Abstract
Objective
Hantavirus infections are emerging infections that cause either Hantavirus pulmonary syndrome or haemorrhagic fever with renal syndrome (HFRS). A recent Swedish outbreak of nephropathia epidemica, a European HFRS, was analysed to study the patient flow and clinical picture and to investigate the value of an early diagnosis in general practice.
Design
In a retrospective design, medical records of verified cases of Hantavirus infection were studied.
Setting
The study was conducted in the county of Norrbotten, Sweden.
Subjects
Data from Hantavirus patients diagnosed between 2006 and 2008 were analysed.
Main outcome measures
Demographic data, level of care, treatment, clinical symptoms, and laboratory findings were obtained.
Results
In total, 456 cases were included (58% males and 42% females). The majority of patients first saw their general practitioner and were exclusively treated in general practice (83% and 56%, respectively). When diagnosed correctly at the first visit, antibiotics and hospitalization were significantly lowered compared with delayed diagnosis (14% vs. 53% and 30% vs. 54%, respectively; p < 0.0001). The clinical picture was diverse. Early thrombocytopenia was found in 65% of the patients, and haemorrhagic manifestations were documented in a few cases. Signs of renal involvement – haematuria, proteinuria, and raised levels of serum creatinine – were found in a majority of patients.
Conclusions
Raised awareness in general practice regarding emerging infections and better diagnostic tools are desirable. This study of a Hantavirus outbreak shows that general practitioners are frontline doctors during outbreaks and through early and correct diagnosis they can reduce antibiotic treatment and hospitalization.
Key Words: Family practice, general practice, Hantavirus, hemorrhagic fever with renal syndrome, nephropathia epidemica, zoonosis
Globally, Hantavirus infection may occur as isolated cases or in large outbreaks and manyof these patients are initially seen by family doctors.
This study analyses a Hantavirus outbreak in Northern Sweden. The majority of patients first saw their general practitioner and were exclusively treated in general practice.
When diagnosed correctly at the first visit, antibiotics and hospitalization were significantly lowered compared with delayed diagnosis.
This highlights the importance of family doctors as frontline doctors in outbreaks of emerging infections. Increased awareness and diagnostic accuracy among family doctors can lower the usage of antibiotics and cut costs for investigation and hospitalization.
Hantavirus infections are rodent-borne zoonoses that are globally recognized as emerging infections with cyclic outbreaks. Hantaviruses cause two significant human syndromes: haemorrhagic fever with renal syndrome (HFRS) in Europe and Asia and Hantavirus pulmonary syndrome (HPS) in the Americas. The American form of Hantavirus infection is a serious cardiopulmonary illness caused by Andes and Sin Nombre viruses that have a case-fatality rate of up to 40% [1–3]. In Asia and Southern Europe, HFRS is caused by Hantaan, Dobrava, and Seoul viruses that have a case-fatality rate of up to 10% [4]. In contrast, the predominant form in Central and Northern Europe, Puumala virus, causes a mild HFRS – nephropathia epidemica (NE) – with a case-fatality rate < 0.5%. Bank voles (Myodes glareolus) are the natural hosts for Puumalavirus, infecting humans mainly by inhalation of virus from rodent excreta [5]. Outbreaks occur throughout Europe [6,7]. The incidence of human cases varies in a cyclical fashion, peaking every third to fourth year, coinciding with peaks in vole populations [8,9]. No transmission between humans has been proven, but Puumala virus has been isolated in human saliva [10].
The regular reported incidence rate is approximately 40 per 100 000 persons per year [9] but the real rate is considered to actually be seven to eight times higher, indicating a high number of unrecognized cases [11]. Known risk factors for contracting NE are agricultural work and activities in peri-domestic areas such as cleaning and wood handling [12].
Usually, NE is a mild haemorrhagic fever with symptoms such as fever, back and abdominal pain, myalgia, headache, vomiting, and oligo- and polyuria [13]. Early laboratory findings are raised levels of C-reactive protein (CRP), thrombocytopenia, proteinuria, and haematuria. In later stages, serum creatinine is elevated, with some cases presenting a tenfold increase compared with reference levels. Despite this, the treatment of HFRS is mainly supportive and in Sweden only a few patients undergo haemodialysis. At present, no vaccine is available. The diagnosis is verified by serology, i.e. detection of specific IgG and IgM antibodies to Puumala virus. Point-of-care tests (POC) for Hantavirus infections are available [14,15]. Nephropathia epidemica is a notifiable disease according to the Swedish Communicable Disease Act, making monitoring possible. During late 2006 to 2008, northern Sweden experienced a large outbreak of NE [11]. During this outbreak, it became obvious that better diagnostic support in general practice was desirable. With this study, we aimed to describe the clinical picture in a larger patient sample, analyse the level of care, and evaluate the importance of an early diagnosis of Hantavirus infections.
Material and methods
Study setting and data collection
All verified cases of NE in the county of Norrbotten during the outbreak (November 2006 to December 2008) were included in the present study. During this period, 516 patients with serologically verified NE were confirmed by the Clinical Microbiology Laboratory at Sunderby hospital. Patients’ medical records were analysed retrospectively. Exclusion criteria were insufficient records (n = 49) and residence outside Norrbotten County (n = 2). Furthermore, medical records of patients treated by private practitioners (n = 9) were not available for analyses for regulatory reasons; these patients were excluded.
Finally, 456 cases (88%) were included. A protocol was created to extract patient data – date of birth, sex, number of visits until diagnosis, level of care, antibiotic treatment, hospitalization, symptoms, and laboratory results. Because samples were not systematically taken, we documented the lowest value of thrombocytes, the highest value of creatinine, and C-reactive protein. Haematuria, proteinuria, and pyuria were noted as positive or negative respectively without quantification due to varying methods of analysis.
Statistical analysis
The material was statistically investigated with help of SPSS 16.0 software (Statistical Package for the Social Sciences version 16; SPSS Inc, Chicago, IL). Frequency distribution of categorical variables such as symptoms, level of care, laboratory findings, sex, and age were established. The mean length of hospitalization was established. Possible differences between sexes and age groups (level of care, symptoms, and laboratory results) and the connection between early diagnosis and hospitalization or antibiotic treatment were tested by Pearson's chi-squared test. The Mann–Whitney U-test was used to compare levels of thrombocytes, creatinine, and C-reactive protein with antibiotic treatment, hospitalization, and age. P-values of ≤0.05 were considered significant. The study was approved by the University Hospital of Umeå Ethical Committee.
Results
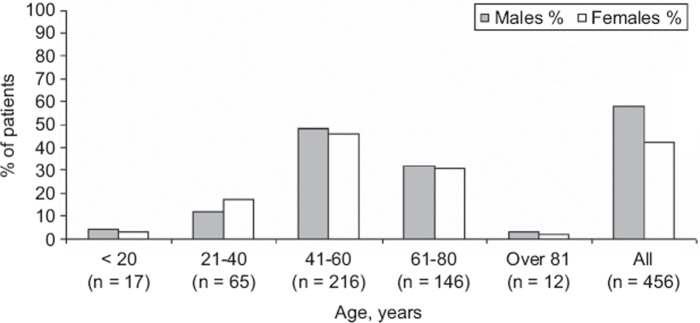
Figure 1 displays the age and sex distribution of 456 Hantavirus cases in northern Sweden during the outbreak. Almost half of the patients were between 41 and 60 years of age; male to female ratio was 1.35 (58% vs. 42%).
Figure 1.
Demographic characteristics of 456 cases of Hantavirus infection in northern Sweden October 2006–December 2008.
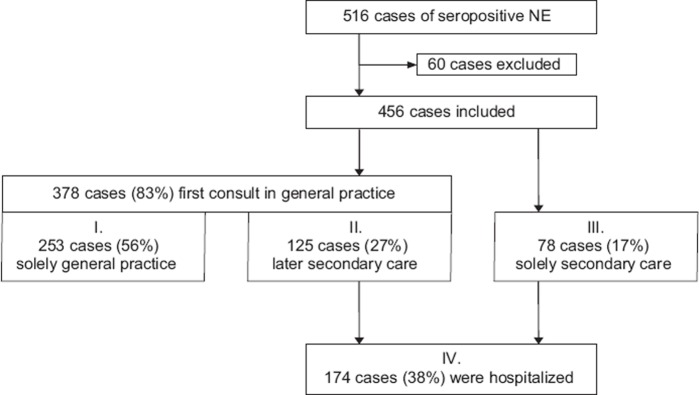
Notably, a majority of patients had their first examination in general practice. More than half of the study population were treated exclusively by GPs; the rest had consultations in secondary care. In total, 38% were hospitalized. The first half (50%) of the verified NE cases in our material had a significantly higher hospitalization rate than patients diagnosed later during the NE epidemic (43% vs. 34% respectively, p < 0.05).
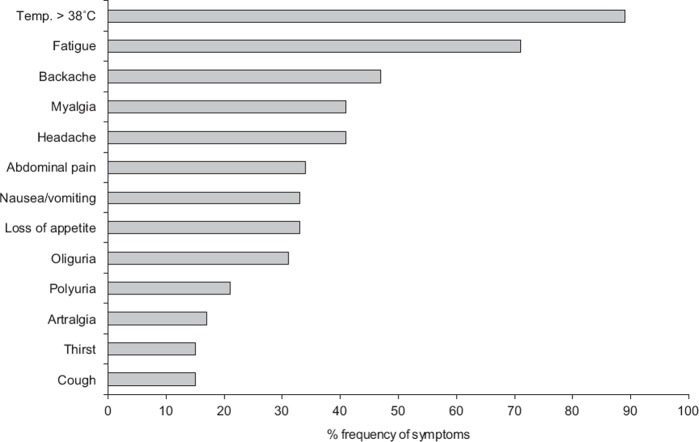
Figure 2 shows an overview of the study population describing the level of care from the first visit in relation to sex. There was a correlation between delayed diagnosis, antibiotic treatment, and hospitalization. When diagnosed correctly at the first examination (n = 297) (based on anamnesis together with clinical picture and laboratory data), antibiotic treatment and hospitalization were significantly lower compared with the patients who had delayed diagnosis (n = 159) (14% vs. 53% and 30% vs. 54%, respectively; p < 0.0001). Figure 3 illustrates the documented symptoms extracted from the medical records. The combination of symptoms and their intensity varied, and sometimes these symptoms were interpreted as the result of other diseases such as urinary tract infection, pyelonephritis, pneumonia, sepsis, or sinusitis. Males and females had similar symptoms and frequency of antibiotic treatment (data not shown).
Figure 2.
Overview of Hantavirus patients in relation to level of care in 456 cases of Hantavirus infection in northern Sweden October 2006–December 2008.
Figure 3.
Frequency of various symptoms among 456 Hantavirus patients in northern Sweden October 2006–December 2008.
Thrombocytopenia, haematuria, and proteinuria were common (Table I). These findings were often present at the first visit. Serum creatinine peaked later during the illness. Most patients presented increased levels of CRP (57% had CRP levels between 50 and 100 mg/L, and 20% had CRP > 100 mg/L). Patients treated with antibiotics had a significantly higher CRP value compared with the untreated (p < 0.0001). Pyuria (15%) was found exclusively among women, of whom 20% received antibiotics. Thrombocytopenia (p < 0.0001), elevated levels of S-creatinine (p < 0.0001), and CRP correlated with hospitalization (p < 0.0001). Among those hospitalized, the mean length of hospital stay was six nights (1–50 nights). Hospitalizations among males were more frequent than in females (see Figure 2, p = 0.097). The hospitalized patients were significantly older than the non-hospitalized (data not shown) (p < 0.05). Despite creatinine levels in many cases being > 1000 mmol/L, only one patient had dialysis. One patient suffered intoxication by lithium carbonate due to delayed diagnosis. Two of the hospitalized patients died in multi-organ failure, which leads to a case-fatality rate of 0.4%.
Table I.
Laboratory findings among 456 Hantavirus patients in northern Sweden October 2006–December 2008.
| Laboratory finding | Reference values | Mean (range) | Proportion of patients with abnormal value |
| C-reactive protein | < 10 mg/L capillary/serum | 69 mg/L (6–325 mg/L) | 92% (n = 420) |
| S-Creatinine | ♂ 60–105 μmol/L | 254 μmol/L | 82% (n = 374) |
| ♀ 45–90 μmol/L | (44–1303 μmol/L) | ||
| Thrombocytes | ♂ 145–348 109/L | 135 × 109/L | 67% (n = 212) |
| ♀ 165–387 109/L | (11–648 × 109/L) | ||
| Proteinuria | Negative | n/a1 | 77% (n = 350) |
| Haematuria | Negative | n/a1 | 68% (n = 310) |
| Pyuria | Negative | n/a1 | 14% (n = 65) |
Note: 1Not applicable.
Discussion
Our main finding was that early diagnosis of the Hantavirus infection nephropathia epidemica significantly reduced inappropriate antibiotic use and hospitalization. The majority of the patients first saw their family doctor, indicating that general practitioners are indeed frontline doctors in outbreaks.
This study's major strength is the large number of included cases. Most diagnosed patients were aged 41 years or older and there was an overall male predominance. Seroprevalence studies have shown equal distribution of antibodies for Puumala virus between sexes [16]. With our retrospective design, we cannot explain these differences. Another design bias is the varying quality of documentation that could misinterpret the frequency of symptoms. Furthermore, laboratory analyses were performed in a heterogenic manner and at different stages of infection, which may have contributed to an underestimation of the frequency of abnormal values.
Our study is, to the best of our knowledge, the first to examine Hantavirus infections in general practice. According to studies conducted in secondary care, oligo- and polyuria (82% vs. 97%) along with fever and fatigue are considered to be the main findings of NE [17]. The general practitioners in our study captured diverse clinical pictures, with lower frequencies of oligo- and polyuria (21% vs. 31%). This could be explained by possibly milder cases in general practice, early examinations with non-fulminant symptomatology, or may simply be a bias from the retrospective study design. Hospitalization was needed for 38% of patients and was significantly lowered when patients were diagnosed correctly at the first visit, reinforcing the value of sharpened diagnostic tools and guidelines.
We found a correlation between thrombocytopenia, high levels of creatinine, and hospitalization, which is probably explained by more clinically affected patients in the hospitalized group. Compared with other Hantavirus infections, NE has a low mortality, but most patients have acute renal impairment. A correct diagnosis is essential when considering reducing or pausing anticoagulants and kidney-dependent drugs, i.e., Metformin, Allopurinol, lithium carbonate, antipsychotics, and non-steroidal anti-inflammatory drugs. The use of antibiotics was significantly lowered when patients were diagnosed correctly at the first visit. Overuse of antibiotics and emergence of resistance have been recognized to be increasing problems [18–21]. A Cochrane review evaluated studies of various interventions to improve antibiotic prescribing [22]. Case definitions, decision aids, and rapid tests can help GPs make a correct diagnosis and consequently reduce prescription of unnecessary antibiotics [23–25]. There was a significantly higher frequency of hospitalization in the beginning of the NE outbreak. It is not likely to be the case that the infection was less aggressive over time; the declining hospitalization rates might be explained by raised consciousness during the ongoing epidemic.
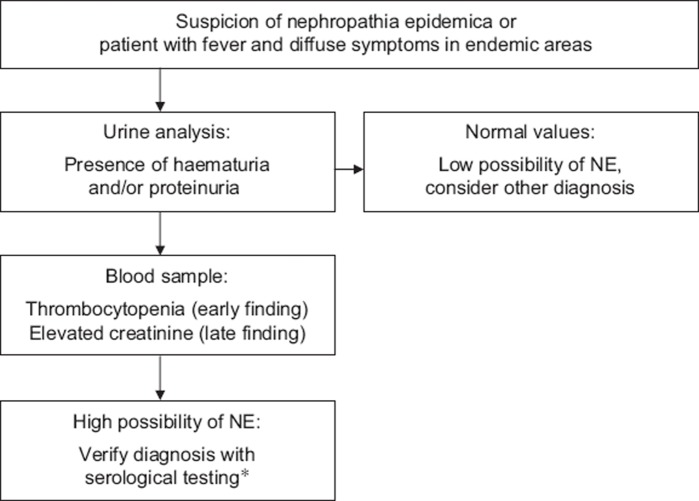
According to our findings, a decision aid for NE is proposed (Figure 4) that includes screening for haematuria, proteinuria, and thrombocytopenia in patients with suspected HFRS. Elevated levels of creatinine can also be a clue to the diagnosis, but are seen later. We encourage doctors to apply these simple tests on patients with diffuse illness in endemic areas. To secure the diagnosis, serology testing is needed, but may be costly and the results can take several days. The point-of-care test (POC) for Hantavirus infection is currently used only in secondary care. Increased clinical recognition of suspected Hantavirus cases and the use of POC could improve the diagnostic accuracy in general practice and consequently lower the use of antibiotics and cut costs for other investigations and hospitalization.
Figure 4.
Proposed decision aid for early diagnosis of nephropathia epidemica (NE). *Point-of-care test or serological testing at clinical microbiological laboratory.
We have proposed simple diagnostic tools to confirm the diagnosis in subtle cases. Most NE patients have different levels of renal impairment, but creatinine levels usually return to subnormal or normal levels. However, there is growing evidence of long-term effects after Hantavirus infections including hypertension and proteinuria [26–28], indicating that follow-ups could be valuable and that further research is desirable.
This study indicates that, in endemic areas, Hantavirus infections are important in general practice. General practitioners are the first care providers who can discover outbreaks of infections. It is important to detect new or emerging infections and syndromic surveillance has been suggested [29]. Increased awareness among general practitioners and a reporting system from family medicine offices could improve public health.
Acknowledgements
For advice on statistics the authors would like to thank Lars Holmgren, County Council of Norrbotten and Hans Stenlund, Umeå University.
Funding support
This work was supported by the County of Norrbotten, the County Councils of Northern Sweden, the Medical Faculty of Umeå University and the Foundation of the National Board of Health and Welfare.
Prior presentations
Poster presentation with preliminary data was presented at the Swedish Medical Association's annual meeting on 27 November 2008 in Gothenburg.
Conflict of interests
None.
References
- 1.Centers for Disease Control and Prevention. Hantavirus pulmonary syndrome—United States: Updated recommendations for risk reduction. MMWR. 2002;51:1–12. [PubMed] [Google Scholar]
- 2.Graziano KL, Tempest B. Hantavirus pulmonary syndrome: A zebra worth knowing. Am Fam Physician. 2002;66:1015–20. [PubMed] [Google Scholar]
- 3.Pierce JR, Jr, Milton JS. Is Hantavirus important to practitioners? South Med J. 2009;102:563–4. doi: 10.1097/SMJ.0b013e3181a4f49e. [DOI] [PubMed] [Google Scholar]
- 4.Kariwa H, Yoshimatsu K, Arikawa J. Hantavirus infection in East Asia. Comp Immunol Microbiol Infect Dis. 2007;30:341–56. doi: 10.1016/j.cimid.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Vapalahti O, Mustonen J, Lundkvist A, Henttonen H, Plyusnin A, Vaheri A. Hantavirus infections in Europe. Lancet Infect Dis. 2003;3:653–61. doi: 10.1016/s1473-3099(03)00774-6. [DOI] [PubMed] [Google Scholar]
- 6.Heyman PCC, Ducoffre G, Mailles A. Haemorrhagic fever with renal syndrome: An analysis of the outbreaks in Belgium, France, Germany, the Netherlands and Luxembourg in 2005. Eurosurveillance. 2007;12:167–71. doi: 10.2807/esm.12.05.00712-en. [DOI] [PubMed] [Google Scholar]
- 7.Winter CH, Brockmann SO, Piechotowski I, Alpers K, an der Heiden M, Koch J, et al. Survey and case-control study during epidemics of Puumala virus infection. Epidemiol Infect. 2009;137:1479–85. doi: 10.1017/S0950268809002271. [DOI] [PubMed] [Google Scholar]
- 8.Tersago K, Verhagen R, Servais A, Heyman P, Ducoffre G, Leirs H. Hantavirus disease (nephropathia epidemica) in Belgium: Effects of tree seed production and climate. Epidemiol Infect. 2009;137:250–6. doi: 10.1017/S0950268808000940. [DOI] [PubMed] [Google Scholar]
- 9.Olsson GE, Hjertqvist M, Lundkvist Å, Hörnfeldt B. Predicting high risk for human Hantavirus infections, Sweden. Emerg Infect Dis. 2009;15:104–6. doi: 10.3201/eid1501.080502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettersson L, Klingstrom J, Hardestam J, Lundkvist A, Ahlm C, Evander M. Hantavirus RNA in saliva from patients with hemorrhagic fever with renal syndrome. Emerg Infect Dis. 2008;14:406–11. doi: 10.3201/eid1403.071242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pettersson L, Boman J, Juto P, Evander M, Ahlm C. Outbreak of Puumala virus infection, Sweden. Emerg Infect Dis. 2008;5:808–10. doi: 10.3201/eid1405.071124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahlm C, Thelin A, Elgh F, Juto P, Stiernström EL, Holmberg S, et al. Prevalence of antibodies specific to Puumala virus among farmers in Sweden. Scand J Work Environ Health. 1998;24:104–8. doi: 10.5271/sjweh.286. [DOI] [PubMed] [Google Scholar]
- 13.Settergren B. Clinical aspects of nephropathia epidemica (Puumala virus infection) in Europe: A review. Scand J Infect Dis. 2000;32:125–32. doi: 10.1080/003655400750045204. [DOI] [PubMed] [Google Scholar]
- 14.Hujakka H, Koistinen V, Eerikainen P, Kuronen I, Laatikainen A, Kauppinen J, et al. Comparison of a new immunochromatographic rapid test with a commercial EIA for the detection of Puumala virus specific IgM antibodies. J Clin Virol. 2001;23:79–85. doi: 10.1016/s1386-6532(01)00191-3. [DOI] [PubMed] [Google Scholar]
- 15.Navarrete M, Barrera C, Zaror L, Otth C. Rapid immunochromatographic test for Hantavirus andes contrasted with capture-IgM ELISA for detection of Andes-specific IgM antibodies. J Med Virol. 2007;79:41–4. doi: 10.1002/jmv.20759. [DOI] [PubMed] [Google Scholar]
- 16.Ahlm C, Juto P, Stegmayr B, Settergren B, Wadell G, Tärnvik A, et al. Prevalence of serum antibodies to Hantaviruses in northern Sweden as measured by recombinant nucleocapsid proteins. Scand J Infect Dis. 1997;29:349–54. doi: 10.3109/00365549709011829. [DOI] [PubMed] [Google Scholar]
- 17.Settergren B, Juto P, Trollfors B, Wadell G, Norrby SR. Clinical characteristics of nephropathia epidemica in Sweden: Prospective study of 74 cases. Rev Infect Dis. 1989;11:921–7. doi: 10.1093/clinids/11.6.921. [DOI] [PubMed] [Google Scholar]
- 18.Cars O, Högberg LD, Murray M, Nordberg O, Sivaraman S, Lundborg CS, et al. Meeting the challenge of antibiotic resistance. BMJ. 2008:337–a1438. doi: 10.1136/bmj.a1438. [DOI] [PubMed] [Google Scholar]
- 19.Enne V. Reducing antimicrobial resistance in the community by restricting prescribing: Can it be done? J Antimicrob Chemother. 2010;65:179–82. doi: 10.1093/jac/dkp443. [DOI] [PubMed] [Google Scholar]
- 20.Arason VA, Sigurdsson JA. The problems of antibiotic overuse. Scand J Prim Health Care. 2010;28:65–6. doi: 10.3109/02813432.2010.487652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Report on infectious diseases 2000: Overcoming antimicrobial resistance. Available at: http://www.who.int/infectious-disease-report/2000/index.html.
- 22.Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev. 2005;((4)):CD003539. doi: 10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low D. Reducing antibiotic use in influenza: Challenges and rewards. Clin Microbiol Infect. 2008;14:298–306. doi: 10.1111/j.1469-0691.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 24.McIsaac WJ, Moineddin R, Ross S. Validation of a decision aid to assist physicians in reducing unnecessary antibiotic drug use for acute cystitis. Arch Intern Med. 2007;167:2201–6. doi: 10.1001/archinte.167.20.2201. [DOI] [PubMed] [Google Scholar]
- 25.Butler CC, Simpson S, Wood F. General practitioners’ perceptions of introducing near-patient testing for common infections into routine primary care: A qualitative study. Scand J Prim Health Care. 2008;26:17–21. doi: 10.1080/02813430701726285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miettinen MH, Makela SM, Ala-Houhala IO, Huhtala HS, Koobi T, Vaheri AI, et al. Ten-year prognosis of Puumala Hantavirus-induced acute interstitial nephritis. Kidney Int. 2006;69:2043–8. doi: 10.1038/sj.ki.5000334. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen MH, Makela SM, Ala-Houhala IO, Huhtala HS, Koobi T, Vaheri AI, et al. Tubular proteinuria and glomerular filtration 6 years after puumala Hantavirus-induced acute interstitial nephritis. Nephron Clin Pract. 2009;112:c115–20. doi: 10.1159/000213899. [DOI] [PubMed] [Google Scholar]
- 28.Pergam SA, Schmidt DW, Nofchissey RA, Hunt WC, Harford AH, Goade DE. Potential renal sequelae in survivors of Hantavirus cardiopulmonary syndrome. Am J Trop Med Hyg. 2009;80:279–85. [PMC free article] [PubMed] [Google Scholar]
- 29.Sloane PD, MacFarquhar JK, Sickbert-Bennett E, Mitchell CM, Akers R, Weber DJ, et al. Syndromic surveillance for emerging infections in office practice using billing data. Ann Fam Med. 2006;4:351–8. doi: 10.1370/afm.547. [DOI] [PMC free article] [PubMed] [Google Scholar]