 included cases, the 70 cases not included because the relative did not respond and the 106 cases where the GP advised against sending the relative a questionnaire. Case data in study arise from the GP and relative-questionnaires and from formal health registers. Case data of relative-non-responders and of the group where the GP advised against sending the relative a questionnaire stem from GP-questionnaires and formal health registers.
| Cases in the study (n=153) | Cases of relative non-responders (n=70) | Cases where the GP advised against sending the relative a questionnaire (n=106) | |
| Data from formal health registers | |||
| Patient's age at time of death (mean years (SD)) | 68.2 (12.8) | 69.6 (12.2) | 70.9 (12.5) |
| Patient's gender (n (%)) | |||
| Male | 95 (62.1) | 33 (47.1)* | 50 (47.2)* |
| Female | 58 (37.9) | 37 (52.9)* | 56 (52.8)* |
| Primary cancer diagnosis (n (%)) | |||
| Bronchus/lung | 25 (16.3) | 18 (25.7) | 22 (20.8) |
| Colon/Rectum | 22 (14.4) | 12 (17.1) | 15 (14.2) |
| Breast | 17 (11.1) | 7 (10.0) | 10 (9.4) |
| Prostate | 22 (14.4) | 6 (8.6) | 11 (10.4) |
| Other | 67 (43.8) | 27 (38.6) | 48 (45.2) |
| Place of death (n (%)) | |||
| Home | 76 (49.7) | 25 (35.7) | 18 (17.0)* |
| Nursing home | 23 (15.0) | 11 (15.7) | 34 (32.0)* |
| Hospital / Hospice | 53 (34.6) | 33 (47.2) | 52 (49.1)* |
| Other(e.g. other institution) | 1 (0.7) | 1 (1.4) | 2 (1.9) |
| Number of GP home-visits during the last 3 months | |||
| (Median (IQI)) | 3 (2;6) | 4 (2;7) | 2 (0;5) |
| (mean (SD)) | 4.2 (3.2) | 4.4 (3.0) | 3.2 (3.3)* |
| GP home-visits during the last 3 months (n (%)) | |||
| No | 11 (7.2) | 5 (7.1) | 27 (25.5)* |
| Yes | 142 (92.8) | 65 (92.9) | 79 (74.5)* |
| Data from relative- questionnaires | |||
| Gender of relative (n (%)) | |||
| Male | 42 (27.4) | – | – |
| Female | 111 (72.6) | ||
| Relation to deceased (n (%)) | |||
| Spouse | 106 (69.3) | ||
| Girlfriend or Boyfriend | 1 (0.7) | ||
| Daughter or son | 39 (25.4) | – | – |
| Sister or brother | 2 (1.3) | ||
| Parent | 2 (1.3) | ||
| Friend | 1 (0.7) | ||
| Daughter-in-law | 2 (1.3) | ||
| Relative lived with patient (n (%)) | |||
| No | 27 (18.2) | – | – |
| Yes | 121 (81.8) | ||
| Relative's vocational education (n (%)) | |||
| 3 years or less | 94 (64.4) | – | – |
| > 3 years | 52 (35.6) | ||
| Relative's evaluation of palliative trajectory (n(%)) | |||
| Really well | 50 (35.5) | ||
| Well | 47 (33.3) | – | – |
| Acceptable | 28 (19.9) | ||
| Bad | 12 (8.5) | ||
| Really bad | 4 (2.8) | ||
| Data from GP-questionnaires | |||
| GP-involvement (n (%)) | |||
| No | 11 (7.3) | 5 (7.3) | 20 (19.4)* |
| Yes | 140 (92.7) | 64 (92.7) | 83 (80.6)* |
| GP knowledge of patient prior to palliative period (n (%)) | |||
| Poor | 14 (9.5) | 7 (10.0) | 23 (22.6)* |
| Well | 133 (90.5) | 63 (90.0) | 79 (77.4)* |
| Unplanned home-visits by GP (n (%)) | |||
| No | 58 (43.9) | 27 (43.6) | 46 (61.3)* |
| Yes | 74 (56.1) | 35 (56.4) | 29 (38.7)* |
| GP gave private number to patient to use in out-of-office hours (n (%)) | |||
| No | 68 (50.7) | 33 (53.2) | 61 (75.3)* |
| Yes | 66 (49.3) | 29 (46.8) | 20 (24.7)* |
| GP had made a plan with the patient for whom to contact in out-of-office hours (n (%)) | |||
| No | 92 (68.2) | 43 (68.3) | 43 (52.4)* |
| Yes | 43 (31.8) | 20 (31.7) | 39 (47.6)* |
| GP had contact with relatives (n (%)) | |||
| No | 8 (5.8) | 5 (7.8) | 26 (34.2)* |
| Yes | 131 (94.2) | 59 (92.2) | 50 (65.8)* |
| Home care nurse involvement (n (%)) | |||
| No | 42 (27.5) | 18 (25.7) | 48 (45.3)* |
| Yes | 111 (72.5) | 52 (74.3) | 58 (54.7)* |
| Specialist team-involvement (n (%)) | |||
| No | 85 (55.6) | 43 (61.4) | 74 (69.8)* |
| Yes | 68 (44.4) | 27 (38.6) | 32 (30.2)* |
*Statistically significantly different from the 153 cases in the study (p-value < 0.05)
The relative questionnaire was pilot-tested among 14 bereaved relatives. Non-responders were sent one reminder. Relevant relative questionnaire data and register data can be seen in Table I. To examine “A successful palliative pathway at home” the relatives were asked to evaluate the palliative pathway, answering the following question: “How, in your own words, was the entire period at home during which the deceased was dying compared with how you felt it should have been?” (Dichotomized into unsuccessful [“Fairly well”, “Bad”, “Very bad”] and successful [“Very well”, “Well”]).
Analysis
“A successful palliative trajectory” was defined as outcome measure, and unadjusted and adjusted associations were calculated. The multivariate model consisted of the variables seen in Table III and “the duration of the palliative period spent at home” (number of weeks as a categorical variable), since it may be associated with the GPs’ ability to provide palliative care. Estimates were adjusted for clustering of patients within practices [15]. Prevalence ratios (PRs) with 95% confidence intervals (95% CI) were used as measure of association. Due to high prevalence of outcome measure (more than 20% of relatives evaluated the palliative trajectory as successful), odds ratios may tend to overestimate the association [16,17]. PRs were calculated using generalised linear models (GLM) with log link and the Bernoulli family and, because the model failed to converge, we used a Poisson regression model [16,18].
Table III.
Associations between a successful palliative course and model variables. A total of 153 cases were included in the analyses. The unadjusted and the adjusted prevalence ratios (PRs) are shown with 95% confidence intervals (95% CIs).
| Unadjusted |
Adjusted |
|||
| Prevalence ratio (95% CI) | p-value | Prevalence ratio (95% CI) | p-value | |
| Gender of relative | ||||
| Male | 1 | 1 | ||
| Female | 1.08 (0.83;1.41) | 0.559 | 1.03 (0.72;1.47) | 0.882 |
| Age of relative | ||||
| 18 – 64 | 1 | 1 | ||
| 65 + | 1.29 (1.06;1.58) | 0.013 | 1.00 (0.76;1.32) | 0.988 |
| Relative living with patient | ||||
| No | 1 | 1 | ||
| Yes | 1.88 (1.16; 3.06) | 0.011 | 1.75 (0.87;3.53) | 0.119 |
| Relative's relation to diseased | ||||
| Not spouse | 1 | Not included because of collinarity with ‘Relative living with patient’ | ||
| Spouse | 1.59 (1.15;2.19) | 0.005 | ||
| Relative's vocational education | ||||
| 3 years or less | 1 | 1 | ||
| > 3 years | 0.80 (0.62:1.04) | 0.102 | 0.88 (0.65;1.18) | 0.397 |
| GP knowledge prior to palliative period | ||||
| Poor | 1 | 1 | ||
| Well | 1.41 (0.82;2.42) | 0.211 | 1.24 (0.74;2.08) | 0.405 |
| GP home-visits | ||||
| No | 1 | 1 | ||
| Yes | 1.11 (0.61;1.92) | 0.719 | 0.97 (0.44;2.26) | 0.951 |
| Unplanned home-visits by GP | ||||
| No | 1 | 1 | ||
| Yes | 1.14 (0.87;1.49) | 0.335 | 1.18 (0.89;1.57) | 0.238 |
| GP gave private number to patient to use in out-of-office hours | ||||
| No | 1 | 1 | ||
| Yes | 1.03 (0.80;1.33) | 0.800 | 0.83 (0.62;1.11) | 0.205 |
| GP had made a plan with the patient for whom to contact in out-of-office hours | ||||
| No | 1 | 1 | ||
| Yes | 0.96 (0.71;1.28) | 0.767 | 0.98 (0.66;1.44) | 0.917 |
| GP had contact with relatives | ||||
| No | 1 | 1 | ||
| Yes | 1.15 (0.55;2.39) | 0.712 | 1.69 (0.55;5.19) | 0.360 |
| Community nurse involvement | ||||
| No | 1 | 1 | ||
| Yes | 0.92 (0.72;1.16) | 0.470 | 0.89 (0.61;1.29) | 0.524 |
| Specialist team-involvement | ||||
| No | 1 | 1 | ||
| Yes | 1.01 (0.81;1.26) | 0.917 | 1.04 (0.78;1.37) | 0.808 |
| Place of death | ||||
| Institution (hospital or hospice) | 1 | 1 | ||
| Nursing home | 0.89 (0.55;1.45) | 0.636 | 1.03 (0.53;1.98) | 0.940 |
| Home | 1.37 (1.05:1.80) | 0.021 | 1.48 (1.04:2.12) | 0.031 |
Note: Significant correlations with a p-value < 0.05 are in bold text.
The variables were assessed for collinearity (Pearson's correlation coefficient >0.4) and multicollinearity (variance inflation factor <10) [19,20]. Due to collinearity, one variable was not included. We adjusted for duration of the palliative period spent at home since it could be associated with GP involvement. Data were analysed using STATA 10 [21].
Results
Of 599 GP questionnaires, 333 questionnaires (231 general practices) were completed. Some 72 were excluded because GPs stated that no home care had been provided (response rate 63.2%) (see Figure 1). General practices completed questionnaires for 1–6 patients (mean: 1.9 (SD 1.1)). Non-responding practices were not statistically significantly different from participating practices with respect to practice organization, patients, and the number of questionnaires we sent per practice. Comparison of the 194 cases from non-responding GPs with included cases are given in Table II.
Table II.
Characteristics of 333 included cases and 194 cases not included because the GP did not respond. Case data obtained from formal health registers.
| Cases of GP responders (n = 333) | Cases of GP non-responders (n = 194) | |
| Patient's age at time of death (mean (SD)) | 69.4 (12.7) | 73.5 (12.7)* |
| Patient's gender (n (%)) | ||
| Male | 181 (54.4) | 113 (58.3) |
| Female | 152 (45.6) | 81 (41.7) |
| Primary cancer diagnosis (n (%)) | ||
| Bronchus/lung | 65 (19.5) | 37 (19.1) |
| Colon/rectum | 50 (15.1) | 32 (16.5) |
| Breast | 34 (10.2) | 22 (11.3) |
| Prostate | 39 (11.7) | 31 (16.0) |
| Other | 145 (43.5) | 72 (37.1) |
| Place of death (n (%)) | ||
| Home | 120 (36.0) | 70 (36.1) |
| Nursing home | 69 (20.7) | 51 (26.3) |
| Hospital/hospice | 140 (42.0) | 70 (36.1) |
| Other (e.g. other institution) | 4 (1.2) | 3 (1.5) |
| Number of home visits by GP during the last 3 months | ||
| (median (IQI)) | 3 (1;6) | 3 (1;5) |
| (mean (SD)) | 3.9 (3.2) | 3.6 (3.1) |
Notes: *Statistically significantly different from cases of GP responders (p-value < 0.05). Not percentages total 100.0% because of round-offs.
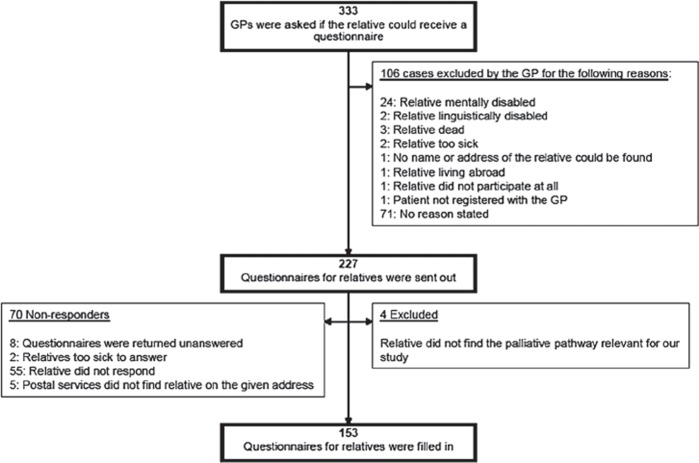
In 106 (31.8%) of 333 cases, GPs advised against contacting the relative (Figure 2). Among 227 relative questionnaires, four cases were excluded since relatives stated that the questionnaire was not relevant at all, and 153 relative questionnaires were completed (response rate 68.6%) (Figure 2). Comparison of included cases with cases where relatives did not respond and cases where GPs advised against sending the relative a questionnaire are given in Table I.
Figure 2.
Flow-chart of questionnaire to bereaved relatives: Responders and non-responders.
Important factors for a successful palliative trajectory
“A successful palliative trajectory” was statistically significantly associated with home death compared with institutional death (PR 1.48 (95% CI 1.04; 2.12)) (Table III). The positive association with “Relative living with the patient” (PR 1.75 (95% CI 0.87; 3.53)) and “GP having contact with the relatives” (PR 1.69 (95% CI 0.55; 5.19)) did not reach statistical significance and should therefore be interpreted with caution. No other associations were found between the relatives’ evaluation and GP services (“GP knowledge prior to palliative period”, “GP home visits”, “Unplanned home visits by GP”, “GP gave private number to patient to use in out-of-office hours”, “GP had made a plan with the patient for whom to contact in out-of-office hours”).
Discussion
Principal findings
In a population who died from cancer having had a palliative trajectory at home, we found that relatives’ positive evaluation of the palliative trajectory was associated with home death. However, we identified no statistically significant associations between evaluations and specific GP services.
Strengths and weaknesses of the study
Strengths of this study are sampling procedure and comprehensive data collection in a field difficult to study. To eliminate differential misclassification, we used official health registers identifying the study population. However, in 25 of 599 cases, GPs returned the questionnaires stating that the patient did not die from cancer. These cases were registered as GP non-responders, which may have introduced bias (including confounding by indication), but direction and importance of this bias are difficult to determine. To minimize recall bias, questionnaires were sent in January 2007 instead of awaiting update of the Danish Registry of Causes of Death. Furthermore, electronic patient records used in general practice help GPs to remember the patients [22].
Selection bias is possibly this study's weakness. We found differences between included cases and cases which were excluded because GPs advised against contacting relatives. Hence, we may have excluded cases where GPs were not as involved and known to the patient as well as in included cases. In excluded cases relatives may evaluate the trajectory as less successful. Thus, we would tend to overestimate associations between successful trajectories and GP-related variables. This would also be the case if GPs excluded unsuccessful cases (which they might have done for several reasons) despite a high level of involvement. As home death is associated with GP involvement [10,23–25], such selection bias would therefore tend to overestimate the association between home death and successful trajectories in this study.
Approximately 1680 patients died from cancer in Aarhus County in 2006, but we recruited only 599 cases during nine months. This may be because we excluded persons under the age of 18 years and persons with non-melanoma skin cancers, and we included only patients registered with cancer diagnoses in Aarhus County hospitals. Furthermore, 1 March–30 November does not include any winter months, which may account for some missing cases since mortality rates are higher during winter months.
Comparison with existing literature
We found a statistically significant association, although minor, between home death and relatives’ positive evaluation of the palliative trajectory. In another study, using other and therefore different data from the same population focusing on CNs’ role, we found a stronger association between relatives’ positive evaluation and home death (PR 2.3 (95% CI 1.2;4.4)) [26]. Furthermore, other studies found home death to be associated with better bereavement response [10,24,25] and overall satisfaction with the palliative trajectory [27].
Some of the difference between bereaved relatives’ evaluation in cases with home versus institutional death may be because patients with severe symptoms are forced into an institutional death, which would affect relatives’ evaluation. However, by adjusting for patients’ contact with palliative specialist teams we may have adjusted for some of the confounding by symptom severity. Eliminating “specialist team involvement” from the model accordingly weakens the association between successful trajectories and home death. One may also argue that caring for dying patients at home in the last few days of life may be distressing for relatives and that institutional death may be preferable even if the palliative trajectory at home has been successful until then. It may even make the trajectory at home look even more successful in retrospect since the distressing and care-demanding last days of the patient's life did not take place at home. In this context, the weak association between a positive evaluation and home death is even more interesting.
To our knowledge, no other population-based study has examined the association between relatives’ or patients’ general evaluation of the palliative trajectory and GP-related factors. Surprisingly, we found no GP-related factors, not even GP home visits, to be statistically significantly associated with relatives’ evaluation even though prior studies have reported that home visits are strongly associated with home death [10,24,25]. Other studies have found that bereaved relatives’ satisfaction with GPs was statistically higher when GPs provided many home visits (> 20) or were willing to perform home-visits [28,29].
Associations between successful palliative trajectories and “relatives living with the patient” and “GPs’ contact with relatives” did not reach statistical significance, which may be because only 100 cases were included in the adjusted analysis. However, the analysis indicates that relationships between specific persons (patient–relative, GP–relative) are important and it would be valuable to try and qualify these relations as they may be important for successful palliative trajectories by comparing with the structural factors we included. Personal relationships with patients and relatives may very well be one of GPs’ strengths and explain why satisfaction with GPs is high despite dissatisfaction with symptom control [6]. Further knowledge on interpersonal relationships and reasons for GPs’ involvement or non-involvement is needed [30].
Implications for future research
More research is needed on how to measure bereaved relatives’ evaluation of palliative trajectories, how GP involvement and personal relationship with patients and families affect this evaluation and what constitutes well-delivered palliative care by GPs.
Conclusion
Our study indicates that home death is positively associated with a higher likelihood that bereaved relatives will evaluate the palliative trajectory at home as successful. We could not identify any specific GP services that were statistically significantly associated with higher satisfaction among relatives, but contact between GPs and relatives seems important and the impact needs further investigation.
Acknowledgements
The authors would like to extend their thanks to the bereaved relatives and the GPs participating in this study.
Funding
The study was funded by the Aarhus County Research Fund for Clinical Development and Research in General Practice and across the Primary and Secondary Health Care Sectors (4-01-3-04), the Danish National Research Foundation for Primary Care (585-457808) and the Multipractice Study Committee (585-04/2072).
Ethical approvals
According to the Regional Research Ethics Committee, the Biomedical Research Ethics Committee System Act does not apply to this study as no active intervention was involved. The study was approved by the Danish Data Protection Agency and the Danish National Board of Health.
Conflicts of interest
The authors have no conflicts of interest.
References
- 1.Thomas C. The place of death of cancer patients: Can qualitative data add to known factors? Soc Sci Med. 2005;60:2597–607. doi: 10.1016/j.socscimed.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Thomas C, Morris SM, Clark D. Place of death: Preferences among cancer patients and their carers. Soc Sci Med. 2004;58:2431–44. doi: 10.1016/j.socscimed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Grande GE, Farquhar MC, Barclay SI, Todd CJ. Valued aspects of primary palliative care: Content analysis of bereaved carers’ descriptions. Br J Gen Pract. 2004;54:772–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Neergaard MA, Vedsted P, Olesen F, Sokolowski I, Jensen AB, Sondergaard J. Associations between home death and GP involvement in palliative cancer care. Br J Gen Pract. 2009;59:671–7. doi: 10.3399/bjgp09X454133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgsteede SD, Deliens L, van der Wal G, Francke AL, Stalman WA, van Eijk JT. Interdisciplinary cooperation of GPs in palliative care at home: A nationwide survey in The Netherlands. Scand J Prim Health Care. 2007;25:226–31. doi: 10.1080/02813430701706501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanratty B. Palliative care provided by GPs: The carer's viewpoint. Br J Gen Pract. 2000;50:653–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Neergaard MA, Olesen F, Jensen AB, Sondergaard J. Palliative care for cancer patients in a primary health care setting: Bereaved relatives’ experience, a qualitative group interview study. BMC Palliat Care. 2008;7:1. doi: 10.1186/1472-684X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weibull A, Olesen F, Neergaard MA. Caregivers’ active role in palliative home care – to encourage or to dissuade? A qualitative descriptive study. BMC Palliat Care. 2008;7:15. doi: 10.1186/1472-684X-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brazil K, Bedard M, Krueger P, Abernathy T, Lohfeld L, Willison K. Service preferences among family caregivers of the terminally ill. J Palliat Med. 2005;8:69–78. doi: 10.1089/jpm.2005.8.69. [DOI] [PubMed] [Google Scholar]
- 10.Aabom B, Kragstrup J, Vondeling H, Bakketeig LS, Stovring H. Population-based study of place of death of patients with cancer: Implications for GPs. Br J Gen Pract. 2005;55:684–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System: A cohort of eight million persons. Dan Med Bull. 2006;53:441–9. [PubMed] [Google Scholar]
- 12.De Fine Olivarius N, Hollnagel H, Krasnik A, Pedersen PA, Thorsen H. The Danish National Health Service Register. Dan Med Bull. 1997;44:449–53. [PubMed] [Google Scholar]
- 13.Neergaard MA, Olesen F, Jensen AB, Søndergaard J. Shared care in basic level palliative home care – organizational and interpersonal challenges. A qualitative group interview study. J Palliat Med. 2010;13 doi: 10.1089/jpm.2010.0036. [DOI] [PubMed] [Google Scholar]
- 14.Neergaard MA. Palliative home care for cancer patients in Denmark – with a particular focus on the primary care sector, GPs and community nurses PhD Thesis. 2009 Faculty of Health Sciences, Aarhus University. Research Unit and Department of General Practice. [Google Scholar]
- 15.Donner A, Klar N. Design and analysis of cluster randomisation trials in health research 1st. London: Hodder Arnold; 2000. [Google Scholar]
- 16.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson ML, Myers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: What is to be done? Occup Environ Med. 1998;55:272–7. doi: 10.1136/oem.55.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 19.Armitage P, Berry G, Matthews JNS. Oxford: Blackwell Science; 2005. Statistical methods in medical research 4th. [Google Scholar]
- 20.O'Brien RM. A caution regarding rules of thumb for variance inflation factors. Quality and Quantity. 2007;41:673–90. [Google Scholar]
- 21.College Station, TX: StataCorp LP; 2007. Stata Statistical Software: Release 10. [Google Scholar]
- 22.Protti D. Comparison of information technology in general practice in 10 countries. Health Q. 2007;10:107–16. [PubMed] [Google Scholar]
- 23.Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ. 2006;332:515–21. doi: 10.1136/bmj.38740.614954.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukui S, Fukui N, Kawagoe H. Predictors of place of death for Japanese patients with advanced-stage malignant disease in home care settings: A nationwide survey. Cancer. 2004;101:421–9. doi: 10.1002/cncr.20383. [DOI] [PubMed] [Google Scholar]
- 25.Howat A, Veitch C, Cairns W. A retrospective review of place of death of palliative care patients in regional north Queensland. Palliat Med. 2007;21:41–7. doi: 10.1177/0269216306072383. [DOI] [PubMed] [Google Scholar]
- 26.Neergaard MA, Vedsted P, Olesen F, Sokolowski I, Jensen AB, Sondergaard J. Associations between successful palliative cancer pathways and community nurse involvement. BMC Palliat Care. 2009;8:18. doi: 10.1186/1472-684X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringdal GI, Jordhoy MS, Kaasa S. Family satisfaction with end-of-life care for cancer patients in a cluster randomized trial. J Pain Symptom Manage. 2002;24:53–63. doi: 10.1016/s0885-3924(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 28.Fakhoury W, McCarthy M, Addington-Hall J. Determinants of informal caregivers’ satisfaction with services for dying cancer patients. Soc Sci Med. 1996;42:721–31. doi: 10.1016/0277-9536(95)00198-0. [DOI] [PubMed] [Google Scholar]
- 29.Lecouturier J, Jacoby A, Bradshaw C, Lovel T, Eccles M. Lay carers’ satisfaction with community palliative care: Results of a postal survey. South Tyneside MAAG Palliative Care Study Group. Palliat Med. 1999;13:275–83. doi: 10.1191/026921699667368640. [DOI] [PubMed] [Google Scholar]
- 30.Aabom B, Pfeiffer P. Why are some patients in treatment for advanced cancer reluctant to consult their GP? Scand J Prim Health Care. 2009;27:58–62. doi: 10.1080/02813430802677817. [DOI] [PMC free article] [PubMed] [Google Scholar]