Abstract
Objectives
Many hospital admissions are due to inappropriate medical treatment, and discharge of fragile elderly patients involves a high risk of readmission. The present study aimed to assess whether a follow-up programme undertaken by GPs and district nurses could improve the quality of the medical treatment and reduce the risk of readmission of elderly newly discharged patients.
Design and setting
The patients were randomized to either an intervention group receiving a structured home visit by the GP and the district nurse one week after discharge followed by two contacts after three and eight weeks, or to a control group receiving the usual care.
Patients
A total of 331 patients aged 78+ years discharged from Glostrup Hospital, Denmark, were included.
Main outcome measures
Readmission rate within 26 weeks after discharge among all randomized patients. Control of medication, evaluated 12 weeks after discharge on 293 (89%) of the patients by an interview at home and by a questionnaire to the GP.
Results
Control-group patients were more likely to be readmitted than intervention-group patients (52% v 40%; p = 0.03). In the intervention group, the proportions of patients who used prescribed medication of which the GP was unaware (48% vs. 34%; p = 0.02) and who did not take the medication prescribed by the GP (39% vs. 28%; p = 0.05) were smaller than in the control group.
Conclusion
The intervention shows a possible framework securing the follow-up on elderly patients after discharge by reducing the readmission risk and improving medication control.
Key Words: Discharge, elderly, family practice, home visit, medication, primary care, readmission
Fewer readmissions and better control of medication is generally strived for. A follow-up programme by the GPs and district nurses after discharge found:
A 23% relative readmission risk reduction within six months after discharge.
Better follow-up including better control of medication after discharge.
A cost-neutral intervention featuring a tendency towards reduced costs.
Because of their often frail condition, discharged, impaired elderly patients face a high risk of readmission [1–5] and they risk being “left in limbo” if a healthcare professional is not assigned explicit responsibility for their bio-psychosocial situation upon their discharge [6]. Readmission can be diminished according to studies of home follow-up on elderly patients involving advanced practice nurses and geriatric teams drawn from hospital staff and other kinds of intervention with improved discharge support for elderly patients with specific diseases [1–3,5,7,8]. Two reviews found that the documented impact of discharge planning without follow-up care was uncertain [4,5]. They concluded that more research and health economic analysis were needed, exploring intervention across the hospital–community interface.
In Denmark (5.5 million inhabitants), the healthcare system is tax-financed with no payment at the point of care. GPs are independent contractors to public health insurance. They act as gatekeepers for 98% of the population. Municipalities run a district nurse system that mainly focuses on care for the frail elderly [9].
Inappropriate medical treatment often has inadvertent effects, and a considerable number of admissions are attributable to inappropriate medical treatment that could be avoided [10,11].
Only a few trials have focused on the effect of improved effort by the existing staff in the primary sector [12]. The aim of this study was to evaluate a simple intervention aimed at improving the interdisciplinary care given by GPs and district nurses.
Material and methods
Participants
The study was conducted at Glostrup Hospital in the Capital Region of Denmark in November 2003 to June 2005. All local GPs and district nurses in seven municipalities were invited to join the study. 63% of the GPs and all the municipalities accepted attendance.
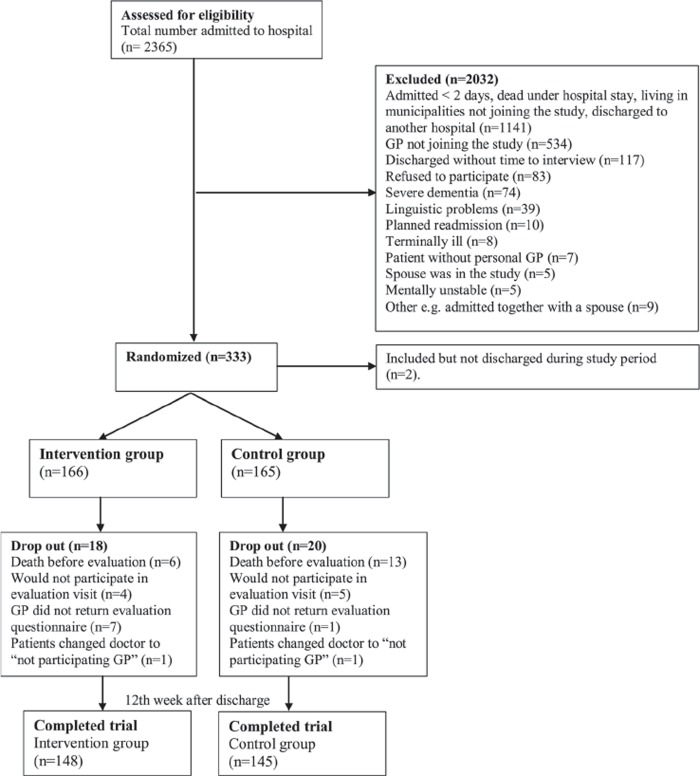
Inclusion criteria were aged 78+ years, discharged from the geriatric or internal medical ward, and hospitalization for a minimum of two days. Exclusion criteria were severe dementia, linguistic problems, and terminal illness. Patients not included in the study are listed in Figure 1. The inclusion was done during daytime on weekdays when discharge was planned.
Figure 1.
Patient flow.
Study design
Patients were enrolled in the study when discharge was planned and participated in a structured interview conducted by a trained research occupational therapist (RT). Informed consent was obtained. Each intervention patient's GP and the local district nurse were contacted by fax in order to arrange the first study visit. Hospital staff were not informed about the randomization.
Randomization was done using a computer-generated algorithm with numbers in closed envelopes which were opened at the inclusion interview.
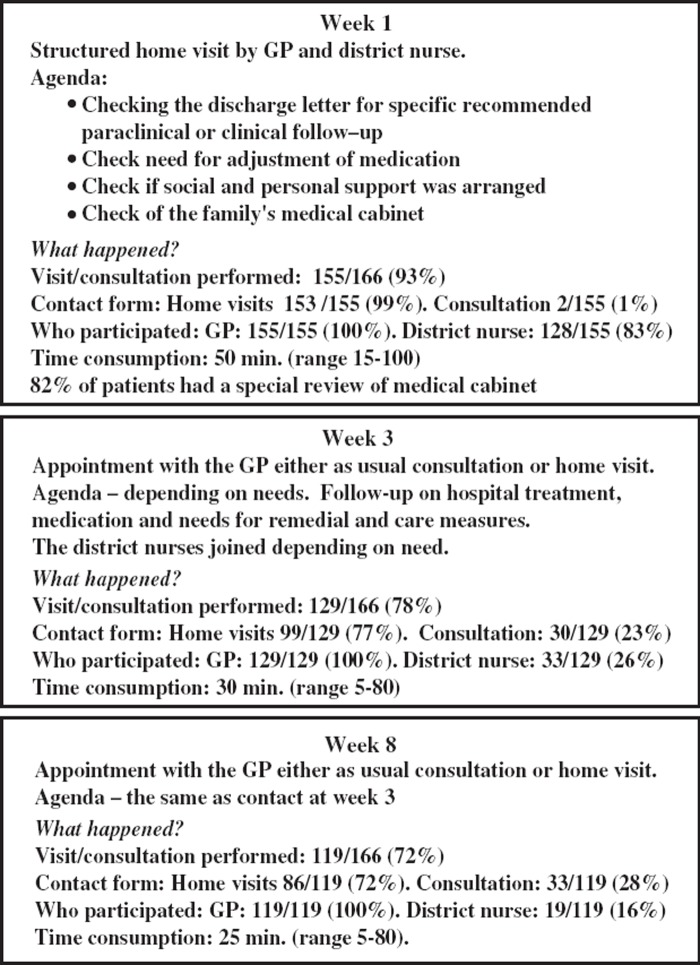
The intervention follow-up consisted of three contacts. The main intervention was a joint home visit involving both the GP and the district nurse. It was conducted approximately one week after discharge and was guided by an agenda (Figure 2). Two more contacts were conducted by the GP in the third and eighth week after discharge either in the GP's clinic or as a home visit depending on the patient's overall condition. The district nurse only attended the two follow-up contacts if required. The GPs had no specific information on enrolment of control patients until the evaluation 12 weeks after discharge. No routines were changed. The contents and routines regarding discharge letters were not changed in either of the groups.
Figure 2.
Agenda for intervention, participants, contact form.
Outcome measures
The primary outcome measures were hospital readmissions of any kind and the concordance between the GP's knowledge of the medical treatment and what the patient was actually taking.
The secondary outcome measures were the degree to which the GP implemented the recommended follow-up as described in the hospital discharge letter, total healthcare costs, functional ability, death rate, patient satisfaction, and self-rated health.
Sample size
The sample size was calculated based on the degree of concordance on medication between the GP and the patient. Previous studies have found a lack of concordance for more than 50% of patients. With an 80% power and a 5% significance level and a possible 15% loss to follow-up, approximately 220 patients should be included in each group to detect a 30% difference.
Data collection
The RT collected baseline data at inclusion. Data were collected from the patient interviews, discharge letters, and patients’ records. 12 weeks after discharge, a structured interview guided by a questionnaire was conducted in the patient's home by a member of the project staff. The GP and the district nurses simultaneously filled in individual questionnaires.
A register-based evaluation of readmissions and healthcare costs was done 26 weeks after discharge. Data on admission to hospital were based on the National Patient Register where costs were calculated on the basis of national DRG rates (Diagnosis Related Groups); costs of ambulatory treatment in hospitals were calculated on the basis of DAGS rates (Danish Ambulant Grouping System). Data on costs in the primary health sector were based on the Health Service Register (e.g. general practice, medication, specialists, and para-clinical activity). The official rate was used to convert Danish Krone into Euros. The National Register of Death provided information in the case of death.
Statistical analysis
The analyses followed the principle of intention to treat. All randomized patients discharged from hospital were included in the register-based analysis. The intervention and the control groups were compared using chi-squared tests and Monte Carlo Estimate for the Exact Test for categorical data and the Wilcoxon rank-sum test for continuous data. Time to first readmission was analysed using a Kaplan–Meier plot and a Cox regression model. Baseline comparisons showed a significant difference between the intervention and control groups for diagnosis (Table I). Logistic and Cox regression analysis was therefore used to adjust for these differences. These results are not shown since they did not differ from the unadjusted analysis. All tests were two-sided and were interpreted at the 5% level for primary and at the 1% level for secondary outcome measures, as multiple tests were made. Bootstrap (10 000 samples) was used in the economic analysis to obtain an empirical 95% confidence interval (CI). All analyses were done in SAS, version 9.1.
Table I.
Sociodemographic and health characteristics in control and intervention groups at baseline.
| Characteristics | Intervention (n = 148) | Control (n = 145) | p-value1 |
| Sex – female | 66% | 66% | 0.9 |
| Age (median) | 84 years | 83 years | 1.02 |
| Housing | 95% living in private home | 97% living in private home | 0.4 |
| Marital status | 59% widow/widower | 57% widow/widower | 0.8 |
| 30% spouse | 29% spouse | ||
| 11% divorced/single | 14% divorced/single | ||
| Contact with family and friends | 61% daily/weekly | 57% daily/weekly | 0.4 |
| 29% monthly | 36% monthly | ||
| 10% more seldom | 7% more seldom | ||
| Days spent in hospital within the last six months before the actual admission | (n = 166) | (n = 165) | |
| 0 days | 95 (57%) | 95 (58%) | 0.52 |
| 1–7 days | 24 (14%) | 39 (24%) | |
| 8–14 days | 16 (10%) | 10 (6%) | |
| 15–28 days | 14 (8%) | 12 (7%) | |
| 29 days or more | 17 (10%) | 9 (5%) | |
| Number of daily medications median (interquartile range) | 6(4.5–9) | 6 (4–8) | 0.4 |
| Receiving help | |||
| From district nurse | 65% | 56% | 0.1 |
| Home helper | 81% | 77% | 0.3 |
| Self-rated health | |||
| Excellent/very good | 12 (8%) | 16 (11%) | 0.5 |
| Good | 73 (49%) | 81 (56%) | |
| Fair/poor | 63 (42%) | 47 (33%) | |
| Dementia (MMSE, Max. 30) [24] | |||
| Light (20–30 points) | 132 (89%) | 120 (83%) | 0.6 |
| Moderate (10–19 points) | 13 (9%) | 23 (16%) | |
| Severe (0–9 points) | 2 (2%) | 2 (1%) | |
| Functional status3 | |||
| Timed up and go [25] | |||
| < 20 seconds | 60 (40%) | 56 (38%) | 1.0 |
| 20–30 seconds | 20 (14%) | 20 (14%) | |
| > 30 seconds | 14 (9%) | 13 (9%) | |
| Could not carry out | 54 (36%) | 56 (38%) | |
| Barthel Index [26] | |||
| Insignificant reduction (80–100 points) | 115 (78%) | 115 (79%) | 1.0 |
| Light reduction (79–50 points) | 24 (16%) | 22 (15%) | |
| Moderate reduction (49–26 points) | 7 (5%) | 6 (4%) | |
| Severe reduction (25–0 points) | 2 (1%) | 2 (1%) | |
| Diagnosis | |||
| Cardiovascular | 45 (30%) | 28 (19%) | 0.02 |
| Other | 103 (70%) | 117 (81%) |
Notes: 1Chi-squared test; 2Wilcoxon rank-sum test on non-grouped data; 3activities of daily living (ADL) measures developed by Avlund [27] were used and showed no differences. Data not shown.
Ethics and approval
The protocol received ethical approval from the National Board of Health and was reported to the Danish Data Protection Agency. Approval from the Scientific Ethical Committee was not needed.
Results
99 (63%) of the local GPs and district nurses in all seven invited municipalities participated in the study. A total of 166 patients were randomized to the intervention group and 165 to the control group. Comparison of participating and non-participating patients showed minor differences on diagnosis, length of hospital stay, and type of housing. More participants in the intervention group than in the control group had a cardiovascular diagnosis (see Table I).
The evaluation in the 12th week was completed by 148 patients in the intervention group and by 145 in the control group. The 38 dropout patients had a significantly lower functional ability (Barthel Index in four groups; p = 0.03) and poorer self-rated health (63% vs. 37% rated their health as poor or less than good; p = 0.03) at inclusion than those who did not drop out. A total of 19 died before the evaluation interview was conducted.
The number of conducted follow-ups, the number of participants, the amount of time consumed, and the distribution among home visits and consultations are shown in Figure 2.
Primary outcomes
Readmission
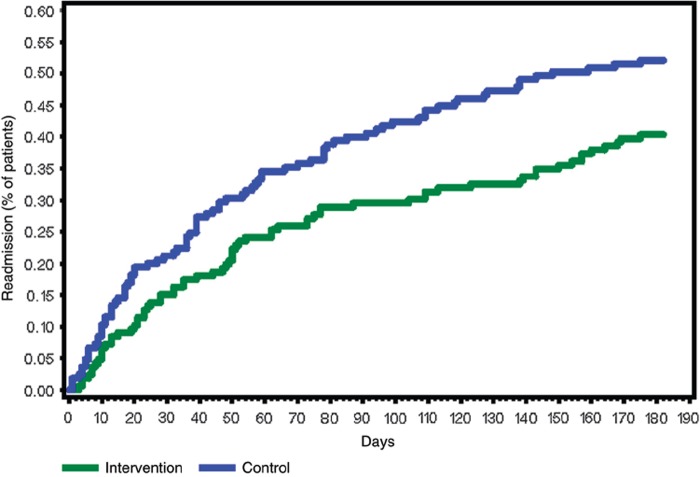
Twenty-six weeks after discharge, 86 (52%) patients in the control group and 67 (40%) in the intervention group had been readmitted (p = 0.03); relative risk reduction (RRR) 23%. A Cox regression analysis of the number of days to first readmission showed a hazard ratio of 0.69 (95% CI: 0.50–0.95. p = 0.02). Figure 3 shows the time to first readmission for any reason. A tendency towards a reduced number of days spent in hospital within 26 weeks was seen in the intervention group (p = 0.07, Wilcoxon rank-sum test).
Figure 3.
Time to first readmission to hospital for any reason (Kaplan–Meyer).
Control of medication
The concordance between the GPs’ knowledge of the patients’ medical treatment and what the patients were actually taking was higher in the intervention group than in the control group. Significantly more changes in medication and a higher number of drugs taken were found in the intervention group than in the control group (Table II).
Table II.
Follow-up on plan, medication, and health economic data.
| Variable | Intervention | Control | p-value3 |
| Follow-up on plan and medication 12 weeks after discharge | (n = 148) | (n = 145) | |
| GPs’ knowledge regarding the actual medication (prescription) | |||
| Patient taking medication that the GP does not know about | 51(34%) | 70(48%) | 0.02 |
| GP reporting medication that patients do not take | 42(28%) | 57(39%) | 0.05 |
| Follow-up on the discharge plan1 | |||
| Planned clinical control was completed as recommended | 41(95%) | 21(72%) | 0.02 |
| Planned para-clinical control was completed as recommended | 38(88%) | 21(68%) | 0.09 |
| Number of drugs taken – median (interquartile range) | 7(5–10) | 6(4–8) | 0.00054 |
| Number of patients with adjusted medication since discharge2 | 84(59%) | 63(44%) | 0.01 |
| Healthcare costs per patient after 26 weeks (€) | (n = 166) | (n = 165) | Extra cost in intervention group |
| Cost of intervention at hospital | 7 | 0 | 7 |
| Cost of GP's 1st home visit5 | 102 | 0 | 102 |
| Costs of district nurses at first home visit | 35 | 0 | 35 |
| Total hospital costs | 4.414 | 5.478 | −1.064 |
| Other cost in the primary sector (public health insurance) | 856 | 667 | 190 |
| Costs of medicine | 208 | 146 | 62 |
| Total | 5.622 | 6.290 | −668 |
| (95% CI € −2.334 to +916 ) |
Notes: 1Clinical control was recommended at 45 vs. 36 patients (I vs. C), para-clinical control was recommended at 51 vs. 33 patients. 2Five intervention patients and two control patients had missing values. 3Chi-squared test. 4Wilcoxon rank-sum test on non-grouped data. 5First visit. Other GP costs from the intervention are included in costs of public health insurance.
Secondary outcomes
There was a trend towards better follow-up on the discharge plan in the intervention group (see Table II). An economic analysis of the total healthcare expenses showed a tendency towards a socioeconomic gain in favour of the intervention (see Table II). Patients in the intervention group felt that their GPs were better informed about their hospitalization (very well-informed 42% vs. 16%; p = 0.01). No significant differences were found in functional ability, self-rated health, or patient satisfaction with the whole admission to hospital or with the services given by the GPs and municipalities in general. 15 patients in the intervention group and 20 in the control group died within 26 weeks after discharge (hazard ratio 0.72 with 95% CI 0.37–1.41).
Discussion
The present study showed that intervention produced a 23% reduction in readmissions and better concordance on prescription information. The intervention was characterized by improved interdisciplinary cooperation between the existing staff in primary healthcare, the GP, and the district nurse. The total number of days spent in hospital within 26 weeks was not significantly lower in the intervention group than in the control group as the intervention primarily eliminated admissions of short duration. The reduction in readmissions was comparable to that reported by other interventions concerning geriatric patients performed to improve discharge [1–2,7,13]. The present study confirms the GP's important but time-consuming role of dealing with elderly patients [14].
The economic analysis indicates that the intervention is cost-neutral, but that it tends to reduce costs. The savings in the intervention group were driven by substantially greater DRG expenses for readmission in the control group that more than compensated for the extra costs in the primary health sector (see Table II). The findings are in accordance with those of other studies [1,5,8,15]. The study confirms the large inconsistency between doctors’ knowledge of medication and what the patients actually use [16–21]. As a considerable number of admissions are attributable to inappropriate medical treatment [10,11], the improved medication control obtained in the present study could be of importance for the outcome. In the present study there was an unexpected, greater consumption of medicine in the intervention group than in the control group. An overrepresentation of patients with cardiac disease in the intervention group, to whom multiple medications are recommended, may have contributed to the higher consumption. Adjustment for this did not entirely eliminate the difference. The study also indicates that better follow-up on the treatment plan specified in the discharge letter is, indeed, possible. Increased collaboration between the GP, the district nurse, and the patient may increase interdisciplinary knowledge and may change the patients’ perspective when something goes wrong – making them more prone to contact their GP or district nurse instead of the hospital or the out-of-hours care staff. As expected, we found no impact on hard-core outcomes such as death [3,5], or on functional ability or self-rated health.
Bias
The baseline analysis showed no important differences between the intervention and the control groups. Data on readmission rate and economic data were based on register data which were obtained for all randomized patients. Good study adherence was found concerning data based on patient interview and questionnaires: only 38 patients dropped out.
The study could not be blinded to the GPs and nurses. They filled in several questionnaires during contact with the intervention patients. This fact may have biased our study towards a better effect than can be obtained in daily practice. On the other hand, the chosen method could have raised the GPs’ attention towards both intervention and control patients and thereby given the control group increased attention. We do not consider that these conditions affect the overall results. Many of the non-included patients were discharged during weekends as the RT included only weekdays. Such patients are generally less impaired than other patients as municipal support cannot be arranged. On the other hand, the inclusion/exclusion criteria excluded many frail patients because the evaluation was partly based on patient interview which would not be possible with patients with, for example, severe dementia. Given the characteristics of these patients, their exclusion was not expected to affect the overall representativeness of our study population.
The data on cost in the primary healthcare sector have been limited by the fact that it was not possible to obtain data on the overall derived expenses in the municipalities.
Perspective
The effect of structured intervention in relation to discharge from hospital on the rate of readmission has already been demonstrated by outgoing hospital teams [1,2,7,8,13,22,23]. We have no knowledge of studies that have investigated strategies to improve control of medical care involving the GP as a key person. Even if the study does demonstrate a potential for savings from an overall perspective, GPs and district nurses have to make an extra effort for such savings to materialise. We conclude that there is a major potential within the existing organizational framework for reducing readmissions, and in a cost-efficient manner.
Authors’ contribution
LR: Conception, design, interpretation, and drafting. HNJ: Conception, design, analysis, interpretation, and revising. FRH: Conception, design, interpretation, and revising. AVH: Analysis. AHA: Analysis, interpretation, and approval. AN: Conception, design, interpretation, and revising. JK: Analysis, interpretation, and approval.
Acknowledgements
The authors would like to thank the following: Eva Jepsen for inclusion and data collection; Karin Stadsgaard for conception and design; Mette Gislum for statistics; Kristine Skovgaard Bossen and Susanne Wilms for data collection; Morten Freil, Rikke Gut, and Stine Schulze for questionnaires and focus-group interview; Anders Rytter Bockhahn, Dea Seidenfaden, and Mette Lehmann Andersen for data collection; Frede Olesen for writing assistance.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
The study was funded by the Danish Centre for Health Technology Assessment, the National Board of Health, the Health Insurance Foundation, the General Practitioners’ Foundation for Development of General Practice (PLU) , the Copenhagen County Health Department, Copenhagen County Quality Committee for General Practice, Copenhagen County Committee on Disease Prevention, and Copenhagen County Health Insurance.
All the authors state their independence from funders. There were no sponsors.
References
- 1.Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: A randomized clinical trial. JAMA. 1999;281:613–20. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 2.Hansen FR, Poulsen H, Sorensen KH. A model of regular geriatric follow-up by home visits to selected patients discharged from a geriatric ward: A randomized controlled trial. Aging (Milano) 1995;7:202–6. doi: 10.1007/BF03324316. [DOI] [PubMed] [Google Scholar]
- 3.Philips CO, Wright SM, Kern DE, Singa RM, Sheppard S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: A meta-analysis. JAMA. 2004;291:1358–67. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 4.Shepperd S, Parkes J, McClaran J, Phillips C. Discharge planning from hospital to home. The Cochrane Database of Systematic Reviews: Reviews 2004. doi: 10.1002/14651858.CD000313.pub2. Issue 1. Art. No. CD000313, UK DOI. 10.1002./14651858.CD000313.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Parker SG, Peet SM, McPherson A, Cannaby AM, Abrams K, Baker R, et al. A systematic review of discharge arrangements for older people. Health Technology Assessment. 2002;6:1–183. doi: 10.3310/hta6040. [DOI] [PubMed] [Google Scholar]
- 6.Preston C, Cheater F, Baker R, Hearnshaw H. Left in limbo: Patients’ views on care across the primary/secondary interface. Qual Health Care. 1999;8:16–21. doi: 10.1136/qshc.8.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen HE, Schultz-Larsen K, Kreiner S, Forchhammer BH, Eriksen K, Brown A. Can readmission after stroke be prevented? Results of a randomized clinical study: A postdischarge follow-up service for stroke survivors. Stroke. 2000;31:1038–45. doi: 10.1161/01.str.31.5.1038. [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: A randomised controlled study. Lancet. 1999;354:1077–83. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 9.Olivarius NF, Hollnagel H, Krasnik A, Pedersen PA, Thorsen H. The Danish National Health Service Registry. A tool for primary care research. Dan Med Bull. 1997;44:449–53. [PubMed] [Google Scholar]
- 10.Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm World Sci. 2002;24:46–54. doi: 10.1023/a:1015570104121. [DOI] [PubMed] [Google Scholar]
- 11.Hanlon JT, Pieper CF, Hajjar ER, Sloane RJ, Lindblad CI, Ruby CM, et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61:511–15. doi: 10.1093/gerona/61.5.511. [DOI] [PubMed] [Google Scholar]
- 12.Hansen FR, Ziebell A, Spedtsberg K, Schroll M. Praktiserende lægers opfølgning i ældres hjem efter hospitalsindlæggelse [Follow-up of elderly patients in their homes by general practitioners after discharge from hospital, English summary] Ugeskr.Laeger. 1991;153:2128–31. [PubMed] [Google Scholar]
- 13.Caplan GA, Williams AJ, Daly B, Abraham K. A randomized, controlled trial of comprehensive geriatric assessment and multidisciplinary intervention after discharge of elderly from the emergency department—the DEED II study. J Am Geriatr Soc. 2004;52:1417–23. doi: 10.1111/j.1532-5415.2004.52401.x. [DOI] [PubMed] [Google Scholar]
- 14.Schoenmakers B, Buntinx F, Delepeleire J. What is the role of the general practitioner towards the family caregiver of a community-dwelling demented relative? Scand J Prim Health Care. 2009;27:31–40. doi: 10.1080/02813430802588907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peleg R, Press Y, Asher M, Pugachev T, Glicensztain H, Lederman M, et al. An intervention program to reduce the number of hospitalizations of elderly patients in a primary care clinic. BMC Health Services Research. 2008;8:36. doi: 10.1186/1472-6963-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forjuoh SN, Reis MD, Couchman GR, Symm B, Mason S, O'Banon R. Physician response to written feedback on a medication discrepancy found with their elderly ambulatory patients. J Am Geriatr Soc. 2005;53:2173–7. doi: 10.1111/j.1532-5415.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 17.Ernst ME, Brown GL, Klepser TB, Kelly MW. Medication discrepancies in an outpatient electronic medical record. Am J Health Syst Pharm. 2001;58:2072–5. doi: 10.1093/ajhp/58.21.2072. [DOI] [PubMed] [Google Scholar]
- 18.Bedell SE, Jabbour S, Goldberg R, Glaser H, Gobble S, Young-Xu Y, et al. Discrepancies in the use of medications: Their extent and predictors in an outpatient practice. Arch Intern Med. 2000;160:2129–34. doi: 10.1001/archinte.160.14.2129. [DOI] [PubMed] [Google Scholar]
- 19.Yang JC, Tomlinson G, Naglie G. Medication lists for elderly patients: Clinic-derived versus in-home inspection and interview. JGIM. 2001;16:112–15. doi: 10.1111/j.1525-1497.2001.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foss S, Schmidt JR, Andersen T, Rasmussen JJ, Damsgaard J, Schaefer K, et al. Congruence on medication between patients and physicians involved in patient course. Eur J Clin Pharmacol. 2004;59:841–7. doi: 10.1007/s00228-003-0708-x. [DOI] [PubMed] [Google Scholar]
- 21.Barat I, Andreasen F, Damsgaard EMS. Drug therapy in the elderly: What doctors believe and patients actually do. Br J Clin Pharmacol. 2001;51:615–22. doi: 10.1046/j.0306-5251.2001.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair AJ, Conroy SP, Davies M, Bayer AJ. Post-discharge home-based support for older cardiac patients: A randomised controlled trial. Age Ageing. 2005;34:338–43. doi: 10.1093/ageing/afi116. [DOI] [PubMed] [Google Scholar]
- 23.Stewart S, Pearson S, Luke CG, Horowitz JD. Effects of home-based intervention on unplanned readmissions and out-of-hospital deaths. J Am Geriatr Soc. 1998;46:174–80. doi: 10.1111/j.1532-5415.1998.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Podsiadlo D, Richardson S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 27.Avlund K, Kreiner S, Schultz-Larsen K. Functional ability scales for the elderly. A validation study. Eur J Public Health. 1996;6:35–42. [Google Scholar]