Abstract
Δ9-Tetrahydrocannabinol (THC), through its action on cannabinoid type-1 receptor (CB1R), is known to activate dopamine (DA) neurotransmission. Functional evidence of a direct antagonistic interaction between CB1R and DA D2-receptors (D2R) suggests that D2R may be an important target for the modulation of DA neurotransmission by THC. The current study evaluated, in rodents, the effects of chronic exposure to THC (1 mg/kg/day; 21 days) on D2R and D3R availabilities using the D2R-prefering antagonist and the D3R-preferring agonist radiotracers [18F]fallypride and [3H]-(+)-PHNO, respectively. At 24 h after the last THC dose, D2R and D3R densities were significantly increased in midbrain. In caudate/putamen (CPu), THC exposure was associated with increased densities of D2R with no change in D2R mRNA expression, whereas in nucleus accumbens (NAcc) both D3R binding and mRNA levels were upregulated. These receptor changes, which were completely reversed in CPu but only partially reversed in NAcc and midbrain at 1 week after THC cessation, correlated with an increased functionality of D2/3R in vivo, based on findings of increased locomotor suppressive effect of a presynaptic dose and enhanced locomotor activation produced by a postsynaptic dose of quinpirole. Concomitantly, the observations of a decreased gene expression of tyrosine hydroxylase in midbrain together with a blunted psychomotor response to amphetamine concurred to indicate a diminished presynaptic DA function following THC. These findings indicate that the early period following THC treatment cessation is associated with altered presynaptic D2/3R controlling DA synthesis and release in midbrain, with the concurrent development of postsynaptic D2/3R supersensitivity in NAcc and CPu. Such D2/3R neuroadaptations may contribute to the reinforcing and habit-forming properties of THC.
Keywords: Δ9-tetrahydrocannabinol, CB1 receptors, dopamine, D2 receptors, D3 receptors, addiction
INTRODUCTION
The main psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol (THC), produces a wide range of psychoactive effects through activation of central cannabinoid type-1 receptors (CB1R). CB1R are widely expressed throughout the brain (Mailleux and Vanderhaeghen, 1992), with a high expression in extended regions of the mesoaccumbens and nigrostriatal dopaminergic systems that are involved in reward, motivation, and locomotor processes. Although CB1R are found exclusively on glutamatergic and GABAergic axon terminals in midbrain (Matyas et al, 2006, 2008), they are localized both presynaptically, on GABAergic and glutamatergic terminals, and postsynaptically in somatodendritic profiles of medium spiny neurons in striatum (review in Fitzgerald et al, 2012). This distribution provides multiple opportunities for functional interactions between the endocannabinoid and ascending dopamine (DA) pathways, and growing evidence indicate that CB1R signaling directly or indirectly modulates DA neurotransmission through either postsynaptic or presynaptic mechanisms (Laviolette and Grace, 2006). It is well established that acute administration of THC activates the firing of DA neurons in the ventral tegmental area (VTA) and substantia nigra (SN; French et al, 1997), and enhances DA release in their respective DA terminal field, the nucleus accumbens (NAcc; Tanda et al, 1997) and caudate-putamen (CPu; Taylor et al, 1988). As with other drugs of abuse, it is believed that the reinforcing properties and abuse liability of THC are related to activation of the mesolimbic DA pathway (Lupica et al, 2004). The mechanisms through which CB1R influence DA neurotransmission remain incompletely understood, but likely involve a trans-synaptic modulation exerted by glutamatergic and GABAergic inputs onto mesoaccumbens and nigrostriatal DA neurons (review in Laviolette and Grace, 2006). Accumulating evidence also indicate the existence of a direct cross talk and modulation of signaling upon heterodimerization between CB1R and the DA D2-receptor (D2R) subtype (Kearn et al, 2005). Behavioral studies support a functional cross talk between the two receptor systems, as acute CB1R blockade potentiates the locomotor stimulant effect of D2R agonists (Giuffrida et al, 1999), whereas acute CB1R stimulation inhibits it (Beltramo et al, 2000). Further evidence indicating antagonistic CB1R/D2R interactions comes from data showing that acute CB1R stimulation reduces the affinity of D2R agonist binding in striatal membranes (Marcellino et al, 2008a). Contrasting with an inhibitory influence of acute CB1R stimulation on D2R signaling, the opposite effect emerged following chronic CB1R stimulation; that is, a potentiation of D2R psychomotor function (Moreno et al, 2005). Taken together, these data suggest that the DA D2R may be an important target for the modulation of DA function by cannabinoids and that this receptor may be involved in the cannabis-reinforcing effects. To date though, the impact of chronic CB1R agonist treatment on D2R is poorly understood and the only few studies performed have given equivocal results (Dalton and Zavitsanou, 2010; Safont et al, 2011; Sevy et al, 2008).
The present study examined the effects on D2/3R signaling of repeated exposure to low doses of THC that appear relevant to human recreational use of cannabis. D2/3R availabilities were evaluated in the rat brain using small-animal positron emission tomography (PET) imaging and [18F]fallypride, a D2R-preferring antagonist radiotracer for which selectivity is Circa 10-fold higher for D2R over D3R (Mukherjee et al, 1999). To investigate the THC effects on D3R, we used ex-vivo autoradiography and the D3R-preferring agonist radiotracer [3H]-(+)-PHNO that possesses more than 50-fold higher selectivity for D3R over D2R (Freedman et al, 1994; Rabiner et al, 2009). Thus, although both radiotracer bindings in D2R-rich regions such as the CPu reflect D2R availabilities, comparison of [3H]-(+)-PHNO and [18F]fallypride bindings in D3R-rich regions such as the midbrain and ventral pallidum (VP) allowed to study the differential effects of THC on D3R vs D2R (Rabiner et al, 2009). In addition, expression of the mRNAs encoding CB1R, D2R, D3R, and tyrosine hydroxylase (TH), the rate-limiting enzyme in DA biosynthesis, were measured to obtain information on whether changes induced by chronic THC dosing would be relatively selective for D2 receptors, or whether it would also influence the expression of other genes important to DA neurotransmission. To further assess the function of pre- and postsynaptic DA signaling, we also investigated the effect of chronic THC on the locomotor stimulant effect of amphetamine and quinpirole. Our data show that, concomitant to a CB1R downregulation, chronic THC elicits a supersensitivity of D2R and D3R in vivo, and induces opposed alterations in the behavioral response to high doses of quinpirole and amphetamine.
MATERIALS AND METHODS
Animals and Treatments
Male Sprague-Dawley rats weighing 250–275 g were used. THC (Sigma-Aldrich) was dissolved in 0.9% saline/ethanol/cremophor (18 : 1 : 1) and administered intraperitoneally (i.p.) at 1 mg/kg/day. Unless otherwise stated, treatment duration was 3 weeks and treatment effects were measured at 24 h after the last THC dose. The dose of THC was chosen based on previous studies, suggesting that at such a low dose, THC has positive reinforcing effects as it stimulates mesolimbic DA activity (Chen et al, 1990; Gessa et al, 1998), enhances brain stimulation reward (Lepore et al, 1996), and can also produce rewarding effects, when they do occur, in the place preference paradigm, whereas higher doses often result in conditioned place aversion (Ghozland et al, 2002; Valjent and Maldonado, 2000; but see also Le Foll et al, 2006).
Quinpirole hydrochloride (Tocris Biosciences) and D-amphetamine (AMPH; Sigma-Aldrich) were administered i.p. at 0.05 or 0.5 and at 2.5 mg/kg, respectively.
Positron Emission Tomography Imaging
Image acquisition
Rats were imaged with [18F]fallypride at 24 h after the last dose of a 3-weeks treatment with THC or vehicle. [18F]Fallypride was obtained from the Advanced Accelerator Applications (Saint-Genis-Pouilly, France). Images were acquired using the YAP-(S)PET tomograph (ISE, Italy). Rats were anaesthetized with 1.5% isoflurane, and 3D-mode emission acquisition was initiated upon i.v. bolus injection of 30 MBq [18F]fallypride (range 23–35 MBq) for 120 min. The average specific radioactivity at the time of injection was 166 GBq/μmol (range: 63–257 GBq/μmol). Sinograms were reconstructed using the expectation maximization algorithm with 20 iterations, which yields an image resolution of 1.9 mm full-width-at-half-maximum (Motta et al, 2002).
Immediately after completion of the PET acquisition, animals were killed by decapitation, and their brain immediately processed for ex-vivo autoradiography as described below.
Image analysis
PET images were processed using the software PMOD V3.2 (PMOD Technologies, Switzerland). Summation PET images were generated over the 10–120-min of dynamic data and manually coregistered to a magnetic resonance imaging (MRI) atlas of the rat brain (Schweinhardt et al, 2003). The resulting transformation was applied to the PET dynamic images, mapping all rat brains into the same reference space.
A region of interest (ROI) template was defined on the MRI atlas, using the rat brain atlas of Paxinos and Watson (1998) and previous guidelines described for analyzing rodent PET data (Dalley et al, 2007). The ROI template included four brain regions: the dorsolateral striatum (DST; wherein CPu is located), the ventral striatum (VST; wherein NAcc is located), the cerebellar cortex, and a ventral midbrain region including both SN and VTA (SN/VTA). ROIs consisted of fixed-size circular ROIs placed bilaterally over the DST and VST (2 mm in diameter), and SN/VTA (1.4 mm in diameter). To minimize partial voluming, ROIs were placed on the central planes on which the structures appeared. A single elliptical ROI (2 mm × 1 mm) was placed on cerebellar cortex, primarily over the central lobule in order to reduce spill-over from bone uptake of [18F]fluoride.
The ROI template was applied to the dynamic images to produce time-activity curves for the target-rich (DST) and reference region (cerebellar cortex), which were then used to generate parametric maps. Voxel-wise parametric maps of the binding potential (BPND) were calculated using the Logan graphical method (Logan et al, 1996). The ROI template was then applied to the individual parametric maps to extract regional BPND estimates. Both hemisphere data were averaged to give a single BPND for each region.
Ex vivo Autoradiography of [18F]Fallypride and [3H]-(+)-PHNO Binding
Rats were treated for either 1 or 3 weeks with THC or vehicle. At 24 h or 1 week after the last injection, rats were i.v. injected with 2.96 MBq of [3H]-(+)-PHNO (45 Ci/mmol; made by AstraZeneca, Wilmington, DE) and killed by decapitation at 60-min post-radiotracer injection.
Brains were cut into series of two adjacent sections (30 μm thick), taken at 150 μm interval along the caudo–rostral extension of the CPu, NAcc, VP, SN, and VTA. The first section was stained for acetylcholinesterase as previously described (Karnovsky and Roots, 1964) and used as histological reference. The second section was either exposed onto γ-sensitive phosphor imaging plates (Fuji BAS-IP MS2325) overnight to reveal [18F]fallypride binding or exposed onto 3H-Hyperfilm (Amersham) for 12-weeks to reveal [3H]-(+)-PHNO binding. Radioactive standard scales for 3H (3H-microscale; Amersham) and 18F were co-exposed along with the tissue sections onto each imaging plate or autoradiographic film to ensure that the radioactive measurement intensities were in the linear range. 18F standards were prepared by pipetting 5 μl drops of a set of 12 different concentrations of [18F]fallypride onto two 25 × 75 mm strips of thin layer chromatography plate (silica gel 60F254; Merck).
[18F]Fallypride autoradiograms were analyzed with the Fujifilm BAS-1800II phosphorimager using Aida Software V4.06 (Raytest Isotopenmessgeräte-GmbH). 3H-Hyperfilms bearing [3H]-(+)-PHNO binding were scanned and log-transformed into relative optical density units. ROIs were delineated on sections stained for acetylcholinesterase and transferred on adjacent autoradiographic sections of [18F]fallypride or [3H]-(+)-PHNO binding. Background film signal values were subtracted from the ROI signal and a mean radioactivity value was calculated for each ROI. For each radioligand, the densitometric measurements fell within the linear range of the standard curves generated from the 3H- or 18F-radioactive scale in all ROIs examined. Ex-vivo binding of both radioligands in target ROIs was quantified using the specific binding ratio (SBR), which is defined as SBR=(ROI−cerebellum)/cerebellum, and where cerebellar cortex is used as reference to estimate free and nonspecifically bound radiotracer.
Quantitative mRNA Estimation by Real-Time RT-PCR
Rats were treated for 3 weeks with THC or vehicle and killed by decapitation at 24 h after the last treatment dose, and their brain was cut serially into 300 μm-thick coronal sections. Tissue samples of the CPu, NAcc, VP, SN/VTA, and cortex were removed bilaterally from all sections they were visible on using a 1.5-mm-diameter stainless-steel puncher. SN and VTA were collected together as the two structures were not easily distinguishable on fresh tissue sections. Tissue punches and tissue remaining in sections, which was to be used as calibrator, were preserved at −80°C.
Total RNA was isolated from tissue punches with Accuzol (Labgene Scientific) and treated with Turbo DNAse (Ambion). RNA quality and yield were assessed by spectrophotometry. Reverse-transcriptions were performed with total RNA (1 μg) using a cDNA synthesis kit (Bioline) with random hexamer (130 ng) and oligos dT (25 ng). RT-PCR reactions were carried out in duplicate on Rotor-Gene (Corbett, Qiagen) according to the Kapa SYBR green protocol (Labgene Scientific). Thermal cycler conditions were 3 min at 95°C followed by 45 cycles of 5 s at 95°C, 20 s at 60°C, and 2 s at 72°C. Primers were evaluated using linear regression analysis. Primer characteristics are given in Table 1. Samples were quantified using the ΔΔCT method with efficiency correction (Pfaffl, 2001), using the calibrator tissue and glyceraldehyde-3-phosphate dehydrogenase and hypoxanthine-guanine phosphoribosyltransferase as references genes for normalization.
Table 1. Primer Sequences used for Semi-Quantitative Real-Time PCR.
| Gene | Gene bank | Primer sequences (5′–3′) | Amplicon size |
|---|---|---|---|
| HPRT | NM_012583.2 | Fwd GACCGGTTCTGTCATGTCG | 61 |
| Rev ACCTGGTTCATCATCACTAATCAC | |||
| GAPDH | XR_009170.1 | Fwd AGGTCAGTGTGAACTGATTTGGT | 61 |
| Rev GAGTAGAAGGCAGCCCTGGT | |||
| D2R | NM_012547 | Fwd AAGCGCCGAGTTACTGTCAT | 111 |
| Rev GGCAATGATACACTCATTCTGGT | |||
| D3R | NM_007877 | Fwd CAGACCTGGCTTCCCTCAG | 71 |
| Rev TGGCCCTTATTGAAAACTGC | |||
| CB1R | NM_012784 | Fwd GGACATGGAGTGCTTTATGATTC | 64 |
| Rev GAGGGACAGTACAGCGATGG | |||
| TH | NM_012740.3 | Fwd TCTCCCTGAGGGGTACAAAA | 75 |
| Rev GAATTTTGGCTTCAAATGTCTCA |
The table lists 19–24 bp forward (Fwd) and reverse (Rev) primer sequences for the appropriate gene, along with the expected PCR amplicon size for each primer sequence.
Effect of THC Exposure on the Locomotor Response to Amphetamine and Quinpirole
Motor activity was measured using the ActiMot System (TSE System) that contained four infrared-beam-operated open fields (48 × 48 × 40 cm). Each open field contained three sets of 16 beams: two sets in the x–y axis to measure horizontal activity and one set in the z-axis to monitor rears.
To determine the longitudinal effect of THC on locomotion, rats were tested at baseline (day 0) and at sequential times throughout the course of chronic THC or vehicle. For baseline data (day 0), animals were given an i.p. injection of saline and placed, 30 min later, into the open field for 60 min. The following day (day 1), rats received their first injection of THC or vehicle and returned to the open field 30 min later for 60 min. The same procedure was repeated on days 7, 14, and 21. Horizontal locomotor activity, expressed as distance travelled (in meters), was calculated either in 5 min blocks or for the entire 60-min testing period.
To determine the effect of THC on the psychomotor response to AMPH, the same animals were tested on days 20 and 22. On day 20, before their 20th treatment injection, animals were placed in the open field for 45 min of habituation. Rats then received a saline injection as a mild stressor and were monitored for an additional 90 min. On day 22 (24 h after the last THC dose), rats were allowed to habituate in the open field for 45 min before AMPH (2.5 mg/kg; i.p.), followed by an additional 90-min testing in the open field.
Two other cohorts of animals were used to determine the effect of chronic THC on the locomotor response to either a postsynaptic (0.5 mg/kg; Eilam and Szechtman, 1989) or a presynaptic (0.05 mg/kg; Eilam and Szechtman, 1989) dose of quinpirole. On day 22, rats in the first cohort were allowed to habituate in the open field for 45 min before quinpirole (0.5 mg/kg; i.p.), followed by an additional 90-min testing in the open field. Rats in the second cohort were tested on day 20 (before their 20th treatment injection) and on day 22 (24 h after the last THC dose). Animals were i.p. injected with saline and with 0.05 mg/kg quinpirole on days 20 and 22, respectively, and immediately placed in the open field for 120 min.
Horizontal locomotor activity was calculated either in 5 or 15 min blocks, or for the entire postinjection time period. Furthermore, the number of rears, the time spent in the center, and at the periphery of the open field were also measured.
To analyze the time-course effect of saline, AMPH, and quinpirole, locomotor activity data were expressed as the percentage of the 15-min epoch pre-injection value.
Statistical Analysis
Between-group differences in BPND, SBRs, and mRNA levels were analyzed with a mixed model repeated measures analysis of variance, with the region as the repeated measure and treatment as the between-subject factor. When significant differences were detected, post hoc comparisons were performed using unpaired two-tailed Student's t-tests. Behavioral data were analyzed using a two-way ANOVA (treatment × time) with a LSD post hoc test. Significance was defined as p<0.05.
RESULTS
Chronic THC Increases Postsynaptic D2/3R Binding
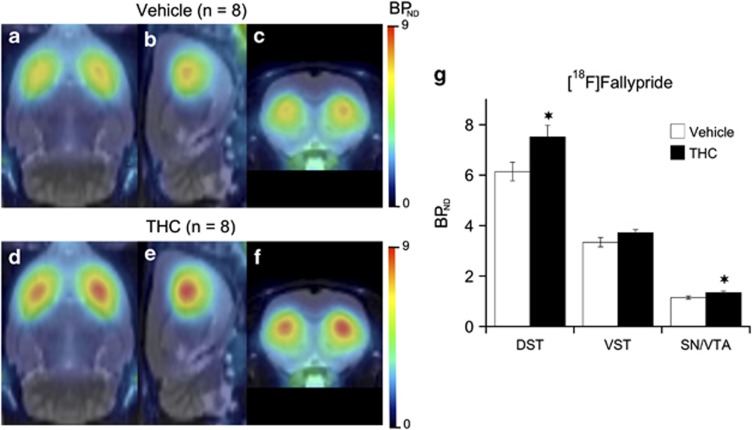
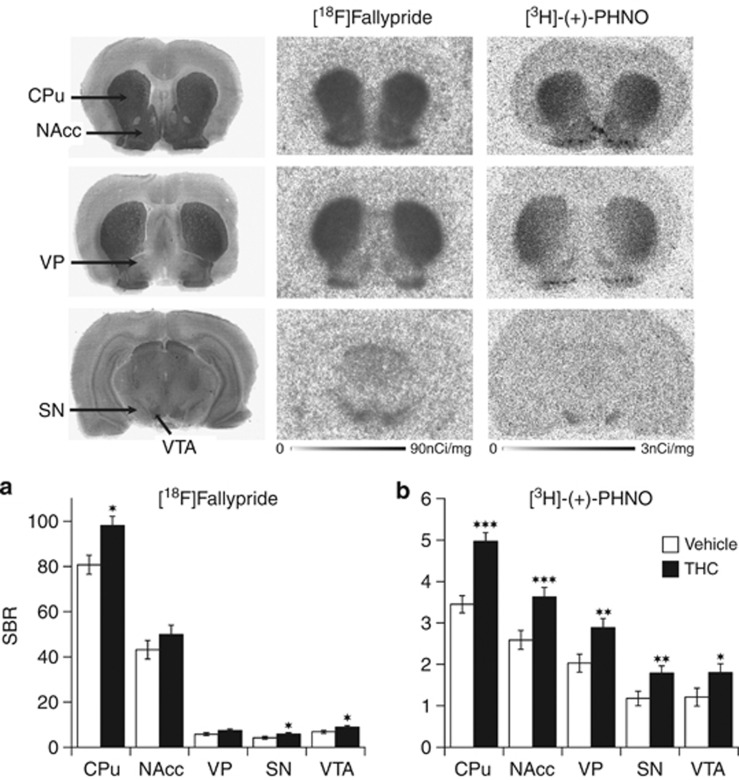
Mean parametric maps of [18F]fallypride BPND by treatment group are shown in Figure 1, projected upon the MRI rat atlas in horizontal, sagittal, and coronal planes. A repeated-measures ANOVA (mixed model) revealed a significant effect of a 3-weeks treatment with THC on [18F]fallypride BPND that was dependent on the brain regions (treatment: F(1,28)=5.4, p<0.05; treatment × region: F(2,28)=4.6, p<0.02). Post hoc testing indicated that, relative to vehicle, THC significantly increased [18F]fallypride BPND in DST (+22% p<0.05; Figure 1). BPND increase (+11%) in VST (wherein NAcc is located) did not reach statistical significance. To control for potential pitfalls in measuring THC effect on D2/3R availability with the relatively low resolution of microPET imaging (∼1.9 mm), animals were killed at the end of the microPET scanning to obtain D2/3R measurements with high spatial resolution (50 μm) ex vivo autoradiography. Ex-vivo autoradiography confirmed and extended the microPET data by showing that chronic THC produced significant elevations in [18F]fallypride binding in CPu (+21% p<0.03), with nonsignificant trends in the same direction in NAcc and VP (Figure 2a).
Figure 1.
Mean parametric maps of [18F]Fallypride BPND obtained in groups (n=8) of animals at 24 h following cessation of a 3-weeks treatment with vehicle (a–c) or THC (d–f; 1 mg/kg/day; 21 days). BPND parametric maps are projected upon the MRI rat atlas (gray scale) and are shown in horizontal (a and d), sagittal (b and e), and coronal (c and f) planes at the level of the DST. [18F]Fallypride BPND estimates in vehicle and THC-treated animals are given in g. Data are presented as means±SEM of eight animals per group in the dorsal striatum (DST), ventral striatum (VST), and ventral midbrain, a region including the substantia nigra and ventral tegmental area (SN/VTA). Significantly different from the vehicle-treated group at *p<0.05 using a mixed model repeated-measures ANOVA with a post hoc t-test.
Figure 2.
Brain regional D2/3R availability (SBR) as measured with ex vivo autoradiography binding of [18F]Fallypride (a) and [3H]-(+)-PHNO (b) at 24 h following cessation of a 3-weeks treatment with either vehicle or THC. Data are shown as mean±SEM (n=8–10) in the caudate/putamen (CPu), nucleus accumbens (NAcc), ventral pallidum (VP), substantia nigra (SN), and ventral tegmental area (VTA). Significantly different from the vehicle-treated group at *p<0.05, **p<0.01, and ***p<0.001 using a mixed model repeated-measures ANOVA with a post hoc t-test. The upper panel shows representative photographs of acetylcholinesterase (Ach) staining and autoradiograms of [18F]Fallypride and [3H]-(+)-PHNO bindings obtained in saline-treated rats in comparable coronal sections taken at 1.6 mm (top row), −0.26 mm (middle row), and −5.60 mm (bottom row) relative to bregma of a rat brain atlas (Paxinos and Watson, 1998).
After 3 weeks of THC treatment, [3H]-(+)-PHNO binding was characterized by widespread elevations across the different postsynaptic components of the mesolimbic and nigrostriatal DA systems when compared with vehicle control animals (treatment: F(1,64)=30.7, p<0.001; treatment × region: F(4,64)=2.9, p<0.05). In CPu, where [3H]-(+)-PHNO binding reflects binding to the high-affinity agonist-binding state (Ginovart et al, 2006), receptor concentration was significantly elevated by 44% (p<0.001; Figure 2b). Contrasting with the lack of change of [18F]fallypride binding in NAcc and VP, [3H]-(+)-PHNO binding was significantly increased in NAcc (+39% p<0.001) and VP (+42% p<0.01), two D3R-rich subregions of the basal ganglia associated with limbic function (Figure 2b).
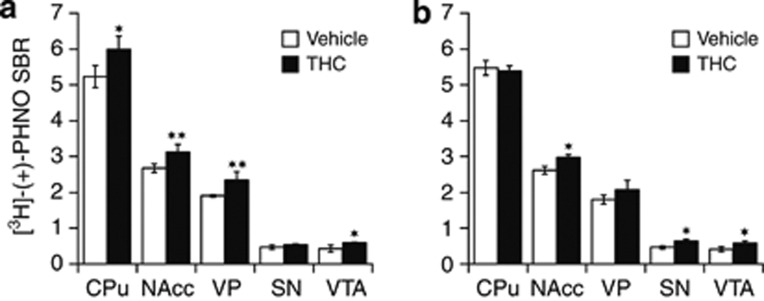
In subsequent studies, we aimed to determine whether these changes in [3H]-(+)-PHNO binding observed at 24 h after cessation of a 3-weeks treatment with THC were readily measurable with a shorter duration of drug exposure and with a longer period of drug discontinuation. To this end, rats were treated with THC or its vehicle for either 1 or 3 weeks, and ex vivo [3H]-(+)-PHNO binding measured at 24 h and at 1 week of abstinence, respectively. At 24 h after cessation of a 1-week treatment with THC (Figure 3a), there was a significant main effect of treatment (F(1,24)=11.4, p<0.015) on [3H]-(+)-PHNO binding and a significant interaction of treatment with regions (F(4,24)=2.8, p<0.05). Significant elevations of [3H]-(+)-PHNO binding were evident in CPu (+15% p<0.05) as well as in NAcc (+17% p<0.01) and VP (+23% p<0.01). At 1 week after cessation of a 3-weeks treatment with THC (Figure 3b), there was no main effect of treatment on [3H]-(+)-PHNO binding (F(1,24)=0.03, p>0.05), but a significant treatment-by-region interaction (F(4,24)=4.51, p<0.01) still existed. Post hoc analysis determined that the concentrations of [3H]-(+)-PHNO binding sites were back to control values in CPu, showed only a trend toward higher values in VP, but remained significantly elevated in NAcc (+14% p<0.05).
Figure 3.
Ex vivo binding of [3H]-(+)-PHNO measured (a) at 24 h following cessation of a 1-week treatment with THC or vehicle, and (b) at 1 week following cessation of a 3-weeks treatment with THC or vehicle. Data are shown as mean±SEM (n=4) in the caudate/putamen (CPu), nucleus accumbens (NAcc), ventral pallidum (VP), substantia nigra (SN), and ventral tegmental area (VTA). Significantly different from the vehicle-treated group at *p<0.05 and **p<0.01 using a mixed model repeated-measures ANOVA with a post hoc t-test.
Chronic THC Increases Presynaptic Inhibitory D2/3 Autoreceptor Binding
Relative to vehicle, and when measured at 24 h after stopping treatment, 3 weeks of daily injections with THC led to a significant increase in [18F]fallypride BPND in the SN/VTA area of the midbrain (+18% p<0.05; Figure 1g). Owing to its higher resolution as compared with microPET, ex vivo autoradiography allowed separate and more accurate quantification of [18F]fallypride SBR in the mesolimbic (ie, VTA) and nigrostriatal (ie, SN) DA cell body containing-structures (Figure 2a). When compared with vehicle, THC-treated animals showed increased [18F]fallypride binding in both VTA (+31% p<0.05) and SN (+39% p<0.05). Despite its limitation in spatial resolution, microPET imaging thus allowed to detect THC-induced D2/3 autoreceptor elevation in the relatively small SN/VTA area of the midbrain.
When measured at 24 h after stopping a 3-week treatment with THC, significant effects of the treatment on [3H]-(+)-PHNO binding (Figure 2b) were also detected in SN (+49% p<0.01) and VTA (+51% p<0.03), midbrain regions within which [3H]-(+)-PHNO binding is almost entirely attributable to D3R (Rabiner et al, 2009). When measured at 1 week after THC cessation (Figure 3b), [3H]-(+)-PHNO binding was still elevated in SN (+38% p<0.01) and VTA (+42% p=0.02) as compared to vehicle controls. Following 1 week only of treatment with THC (Figure 3a), [3H]-(+)-PHNO binding was significantly elevated in VTA (+37% p<0.02), but not in SN.
Regulation of CB1R, D2R, D3R, and TH mRNA Levels by Chronic THC
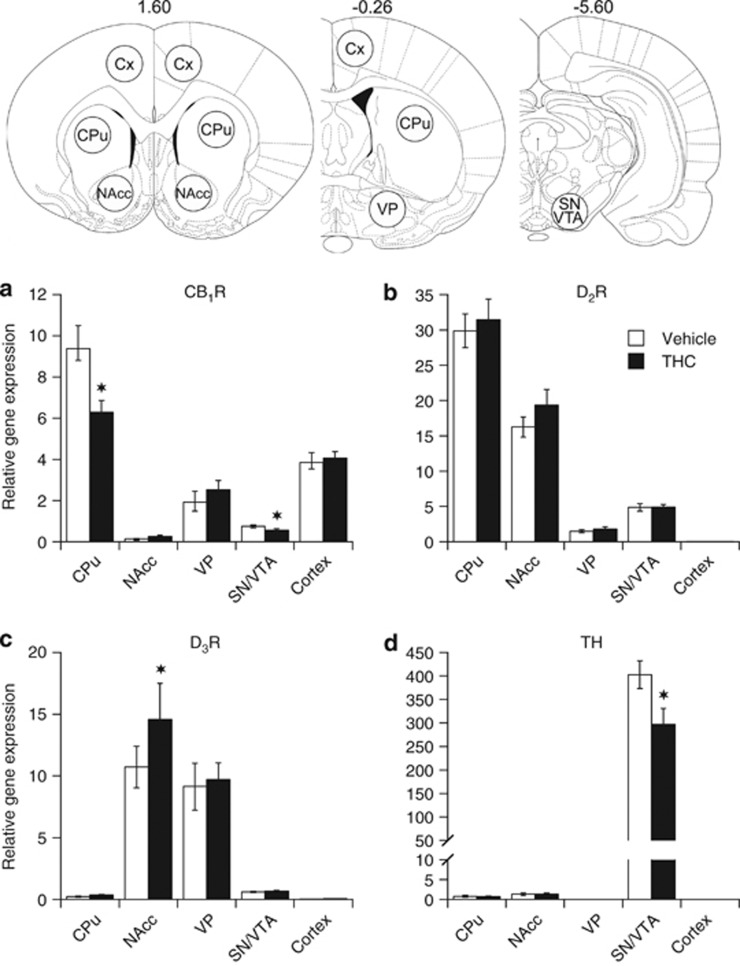
The use of quantitative real-time PCR gave reliable estimates of CB1R, D2R, D3R, and TH mRNA levels within the six different regions examined, and the relative abundance of each mRNA transcript in vehicle- and THC-treated animals is shown in Figure 4.
Figure 4.
Relative mRNA levels for CB1R (a), D2R (b), D3R (c), and tyrosine hydroxylase (d) in caudate/putamen (CPu), nucleus accumbens (NAcc), ventral pallidum (VP), substantia nigra (SN), ventral tegmental area (VTA), and cortex of rats as measured at 24 h following cessation of a 3-weeks treatment with either vehicle or THC. Levels of mRNA were measured by RT-qPCR, quantitated using the ΔΔCT method, and the results evaluated as the relative ratio of the expression level of each mRNA to that of both glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and hypoxanthine-guanine phosphoribosyltransferase (HPRT). Data are presented as mean±SEM (n=15). Significantly different from the vehicle-treated group at *p<0.05 using a mixed model repeated-measures ANOVA followed, when appropriate, by a post hoc t-test. Shown on the upper panel are schematic coronal sections adapted from a rat brain atlas (Paxinos and Watson, 1998) displaying the positions of the 1.5-mm-diameter circular tissue punches used for mRNA extraction.
In control vehicle animals, the anatomical brain distribution of CB1R mRNA (Figure 4a) was consistent with and extended previous in situ hybridization studies. Highest expression of CB1R mRNA was found in CPu followed by the neocortex, whereas moderate expression was detected in VP. Contrasting with previous in situ hybridization studies (Berrendero et al, 1999; Mailleux and Vanderhaeghen, 1992), low but clearly detectable levels of CB1R mRNA were found in NAcc and in SN/VTA, a finding most likely reflecting the higher sensitivity of RT-PCR compared to in situ hybridization. The highest concentrations of D2R mRNA were found in the CPu, where its concentration was twofold higher than that in the NAcc. D2R mRNA was least abundant in VP, with intermediate levels of expression in the SN/VTA (Figure 4b). In contrast to the D2R gene, the expression of the D3R gene was most and approximately equally abundant in NAcc, and VP where its expression was ∼30–50-fold higher than that in the CPu and SN/VTA (Figure 4c). Low levels of D2R and D3R mRNAs were detected in neocortex. Levels of TH mRNA in SN/VTA DA-containing cell bodies were ∼400-fold higher than in the DA terminal fields of the CPu and NAcc (Figure 4d).
Although there was no main effect of treatment on CB1R mRNA levels (F(1,76)=2.39, p>0.05), there was a highly significant effect of region (F(4,76)=62.44, p<0.001) and a significant treatment-by-region interaction (F(4,76)=4.77, p<0.002). Chronic THC treatment induced significant decreases in CB1R gene expression in both CPu (−33%, p<0.05; Figure 4a) and SN/VTA (−25%, p<0.05; Figure 4a) when compared with vehicle treatment. No THC effect was found in any other regions examined. Chronic THC treatment did not induce significant alteration in D2R gene expression (treatment: F(1,76)=0.13, p>0.05; treatment × region: F(4,76)=0.54, p>0.05; Figure 4b) but did induce a significant increase in D3R gene expression (treatment: F(1,72)=6.44, p=0.02; treatment × region: F(4,72)=3.57, p=0.01; Figure 4c). D3R mRNA levels were increased in the NAcc (+36% p<0.05) without significant change in any other region (Figure 4c). Importantly, there was a main effect of treatment on TH gene expression (treatment: F(1,38)=4.52, p<0.05; treatment × region: F(2,38)=4.54, p<0.02; Figure 4d). TH mRNA levels were significantly decreased (−25% p<0.05) by THC treatment in SN/VTA (Figure 4d).
Chronic THC Induces Functional Tolerance
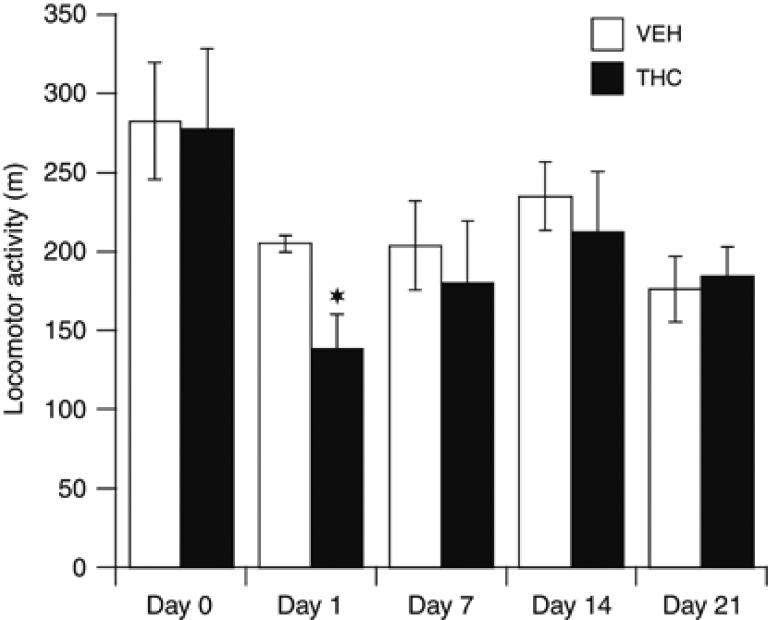
Figure 5 shows the time-course of alterations in THC-induced locomotor activity on days 1, 7, 14, and 21 of treatment as measured 30 min after injection. Two-way repeated ANOVA analysis showed that the effect of THC on locomotion was dependent on the day of testing (F(4,672)=2.84, p<0.05). When compared with vehicle treatment, THC significantly reduced (−32% p<0.015) locomotor activity at 1 day but not at 7, 14, or 21 days of treatment. By the 7th injection, the acute depressive effect of THC on locomotor activity was thus no longer apparent, a result indicative of functional tolerance and consistent with a desensitization of CB1R.
Figure 5.
Longitudinal effect of a chronic treatment with THC (1 mg/kg/day; 21 days) or its vehicle on locomotor activity. Locomotor activity was measured as the distance travelled in meters and monitored at 30 min postinjection of THC or vehicle for 60 min. Measurements were done at baseline conditions (day 0) and after the 1st (day 1), 7th (day 7), 14th (day 14), and last (day 21) injection of THC or VEH. Data are presented as mean±SEM (n=8). Significantly different from the vehicle-treated group at *p<0.05, using a two-way ANOVA followed by a LSD post hoc test.
Locomotor Activity Response to Amphetamine and Quinpirole Challenges
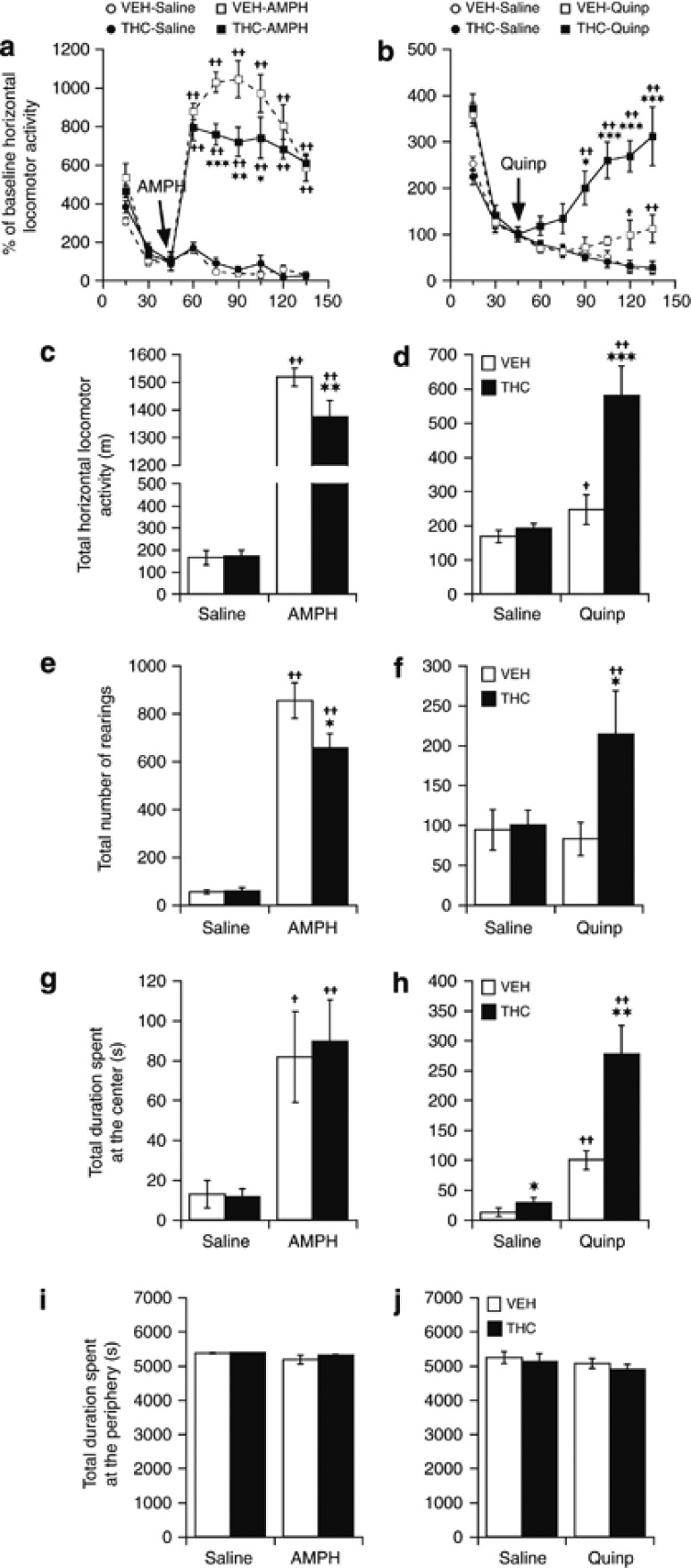
Spontaneous locomotor activity measured after a challenge dose of saline in rats treated chronically with THC was not different from that of vehicle-treated animals (Figure 6a). Administration of AMPH (2.5 mg/kg i.p.) produced a marked and sustained rise in locomotor activity, which reached maximal levels at 15 min postinjection and remained elevated above baseline levels over the 90-min of monitoring (Figure 6a). When compared with control animals, THC-treated rats exhibited a lower, time-dependent locomotor response to AMPH (treatment: F(1,126)=35.89, p<0.001; treatment × time: F(8,126)=2.55, p<0.01; Figure 6a). When data were binned over the 90-min postinjection time period (Figure 6c), THC treatment decreased the horizontal and vertical (rearings) locomotor response to AMPH by ∼15% (p<0.01; Figure 6c) and 23% (p<0.05; Figure 6e), respectively. AMPH did not affect the time spent by any animals at the periphery of the open field (Figure 6i) but significantly increased the time spent at the center in both the VEH- and THC-treated animals, with no difference between the two groups (Figure 6g).
Figure 6.
Effect of a chronic (21 days) treatment with THC or vehicle (VEH) on the locomotor response to (a, c, e, g, and i) amphetamine (AMPH; 2.5 mg/kg; i.p.) and (b, d, f, h, and j) to quinpirole (Quinp; 0.5 mg/kg; i.p.) as measured 24 h after the last treatment dose. Locomotor activity is illustrated as horizontal travel distance (in meters) during each 15 min block (a and b) and during the 90-min postdrug time period (c and d). Vertical activity is expressed as number of rearings during the 90-min postdrug time period (e and f). The times (in seconds) spent in the center and at the periphery of the open field during the 90-min postdrug time period are given in (g and h) and (i and j), respectively. Data are presented as mean±SEM (n=8). Significantly different from the respective VEH-treated group at *p<0.05, **p<0.01, and ***p<0.001, and significantly different from the respective saline-treated group at +p<0.05 and ++p<0.01, using a two-way ANOVA followed by a LSD post hoc test.
Rats treated with THC exhibited a marked increased response to a postsynaptic dose (0.5 mg/kg; Eilam and Szechtman, 1989) of the direct-acting D2/3R agonist quinpirole when compared with vehicle-treated rats (treatment: F(1,125)=38.45, p<0.001; treatment × time: F(8,125)=4.36, p<0.001; Figure 6b). When data were binned over the 90-min postinjection time period, the horizontal and vertical (rearings) locomotor responses to a postsynaptic dose of quinpirole were ∼twofold higher in THC-treated than in vehicle-treated animals (Figure 6d and f; p<0.001). As AMPH, quinpirole significantly increased the time spent at the center of the open field in both VEH- and THC-treated animals (Figure 6h), but this effect was significantly more pronounced in THC-treated animals. No effect of quinpirole was observed on the time spent at the periphery of the area (Figure 6j).
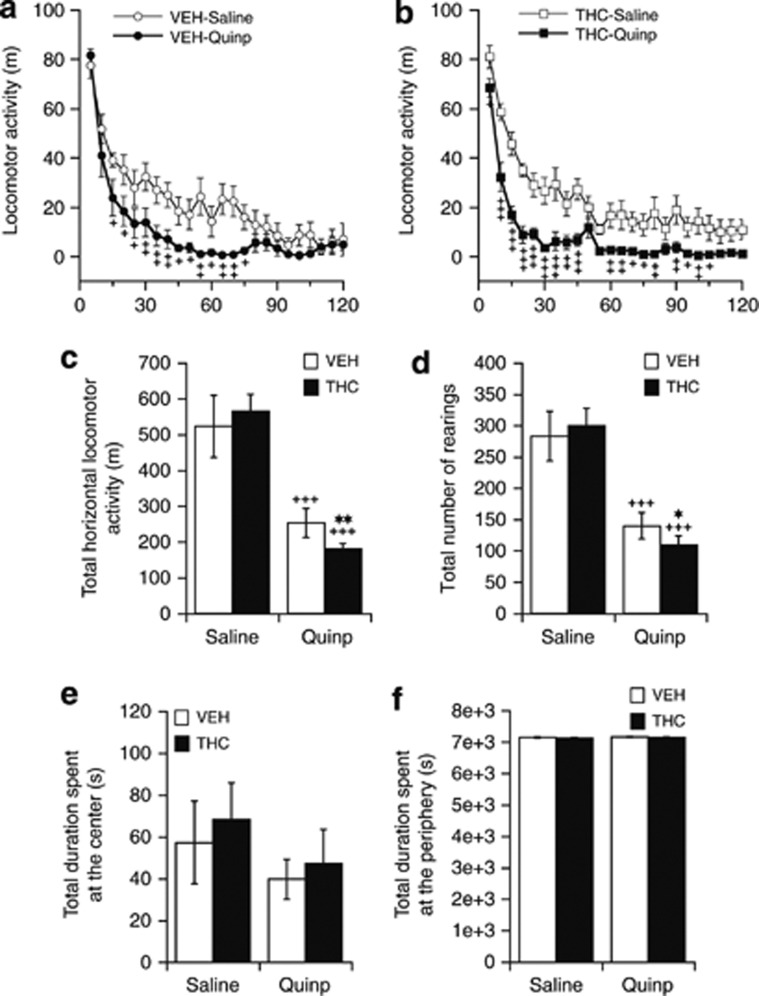
When compared with saline, administration of a low, presynaptic, dose of quinpirole (0.05 mg/kg; Eilam and Szechtman, 1989) produced a marked decrease in locomotor activity in both VEH- (treatment: F(1,336)=69.20, p<0.001; treatment × time: F(23,336)=2.12, p<0.01; Figure 7a) and THC-treated animals (treatment: F(1,336)=199.03, p<0.001; treatment × time: F(23,336)=1.62, p<0.05; Figure 7b). The numbers of rears were also significantly reduced following quinpirole in both VEH- (treatment: F(1,336)=42.16, p<0.001; treatment × time: F(23,336)=1.69, p<0.05) and THC-treated animals (treatment: F(1,336)=25.48, p<0.001; treatment × time: F(23,336)=1.60, p<0.05). When compared with vehicle treatment, rats treated with THC exhibited an increased suppressive effect of 0.05 mg/kg quinpirole both on locomotion (treatment: F(1,336)=6.64, p<0.01; treatment × time: F(23,336)=1.13, p>0.05; Figure 7a and b) and numbers of rears (treatment: F(1,336)=5.21, p<0.02; treatment × time: F(23,336)=1.07, p>0.05; data not shown). When data were binned over the 120-min of testing and compared with the respective saline-treated control data, a 0.05-mg/kg challenge dose of quinpirole caused significantly higher reductions of both horizontal (p<0.01) and vertical (p<0.02) locomotor activity in THC-treated (−67.9% and −63.5%, respectively; Figure 7c) than in vehicle-treated animals (−51.7% and −50.6%, respectively; Figure 7d). A low dose of quinpirole had no effect on the time spent at the center (Figure 7e) or at the periphery of the open field (Figure 7f).
Figure 7.
Effects of chronic (21 days) treatment with THC or vehicle (VEH) on the locomotor response to an acute challenge with a presynaptic dose of quinpirole (Quinp, 0.05 mg/kg, i.p.) as measured 24 h after the last treatment dose. Locomotor activity is illustrated as horizontal travel distance (in meters) during each 5 min block (a and b) and during the 120-min postdrug time period (c). Vertical activity is expressed as number of rearings during the 120-min postdrug time period (d). The times (in seconds) spent in the center and at the periphery of the open field during the 120-min postdrug time period are given in (e) and (f), respectively. Data are presented as mean±SEM (n=8). Significantly different from the respective VEH-treated group at *p<0.05 and **p<0.01, and significantly different from the respective saline-treated group at +p<0.05, ++p<0.01, and +++p<0.001 using a two-way ANOVA followed by a LSD post hoc test.
DISCUSSION
To our knowledge, this is the first study to investigate the effects of THC on D2R and D3R. Our results indicate that a 3-weeks treatment with relatively low doses of THC has a pervasive effect on D2R and D3R in the nigrostriatal and mesolimbic DA pathways. When measured at 24 h after THC treatment cessation, both D2R and D3R were upregulated in the SN and VTA, indicating a supersensitivity of the midbrain D2/3R that was associated with a concurrent decrease in TH gene expression. THC exposure also increased D2R availability in CPu and upregulated D3R in NAcc. These receptor changes correlated with an increased functionality of pre- and postsynaptic D2/3R in vivo, as indicated by findings of increased locomotor suppressive effects of a low, presynaptic dose of quinpirole and enhanced locomotor activation produced by a higher, postsynaptic dose of quinpirole. Concomitantly, the psychomotor response to the presynaptic DA releaser AMPH was diminished following THC. Our results support the notion of a hypodopaminergic state resulting from altered presynaptic mechanisms controlling DA synthesis and release during the early period after THC cessation, with the concurrent development of postsynaptic DA receptor supersensitivity.
The results of the present study moreover demonstrate that dysregulations of D2R and D3R receptors occur rapidly following THC exposure (ie, within the first week) and that these dysregulations persist during the initial phase of abstinence (7 days) of a 3-weeks THC exposure. Furthermore, our data also indicate that recovery of D2R and D3R densities during abstinence exhibits regional brain differences, with D2R in CPu showing more rapid recovery than D3R in NAcc and midbrain. These data suggest that chronic THC exposure may not produce permanent alterations in the DA D2-like receptor system, but that recovery within the mesolimbic system may require prolonged abstinence from drug use. Owing to its high lipophilicity and sequestration in fat, the tissue half-life of THC is long (∼5 days; Kreuz and Axelrod, 1973) and the drug likely persists at brain CB1R for days after discontinuing treatment. This makes difficult to distinguish whether THC effects on D2/3R arise from indirect effects of residual drug or from the effects of drug withdrawal.
In addition to increased D2/3R binding in midbrain, further evidence for a THC-induced sensitization of midbrain DA autoreceptors was obtained based on findings of a heightened locomotor suppressive effect of low dose of quinpirole, an effect that has been attributed to presynaptic activation of D2/3 autoreceptors (Aghajanian and Bunney, 1977; White and Wang, 1984). Midbrain D2/3 autoreceptors inhibit DA cell firing, thereby decreasing DA release in terminal fields (Westerink et al, 1996). The development of supersensitive midbrain DA autoreceptors following chronic THC is thus expected to result in increased self-inhibition and hypoactivity of DA neurons. This hypothesis concurs with the substantial decrement in spontaneous electrical activity of NAcc-projecting DA neurons observed in VTA following repeated THC (Diana et al, 1998) and with the abrupt reductions in mesolimbic DA neuronal activity and accumbal DA levels following SR 141716A-precipitated THC withdrawal (Diana et al, 1998; Tanda et al, 1999). Further evidence for a diminished presynaptic DA function is the decreased gene expression of TH in SN/VTA, a finding that ties up with the reduced TH activity reported in striatum following repeated CB1R stimulation (Moranta et al, 2009). Such effects are expected to diminish the maximal capacity of midbrain neurons to synthesize and release DA and likely underlie the diminished locomotor response to AMPH observed in THC-treated animals. A THC-induced presynaptic DA hypofunction is consistent with the reduced release of accumbal DA observed in response to alcohol and morphine in rats chronically pre-exposed to cannabinoid agonists (Cadoni et al, 2008; Lopez-Moreno et al, 2008). Such an attenuated response of mesolimbic DA release to drug challenge has also been observed during the early phase of withdrawal (ie, within the first week) of chronic exposure to several psychostimulants and opiates (Rossetti et al, 1992; Spanagel et al, 1994). Taken together, the decreased expression of TH and supersensitive D2/3R observed in midbrain combined with the blunted locomotor response to AMPH suggests that, as observed with other drugs of abuse, chronic THC exposure causes a presynaptic mesolimbic DA hypofunction during the early period following THC cessation.
To date, few studies have investigated the effects of chronic cannabinoid exposure on D2/3R in striatum. Using [11C]raclopride, two independent studies (Sevy et al, 2008; Stokes et al, 2012) found normal levels of striatal D2/3R in detoxified cannabis users. However, given the length of cannabis abstinence (>12 weeks in Sevy et al (2008); 18 months on average in Stokes et al (2012)), abnormal D2/3R density may have had recovered at time of measurement. After shorter periods of cannabinoid abstinence (ie, 24 h), higher striatal D2R binding have been reported in cannabis users with first-episode psychosis than in nonusers and in healthy controls, although the difference was not significant due to insufficient statistical power (Safont et al, 2011). Lastly, using [3H]raclopride in rats chronically exposed to the synthetic CB1R agonist HU-210, Dalton and Zavitsanou (2010) found an overall increase in D2R across all cerebral regions examined (which included CPu and NAcc) relative to controls, although region-by-region comparisons failed to reach significance in any individual brain area. In light of these studies, it thus remained unclear whether any change in striatal D2/3R density, even subtle, occurs following chronic cannabinoid exposure. Here, we provided evidence that postsynaptic DA receptor supersensitization mechanisms involving an increased density and enhanced agonist recognition of D2/3R developed in striatum over the course of THC treatment. In NAcc, which contains similar amount of D2R and D3R, chronic THC produced an increase in [3H]-(+)-PHNO binding, whereas [18F]fallypride binding was unaltered. Together with the concurrent elevation of accumbal D3R mRNA levels, this finding suggests a selective upregulation of D3R vs D2R in NAcc following THC. However, concurrent behavioral data indicated that the locomotor activation produced by a 0.5-mg/kg dose of quinpirole was dramatically enhanced following THC. Although quinpirole has high affinity for both D2R and D3R (Freedman et al, 1994), the drug is known to produce locomotor activation through stimulation of postsynaptic D2R in NAcc (Breese et al, 1987; Kling-Petersen et al, 1995), with no significant contribution of D3R (Marcellino et al, 2008b). The enhanced locomotor-activating effect of quinpirole thus indicated that THC treatment may also increase the affinity of agonist binding to D2R or their coupling to G-protein, in a manner similar to the effects recently seen with cocaine (Ferraro et al, 2012; Marcellino et al, 2010). In CPu, where D2R are much more abundant than D3R, THC increased both [18F]fallypride and [3H]-(+)-PHNO bindings, indicating that D2R are upregulated and their agonist binding affinity increased in this region. As discussed below, the mechanism responsible for a D2R supersensitization is unclear but it is not mediated by increased receptor transcription, as it was not accompanied with any change in D2R mRNA levels. It is noteworthy that D2/3R availability as measured in vivo and ex vivo can be influenced by competition with endogenous DA, depending on the radioligand used. As [18F]fallypride is insensitive to decreased levels of competing DA (Rominger et al, 2010), the most likely explanation for the significant elevation in [18F]fallypride binding measured in midbrain and CPu following THC is an increase in D2R number. Ex vivo [3H]-(+)-PHNO binding, on the other hand, is sensitive to DA depletion (Wilson et al, 2005) and might be confounded by low levels of endogenous DA. However, given that [3H]-(+)-PHNO binding elevation in NAcc is associated with increased mRNA D3R levels and with a behavioral sensitization to a postsynaptic dose of quinpirole, it is likely that this effect reflects, at least in part, a supersensitization of D2/3R. Thus, in addition to providing convergent evidence to support a THC-induced sensitization of the D2R, our study indicates that D3R upregulate in NAcc in response to repeated THC in a manner similar to other drugs of abuse (Le Foll et al, 2002, 2003; Staley and Mash, 1996). Those results concur to indicate a postsynaptic hyperdopaminergic state following chronic THC.
The mechanism by which THC induces supersensitization of postsynaptic D2R remains to be clarified. Besides a compensatory increase in D2R resulting from a presynaptic DA deficiency, a molecular mechanism involving modulation of D2R signaling by desensitized CB1R could be invoked. Indeed, CB1R undergo downregulation and functional desensitization during ongoing treatment with THC (review in Sim-Selley, 2003). These adaptations, which have been consistently demonstrated in CPu but inconsistently in other regions including the NAcc, pallidum, and ventral midbrain (Breivogel et al, 1999; Rodriguez de Fonseca et al, 1994; Romero et al, 1998), are believed to contribute to behavioral tolerance to the drug. Most studies investigating the effects of chronic THC on CB1R have used relatively high doses (ie, typically 10 mg/kg/day and up), and the efficacy of low dose of THC has been questioned based on the dose-dependent effect of cannabinoids on CB1R adaptation (McKinney et al, 2008; Sim-Selley, 2003). Here, we show that chronic THC doses as low as 1 mg/kg/day indeed induce downregulation of CB1R mRNA in CPu and ventral midbrain, a result that together with the development of tolerance to the acute hypomotility effect of the drug concurs to indicate a desensitization of CB1R. Compelling evidence suggest that such a CB1R desensitization may affect D2R signaling. Indeed, CB1R and D2R, which colocalized in dendritic spines of the medium spiny neurons of the striatum (Fitzgerald et al, 2012; Pickel et al, 2006), interact in an antagonistic and reciprocal manner and form CB1R/D2R heteromeric complexes in the brain (Kearn et al, 2005). One consequence of CB1R/D2R heteromerization is a decreased affinity of D2R agonist binding sites in striatum upon CB1R stimulation (Marcellino et al, 2008a), a finding providing a potential mechanism for the well-known inhibitory effect of acute cannabinoid dosing on D2R-mediated behaviors (Beltramo et al, 2000; Giuffrida et al, 1999). In addition to modulating D2R agonist affinity, accumulating evidence indicate that CB1R may also modulate the membrane expression of D2R and vice versa. For instance, chronic D2R blockade upregulate striatal levels of CB1R (Mailleux and Vanderhaeghen, 1993). Moreover, mice lacking CB1R show increased densities of striatal D2R (Houchi et al, 2005), whereas mice lacking D2R show an upregulation of CB1R in basal ganglia (Thanos et al, 2011), indicating that CB1R and D2R may exert a reciprocal inhibition of their cell-surface expression. Taken together, these observations support the hypothesis that, subsequent to persistent CB1R activation by chronic THC, CB1R desensitization may alleviate a tonic inhibitory action of CB1R onto D2R agonist affinity and cell-surface expression, leading to a postsynaptic D2R supersensitivity. This hypothesis remains speculative and warrants further investigation. Nevertheless, alterations in D2/3R signaling during acute THC cessation may represent early neuroadaptative changes and be a recurrent aspect of intermittent THC exposure that may contribute significantly to the development of addiction. In view of the role of mesolimbic DA in attributing incentive salience to reward-related stimuli (Robinson and Berridge, 2008), a supersensitivity of D2R in NAcc may counteract a diminished presynaptic DA function and serve as the molecular substrate for the acquisition of drug-seeking behavior during the early phase of THC cessation.
Besides its addictive potential, cannabis is increasingly recognized as a drug that may precipitate or exacerbate psychosis in vulnerable individuals (Arseneault et al, 2004). In patients with schizophrenia, its use is associated with an earlier onset of psychotic illness (Large et al, 2011), more severe and frequent psychotic episodes (Johns, 2001), and accumulating evidence suggests that cannabis may be a risk factor for psychosis in predisposed individuals (Moore et al, 2007). The mechanism by which cannabis can induce psychosis is not known but recent data indicate that D2R mechanisms may well have a role in mediating the psychotomimetic effect of THC as this effect is reduced by clinically relevant dose of haloperidol (Liem-Moolenaar et al, 2010). Moreover, there are several reports suggesting that blocking D2R with antipsychotic drugs, particularly second-generation drugs, may improve cannabis use disorder (Green et al, 2003; van Nimwegen et al, 2008). Considering that a mesolimbic hyperdopaminergic function has been proposed as a possible cause of psychosis (Davis et al, 1991), a THC-induced supersensitivity of striatal D2R may offer a neurochemical basis to better understand how cannabis could potentially contribute to the development of psychosis in predisposed individuals.
In conclusion, the present study indicates that repeated exposure to THC, even at relatively low doses, leads to a neurochemical and functional supersensitivity of D2/3R that affect DA neurons in the midbrain and extend to their terminal fields in the dorsal and ventral portions of the striatum. These findings extend the accumulating evidence on cannabinoid modulation of D2/3R signaling and suggest that a presynaptic DA hypofunction conjoined with a postsynaptic DA hyperfunction in the nigrostriatal and mesolimbic circuitry may contribute to the mechanism of cannabis addiction.
Acknowledgments
We thank Pr Alan Wilson from the Centre for Addiction and Mental Health in Toronto (Canada) for kindly providing cold precursor of [3H]-(+)-PHNO, and Dr Peter Dorff and colleagues from the Department of Chemistry of AstraZeneca in Wilmington (US), for tritiating the precursor of [3H]-(+)-PHNO. We are also grateful to the contributions of the BioPark Platform in Archamps, the Fondation Caisse d'Epargne Rhône-Alpes, and the ABC laboratory of the European Scientific Institute (ESI). This work was supported by the Swiss National Science Foundation (Grant 31003A-122352).
The authors declare no conflict of interest.
References
- Aghajanian GK, Bunney BS. Dopamine ‘autoreceptors': pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- Beltramo M, de Fonseca FR, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G, et al. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci. 2000;20:3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Breese GR, Duncan GE, Napier TC, Bondy SC, Iorio LC, Mueller RA. 6-Hydroxydopamine treatments enhance behavioral responses to intracerebral microinjection of D1- and D2-dopamine agonists into nucleus accumbens and striatum without changing dopamine antagonist binding. J Pharmacol Exp Ther. 1987;240:167–176. [PMC free article] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Valentini V, Di Chiara G. Behavioral sensitization to delta 9-tetrahydrocannabinol and cross-sensitization with morphine: differential changes in accumbal shell and core dopamine transmission. J Neurochem. 2008;106:1586–1593. doi: 10.1111/j.1471-4159.2008.05503.x. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton VS, Zavitsanou K. Differential treatment regimen-related effects of cannabinoids on D1 and D2 receptors in adolescent and adult rat brain. J Chem Neuroanat. 2010;40:272–280. doi: 10.1016/j.jchemneu.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci USA. 1998;95:10269–10273. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D, Szechtman H. Biphasic effect of D-2 agonist quinpirole on locomotion and movements. Eur J Pharmacol. 1989;161:151–157. doi: 10.1016/0014-2999(89)90837-6. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Frankowska M, Marcellino D, Zaniewska M, Beggiato S, Filip M, et al. 2012A novel mechanism of cocaine to enhance dopamine D(2)-like receptor mediated neurochemical and behavioral effects. An in vivo and in vitro study Neuropsychopharmacologye-pub ahead of print 28 March 2012. doi: 10.1038/npp.2012.33 [DOI] [PMC free article] [PubMed]
- Fitzgerald ML, Shobin E, Pickel VM.2012Cannabinoid modulation of the dopaminergic circuitry: Implications for limbic and striatal output Prog Neuropsychopharmacol Biol Psychiatrye-pub ahead of print 11 January 2012. doi: 10.1016/j.pnpbp.2011.12.004 [DOI] [PMC free article] [PubMed]
- Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, et al. Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther. 1994;268:417–426. [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, et al. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD. Alcohol and cannabis use in schizophrenia: effects of clozapine vs. risperidone. Schizophr Res. 2003;60:81–85. doi: 10.1016/s0920-9964(02)00231-1. [DOI] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Johns A. Psychiatric effects of cannabis. Br J Psychiatry. 2001;178:116–122. doi: 10.1192/bjp.178.2.116. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A ‘direct-coloring' thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk. Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Kling-Petersen T, Ljung E, Svensson K. Effects on locomotor activity after local application of D3 preferring compounds in discrete areas of the rat brain. J Neural Transm Gen Sect. 1995;102:209–220. doi: 10.1007/BF01281155. [DOI] [PubMed] [Google Scholar]
- Kreuz DS, Axelrod J. Delta-9-tetrahydrocannabinol: localization in body fat. Science. 1973;179:391–393. doi: 10.1126/science.179.4071.391. [DOI] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68:555–561. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wiggins M, Goldberg SR. Nicotine pre-exposure does not potentiate the locomotor or rewarding effects of Delta-9-tetrahydrocannabinol in rats. Behav Pharmacol. 2006;17:195–199. doi: 10.1097/01.fbp.0000197460.16516.81. [DOI] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, Gardner EL. Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sci. 1996;58:PL365–PL372. doi: 10.1016/0024-3205(96)00237-8. [DOI] [PubMed] [Google Scholar]
- Liem-Moolenaar M, te Beek ET, de Kam ML, Franson KL, Kahn RS, Hijman R, et al. Central nervous system effects of haloperidol on THC in healthy male volunteers. J Psychopharmacol. 2010;24:1697–1708. doi: 10.1177/0269881109358200. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Scherma M, Rodriguez de Fonseca F, Gonzalez-Cuevas G, Fratta W, Navarro M. Changed accumbal responsiveness to alcohol in rats pre-treated with nicotine or the cannabinoid receptor agonist WIN 55,212-2. Neurosci Lett. 2008;433:1–5. doi: 10.1016/j.neulet.2007.11.074. [DOI] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Dopaminergic regulation of cannabinoid receptor mRNA levels in the rat caudate-putamen: an in situ hybridization study. J Neurochem. 1993;61:1705–1712. doi: 10.1111/j.1471-4159.1993.tb09807.x. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008a;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferre S, Casado V, Cortes A, Le Foll B, Mazzola C, et al. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J Biol Chem. 2008b;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Navarro G, Sahlholm K, Nilsson J, Agnati LF, Canela EI, et al. Cocaine produces D2R-mediated conformational changes in the adenosine A(2A)R-dopamine D2R heteromer. Biochem Biophys Res Commun. 2010;394:988–992. doi: 10.1016/j.bbrc.2010.03.104. [DOI] [PubMed] [Google Scholar]
- Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, Freund TF, et al. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. 2008;54:95–107. doi: 10.1016/j.neuropharm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, et al. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Moranta D, Esteban S, Garcia-Sevilla JA. Chronic treatment and withdrawal of the cannabinoid agonist WIN 55,212-2 modulate the sensitivity of presynaptic receptors involved in the regulation of monoamine syntheses in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:61–72. doi: 10.1007/s00210-008-0337-0. [DOI] [PubMed] [Google Scholar]
- Moreno M, Lopez-Moreno JA, Rodriguez de Fonseca F, Navarro M. Behavioural effects of quinpirole following withdrawal of chronic treatment with the CB1 agonist, HU-210, in rats. Behav Pharmacol. 2005;16:441–446. doi: 10.1097/00008877-200509000-00017. [DOI] [PubMed] [Google Scholar]
- Motta A, Damiani C, Del Guerra A, Di Domenico G, Zavattini G. Use of a fast EM algorithm for 3D image reconstruction with the YAP-PET tomograph. Comput Med Imaging Graph. 2002;26:293–302. doi: 10.1016/s0895-6111(02)00034-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Yang ZY, Brown T, Lew R, Wernick M, Ouyang X, et al. Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl Med Biol. 1999;26:519–527. doi: 10.1016/s0969-8051(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego; 1998. [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63:782–793. doi: 10.1002/syn.20658. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA. Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav. 1994;47:33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Romero J, Berrendero F, Manzanares J, Perez A, Corchero J, Fuentes JA, et al. Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by repeated exposure to delta9-tetrahydrocannabinol. Synapse. 1998;30:298–308. doi: 10.1002/(SICI)1098-2396(199811)30:3<298::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rominger A, Wagner E, Mille E, Boning G, Esmaeilzadeh M, Wangler B, et al. Endogenous competition against binding of [(18)F]DMFP and [(18)F]fallypride to dopamine D(2/3) receptors in brain of living mouse. Synapse. 2010;64:313–322. doi: 10.1002/syn.20730. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Safont G, Corripio I, Escarti MJ, Portella MJ, Perez V, Ferrer M, et al. Cannabis use and striatal D2 receptor density in untreated first-episode psychosis: an in vivo SPECT study. Schizophr Res. 2011;129:169–171. doi: 10.1016/j.schres.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JL. A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods. 2003;129:105–113. doi: 10.1016/s0165-0270(03)00192-4. [DOI] [PubMed] [Google Scholar]
- Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, et al. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 2008;197:549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl) 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes PR, Egerton A, Watson B, Reid A, Lappin J, Howes OD, et al. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol. 2012;26:144–149. doi: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- Tanda G, Loddo P, Di Chiara G. Dependence of mesolimbic dopamine transmission on delta9-tetrahydrocannabinol. Eur J Pharmacol. 1999;376:23–26. doi: 10.1016/s0014-2999(99)00384-2. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Sitaram BR, Elliot-Baker S.1988Effect of 9-tetrahydrocannabinol on release of dopamine in the corpus striatum of the ratIn: Chesher G, Consroe P, Musty R (eds).Marijuana: An International Research Report Australian Govt. Publ. Service: Canberra; 405–408. [Google Scholar]
- Thanos PK, Gopez V, Delis F, Michaelides M, Grandy DK, Wang GJ, et al. Upregulation of cannabinoid type 1 receptors in dopamine D2 receptor knockout mice is reversed by chronic forced ethanol consumption. Alcohol Clin Exp Res. 2011;35:19–27. doi: 10.1111/j.1530-0277.2010.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 2000;147:436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- van Nimwegen LJ, de Haan L, van Beveren NJ, van der Helm M, van den Brink W, Linszen D. Effect of olanzapine and risperidone on subjective well-being and craving for cannabis in patients with schizophrenia or related disorders: a double-blind randomized controlled trial. Can J Psychiatry. 2008;53:400–405. doi: 10.1177/070674370805300610. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Wang RY. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther. 1984;231:275–280. [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9 -ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–4160. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]