Abstract
Diphenyliodonium (DPI) is known to irreversibly inactivate flavoproteins. We have found that DPI inhibits both membrane-bound methane monooxygenase (pMMO) from Methylococcus capsulatus and ammonia monooxygenase (AMO) of Nitrosomonas europaea. The effect of DPI on NADH-dependent pMMO activity in vitro is ascribed to inactivation of NDH-2, a flavoprotein which we proposed catalyzes reduction of the quinone pool by NADH. DPI is a potent inhibitor of type 2 NADH:quinone oxidoreductase (NDH-2), with 50% inhibition occurring at ≈5 μM. Inhibition of NDH-2 is irreversible and requires NADH. Inhibition of NADH-dependent pMMO activity by DPI in vitro is concomitant with inhibition of NDH-2, consistent with our proposal that NDH-2 mediates reduction of pMMO. Unexpectedly, DPI also inhibits pMMO activity driven by exogenous hydroquinols, but with ≈100 μM DPI required to achieve 50% inhibition. Similar concentrations of DPI are required to inhibit formate-, formaldehyde-, and hydroquinol-driven pMMO activities in whole cells. The pMMO activity in DPI-treated cells greatly exceeds the activity of NDH-2 or pMMO in membranes isolated from those cells, suggesting that electron transfer from formate to pMMO in vivo can occur independent of NADH and NDH-2. AMO activity, which is known to be independent of NADH, is affected by DPI in a manner analogous to pMMO in vivo: ≈100 μM is required for 50% inhibition regardless of the nature of the reducing agent. DPI does not affect hydroxylamine oxidoreductase activity and does not require AMO turnover to exert its inhibitory effect. Implications of these data for the electron transfer pathway from the quinone pool to pMMO and AMO are discussed.
Ammonia monooxygenase (AMO) and the membrane-bound form of methane monooxygenase (pMMO) are two of the three members of a novel family of membrane-bound monooxygenases capable of oxidizing small hydrocarbons (3, 33). These two enzymes are similar in their putative subunit composition, inhibitor profiles, and the DNA sequence of the genes encoding the subunits (19, 20, 22). The membrane-bound butane monooxygenase from Nocardioides sp. strain CF8 was recently identified as the third member of this class of enzymes, sharing many of the characteristics of pMMO and AMO (18). These enzymes are known to require copper, because inhibition by metal chelators is reversed only by addition of copper (14, 17). In addition, all three are irreversibly inhibited by acetylene, and when radiolabeled acetylene is used, a ≈27-kDa peptide is specifically labeled (5, 18, 26, 37).
Enzymes of this class can oxidize small alkanes and halogenated solvents, hence their potential application in bioremediation (9, 25, 29, 45). Although there are other enzymes that are able to oxidize small alkanes (32, 46, 47), they are distinct in structure and properties from AMO and pMMO. These two enzymes thus represent a novel mechanism for alkane oxidation, one that has yet to be elucidated due to the difficulty in isolating pure samples of either enzyme with high activity (4, 17, 28, 31, 49, 51).
AMO and pMMO initiate oxidative metabolism of NH3 and CH4 in nitrifying and methanotrophic bacteria, respectively (3, 19). Both types of bacteria obtain energy solely from the subsequent oxidation of the products of the monooxygenase reaction. In methanotrophs, energy needs are satisfied by the oxidation of methanol to CO2 by periplasmic and cytosolic dehydrogenases specific for methanol, formaldehyde, and formate (1). In nitrifying bacteria, the 4-electron oxidation of hydroxylamine to nitrite by hydroxylamine oxidase (HAO) provides reductant for ammonia oxidation and all other cellular processes (8).
AMO and pMMO are notoriously refractory to purification. AMO has not been purified to homogeneity with activity, and although several laboratories have reported purification of active pMMO (4, 31, 34, 49, 51), the specific activity of the purified enzyme is a small fraction of the activity in vivo. Disruption of the physiological electron transfer pathways to AMO and pMMO may be in part responsible for the deterioration of activity in vitro (40, 41). These pathways are poorly characterized (1, 48), and thus, the reducing agents used in vitro may not be physiologically relevant, resulting in inefficient electron transfer to their active sites.
NADH generated from oxidation of formaldehyde and formate is believed to be the source of reducing equivalents for methane oxidation (2). It is well established that NADH directly reduces the soluble form of methane monooxygenase (sMMO), mediated by an iron-sulfur flavoprotein reductase (32). NADH is also the optimal reductant for pMMO in isolated membrane fractions (42). However, we and others have reported that NADH is ineffective with detergent-solubilized or purified pMMO, which can only be reduced by exogenous hydroquinols, such as duroquinol (4, 40, 41, 51). We recently reported isolation of a 36-kDa flavoprotein from membranes of Methylococcus capsulatus that has characteristics of a type 2 NADH:quinone oxidoreductase (NDH-2) and suggested that it mediates electron transfer to pMMO in conjunction with the endogenous quinone pool (solid lines in Fig. 1A) (13). Support for this suggestion comes from a recent report in which high pMMO activity was sustained by NADH and exogenous quinones only after addition of purified NDH-2 (11). Furthermore, the specific activity in the presence of duroquinone, NADH, and NDH-2 was ≈40% greater than that with duroquinol alone (11). However, Chan and coworkers recently reported pMMO activity in detergent-solubilized membranes with either NADH or duroquinol as the reductant but 10-fold greater specific activity with the quinol (49). Their purified pMMO samples had similar specific activity with either NADH or duroquinol as the reductant (49), but 4- to 10-fold lower than reported by others (4, 11). Clearly, more work is necessary to elucidate electron transfer to pMMO both in vivo and in vitro.
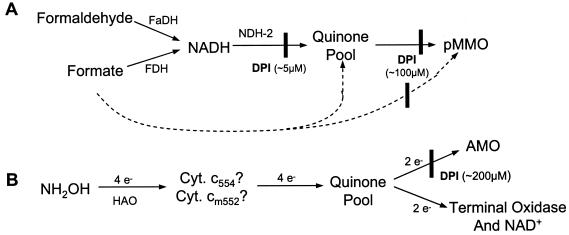
FIG. 1.
Proposed electron transfer pathways to pMMO in M. capsulatus (A) and to AMO in N. europaea (B). The solid lines in A depict the pathway proposed in reference 13, and the dashed lines represent the NADH-independent pathway we propose for in vivo reduction of pMMO based on the data presented in this work. The pathway depicted in part B is adapted from reference 48. In both pathways, proposed sites of inhibition by DPI are indicated by a vertical bar.
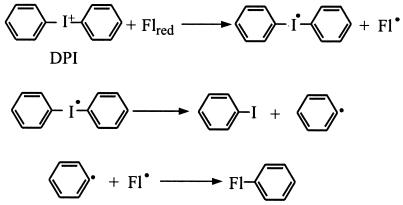
In the present study we used diphenyliodonium (DPI) to further investigate the electron transfer pathways to pMMO and AMO. DPI and other diaryl-iodonium compounds irreversibly inactivate many redox-active proteins, primarily flavoproteins (10, 15, 35, 36, 43), but DPI may also react with some low-potential b-type cytochromes and quinoproteins (6, 16). The proposed mechanism for DPI inactivation of flavoproteins (10, 35) is shown in Fig. 2: DPI accepts one electron from the fully reduced flavin, generating a DPI free radical and the flavin semiquinone. Spontaneous cleavage of the DPI radical yields a benzene radical which combines with the flavin semiquinone, resulting in covalent modification of the cofactor and irreversible inactivation of its redox cycle. DPI is thought to inactivate other redox-active proteins by a similar mechanism involving reduction of DPI and radical recombination with the cofactor (16).
FIG. 2.
Proposed mechanism for inhibition of flavoproteins by DPI, adapted from references 10 and 34. Fl represents the protein-bound flavin cofactor.
In this work we show that DPI irreversibly inactivated the NDH-2 from M. capsulatus, leading to irreversible inhibition of NADH-dependent pMMO activity in vitro, as expected (solid lines in Fig. 1A). However, DPI inhibited pMMO activity in vitro even when reducing equivalents were provided downstream of NDH-2 by hydroquinols, suggesting that a second DPI-sensitive protein is required for reduction of pMMO. We show that DPI also inhibited pMMO activity in whole cells in a manner that is apparently independent of NDH-2 (dashed lines in Fig. 1A). DPI irreversibly inhibited the activity of AMO in whole cells of Nitrosomonas europaea as well.
Electron transfer to AMO is independent of NADH (Fig. 1B) (22, 48), so the site of DPI inhibition cannot be analogous to the NDH-2 of M. capsulatus. AMO is inhibited by DPI whether reducing equivalents are donated through HAO by hydroxylamine or hydrazine or at the level of the quinone pool by duroquinol. Furthermore, DPI does not affect electron transfer from HAO to the terminal oxidase and does not have the characteristics of a mechanism-based inhibitor of AMO. Thus, we propose that DPI acts to interrupt electron transfer from the quinone pool to AMO (Fig. 1B), perhaps by irreversibly binding to a site analogous to the NDH-2-independent site in M. capsulatus.
MATERIALS AND METHODS
Growth of bacteria.
M. capsulatus (strain Bath) was maintained at 45°C on 1.8% agar plates in an atmosphere of ≈30% methane in air, and cultures in liquid medium were grown at 45°C in NMS medium supplemented with phosphate buffer, vitamins, and copper, as described in previous reports (13, 40). M. capsulatus was harvested at late log phase by centrifugation for 10 min at 8,000 × g from either 500-ml batch cultures in 2-liter flasks or 10-liter batch cultures grown in a 14-liter fermenter according to published procedures (40). Cells were washed one to three times in a volume of 10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES, pH 7.2) equal to 2% of the culture volume. The washed cells were suspended in 50 mM PIPES, pH 7.2, in a final volume that was 0.5% of the culture volume. Cells were stored at 4°C under argon and typically used within 5 days of harvesting.
N. europaea was grown in batch cultures at 30°C with shaking in a minimal medium, as previously described (17, 29). The cells were harvested by centrifugation at 8,000 × g from 1.5-liter cultures at late log phase. Cells were washed three times with 50 mM phosphate, pH 8.0, and suspended in the same buffer at a volume equivalent to 0.1% of the culture volume. Cells were stored at 4°C and used within 3 days.
Membrane isolation.
Similar procedures were used to isolate membrane fractions from M. capsulatus and N. europaea. Cells were suspended in buffer (50 mM PIPES, pH 7.2, for M. capsulatus and 50 mM phosphate, pH 8.0, for N. europaea) at 15 to 30 mg/ml total protein concentration. Both types of cell suspension were supplemented with 1 mM phenylmethylsulfonyl fluoride and 200 μM Cu(SO4), and N. europaea also contained 10 mg of bovine serum albumin per ml. Cell suspensions were passed three times through a French press cell at 18,000 to 20,000 lb/in2. The cell lysate was centrifuged at 8,000 × g for 10 min to remove unbroken cells. Sufficient NaCl was added to the supernatant to bring the concentration to 1 M, to facilitate release of peripheral proteins from the membrane. The supernatant was then centrifuged at 106,000 × g for 60 to 90 min. The pellet was suspended with a Dounce homogenizer in 50 mM buffer containing 100 μM Cu(SO4), centrifuged again at 106,000 × g for 60 min, and suspended in a volume of 50 mM buffer equivalent to half of the original volume of cell suspension. Membrane samples were stored at 4°C and used for experiments within 48 h of isolation.
Detergent solubilization of pMMO and NDH-2 and purification of NDH-2.
NDH-2 and pMMO were solubilized from the M. capsulatus membrane fraction by addition of a 20% (wt/vol) stock solution of lauryl maltoside. The detergent was added with vortexing to a final detergent-to-protein ratio of 0.9 (wt/wt). The sample was incubated on ice for 30 to 45 min with frequent vortexing, then centrifuged at 106,000 × g for 60 min. The supernatant was used immediately for pMMO or NDH assays or NDH-2 isolation. The NDH-2 was purified by column chromatography, and purity was determined by silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, as described previously (13). The purified protein was stored at 4°C and used for experiments within 1 week. No loss of activity was observed over this time.
Enzyme assays.
The activity of pMMO was assayed by measuring the rate of propylene oxidation, as previously described (12, 13). Propylene oxide was measured by injecting either 2 μl of solution or 100 μl of headspace into a SRI-8610 gas chromatograph (SRI Instruments, Torrance, Calif.) equipped with an Rtx-1 capillary column (30 m by 0.53 mm; Restek Corp., Bellefonte, Pa.) and flame ionization detector. The concentration of propylene oxide was determined from the integrated peak area relative to a standard curve. Formate, formaldehyde, or duroquinol was used as the reductant for whole-cell assays, as indicated in the figure legends, whereas NADH or hydroquinols were used to assay membrane and detergent-solubilized samples, according to previously published methods (13, 40).
AMO activity was determined from the rate of ethylene oxidation or the rate of ammonia-dependent oxygen uptake (27, 38). In the former assay, 1 ml of sample was incubated at 30°C for 3 min in a septum-sealed 6-ml vial. The assay was initiated by adding 5 to 10 μl of reductant (hydrazine, hydroxylamine, or duroquinol) from a concentrated stock solution, followed by 3 ml of ethylene. Ethylene oxide concentration was measured by injecting 100 μl of headspace into a Shimadzu model GC-8A gas chromatograph (Shimadzu Corp., Kyoto, Japan) equipped with a Porapak-Q column (0.3 cm inner diameter by 40 cm long; Waters Associates, Framingham, Mass.) and flame-ionization detector and referenced to a standard curve. The oxygen uptake assay was performed with a polarographic oxygen electrode (Yellow Springs Instruments) and a cell maintained at 30°C. The assay was initiated by adding 6 μl of 1 M (NH4)2SO4 and the change in percent oxygen saturation over time was recorded on a chart recorder. The rate of oxygen uptake in micromoles per minute was calculated based on a concentration of 230 μM O2 in air-saturated buffer (44).
HAO activity was also measured polarographically as the rate of hydroxylamine-dependent oxygen uptake (30). Samples were prepared as above, and the assay was initiated by adding 10 μl of a 0.25 M solution of hydroxylamine. To determine AMO and HAO activity on the same sample, AMO activity was measured as above, but when the oxygen concentration reached ≈40% saturation, 10 μl of 100 mM allylthiourea was added to completely inhibit O2 uptake by AMO. HAO activity was then measured by adding hydroxylamine, as described above.
NDH activity of membrane fractions and purified NDH-2 was measured as either the rate of quinone reduction in the presence of NADH or the rate of quinone-dependent NADH oxidation, according to methods described previously (13). The rate of absorbance change was measured with an Aminco DW-2000 spectrometer, equipped with a stirred, thermostatted cell holder.
In some cases the effect of DPI on enzyme activity was determined by performing the assay in the presence of the inhibitor (as in Fig. 3 and 5A), which was prepared as a 10 mM stock solution in aqueous buffer. In these assays the presence of DPI and reductant caused the enzyme activity to decrease steadily during the assay. Therefore, activity was determined from the initial linear portion of the assay: the first 60 s for NDH-2 and the first 3 min for pMMO. In all other cases the cells or membranes were incubated with DPI, followed by extensive washing to remove the unreacted inhibitor prior to performing the assays, as described above.
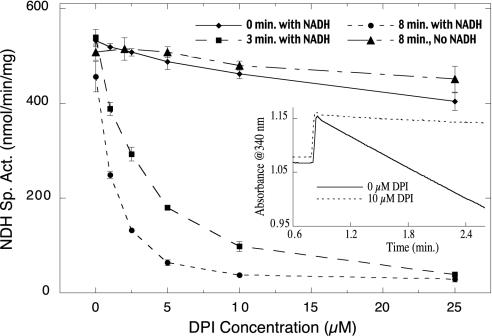
FIG.3.
Inhibition of NDH-2 from M. capsulatus by DPI is enhanced by incubation with NADH. Membrane samples were incubated at 25°C with 0 to 25 μM DPI and 1 mM NADH for 0, 3, or 8 min. The assay was initiated by addition of 1 mM 2,3-dimethoxy-5-methyl-1,4-benzoquinone (Q-zero), and the rate of Q-zero reduction was monitored by the decrease in absorbance at 410 nm during the initial 45 s of the assay. As a control, membrane samples were incubated for 8 min with 0 to 25 μM DPI in the absence of NADH before initiating the assay. Each data point is reported as the mean value ± standard deviation of three or four measurements. The inset shows the effect of 10 μM DPI on the activity of pure NDH-2. DPI and 0.2 mM NADH were added to the enzyme sample at time zero and incubated at 25°C for 45 s before initiating NDH-2-catalyzed NADH oxidation with 0.25 mM Q-zero.
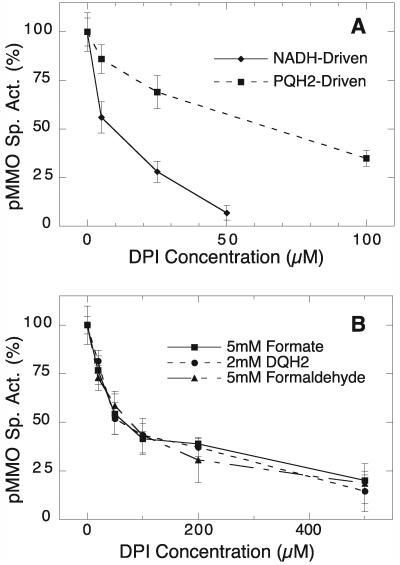
FIG. 5.
DPI inhibition profiles of hydroquinol- and NADH-driven pMMO activities in vitro (A) and hydroquinol- formaldehyde-, and formate-driven pMMO activities in vivo (B). In A, the activity of detergent-solubilized pMMO was measured in the presence of DPI with 5 mM NADH plus 350 μM PQ or 3 mM PQH2 as the reductant. Activities are expressed as a percentage of the specific activity in the absence of DPI (17.8 and 6.4 nmol min−1 mg−1 for NADH and PQH2-driven controls, respectively). (B) M. capsulatus cells were incubated for 5 min at 45°C with DPI and 5 mM formate, washed three times, and assayed for pMMO activity with either 5 mM formate, 5 mM formaldehyde, or 2 mM duroquinol as the reductant. Activities are expressed as a percentage of that of the 0 μM DPI control (205.4, 225.1, and 130.8 nmol min−1 mg−1 for the formate-, formaldehyde-, and duroquinol-driven controls, respectively). Each data point is reported as the mean value ± standard deviation of three or four measurements.
Protein concentrations were determined with the bicinchoninic acid assay with reagents from Pierce Chemical Corp. and bovine serum albumin as the standard. Whole cells and membrane fractions were digested in 1.0 M NaOH at 90°C for 10 min prior to the protein assay.
Materials.
Ethanol was removed from NADH by two cycles of dissolving it in water and lyophilizing to dryness. Quinones and hydroquinols were prepared as concentrated stock solutions (≈50 mM) in ethanol or dimethyl sulfoxide. The dimethyl sulfoxide solutions were added directly to the protein samples. The quinones in ethanol solution were placed in a vial and dried under vacuum to remove the solvent to prevent interference with GC detection of assay products. The protein sample was added to this vial and vortexed vigorously prior to addition of the substrate.
Hydroquinols were prepared by reducing the quinone with zinc dust in either ethanol or dimethyl sulfoxide containing 50 mM HCl. For a typical reaction, 1 ml of quinone (≈50 mM in ethanol or dimethyl sulfoxide) was placed in a 1.5-ml microcentrifuge tube, and 20 mg of Zn dust was added, followed by 50 μl of 1.0 M HCl. This sample was shaken at room temperature until it became colorless. The excess Zn dust was removed by centrifugation at 14,000 × g for 3 min. Water-soluble contaminants (principally Zn2+) were removed by adding the supernatant to 15 ml of 10 mM HCl. The hydroquinol formed a white precipitate that was collected by centrifugation, washed twice with 10 mM aqueous HCl, and dissolved in ≈0.5 ml of ethanol or dimethyl sulfoxide containing 10 mM HCl. The stock solutions of hydroquinol were stored at −80°C to prevent oxidation. The concentrations of quinone and hydroquinol stock solutions were determined spectrophotometrically, with published extinction coefficients (40). All other materials were used as supplied by the manufacturer without further purification.
RESULTS
DPI inhibition of NDH-2.
NDH-2 from M. capsulatus catalyzes NADH-dependent quinone reduction (13). DPI was a potent inhibitor of NADH-dependent quinone reduction in M. capsulatus membranes (Fig. 3). This inhibition required reduction by NADH; longer incubation of the membrane samples with DPI and NADH (0, 3, and 8 min) resulted in a dramatic increase in the extent of inhibition (Fig. 3). In contrast, little or no effect on quinone reductase activity was observed after incubating membranes for 8 min with DPI in the absence of NADH (Fig. 3). Incubation of purified NDH-2 for 45 s with 10 μM DPI and 200 μM NADH results in >97% inhibition of the rate of duroquinone-dependent NADH oxidation (Fig. 3, inset). The effect of DPI on NDH-2 activity was irreversible; overnight dialysis of purified, DPI-treated NDH-2 resulted in no change in the extent of inhibition (data not shown), and extensive washing of DPI-treated membranes did not reverse the inhibitory effect of DPI (Fig. 4).
FIG.4.
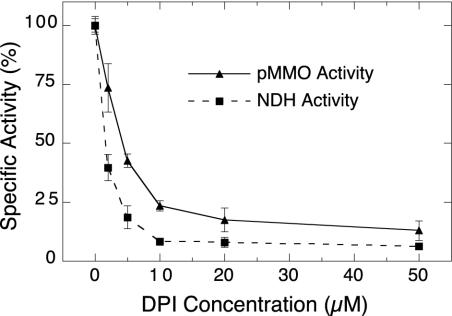
DPI inhibition of pMMO and NDH-2 in membranes from M. capsulatus. Membrane samples were incubated for 3 min at 45°C with 2 mM NADH and 0 to 50 μM DPI and washed three times by centrifugation before measuring pMMO and NDH activity. The pMMO activity was measured as the rate of NADH-driven propylene epoxidation. NDH activity was measured as the rate of NADH oxidation in the presence of 1 mM duroquinone and 125 μM NADH. Activities are expressed as a percentage of that of the 0 μM DPI controls (154 nmol min−1 mg−1 for pMMO and 930 nmol min−1 mg−1 for NDH-2). Each data point is reported as the mean value ± standard deviation of three measurements.
DPI inhibition of pMMO.
To determine if DPI affects pMMO activity, membrane samples from M. capsulatus were incubated with increasing concentrations of DPI in the presence of 2 mM NADH. The results of this titration with DPI (Fig. 4) demonstrated that NADH-driven pMMO activity was extensively inhibited by DPI. Only ≈8% of the original pMMO activity remained after treatment with 50 μM DPI. Measurement of NDH-2 activity in the same DPI-treated membrane samples demonstrated a comparable response to DPI, such that 50% inhibition was observed at ≈2 μM and ≈5 μM for NDH-2 and pMMO activities, respectively (Fig. 4). In this experiment, the activities of both NDH-2 and pMMO were measured after the membranes were washed to remove unreacted DPI and NADH, suggesting that the effect of DPI is irreversible. When NADH was absent from the incubation with DPI, little or no inhibition of either NDH-2 or pMMO activity was observed (data not shown).
As was observed for NDH-2 activity, the extent of DPI inhibition of pMMO activity depended on the length of the incubation with NADH (Table 1). In this experiment, the effect of DPI was determined without washing the membranes. Therefore, significant inhibition is observed for the time zero incubation, since DPI and NADH are present throughout the assay (Table 1).
TABLE 1.
Effect of incubation time on DPI inhibition of pMMO activity in membranes and protection by PQa
| Incubation conditions | Mean pMMO sp act (nmol min−1 mg−1) ± SD (% of control) |
|---|---|
| 5 μM DPI, 5 mM NADH for 0 min | 11.9 ± 3.4 (17.2) |
| 5 μM DPI, 5 mM NADH for 3 min | 5.6 ± 2.6 (8.1) |
| 5 μM DPI, 5 mM NADH for 6 min | 3.9 ± 0.8 (5.6) |
| 5 μM DPI, 5 mM NADH for 15 min | 2.8 ± 1.3 (4.0) |
| 0 μM DPI, 5 mM NADH for 0 min | 69.1 ± 4.1 (100) |
| 0 μM PQ, 5 μM DPI | 8.2 ± 1.4 (11.7) |
| 25 μM PQ, 5 μM DPI | 22.1 ± 2.8 (31.5) |
| 50 μM PQ, 5 μM DPI | 25.9 ± 1.1 (37.0) |
| 100 μM PQ, 5 μM DPI | 34.7 ± 3.6 (49.5) |
| 0 μM PQ, 0 μM DPI | 70.1 ± 5.7 (100) |
M. capsulatus membranes were incubated at 45°C for the indicated time in the presence of 5 mM NADH and 0 or 5 μM DPI before initiating the assay with propene. M. capsulatus membranes were incubated for 3 min at 45°C with DPI, PQ, and 1 ml of propene before initiating the assay with NADH. Values represent the means of three or four measurements. Numbers in parentheses are the activities expressed as a percentage of that of the 0 μM DPI control.
Some DPI-sensitive enzymes are protected from inactivation in the presence of an electron acceptor. For example, increasing concentrations of cytochrome c resulted in reduced sensitivity of cytochrome P450 reductase to DPI, ascribed to decreased availability of the reduced flavin to DPI. Decyl-plastoquinone (PQ) is an effective electron acceptor for NDH-2 (13), and therefore we examined the ability of PQ to protect pMMO from DPI inhibition. Increasing concentrations of PQ resulted in partial protection of pMMO activity from DPI inhibition (i.e., 88% inhibition in the absence of PQ, relative to 50% inhibition in the presence of 100 μM PQ) (Table 1).
According to our proposed electron transfer pathway (Fig. 1A) and the mechanism in Fig. 2, DPI is expected to inhibit pMMO turnover by inactivating NDH-2, thereby disrupting electron transfer from NADH to the monooxygenase. To further elucidate the role of DPI in blocking the electron transfer pathway (Fig. 1A), we examined its effect on hydroquinol-dependent pMMO activity. For this experiment we used detergent-solubilized pMMO, which is not active with NADH alone (40), but is active with exogenous hydroquinols or with NADH plus exogenous quinones and NDH-2 (13, 40).
As expected, the NADH-dependent activity of detergent-soluble pMMO was very sensitive to DPI. Only ≈5 μM DPI was required to inhibit 50% of the activity (Fig. 5A), which was similar to the amount required to inhibit membrane-bound pMMO activity (Fig. 4). Unexpectedly, the pMMO activity driven by exogenous decyl-plastoquinol (PQH2) was also inhibited by DPI (Fig. 5A). However, the PQH2-dependent pMMO activity was markedly less sensitive to DPI than the NADH-dependent activity. For example, 50% inhibition of the PQH2-dependent activity required ≈70 μM DPI, relative to ≈5 μM DPI for the NADH-dependent activity (Fig. 5A).
To determine the effect of DPI on pMMO activity in whole cells, we treated M. capsulatus cells with DPI in the presence of formate, washed the cells thoroughly, then assayed pMMO activity with either formate, formaldehyde, or duroquinol as the electron donor. DPI did inhibit whole-cell pMMO activity in a concentration-dependent manner (Fig. 5B). Furthermore, the inhibition was independent of the electron donor; the same extent of inhibition is observed with formate, formaldehyde, and duroquinol (Fig. 5B). The concentration of DPI required for 50% inhibition of whole-cell pMMO activity was similar to that needed for inhibition of the hydroquinol-driven activity of detergent-solubilized pMMO (Fig. 5) and much higher than the concentration required to inhibit the NADH-dependent activities of pMMO and NDH-2 in vitro. For example, 50% inhibition of whole-cell pMMO activity required ≈70 μM DPI (Fig. 5B), whereas the NADH-dependent in vitro activities required less than 5 μM DPI to reach 50% inhibition (Fig. 4).
To determine if the effect of DPI on whole-cell pMMO activity resulted from NDH-2 inhibition, we measured pMMO activity in cells treated with 0, 50, or 500 μM DPI, lysed the cells, and isolated membrane fractions to measure NADH-dependent NDH-2 and pMMO activities in vitro (Table 2). Most (83%) of the whole-cell pMMO activity remained after treatment with 50 μM DPI, whereas only 27% of NDH-2 activity was recovered in the membrane fraction, relative to an untreated control (Table 2). The percentage of NADH-driven pMMO activity recovered in the membrane fraction (16%) was comparable to the recovered NDH-2 activity (27%), rather than the whole-cell pMMO activity (83%) (Table 2). Similar trends were observed for whole cells treated with 500 μM DPI; 28% of whole-cell pMMO activity remained, whereas only 4% and 2% of NDH-2 and pMMO activity, respectively, remained in the membrane fraction (Table 2).
TABLE 2.
NDH-2 and pMMO activity after DPI treatment of M. capsulatus cellsa
| Incubation conditions | Mean sp act (nmol min−1 mg−1) ± SD (% of control)
|
||
|---|---|---|---|
| Formate-dependent pMMO | NADH-dependent pMMO | NDH | |
| 0 μM DPI, 5 mM formate | 185.9 ± 8.4 (100) | 201.5 ± 21.9 (100) | 583.8 ± 84.1 (100) |
| 50 μM DPI, 5 mM formate | 154.3 ± 27.1 (83) | 32.4 ± 3.2 (16.2) | 158.1 ± 48.7 (27.1) |
| 500 μM DPI, 5 mM formate | 52.6 ± 18.1 (28.3) | 4.3 ± 1.2 (2.1) | 24.6 ± 6.3 (4.2) |
| 50 μM DPI, no formate | 155.6 ± 12.7 (83.7) | 152.3 ± 11.0 (75.6) | 517.7 ± 34.3 (88.7) |
Cells were incubated for 10 min at 45°C under the conditions described and then washed three times with 50 mM PIPES (pH 7.2). A portion of each sample was saved for in vivo pMMO assays, and the remainder was lysed and the membranes were isolated and assayed for in vitro NADH-driven pMMO and NDH activities. Values reported are the mean of 8 to 14 measurements. Numbers in parentheses are the activities expressed as a percentage of that of the 0 μM DPI control.
To ensure that the effect of DPI on the membrane fraction was not enhanced by cell lysis fostering access of adventitiously bound DPI to its target sites, the cells were washed extensively prior to lysis. In addition, a control sample was prepared by incubating cells with 50 μM DPI in the absence of formate. The NDH-2 and pMMO activities in membranes from this control were four- to fivefold greater than in the sample treated with 50 μM DPI and formate and only slightly lower than that of the untreated control (Table 2). Thus, the difference between whole-cell and membrane activities is not due to the presence of adventitious DPI during lysis.
DPI inhibition of AMO.
DPI also inhibited NH3-dependent oxygen uptake and ethylene oxidation catalyzed by AMO in whole cells of N. europaea, with 50% inhibition observed at ≈100 μM DPI (Fig. 6). In these experiments, N. europaea cells were incubated with DPI and then washed thoroughly to remove unreacted DPI before enzyme activity was measured. The extensive washing did not restore AMO activity (Fig. 6), suggesting that the inhibition was irreversible. NH3-dependent O2 uptake by AMO in cell-free lysates was completely inhibited by 50 μM DPI, but detailed characterization was prevented by the instability of AMO in vitro.
FIG. 6.
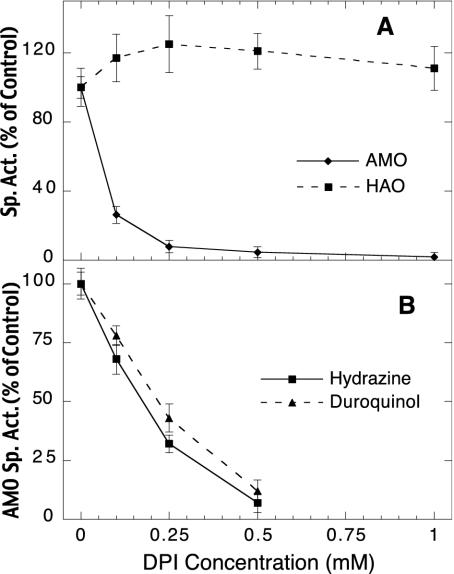
Effect of DPI on HAO and AMO activities in whole cells of N. europaea. Cells were incubated with DPI for 5 min at room temperature, washed five times, and assayed for AMO and HAO activity. (A) AMO activity was measured as the rate of oxygen uptake in the presence of 3.75 mM NH4+ and is expressed as a percentage of the 0 μM DPI control activity (1.05 μmol min−1 mg−1). HAO activity was measured after adding 0.6 mM allylthiourea to inhibit AMO, followed by 1.25 mM NH2OH. Activity is expressed as a percentage of the 0 μM DPI control (256 nmol min−1 mg−1). (B) AMO activity of the DPI-treated cells was measured as the rate of ethylene epoxidation with either 500 μM hydrazine or 500 μM duroquinol as the reductant. Activities are expressed as a percentage of the 0 μM DPI control sample (348 and 165 nmol min−1 mg−1 for the hydrazine-driven and duroquinol-driven controls, respectively). Each data point is reported as the mean value ± standard deviation of four measurements.
As discussed above, ammonia oxidation in N. europaea is dependent on HAO to supply reducing equivalents from the oxidation of hydroxylamine (3). Hydroxylamine-dependent O2 uptake was unaffected by DPI (Fig. 6A), indicating that HAO and the electron transfer pathway from HAO to the terminal oxidase were not disrupted by DPI. DPI also inhibited both hydrazine- and duroquinol-driven ethylene oxidation activities of AMO in whole cells (Fig. 6B). Furthermore, nearly identical inhibition profiles were observed for these two reductants, with 50% inhibition at ≈200 μM DPI (Fig. 6B). This is similar to the concentration required for 50% inhibition of ammonia-dependent O2 uptake (Fig. 6A), as well as pMMO activity in whole cells (≈70 μM, Fig. 5B), and at least an order of magnitude greater than required for inhibition of NADH-dependent activities of NDH-2 and pMMO in membrane fractions (less than 5 μM, Fig. 4).
In view of the much broader substrate range of AMO relative to pMMO (24, 27, 29), we considered the possibility that hydroxylation of DPI caused the inactivation of AMO, as is the case for certain alkynes, cyclopropanes, and aniline derivatives (30). Inactivation by these mechanism-based inhibitors requires turnover of AMO and is thereby prevented by anaerobic conditions or the presence of alternative substrates that compete for the hydroxylation site of AMO (23, 26, 30). We therefore investigated whether the presence of hydrocarbon substrates or removal of oxygen could similarly protect AMO from DPI. Control samples were incubated in buffer alone, whereas experimental samples included either DPI or DPI plus the hydrocarbon substrate. All samples were washed extensively prior to measuring ethylene oxidation activity of AMO.
The AMO substrates methane, ethylene, toluene, and phenol had no protective effect; nearly identical levels of AMO activity were recovered after incubation with DPI regardless of the presence of these hydrocarbons (Table 3). Incubation of N. europaea cells with DPI under anaerobic conditions also failed to protect AMO from inhibition. In fact, removal of oxygen resulted in recovery of ≈50% less AMO activity relative to incubation in the presence of oxygen (Table 3). The increased sensitivity to DPI under anaerobic conditions could be due to enhanced reduction of the DPI binding site without O2 to serve as the terminal electron acceptor. This explanation is consistent with similar enhancement of DPI inhibition observed in the presence of the electron donor hydrazine (Table 3), suggesting that reduction is a precursor to DPI binding, as expected from its proposed mechanism of inactivation (Fig. 2).
TABLE 3.
Protection of AMO by allylthiourea but not by AMO substrates or anaerobic conditionsa
| Protectant | Mean AMO activity with DPI only (% of control) ± SD | Mean AMO activity with DPI and protectant (% of control) ± SD |
|---|---|---|
| Allylthiorrea (1 mM) | 47.6 ± 5.7 | 90.4 ± 6.8 |
| Methane (3 ml) | 27.0 ± 8.4 | 22.1 ± 7.4 |
| Ethane (3 ml) | 27.2 ± 6.2 | 23.2 ± 5.1 |
| Phenol (1 mM) | 23.3 ± 3.9 | 26.5 ± 4.9 |
| Toluene (1 mM) | 32.9 ± 5 | 26.7 ± 6.6 |
| Anaerobic | 36.8 ± 7.1 | 19.2 ± 9.3 |
| Hydrazineb (500 μM) | 77.4 ± 5.9 | 43.3 ± 4.8 |
For each protectant, three samples of N. europaea cells were prepared: one with 200 μM DPI, one with 200 μM DPI and the indicated protectant, and a control with no DPI and no protectant. These samples were incubated at room temperature for 5 min, washed four times with 50 mM phosphate (pH 8), and assayed for AMO activity by measuring the rate of ethylene oxidation as described in the text. Activities are given relative to the activity of the control sample and represent the mean of three measurements.
These samples contained 100 μM DPI rather than 200 μM as in all the other samples.
The only agent found to protect AMO from DPI inhibition was allylthiourea, which inhibits AMO by chelating copper (21). A similar chelator, thiourea, is known to protect AMO from mechanism-based inactivation by alkynes, anilines, and cyclopropanes (30). We found that 90% of AMO activity was recovered after incubation with 200 μM DPI in the presence of 1 mM allylthiourea, whereas only 48% remained after DPI treatment in the absence of allylthiourea (Table 3). In this experiment, two control samples were prepared, with no allylthiourea and no DPI or with 1 mM allylthiourea and no DPI. The experimental samples contained no allylthiourea and 200 μM DPI or 1 mM allylthiourea and 200 μM DPI. The activities reported in Table 3 for samples with DPI alone are relative to the control with no DPI or allylthiourea. Similarly, the activities of the samples incubated with both allylthiourea and DPI are reported relative to the control treated with allylthiourea alone (Table 3). The buffer used to wash these samples contained 50 μM CuSO4 to reconstitute AMO activity lost due to copper chelation by allylthiourea. In this way we determined that the loss of activity due to incubation with allylthiourea alone is almost fully reversible. The allylthiourea-treated sample retained 93% of the AMO activity in an untreated control.
DISCUSSION
Inactivation of NDH-2 by DPI and its effect on pMMO activity.
The substrate oxidation mechanism of pMMO and AMO remains poorly characterized due to their low and unstable activity in vitro (27, 31, 51). The lack of robust activity in vitro may arise from the use of nonphysiological reducing agents that are unable to efficiently reduce the monooxygenases. Hence, a better understanding of the physiological mechanism for reduction of pMMO and AMO may lead to increased stability and activity in vitro and facilitate characterization of these novel enzymes.
In previous publications we proposed the electron transfer pathway to pMMO that is illustrated by the solid lines in Fig. 1A. This proposal was based on the observation that pMMO activity could be driven by either exogenous hydroquinols or exogenous quinones plus NADH and NDH-2 under conditions in vitro where NADH alone is ineffective as a reductant (13, 40). This proposal could be tested by determining if inhibition of NDH-2 prevents turnover of pMMO. However, there are no general inhibitors of NDH-2 enzymes; flavone inhibits some NDH-2 enzymes but is ineffective against the NDH-2 from M. capsulatus (13).
In the present study we demonstrated that DPI is a potent, irreversible inhibitor of purified M. capsulatus NDH-2: >97% inhibition was observed in the presence of 10 μM DPI (Fig. 3, inset). DPI also irreversibly inhibited NDH-2 activity in isolated membrane fractions. This inhibition required reduction of NDH-2, since DPI had little effect on enzyme activity in the absence of NADH (Fig. 3). Thus, inhibition of NDH-2 by DPI has the characteristics one would expect from its typical mechanism of action on flavoproteins (Fig. 2) (10, 35, 43), in that inhibition was irreversible and required reduction by NADH (Fig. 3 and 4). We conclude that DPI reacts only with the reduced form of NDH-2, resulting in covalent modification of its flavin cofactor and inactivation of its normal redox cycle.
Having shown that DPI will inactivate NDH-2, we investigated whether NADH-coupled pMMO activity is also inhibited by DPI. We found that titration of membrane fractions with DPI in the presence of NADH resulted in similar inhibition profiles for NADH-dependent pMMO and NDH-2 activities (Fig. 4). Furthermore, the inhibitory effect of DPI on pMMO activity was irreversible and dependent on the presence of NADH, consistent with inactivation of NDH-2 as the cause of pMMO inhibition. Protection of pMMO activity by PQ (Table 1) is also consistent with inhibition caused by inactivation of NDH-2. PQ is an efficient electron acceptor for NDH-2 (13), so increased concentrations of PQ would decrease the steady-state concentration of reduced NDH-2, providing less opportunity for reaction with DPI and therefore less disruption of electron flow to pMMO. A similar result was observed for DPI inhibition of cytochrome P450 reductase (43), whereby increased concentrations of the electron acceptor (cytochrome c) resulted in decreased sensitivity to DPI. We therefore conclude that electron transfer from NADH to pMMO in vitro requires NDH-2 and that DPI inhibits pMMO by blocking this pathway (Fig. 1A).
DPI inhibition of pMMO and AMO that is independent of NDH-2.
We have proposed that NADH, NDH-2, and the quinone pool convey reducing equivalents from formaldehyde and formate to pMMO in vivo (solid lines in Fig. 1A) (13, 40). On this basis, we expected DPI to inhibit formate- or formaldehyde-driven pMMO activity in whole cells at a concentration similar to that observed for inhibition of NADH-driven activity in vitro (≈10 μM). We found that formate- and formaldehyde-driven pMMO activities were both inhibited by DPI (and with identical sensitivity), but these activities were less sensitive than NADH-driven in vitro activity by at least an order of magnitude (cf. Fig. 4 and 5B). The obstacle of crossing the cell membrane might account for some of the reduced sensitivity of pMMO in whole cells to DPI. However, treatment of whole cells with 50 μM DPI caused inactivation of 73% of the NDH-2 activity but only 17% of the pMMO activity (Table 2), indicating that DPI can readily interact with and substantially inhibit NDH-2 in whole cells without significantly affecting pMMO activity.
It is possible that the turnover rate of NDH-2 is much greater than that of pMMO in vivo, such that NDH-2 activity remaining after DPI treatment was sufficient to support the relatively high level of whole-cell pMMO activity observed (Table 2). However, if the residual NDH-2 activity is sufficient to support significant pMMO activity in vivo, it should support similar levels of pMMO activity in vitro. In fact, the NADH-driven pMMO activity recovered from DPI-treated cells was ≈5-fold lower than pMMO activity in vivo (Table 2). Furthermore, the percentage of NADH-driven pMMO activity was comparable to the percentage of recovered NDH-2 activity rather than the whole-cell pMMO activity (Table 2). Thus, it appears that electron transfer from formate to pMMO can substantially continue in the presence of DPI concentrations sufficient to inactivate NDH-2. This result suggests the existence of an additional electron transfer pathway from formate to pMMO that is independent of NDH-2 but also contains a DPI-sensitive redox site (dashed lines in Fig. 1A). This second DPI-binding site has a lower affinity for the inhibitor than NDH-2, since ≈15-fold more DPI is required to inhibit formate-driven pMMO activity relative to NDH-2 activity (Fig. 5B and Table 2).
The redox cofactors and proteins that constitute this additional electron transfer pathway from formate to pMMO remain unknown at present. Both pathways illustrated in Fig. 1A appear to operate simultaneously in vivo, since treatment of whole cells with formate and DPI results in inhibition of NDH-2-dependent as well as NDH-2-independent pathways (Table 2). The NDH-2-independent pathway could consist of a single DPI-sensitive protein that transfers electrons from formate directly to pMMO. A pyrroloquinoline quinone (PQQ)-containing formaldehyde dehydrogenase was recently isolated from M. capsulatus membranes that appears to transfer reducing equivalents specifically from formaldehyde to the cytochrome bc1 complex (50). It was suggested that the reduced cytochrome bc1 complex subsequently transfers these reducing equivalents to pMMO (50). This proposed electron transfer chain could be responsible for the NDH-2 independent DPI inhibition of pMMO, since both PQQ and low-potential b-hemes are reportedly sensitive to DPI (6, 16). However, the response of whole-cell pMMO activity to DPI was independent of the source of reducing equivalents (Fig. 5B), suggesting that a single DPI-binding site blocks electron transfer to pMMO from formate, formaldehyde, and duroquinol, and that this site is located downstream of the quinone pool. The PQQ-containing dehydrogenase is not likely to be the DPI-binding site, since it is specific for formaldehyde (50) and therefore could not be responsible for inhibition of formate- or duroquinol-driven pMMO activity. Similarly, binding of DPI to the cytochrome bc1 complex could account for inhibition of pMMO only if it mediates electron transfer from formate, formaldehyde, and duroquinol. This seems unlikely, since several laboratories have reported that duroquinol supports the activity of pure pMMO in the absence of any cytochrome bc1 (4, 11, 31, 51).
It was recently reported that maximal activity of purified pMMO was observed with NADH, NDH-2, and duroquinol (11), suggesting that no other proteins are required to mediate electron transfer from hydroquinols to pMMO. Consistent with this proposal is DPI inhibition of the PQH2-driven activity of detergent-solubilized pMMO (Fig. 5A). Significantly, the PQH2-driven activity is ≈15-fold less sensitive to DPI than the NADH-driven activity (Fig. 5). Given their similar response to DPI, we propose that a single DPI-binding site is responsible for inhibiting formaldehyde-, formate-, and duroquinol-driven pMMO activity in vivo as well as plastoquinol-driven activity in vitro. This implies that the NDH-2-independent pathway includes the quinone pool and that binding of DPI to this relatively low-affinity site blocks electron transfer from hydroquinols to pMMO (Fig. 1A), perhaps within the pMMO complex itself (see below). In contrast, NDH-2 activity is essential for NADH-driven pMMO activity, and the relatively greater sensitivity of this activity is due to the high affinity of NDH-2 for DPI (Fig. 3).
DPI also inhibited AMO activity in cells of N. europaea (Fig. 6). This inhibition appeared to be irreversible, since extensive washing of the cells after incubation with DPI did not restore activity. Mechanism-based inhibition resulting from hydroxylation of DPI at the AMO active site does not appear to be responsible for the effect of DPI on AMO activity (Table 3). In addition, it is unlikely that inactivation of an NDH-2 enzyme by DPI is responsible for inhibition of AMO activity, since the electron transfer pathway from HAO to AMO in N. europaea is thought to be independent of NADH (the redox potential of the NH2OH/NO2− couple is ≈300 mV higher than that of NADH/NAD+) (8, 48). However, it is possible that an enzyme similar to NDH-2 mediates reduction of the quinone pool (and ultimately AMO) in N. europaea, with an electron donor other than NADH (a reduced cytochrome, for example). A search of the N. europaea genome revealed a single gene with sequence homology (26% identity, 41% similarity) to the NDH-2 of Escherichia coli (7), but a knockout mutation of this gene had no effect on AMO activity or inhibition by DPI (data not shown). We propose instead that DPI disrupts electron transfer from the quinone pool to AMO in a manner analogous to that suggested above for the NDH-2-independent inhibition of pMMO (Fig. 1B).
DPI appears to specifically inhibit AMO activity, having no effect on hydroxylamine-dependent oxygen uptake by N. europaea cells (Fig. 6A). Therefore, neither HAO turnover nor the electron transfer pathway from HAO to the terminal oxidase is affected by DPI. If we assume that the quinone pool is a common source of reducing equivalents for both AMO and the terminal oxidase (as suggested by Hooper and coworkers [48] and illustrated in Fig. 1B), then the specificity of DPI inhibition of AMO is consistent with disruption of electron flow from reduced quinones to the monooxygenase. Furthermore, identical levels of AMO inhibition by DPI were observed with either hydrazine or duroquinol as the reductant (Fig. 6B). HAO and the quinone pool are intermediates when hydrazine donates electrons to AMO and the terminal oxidase (3), whereas duroquinol is thought to directly reduce AMO (or perhaps the endogenous quinone pool) (39, 48). DPI could disrupt electron transfer to AMO from hydrazine and duroquinol by reacting with redox components unique to each reductant. However, the identical sensitivity to DPI is consistent with disruption of electron flow at a point common to both reductants, i.e., between the quinone pool and AMO (Fig. 1B).
The precise identity of the DPI- binding site in N. europaea (and by analogy the low-affinity DPI-binding site in M. capsulatus) remains unknown. However, if hydroquinols react directly with AMO and pMMO, as has been proposed (13, 40, 48), then DPI must inhibit electron transfer between the hydroquinol-binding site and the hydroxylation site, i.e., within the monooxygenase itself. Although there is no evidence for a flavin-binding site in either AMO or pMMO, DPI has been reported to react with redox cofactors other than flavins (6, 16). Protection of AMO by the copper chelator allylthiourea (Table 3) could be significant in this regard. Specific chelation of copper has long been known to completely inhibit AMO (5, 21) and pMMO (14, 42) in whole cells and membrane fractions, but the precise role and location of the labile copper was unknown. More recently, several laboratories have reported that purified pMMO is also inhibited by chelation of copper (4, 31, 51), suggesting that this labile copper is an obligatory cofactor of the catalytic complex of pMMO and, by homology, AMO as well. Since removal of this labile copper protects AMO from inhibition by DPI, we tentatively suggest that it is required for electron transfer from the physiological electron donor (presumably hydroquinol) to the hydroxylation site. Future investigations into the mechanism of DPI inhibition of AMO and pMMO could provide significant information regarding the redox chemistry of these novel monooxygenases.
Acknowledgments
This work was supported by grant DE-FG03-97ER20266 from the U.S. Department of Energy (to D.J.A.). Sabbatical support was provided to A.K.S. from the West Virginia University Faculty Senate and the office of the Office of the Associate Dean for Research of the WVU School of Medicine.
REFERENCES
- 1.Anthony, C. 1986. Bacterial oxidation of methane and methanol. Adv. Microb. Physiol. 27:113-210. [DOI] [PubMed] [Google Scholar]
- 2.Anthony, C. 1982. The biochemistry of methanotrophs. Academic Press, London, England.
- 3.Arp, D. J., L. A. Sayavedra-Soto, and N. G. Hommes. 2002. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch. Microbiol. 178:250-255. [DOI] [PubMed] [Google Scholar]
- 4.Basu, P., B. Katterle, K. K. Andersson, and H. Dalton. 2003. The membrane-associated form of methane mono-oxygenase from Methylococcus capsulatus (Bath) is a copper/iron protein. Biochem. J. 369:417-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard, C., and R. Knowles. 1989. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 53:68-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop, A., M. A. Paz, P. M. Gallop, and M. L. Karnovsky. 1995. Inhibition of redox cycling of methoxatin (PQQ), and of superoxide release by phagocytic white cells. Free Radic. Biol. Med. 18:617-620. [DOI] [PubMed] [Google Scholar]
- 7.Bjorklof, K., V. Zickermann, and M. Finel. 2000. Purification of the 45 kDa, membrane bound NADH dehydrogenase of Escherichia coli (NDH-2) and analysis of its interaction with ubiquinone analogues. FEBS Lett. 467:105-110. [DOI] [PubMed] [Google Scholar]
- 8.Bock, E., H.-P. Koops, H. Harms, and B. Ahlers. 1991. The biochemistry of nitrifying organisms, p. 171-200. In J. M. Shively and L. L. Barton (ed.), Variations in autotrophic life. Academic Press Limited, San Diego, Calif.
- 9.Burrows, K. J., A. Cornish, D. Scott, and I. J. Higgins. 1984. Substrate specificities of the soluble and particulate methane monooxygenase of Methylosinus trichosporium OB3b. J. Gen. Microbiol. 130:3327-3333. [Google Scholar]
- 10.Chakraborty, S., and V. Massey. 2002. Reaction of reduced flavins and flavoproteins with diphenyliodonium chloride. J. Biol. Chem. 277:41507-41516. [DOI] [PubMed] [Google Scholar]
- 11.Choi, D. W., R. C. Kunz, E. S. Boyd, J. D. Semrau, W. E. Antholine, J. I. Han, J. A. Zahn, J. M. Boyd, A. M. de la Mora, and A. A. DiSpirito. 2003. The membrane-associated methane monooxygenase (pMMO) and pMMO-NADH:quinone oxidoreductase complex from Methylococcus capsulatus Bath. J. Bacteriol. 185:5755-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colby, J., D. I. Stirling, and H. Dalton. 1977. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem. J. 165:395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook, S. A., and A. K. Shiemke. 2002. Evidence that a type-2 NADH:quinone oxidoreductase mediates electron transfer to particulate methane monooxygenase in Methylococcus capsulatus. Arch. Biochem. Biophys. 398:32-40. [DOI] [PubMed] [Google Scholar]
- 14.Cook, S. A., and A. K. Shiemke. 1996. Evidence that copper is a required cofactor for the membrane-bound form of methane monooxygenase. J. Inorg. Biochem. 63:273-284. [Google Scholar]
- 15.Coves, J., C. Lebrun, G. Gervasi, P. Dalbon, and M. Fontecave. 1999. Overexpression of the FAD-binding domain of the sulphite reductase flavoprotein component from Escherichia coli and its inhibition by iodonium diphenyl chloride. Biochem. J. 342:465-472. [PMC free article] [PubMed] [Google Scholar]
- 16.Doussiere, J., J. Gaillard, and P. V. Vignais. 1999. The heme component of the neutrophil NADPH oxidase complex is a target for aryliodonium compounds. Biochemistry 38:3694-3703. [DOI] [PubMed] [Google Scholar]
- 17.Ensign, S. A., M. R. Hyman, and D. J. Arp. 1993. In vitro activation of ammonia monooxygenase from Nitrosomonas europaea by copper. J. Bacteriol. 175:1971-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamamura, N., C. M. Yeager, and D. J. Arp. 2001. Two distinct monooxygenases for alkane oxidation in Nocardioides sp. strain CF8. Appl. Environ. Microbiol. 67:4992-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson, R. S., and T. E. Hanson. 1996. Methanotrophic bacteria. Microbiol. Rev. 60:439-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes, A. J., A. Costello, M. E. Lidstrom, and J. C. Murrell. 1995. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol. Lett. 132:203-208. [DOI] [PubMed] [Google Scholar]
- 21.Hooper, A. B., and K. R. Terry. 1973. Specific inhibitors of ammonia oxidation in Nitrosomonas. J. Bacteriol. 115:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper, A. B., T. Vannelli, D. J. Bergmann, and D. M. Arciero. 1997. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie Van Leeuwenhoek 71:59-67. [DOI] [PubMed] [Google Scholar]
- 23.Hyman, M. R., and D. J. Arp. 1988. Acetylene inhibition of metalloenzymes. Anal. Biochem. 173:207-220. [DOI] [PubMed] [Google Scholar]
- 24.Hyman, M. R., I. B. Murton, and D. J. Arp. 1988. Interaction of ammonia monooxygenase from Nitrosomonas europaea with alkanes, alkenes, and alkynes. Appl. Environ. Microbiol. 54:3187-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyman, M. R., and P. M. Wood. 1984. Ethylene oxidation by Nitrosomonas europaea. Arch. Microbiol. 137:155-158. [Google Scholar]
- 26.Hyman, M. R., and P. M. Wood. 1985. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem. J. 227:719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juliette, L. Y., M. R. Hyman, and D. J. Arp. 1993. Mechanism-based inactivation of of ammonia monooxygenase in Nitrosomonas europaea by allylsulfide. Appl. Environ. Microbiol. 59:3728-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juliette, L. Y., M. R. Hyman, and D. J. Arp. 1995. Roles of bovine serum albumin and copper in the assay and stability of ammonia monooxygenase activity in vitro. J. Bacteriol. 177:4908-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keener, W. K., and D. J. Arp. 1994. Transformations of aromatic compounds by Nitrosomonas europaea. Appl. Environ. Microbiol. 60:1914-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keener, W. K., S. A. Russell, and D. J. Arp. 1998. Kinetic characterization of the inactivation of ammonia monooxygenase in Nitrosomonas europaea by alkyne, aniline and cyclopropane derivatives. Biochim. Biophys. Acta 1388:373-385. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman, R. L., D. B. Shrestha, P. E. Doan, B. M. Hoffman, T. L. Stemmler, and A. C. Rosenzweig. 2003. Purified particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a dimer with both mononuclear copper and a copper-containing cluster. Proc. Natl. Acad. Sci. 100:3820-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipscomb, J. D. 1994. Biochemistry of the soluble methane monooxygenase. Annu. Rev. Microbiol. 48:371-399. [DOI] [PubMed] [Google Scholar]
- 33.Murrell, J. C., I. R. McDonald, and B. Gilbert. 2000. Regulation of expression of methane monooxygenases by copper ions. Trends Microbiol. 8:221-225. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen, H. H., S. J. Elliott, J. H. Yip, and S. I. Chan. 1998. The particulate methane monooxygenase from Methylococcus capsulatus (Bath) is a novel copper-containing three-subunit enzyme. Isolation and characterization. J. Biol. Chem. 273:7957-7966. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell, B. V., D. G. Tew, O. T. Jones, and P. J. England. 1993. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 290:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell, V. B., G. C. Smith, and O. T. Jones. 1994. Involvement of phenyl radicals in iodonium inhibition of flavoenzymes. Mol. Pharmacol. 46:778-785. [PubMed] [Google Scholar]
- 37.Prior, S. D., and H. Dalton. 1985. Acetylene as a suicide substrate and active site probe for methane monooxygenase from Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 29:105-109. [Google Scholar]
- 38.Rasche, M. E., R. E. Hicks, M. R. Hyman, and D. J. Arp. 1990. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J. Bacteriol. 172:5368-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shears, J. H., and P. M. Wood. 1986. Tri- and tetramethylhydroquinone as electron donors for ammonia monooxygenase in whole cells. FEMS Microbiol. Lett. 33:281-284. [Google Scholar]
- 40.Shiemke, A. K., S. A. Cook, T. Miley, and P. Singleton. 1995. Detergent solubilization of membrane-bound methane monooxygenase requires plastoquinol analogs as electron donors. Arch. Biochem. Biophys. 321:421-428. [DOI] [PubMed] [Google Scholar]
- 41.Smith, D. D., and H. Dalton. 1989. Solubilisation of methane monooxygenase from Methylococcus capsulatus (Bath). Eur. J. Biochem. 182:667-671. [DOI] [PubMed] [Google Scholar]
- 42.Stanley, S. H., S. D. Prior, D. J. Leak, and H. Dalton. 1983. Copper stress underlies the fundamental change in intracellular location of methane monooxygenase in methane oxidizing organisms. Biotechnol. Lett. 5:487-492. [Google Scholar]
- 43.Tew, D. G. 1993. Inhibition of cytochrome P450 reductase by the diphenyliodonium cation. Kinetic analysis and covalent modifications. Biochemistry 32:10209-10215. [DOI] [PubMed] [Google Scholar]
- 44.Truesdale, G. A., and A. L. Downing. 1954. Solubility of oxygen in water. Nature 173:1236. [Google Scholar]
- 45.Tsien, H. C., G. A. Brusseau, R. S. Hanson, and L. P. Wackett. 1989. Biodegradation of trichloroethylene by Methylosinus trichosporium OB3b. Appl. Environ. Microbiol. 55:3155-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wackett, L. P., G. A. Brusseau, S. R. Householder, and R. S. Hanson. 1989. Survey of microbial oxygenases: trichloroethylene degradation by propane-oxidizing bacteria. Appl. Environ. Microbiol. 55:2960-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallar, B. J., and J. D. Lipscomb. 1996. Dioxygen activation by enzymes containing binuclear non-heme iron clusters. Chem. Rev. 96:2625-2658. [DOI] [PubMed] [Google Scholar]
- 48.Whittaker, M., D. Bergmann, D. Arciero, and A. B. Hooper. 2000. Electron transfer during the oxidation of ammonia by the chemolithotrophic bacterium Nitrosomonas europaea. Biochim. Biophys. Acta 1459:346-355. [DOI] [PubMed] [Google Scholar]
- 49.Yu, S. S., K. H. Chen, M. Y. Tseng, Y. S. Wang, C. F. Tseng, Y. J. Chen, D. S. Huang, and S. I. Chan. 2003. Production of high-quality particulate methane monooxygenase in high yields from Methylococcus capsulatus (Bath) with a hollow-fiber membrane bioreactor. J. Bacteriol. 185:5915-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zahn, J. A., D. J. Bergmann, J. M. Boyd, R. C. Kunz, and A. A. DiSpirito. 2001. Membrane-associated quinoprotein formaldehyde dehydrogenase from Methylococcus capsulatus Bath. J. Bacteriol. 183:6832-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zahn, J. A., and A. A. DiSpirito. 1996. Membrane-associated methane monooxygenase from Methylococcus capsulatus (Bath). J. Bacteriol. 178:1018-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]