Abstract
A number of lines of evidence suggest that negative emotional symptoms of withdrawal involve reduced activity in the mesolimbic dopamine system. This study examined the contribution of dopaminergic signaling in structures downstream of the ventral tegmental area to withdrawal from acute morphine exposure, measured as potentiation of the acoustic startle reflex. Systemic administration of the general dopamine receptor agonist apomorphine or a cocktail of the D1-like receptor agonist SKF82958 and the D2-like receptor agonist quinpirole attenuated potentiated startle during morphine withdrawal. This effect was replicated by apomorphine infusion into the nucleus accumbens shell. Finally, apomorphine injection was shown to relieve startle potentiation during nicotine withdrawal and conditioned place aversion to morphine withdrawal. These results suggest that transient activation of the ventral tegmental area mesolimbic dopamine system triggers the expression of anxiety and aversion during withdrawal from multiple classes of abused drugs.
Keywords: dopamine, opioids, addiction and substance abuse, withdrawal, startle, nucleus accumbens
INTRODUCTION
Negative affective signs and symptoms, including anxiety, irritability, anhedonia, and dysphoria, are common to withdrawal from all classes of abused drugs, are manifested after the very first exposure to a drug, and increase in intensity with repeated exposures (Koob and Le Moal, 1997; Haertzen and Hooks, 1969). Negative reinforcement theories of drug dependence, such as the ‘opponent process' and ‘hedonic allostasis' theories (Koob and Bloom, 1988; Solomon and Corbit, 1974), hypothesize that the emotional signs and symptoms of withdrawal contribute to the acquisition and maintenance of drug-taking behavior as well as to relapse following periods of prolonged abstinence.
One interesting prediction of the opponent process view of addiction is that activity in the neural circuits responsible for withdrawal is dependent on prior activation of reward-related circuitry (Koob and Bloom, 1988). Recent work in our laboratory (Radke et al, 2011) supported this prediction by demonstrating that targeted infusion of morphine in the ventral tegmental area (VTA) was sufficient to induce anxiety-like withdrawal behaviors. In the same study, we also found that systemic injection of the general dopamine receptor agonist apomorphine relieved withdrawal-induced anxiety (Radke et al, 2011). Taken together, this evidence led us to hypothesize that negative affective withdrawal behaviors are triggered by declining levels of dopamine in structures downstream of the VTA. The current studies address this hypothesis by examining the contribution of dopaminergic signaling in these structures to withdrawal from acute morphine exposure.
The hypothesis that expression of negative emotional withdrawal signs involves dopaminergic signaling is supported by further evidence. First, VTA dopamine neurons project to structures known to be involved in negative emotional signs of withdrawal, including the nucleus accumbens (NAc), basolateral amygdala (BLA), central amygdala (CeA), and lateral portion of the bed nucleus of the stria terminalis (lBNST) (Fallon et al, 1978; Hasue and Shammah-Lagnado, 2002; Meloni et al, 2006). Cellular activity in the CeA and lBNST following drug exposure has also been shown to depend on the activation of dopamine receptors (Valjent et al, 2004; Kash et al, 2008). Furthermore, dopamine release in these target structures falls below baseline levels during spontaneous opiate withdrawal (Acquas et al, 1991; Crippens and Robinson, 1994) and following administration of an opioid receptor antagonist such as naloxone (Rossetti et al, 1992; Pothos et al, 1991; Spanagel et al, 1994). Treatments that attenuate withdrawal behaviors have been shown to reverse this decrease in dopaminergic signaling, while treatments that exacerbate withdrawal potentiate it (Spanagel et al, 1994; Georges and Aston-Jones, 2003). Finally, studies examining the role dopamine plays in opiate withdrawal behaviors (Bechara et al, 1995; Laviolette et al, 2002; Chartoff et al, 2006, 2009; Radke et al, 2011) have found that dopaminergic signaling contributes to increased aversion, aggression, and anxiety during withdrawal (but see, Caillé et al, 2003).
To better understand how drug-induced changes in dopaminergic signaling contribute to withdrawal behaviors, we evaluated the effects of intracerebrally infused dopamine receptor agonists on potentiation of the acoustic startle reflex (‘withdrawal-potentiated startle') during opiate withdrawal (Harris and Gewirtz, 2004; Rothwell et al, 2009; Cabral et al, 2009). Increases in startle following drug exposure likely represent the anxiety-like component of the withdrawal state. For example, potentiated startle during withdrawal is relieved by a second drug exposure or administration of anxiolytic compounds (Harris and Gewirtz, 2004; Rothwell et al., 2009; Engelmann et al, 2009, Radke et al, 2011). The timing of the withdrawal-potentiated startle effect following morphine also coincides with the decrease in drug levels in the brain (Barjavel et al, 1995; Hipps et al, 1976).
Previous work from our laboratory and others (Harris and Aston-Jones, 1994; Chartoff et al, 2006; Chartoff et al, 2009; Radke et al, 2011) has demonstrated that dopamine receptor agonists attenuate withdrawal behaviors. The current experiments used two subtype-specific dopamine receptor agonists, SKF82958 and quinpirole, to examine the individual contributions of D1- and D2-like dopamine receptors, respectively, to this effect. These two classes of dopamine receptors have opposite effects on cell excitability and glutamatergic signaling (Neve et al, 2004; Surmeier et al, 2007), but have both been shown to contribute to aversive and withdrawal behaviors (Carlezon and Thomas, 2009). We next investigated the location of the dopamine receptors involved in withdrawal-induced anxiety by locally infusing the D1/D2 agonist apomorphine into the shell of the NAc, lBNST, CeA, and VTA. The dorsolateral component of the BNST (dlBNST) was specifically targeted because this region contains the most dense distribution of dopaminergic fibers (Freedman and Cassell, 1994). Two final experiments examined whether reduced dopaminergic signaling is similarly involved in nicotine withdrawal-potentiated startle and conditioned place aversion to morphine withdrawal.
MATERIALS AND METHODS
Subjects
Male Sprague–Dawley rats (Harlan, Indianapolis, IN), weighing between 225 and 400 g at the start of the experiment, were housed in groups of four in metal cages with a 12 h light–dark cycle and free access to food and water, except during testing. Animals were acclimated to housing conditions for 2 weeks and then gently handled for two consecutive days. Rats that underwent intracranial cannulation surgery were subsequently housed individually in metal cages. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Drugs
Morphine sulfate was purchased from Mallinckrodt (Hazelwood, MO). SKF82958, quinpirole, and (−)-nicotine hydrogen tartrate salt were purchased from Sigma-Aldrich (St Louis, MO). Apomorphine was purchased from Tocris (Ellisville, MO). Systemically administered drugs were dissolved in 0.9% saline and injected subcutaneously. Intracerebrally infused apomorphine was dissolved in 25%. DMSO in sterile saline. The nicotine solution was titrated to a pH of approximately 7.1 using sodium hydroxide. Throughout the text, 0 mg/kg and 0 μg denote groups given vehicle injection or infusion, respectively. All drug doses are expressed as the weight of the salt, except nicotine which is expressed as the weight of the base.
Intracranial Cannulation and Infusion
Animals were anesthetized with Nembutal (sodium pentobarbital, 75 mg/kg, intraperitoneally), injected with atropine (1 mg/kg, subcutaneously), and secured in a Kopf stereotaxic instrument. Following exposure of the skull, 22-gauge guide cannulae (Plastics One Products, Roanoke, VA) were lowered to the appropriate stereotaxic coordinates measured in mm from Bregma. Jeweler screws were anchored to the skull, and the entire assembly was cemented into place using Loctite 444 Tak Pak Instant Adhesive (Henkel Corporation, Düsseldorf, Germany) and Perm Reline & Repair Resin (Hygenic Corporation, Akron, OH). ‘Dummy' cannulae (model C232DC or C313DC; Plastics One Products) were inserted to maintain patency, with the tips flush with the end of the guide cannulae. When necessary, dust caps (model 303DC/1; Plastics One Products) were secured over the dummy cannulae to prevent their removal.
22-gauge guide cannulae (model C313G; Plastics One Products) were implanted bilaterally into the NAc shell (AP: 1.7 mm; ML: ±1.5 mm; DV: −7.2 mm from Bregma), dlBNST (AP: −0.4 mm; ML: ±3.7 mm; DV: −4.8 mm from Bregma, inserted at a 15° angle), CeA (AP: −2.2 mm; ML: ±4.0 mm; DV: −6.4 mm from Bregma), or VTA (AP: −5.3 mm; ML: ±1.0 mm; DV: −7.2 mm from Bregma). Infusions of 0.3 μl were made over the course of 2 min through 28-gauge infusion cannulae (model C313I; Plastics One Products), with tips that extended 1 mm past the end of the guide. Infusion cannulae were attached with polyethylene tubing to a 5 μl Hamilton microsyringe and were left in place for 1 min following infusions. Infusions were given in a room distinct from the colony and behavioral testing rooms.
Acoustic Startle and Activity
Acoustic startle and activity levels were tested in four identical plastic cages (17 × 8.5 × 11 cm3) resting on compression springs and located within individual ventilated sound-attenuating chambers. Cage movement resulted in the displacement of a piezoelectronic accelerometer (Model ACH-01, Measurement Specialties, Valley Forge, PA) attached to each cage. Voltage output from the accelerometer was filtered and amplified by a custom-built signal processor, digitized on a scale of arbitrary units ranging from 0 to 1000 (National Instruments SCB100 and PCI-6071E boards), and recorded using Matlab (The MathWorks, Natick, MA). Startle amplitude was defined as the peak accelerometer voltage during the first 200 ms after onset of the startle stimulus. High-frequency speakers (Radio Shack Supertweeters, range=5–40 kHz) located 10 cm beside each cage delivered the startle stimuli, which were 50 ms bursts of filtered white noise (low pass: 22 kHz, rise decay <5 ms) at intensities of 95 or 105 dB. Ventilating fans elevated background noise to approximately 60 dB.
Each startle test session consisted of a 5 min acclimation period, followed by presentation of 40 startle stimuli (20 each at 95 or 105 dB in semi-random order) with a 30 s fixed inter-stimulus interval. Activity levels were monitored during the acclimation period and throughout the session. For each experiment, acoustic startle was first tested on 2 consecutive drug-free days. After the second day, average startle amplitudes were used to match animals into groups with similar overall mean startle amplitude. Each test day began with a pre-drug exposure, baseline startle session (pretest), and concluded with a final post-drug exposure startle session (post-test).
Conditioned Place Aversion
The place conditioning apparatus consisted of a rectangular plastic cage (40 × 20 × 20 cm3) divided into two sides by a central partition. Each side had a distinct floor texture and wall color: metal bars paired with white walls and wire mesh paired with black striped walls. Each rat's position within the conditioning chamber was monitored by an overhead video camera connected to a computer running ANY-Maze software (Stoelting, Wood Dale, IL).
Rats were acclimated to the conditioning room for 10 min before each experimental session. The experiment began with a 10 min baseline session in which rats were free to move between both sides of the conditioning chamber. Rats with >75% baseline preference for one side were excluded from further study. The side of the chamber paired with drug treatment was counterbalanced within each experiment, yielding an unbiased procedure in which rats spent on an average 50% of the baseline session on the drug-paired side. Two, daily, 30-min conditioning sessions followed the baseline session. During these sessions, rats were injected with vehicle or drug and confined to one side of the conditioning chamber. A final 10-min test session in which rats were free to move between both sides of the chamber was conducted 24 h after the second conditioning session.
Histology
Animals were deeply anesthetized with Beuthanasia (sodium pentobarbital, 390 mg/kg, intraperitoneally) and perfused intracardially with 0.9% saline, followed by 10% formalin. Brains were subsequently removed and immersed in a 30% sucrose–formalin solution for at least 3 days. Coronal sections (30 μm) from the relevant brain regions were cut, mounted onto gelatin-coated slides, stained with cresyl violet, and scored for correct cannulae placement by an observer who was blind to group assignments.
Experimental Design
Experiment 1: SKF82958 or quinpirole injection during withdrawal from systemic morphine
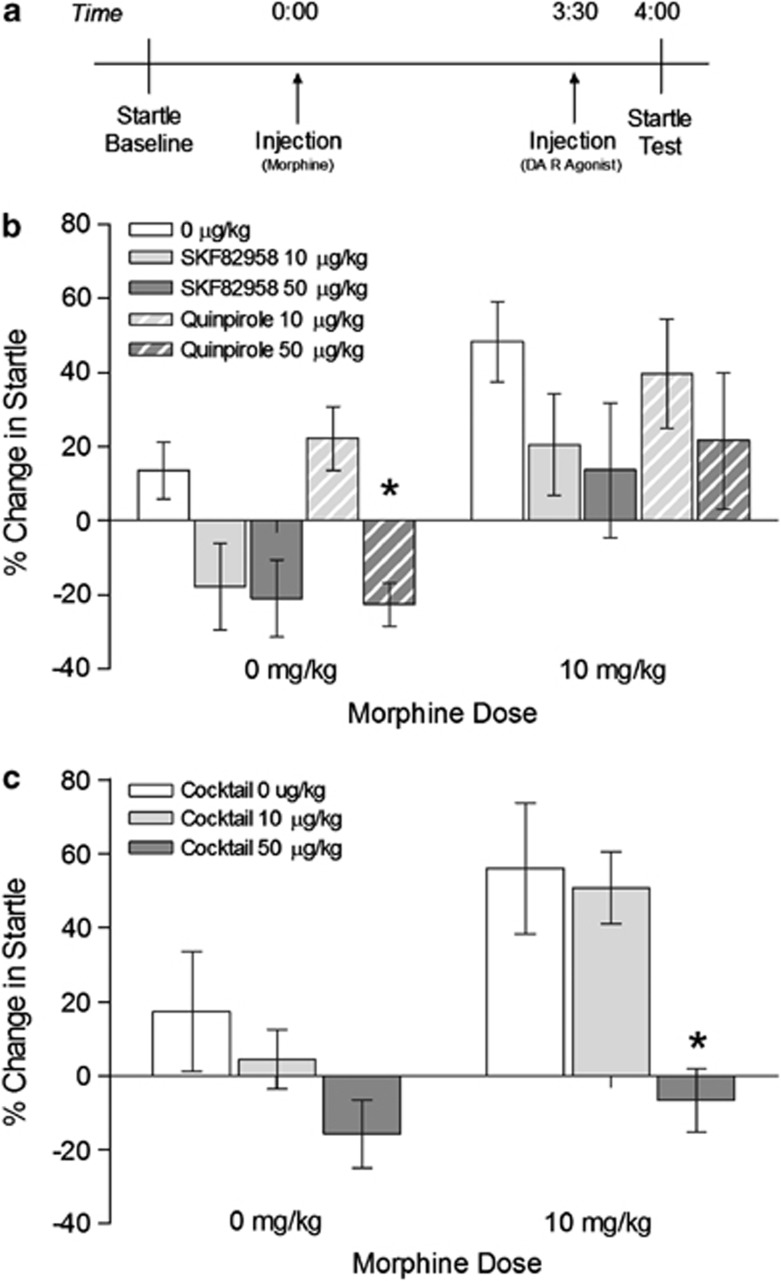
Rats were injected with either 0 or 10 mg/kg of morphine at 0 h, followed by vehicle (N=12) or 10 (N=8) or 50 μg/kg (N=8) of the D1-like receptor agonist SKF82958 or 10 (N=11) or 50 μg/kg (N=8) of the D2-like receptor agonist quinpirole 3 h and 30 min later. Startle was tested at 4 h. A crossover design was used so that each rat was injected with the two doses of morphine in a random order over two consecutive test days.
Experiment 2: SKF82958 and quinpirole cocktail injection during withdrawal from systemic morphine
Rats were injected with either 0 (N=12) or 10 mg/kg (N=11) of morphine at 0 h and received an injection of a cocktail of SKF82958 and quinpirole (0, 10, or 50 μg/kg of each agonist) 3 h and 30 min later. Startle was tested at 4 h. A Latin square design was used so that each rat was injected with the three doses of the dopamine receptor agonist cocktail in a random order over three consecutive test days.
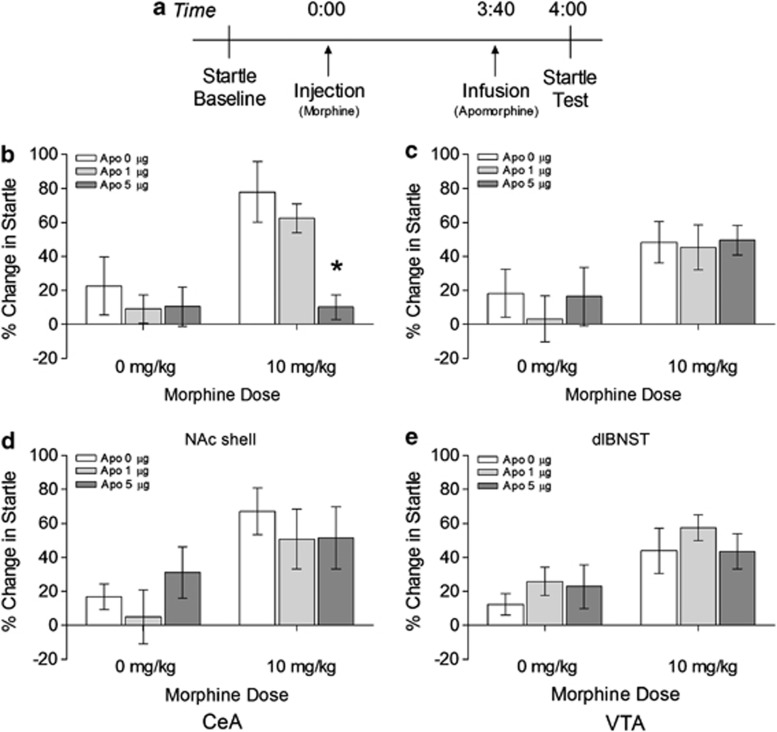
Experiment 3: Apomorphine infusion in local brain structures during withdrawal from systemic morphine
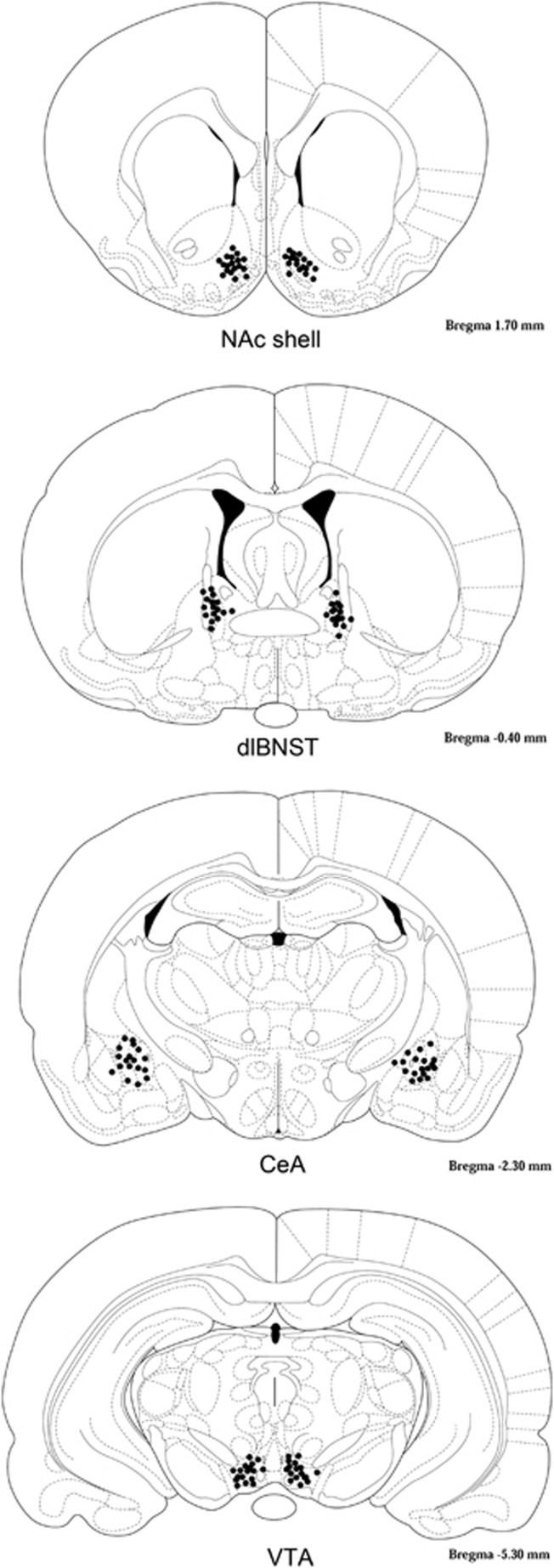
Following bilateral implantation of cannulae into the shell of the NAc, dlBNST, CeA, or VTA, rats were injected with either 0 or 10 mg/kg of morphine at 0 h and received an infusion of apomorphine (0, 1, or 5 μg per side; Willner et al, 1985; Hull et al, 1986) 3 h and 40 min later. Startle was tested at 4 h. A Latin square design was used so that each rat was infused with the three doses of apomorphine in a random order over three test days. Rats received a total of three test days, each separated by two intervening days to prevent tissue damage. In the NAc, 3 animals were removed for misplaced cannulae or large lesions at the infusion site and 1 animal was removed as an outlier, leaving final sample sizes of 7 (0 mg/kg) and 11 (10 mg/kg). In the dlBNST, 10 animals were removed for misplaced cannulae or large lesions at the infusion site and 1 animal was removed because of a blocked cannula, leaving final sample sizes of 8 (0 mg/kg) and 8 (10 mg/kg). In the CeA, 8 animals were removed for misplaced cannulae or large lesions at the infusion site and 1 animal was removed as an outlier, leaving final sample sizes of 7 (0 mg/kg) and 11 (10 mg/kg). In the VTA, 4 animals were removed for misplaced cannulae, leaving final sample sizes of 8 (0 mg/kg) and 9 (10 mg/kg).
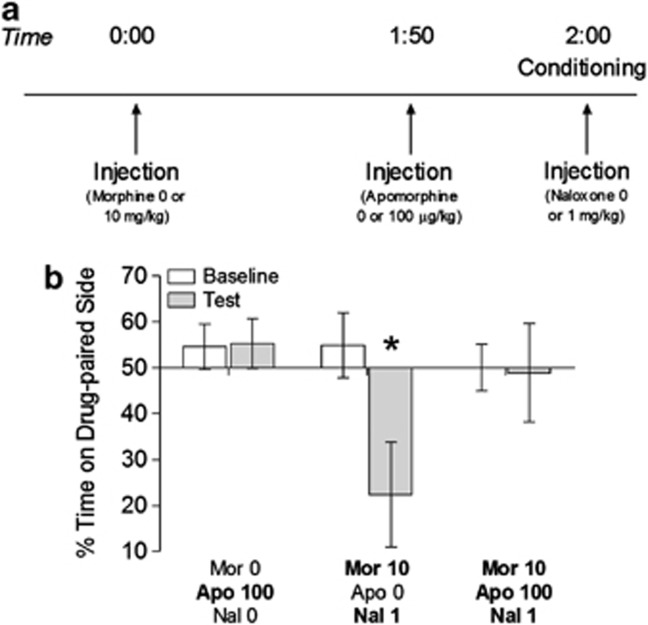
Experiment 4: Apomorphine injection before induction of morphine conditioned place aversion
To determine whether activation of dopamine receptors can relieve another negative affective sign of opiate withdrawal, rats were administered apomorphine (0 or 100 μg/kg) during a 2-day, naloxone-precipitated place conditioning paradigm (Rothwell et al, 2009). One vehicle and one drug conditioning session were conducted over 2 days. There were a total of three groups in this study. To test whether apomorphine alone could produce a place preference, the first group received 0 mg/kg morphine, 100 μg/kg apomorphine, and 0 mg/kg naloxone on the drug-paired side of the conditioning chamber (N=8). A second group went through precipitated morphine withdrawal on the drug-paired side (10 mg/kg morphine, 0 μg/kg apomorphine, and 1 mg/kg naloxone) (N=5) and a third received apomorphine 10 min before this conditioning procedure (10 mg/kg morphine, 100 μg/kg apomorphine, and 1 mg/kg naloxone) (N=8). The second and third groups also received naloxone on the vehicle-paired side of the conditioning chamber to control for any nonspecific aversive effects of naloxone. The timing of the injections is indicated in Figure 4a.
Experiment 5: Apomorphine injection during withdrawal from systemic nicotine
Because repeated nicotine exposure is necessary to observe withdrawal-potentiated startle (Engelmann et al, 2009), rats were injected with 0 (N=10) or 0.25 mg/kg (N=10) nicotine for 7 days. On days 8, 9, and 10, animals were injected with nicotine or saline at 0 h followed by 0, 50, or 100 μg/kg apomorphine hydrochloride 1 h and 50 min later. Startle was tested at 2 h. A Latin square design was used so that rats received each dose of apomorphine once over a series of three consecutive test days.
Data Analysis
Throughout the text and figures, all data are expressed as mean±SEM. Startle data were collapsed across both intensities (95/105 dB) before further statistical analysis, as the magnitude of withdrawal-potentiated startle does not depend on startle stimulus intensity (Harris and Gewirtz, 2004). As there were no effects of order of treatment in any experiment, data were also collapsed across test days. In each experiment, one-way analysis of variance (ANOVA) was conducted to verify that animal weights and baseline startle amplitudes did not differ between experimental groups. Changes in startle or activity after experimental treatment were calculated as the percent change from baseline on the same day, that is, percent change=((test−baseline)/baseline) × 100 (Harris and Gewirtz, 2004). Data were evaluated for outliers with the Grubb's extreme studentized deviate test with a significance level of α=0.01.
Data from all experiments were analyzed with repeated measures ANOVA followed by t-tests corrected for multiple comparisons. Bonferroni adjusted α-levels for each experiment are reported with the results. All statistical analyses were conducted using SPSS (version 17.0) with a type I error rate of α=0.05 (two-tailed).
RESULTS
Experiment 1: SKF82958 or quinpirole injection during withdrawal from systemic morphine
To determine whether the effect of apomorphine seen in our previous experiments (Radke et al., 2011) was mediated by D1- or D2-like dopamine receptors, rats were injected systemically with the D1-like receptor agonist SKF82958 or the D2-like receptor agonist quinpirole (0, 10, or 50 μg/kg) during spontaneous withdrawal from morphine, and startle was tested 10–30 min later (Figure 1a). Repeated measures ANOVA revealed a significant main effect of morphine (F1,42=22.904, p <0.001) and a significant main effect of the dopamine receptor agonists (F4,42=3.562, p=0.014). Neither SKF82958 nor quinpirole significantly decreased morphine withdrawal-potentiated startle at any dose tested (Figure 1b). Quinpirole did, however, significantly reduce startle amplitude on its own (0 μg/kg vs quinpirole 50 μg/kg: t18=3.360, p=0.003; quinpirole 10 μg/kg vs quinpirole 50 μg/kg: t17=3.948, p=0.001; Bonferroni-adjusted α-level=0.0083).
Figure 1.
Activation of both D1- and D2-like receptors relieves withdrawal from systemic morphine. (a) Timeline of test day for Experiments 1 and 2. (b) Withdrawal-potentiated startle was not significantly reduced by SKF82958 or quinpirole treatment, although these treatments did have effects on baseline startle. (c) A cocktail of SKF82958 and quinpirole significantly reduced startle potentiation. *p<0.05 compared to 0 and 10 μg/kg groups.
Experiment 2: SKF82958 and quinpirole cocktail injection during withdrawal from systemic morphine
Because neither SKF82958 nor quinpirole significantly attenuated withdrawal-potentiated startle on its own, the hypothesis that activation of both D1- and D2-like receptors is necessary for dopamine's anxiolytic effects during withdrawal was tested. The animals received a systemic injection of a cocktail of both SKF82958 and quinpirole (0, 10, or 50 μg/kg of each) 3 h and 30 min after morphine or vehicle injection. Startle was tested at 4 h (Figure 1a). Repeated measures ANOVA revealed a significant main effect of morphine (F1,21=8.633, p=0.008) and a significant main effect of the agonist cocktail (F1.726,36.246=9.426, p=0.001). Potentiated startle was significantly decreased in animals given 10 mg/kg of morphine, followed by 50 μg/kg of the agonist cocktail when compared with the 0 μg/kg (t10=3.067, p=0.012) and 10 μg/kg groups (t10=5.271, p <0.001; Bonferroni-adjusted α-level=0.0167) (Figure 1c). Although the 50 μg/kg dose of the cocktail also slightly decreased startle amplitude in animals given 0 mg/kg morphine, this effect was not significant.
Experiment 3: Apomorphine infusion in local brain structures during withdrawal from systemic morphine
Experiments 1 and 2 demonstrated that activation of D1- and D2-like receptors attenuates opiate withdrawal-induced anxiety. To identify the location of the receptors involved in mediating this effect, rats were bilaterally implanted with chronically indwelling cannulae targeted at the NAc shell, dlBNST, CeA, or VTA (Figure 2). On the test day, animals were injected with 0 or 10 mg/kg morphine and 3 h and 40 min later infused with apomorphine (0, 1, or 5 μg per side) (Figure 3a). In the NAc shell, repeated measures ANOVA revealed a significant main effect of morphine (F1,16=10.954, p=0.004) and a significant main effect of apomorphine (F1.519,24.309=5.204, p=0.020). Potentiated startle was significantly decreased in animals that received 10 mg/kg of morphine, followed by 5 μg of apomorphine in the NAc shell when compared to the 0 μg (t10=3.746, p=0.004) and 1 μg groups (t10=5.752, p<0.001; Bonferroni-adjusted α-level=0.0167) (Figure 3b). Repeated measures ANOVA also revealed a significant main effect of morphine in animals treated with apomorphine in the dlBNST (F1,14=8.012, p=0.013), CeA (F1,16=24.707, p <0.001), or VTA (F1,15=8.822, p=0.010). No other significant effects were observed in these groups (Figure 3c and d).
Figure 2.
Cannula tip placements for Experiment 3. Tip locations for animals with correct placements are indicated with black circles.
Figure 3.
Withdrawal from systemic morphine is dependent on dopaminergic signaling in the shell of the nucleus accumbens. (a) Timeline of test day for Experiment 3. (b) Startle potentiation was attenuated by apomorphine infusion into the NAc shell (*p<0.05 compared to 0 and 1 μg groups). (c) Startle potentiation was not reduced by apomorphine infusion into the dorsolateral component of the bed nucleus of the stria terminal (dlBNST). (d) Startle potentiation was not reduced by apomorphine infusion into the central amygdala (CeA). (e) Startle potentiation was not reduced by apomorphine infusion into the ventral tegmental area (VTA).
Because dopamine is also involved in the production of motor behaviors, activity levels (ie, cage displacement during the 5 min acclimation period before startle stimulus presentation) were measured during each startle session. Changes in activity after agonist infusion were calculated as the percent change from baseline on the same day. No increases in activity were observed following infusion of apomorphine into the dlBNST, CeA, or VTA. In the NAc, there was a main effect of infusion that approached significance (F1.449,23.190=2.923; p=0.088). Activity levels following infusion of 5 μg apomorphine into the NAc were increased equally in animals treated with saline and morphine (saline=16.41% morphine=16.91%).
Experiment 4: Apomorphine injection before induction of morphine conditioned place aversion
To determine whether activation of dopamine receptors can relieve other signs of opiate withdrawal, rats were place-conditioned during naloxone-precipitated morphine withdrawal following 0 or 100 μg/kg apomorphine (Figure 4a). Repeated measures ANOVA revealed a significant session × group interaction (F2,18=3.621, p=0.048). Follow-up analyses revealed that animals that went through precipitated morphine withdrawal with 0 μg/kg apomorphine developed a significant aversion to the drug-paired side of the chamber, whereas those receiving 100 μg/kg apomorphine did not (0 μg/kg: t4=3.192, p=0.033; 100 μg/kg: t7=0.117, p=0.910) (Figure 4b). The 100 μg/kg dose of apomorphine also did not produce a place preference when administered alone (t7=0.099, p=0.924).
Figure 4.
Dopamine receptor activation prevents conditioned place aversion to morphine withdrawal. (a) Timeline of conditioning days for Experiment 4. (b) Aversion to the drug-paired side of the conditioning chamber was relieved by apomorphine injection (*p<0.05 compared to baseline).
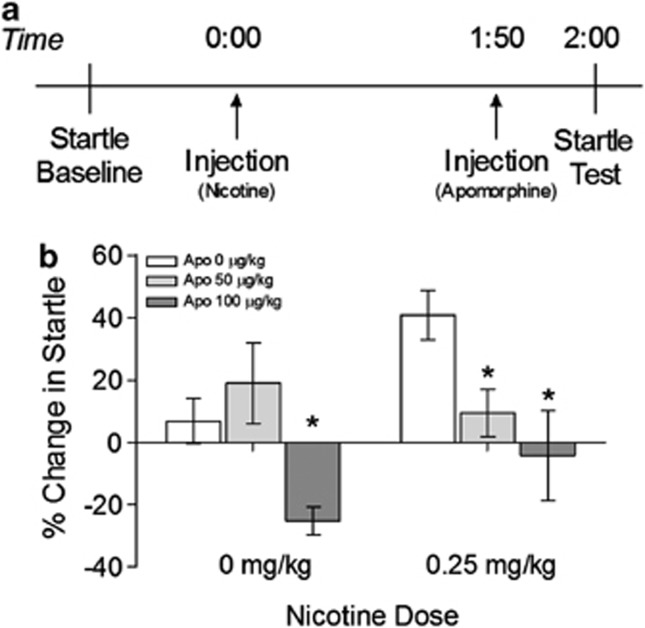
Experiment 5: Apomorphine injection during withdrawal from systemic nicotine
To test whether dopaminergic mechanisms also contribute to withdrawal from another drug of abuse, nicotine withdrawal-potentiated startle was induced by systemically injecting rats with 0 or 0.25 mg/kg of nicotine for 7 days (Engelmann et al, 2009). On the test day, animals received nicotine, followed by a systemic injection of apomorphine hydrochloride (0, 50, or 100 μg/kg) 1 h and 50 min later and startle was tested at 2 h (Figure 5a). Repeated measures ANOVA revealed a significant main effect of apomorphine (F2,36=9.494, p<0.001). Potentiated startle was significantly decreased in animals given 0.25 mg/kg of nicotine, followed by injection of 50 μg/kg (t9=3.124, p=0.012) and 100 μg/kg apomorphine (t9=3.151, p=0.012; Bonferroni-adjusted α-level=0.0167) (Figure 5b). There were no significant differences between the 50 and 100 μg/kg nicotine-apomorphine groups. Startle was also decreased in animals receiving 0 mg/kg nicotine and 100 μg/kg apomorphine when compared to the 0 μg/kg (t9=4.399, p=0.002) and 50 μg/kg groups (t9=3.253, p=0.010; Bonferroni-adjusted α-level=0.0167). Administration of 50 μg/kg apomorphine following 0 mg/kg nicotine did not significantly change startle, demonstrating that the lower dose of apomorphine attenuated withdrawal-potentiated startle without affecting baseline startle.
Figure 5.
Withdrawal from systemic nicotine is dependent on dopaminergic signaling. (a) Timeline of test day for Experiment 5. (b) Startle potentiation was attenuated by apomorphine injection (*p<0.05 compared to 0 μg/kg group). See text for discussion of significant effects in 0 mg/kg group.
DISCUSSION
The experiments described here investigated the role of dopamine in opiate, as well as nicotine, withdrawal. Our previous studies demonstrated that anxiety during withdrawal from acute opiate exposure is dependent on reduced opioid receptor activity in the VTA (Radke et al, 2011). Collectively, the results of the present experiments support the hypothesis that a drop in dopamine receptor activation is the next step in this process. The ability of the general dopamine receptor agonist apomorphine and a cocktail of SKF82958 and quinpirole to prevent completely the expression of opiate withdrawal-potentiated startle suggests that this behavior is dependent on reduced activity at both D1- and D2-like receptors. The effects of apomorphine on nicotine withdrawal-potentiated startle also raise the possibility that changes in dopaminergic activity may represent a shared mechanism involved in the withdrawal syndromes of other classes of abused drugs. Finally, the prevention of conditioned place aversion with the same dose of apomorphine used to reduce morphine withdrawal-potentiated startle (Radke et al, 2011) confirms that dopamine's involvement extends to multiple facets of the opiate withdrawal syndrome.
Because systemic administration of dopamine receptor agonists decreased baseline startle in Experiment 1, a unique contribution of either dopamine receptor subtype alone cannot be ruled out. The effects on baseline startle were surprising, as previous reports have shown dopamine receptor agonists to increase startle (Davis and Aghajanian, 1976; Meloni and Davis, 1999; Meloni and Davis, 2000). Decreases in baseline likely do not account for the results of Experiment 4, as the effect of apomorphine on nicotine withdrawal-potentiated startle was apparent following 50 μg/kg apomorphine, a dose that did not affect baseline startle. In addition, the tendency of the dopamine receptor agonists to decrease baseline startle was modest when compared to the decrease in potentiated startle observed in the morphine/nicotine animals, and this difference was not likely due to a floor effect, as other treatments (eg, presentation of a ‘prepulse') can cause much greater inhibition of startle than was observed here (Gewirtz and Davis, 1995).
In addition to the systemic effects observed, local infusion of apomorphine into the shell of the NAc attenuated withdrawal-potentiated startle in Experiment 3. Apomorphine infusion into the dlBNST, the CeA, or the VTA, on the other hand, did not affect withdrawal-potentiated startle. This result implicates changes in dopaminergic signaling in the shell of the NAc in the expression of withdrawal following systemic morphine exposure. There was a trend for infusion of apomorphine into the NAc shell to increase locomotor activity as well. This increase in locomotor activity cannot explain the decreased startle potentiation seen in these animals, as treatments that increase locomotor activity in saline-injected animals to a similar or greater degree (eg, intra-NAc apomorphine in the current experiment or intra-VTA morphine infusion in previous studies; Radke et al, 2011) do not cause significant decreases in baseline startle. In addition, Cousens et al. (2011) have recently shown that increased locomotor activity immediately before presentation of a startle stimulus does not affect startle amplitude.
Our results suggest that expression of anxiety and aversion during opiate withdrawal likely coincides with a relative decrease in activation of the mesolimbic dopamine system. Decreased dopaminergic activity has been shown to contribute to the production of negative emotional states (Stinus et al, 1990; Liu et al, 2008; Nestler and Carlezon, 2006) and manipulation of dopaminergic signaling attenuates conditioned place aversion (Bechara et al, 1995; Laviolette et al, 2002; Chartoff et al, 2006; but see Caillé et al, 2003) and other signs of opiate withdrawal (Harris and Aston-Jones, 1994; Rodríguez-Arias et al, 1999). Importantly, the initial increase in NAc dopamine release following acute exposure to 10 mg/kg of morphine would have largely dissipated at the time at which spontaneous withdrawal-potentiated startle is observed (Di Chiara and Imperato, 1988; Rothwell et al, 2009). The current finding that apomorphine infusion into the NAc shell prevents the expression of withdrawal-induced anxiety also agrees with the idea that reduced activity within the VTA-to-NAc dopamine projection contributes to negative emotional signs of withdrawal (Diana et al, 1995, 1999).
The results of the SKF82958 and quinpirole studies support the conclusion that diminishing activity at both D1- and D2-like receptors, probably in the NAc shell, is involved in the expression of withdrawal. Both of these receptor subtypes are found in the NAc, although D1-like receptors are more abundant than D2-like receptors (Boyson et al, 1986). While investigations of the contributions of D1- vs D2-like receptors are limited, there is evidence that activation of either receptor subtype can attenuate the somatic signs of opiate withdrawal (Harris and Aston-Jones, 1994; Walters et al, 2000; Chartoff et al, 2006). D1-like receptor agonists also prevent opiate withdrawal-induced aggression (Tidey and Miczek, 1992; Rodríguez-Arias et al, 1999) and conditioned place aversion (Chartoff et al, 2006), although this latter effect appears to be mediated by receptors in the VTA (Chartoff et al, 2009). Combined activation of both D1- and D2-like receptors has also recently been shown to prolong the pauses between bouts of cocaine intake in self-administering rats (Suto and Wise, 2011). This finding suggests that anxiety during periods of drug withdrawal and the motivation to consume additional drug may rely on similar mechanisms. One intriguing possibility is that the cooperative effects of D1- and D2-like receptor agonists on opiate withdrawal and cocaine self-administration are mediated by a heterodimeric D1–D2 dopamine receptor signaling complex. A D1–D2 heterodimer found in the NAc requires activation of both receptors to stimulate intracellular signaling (Rashid et al, 2007), which could explain why only the dopamine receptor agonist cocktail was effective both in the current study and in the experiments by Suto and Wise (2011).
The development of negative emotional signs of withdrawal involves ‘between-systems' adaptations in neural structures mediating negative affective states, such as the amygdala and BNST (Koob and Bloom, 1988; Stinus et al, 1990; Harris et al, 2006; Smith and Aston-Jones, 2008). These between-systems adaptations may be initiated by morphine's effects on the mesolimbic dopamine system. Although infusion of a dopamine receptor agonist into the CeA and dlBNST did not attenuate opiate withdrawal-potentiated startle, these results only demonstrate that a reduction in dopaminergic activity is not necessary for anxiety. It is therefore possible that increased dopamine release in one or both of these structures following morphine exposure is responsible for their recruitment during opiate withdrawal (Stinus et al, 1990; Nakagawa et al, 2005; Harris et al, 2006). Dopaminergic signaling in the BLA, which is necessary for the acquisition of fear-potentiated startle (Nader and LeDoux, 1999; Greba and Kokkinidis, 2000; Greba et al, 2001; Fadok et al, 2009), may also trigger extended amygdala activity. Following opiate exposure, dopamine levels rise and fall in portions of the extended amygdala (Di Chiara and Imperato, 1988; Acquas and Di Chiara, 1992; Spanagel et al, 1992; Wise et al, 1995; Carboni et al, 2000), possibly triggering the release of corticotropin-releasing factor and norepinephrine (Guiard et al, 2008; Kash et al, 2008).
Alternatively, changes in dopaminergic signaling in the NAc may be responsible for the recruitment of the amygdala and the BNST during opiate withdrawal. This could occur via direct projections from the NAc shell to the BNST (Nauta et al, 1978; Usuda et al, 1998) or an indirect pathway from the NAc shell to the ventral pallidum to the amygdala (Nauta et al, 1978; Haber et al, 1985; Usuda et al, 1998). Consistent with this latter possibility, electrical stimulation of the ventral pallidum modulates the amplitude of the acoustic startle reflex (Li et al, 1999). In addition, both the NAc shell and ventral pallidum project to the pedunculopontine tegmental nucleus, a structure that projects to the startle circuit and has been shown to play a role in spontaneous morphine withdrawal (Swanson et al, 1984; Koch et al, 1993; Usuda et al, 1998; Vargas-Perez et al, 2009). A role for the ventral pallidum seems particularly likely given that it is a target of the NAc shell neurons that co-express both D1- and D2-like receptors (Haber et al, 1985; Lu et al, 1997). Clearly, further research is necessary to determine the involvement of dopaminergic coupling of the VTA and extended amygdala in the production of anxiety following acute opiate exposure.
Our final finding that apomorphine attenuated nicotine withdrawal-potentiated startle raises the possibility that the mesolimbic dopamine system may play an important role in the emotional component of withdrawal from a wider range of drugs of abuse, a hypothesis that is supported by a number of findings in the literature. Activation of the mesolimbic dopamine system is a feature shared by all classes of abused drugs (Di Chiara and Imperato, 1988) and, much like opiate withdrawal, the electrophysiological activity and neurochemical output of dopamine neurons is reduced during withdrawal from ethanol (Rossetti et al, 1991, 1992; Diana et al, 1993; Shen 2003; Rada et al, 2004), nicotine (Hildebrand et al, 1998; Rada et al, 2001; Liu and Jin, 2004), and other stimulants (Parsons et al, 1991; Robertson et al, 1991; Rossetti et al, 1992). Furthermore, reduced dopaminergic signaling in the NAc is associated with emotional signs of nicotine withdrawal (Cryan et al, 2003; Paterson et al, 2007). Further confirmation that the mesolimbic dopaminergic system contributes to withdrawal from other addictive drugs would cohere with a growing body of literature demonstrating that it is also involved in a wide variety of aversive behaviors, including conditioned place aversion (Acquas et al, 1989; Calcagnetti and Schechter, 1991; Schechter and Meechan, 1994; Liu et al, 2008), fear conditioning (Borowski and Kokkinidis, 1996; Nader and LeDoux, 1999; Pezze and Feldon, 2004; Fadok et al, 2009; Muschamp et al, 2011), anxiety (Fride and Weinstock, 1988; Barrot et al, 2002; Barrot et al, 2005; Meloni et al, 2006; Rezayof et al, 2009; Richard and Berridge, 2011; Zweifel et al, 2011), intrinsic aversion to gustatory cues (Roitman et al, 2008), conditioned taste aversion (Mark et al, 1991; Fenu et al, 2001), responses to stress (Geyer and Segal, 1974; Herman et al, 1982; Inglis and Moghaddam, 1999; Belda and Armario, 2009), and responses to nociceptive stimulation (Gear et al, 1999; Becerra et al, 2001; Barrot et al, 2002).
Drug exposure involves intrinsic withdrawal episodes that likely contribute to the development of dependence, and these ‘daily' withdrawals occur spontaneously after every drug exposure (Dole et al, 1966; Koob and Le Moal, 1997; Kreek 2000; Baker et al, 2004). The current experiments simulated these conditions by administering discrete injections of morphine and allowing withdrawal to occur spontaneously. These studies therefore offer insight into the neural mechanisms of the type of withdrawal states that participate in the acquisition and maintenance of addictive behavior. The finding that anxiety during withdrawal is dependent on changes in dopamine signaling suggests that the positive reinforcing effects of drugs and the negative affective state that develops as the effects of the drug wear off are initiated by the same circuitry. These results therefore imply that positive and negative sources of reinforcement are mutually interdependent components of drug-taking behavior. Dopamine is hypothesized to serve to make neutral stimuli motivationally relevant (Berridge and Robinson, 1998; Wise, 2004) and these studies emphasize that this is true regardless of whether a stimulus carries a positive or negative valence.
Acknowledgments
We thank Sofiya Hupalo, Jacob Leslie for technical assistance and Dr Mark Thomas for the use of the place conditioning apparatus. This work was funded by grants from NICHD (HD007151 to AKR) and NIDA (DA007097 to AKR and DA018784 to JCG).
The authors declare no conflict of interest.
References
- Acquas E, Carboni E, Di Chiara G. Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol. 1991;193:133–134. doi: 10.1016/0014-2999(91)90214-b. [DOI] [PubMed] [Google Scholar]
- Acquas E, Carboni E, Leone P, Di Chiara G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade. Psychopharmacology (Berl) 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- Acquas E, Di Chiara G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J Neurochem. 1992;58:1620–1625. doi: 10.1111/j.1471-4159.1992.tb10033.x. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barjavel MJ, Scherrmann JM, Bhargava HN. Relationship between morphine analgesia and cortical extracellular fluid levels of morphine and its metabolites in the rat: a icrodialysis study. Br J Pharmacol. 1995;116:3205–3210. doi: 10.1111/j.1476-5381.1995.tb15125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Wallace DL, Bolanos CA, Graham DL, Perrotti LI, Neve RL, et al. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc Natl Acad Sci USA. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Nader K, van der Kooy D. Neurobiology of withdrawal motivation: evidence for two separate aversive effects produced in morphine-naive versus morphine-dependent rats by both naloxone and spontaneous withdrawal. Behav Neurosci. 1995;109:91–105. doi: 10.1037//0735-7044.109.1.91. [DOI] [PubMed] [Google Scholar]
- Belda X, Armario A. Dopamine D1 and D2 dopamine receptors regulate immobilization stress-induced activation of the hypothalamus–pituitary–adrenal axis. Psychopharmacology (Berl) 2009;206:355–365. doi: 10.1007/s00213-009-1613-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience. Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Borowski TB, Kokkinidis L. Contribution of ventral tegmental area dopamine neurons to expression of conditional fear: effects of electrical stimulation, excitotoxin lesions, and quinpirole infusion on potentiated startle in rats. Behav Neurosci. 1996;110:1349–1364. doi: 10.1037//0735-7044.110.6.1349. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral A, Ruggiero RN, Nobre MJ, Brandao ML, Castilho VM. GABA and opioid mechanisms of the central amygdala underlie the withdrawal-potentiated startle from acute morphine. Prog Neuro-Psychoph. 2009;33:334–344. doi: 10.1016/j.pnpbp.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Caillé S, Espejo EF, Rodríguez-Arias M, Minarro J, Cador M, Stinus L. Changes in dopaminergic neurotransmission do not alter somatic or motivational opiate withdrawal-induced symptoms in rats. Behav Neurosci. 2003;117:995–1005. doi: 10.1037/0735-7044.117.5.995. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Conditioned place aversion following the central administration of a novel dopamine release inhibitor CGS 10746B. Pharmacol Biochem Behav. 1991;40:255–259. doi: 10.1016/0091-3057(91)90548-g. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Rolando MTP, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20:RC102 (1–5). doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56 (Suppl 1:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Barhight MF, Mague SD, Sawyer AM, Carlezon WA. Anatomically dissociable effects of dopamine D1 receptor agonists on reward and relief of withdrawal in morphine-dependent rats. Psychopharmacology (Berl) 2009;204:227–239. doi: 10.1007/s00213-008-1454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA. Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26:6450–6457. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens GA, Skrobacz CG, Blumenthal A. Nucleus accumbens carbachol disrupts olfactory and contextual fear-potentiated startle and attenuates baseline startle reactivity. Behav Brain Res. 2011;216:673–680. doi: 10.1016/j.bbr.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Crippens D, Robinson TE. Withdrawal from morphine or amphetamine: different effects on dopamine in the ventral–medial striatum studied with microdialysis. Brain Res. 1994;650:56–62. doi: 10.1016/0006-8993(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Davis M, Aghajanian GK. Effects of apomorphine and haloperidol on the acoustic startle response in rats. Psychopharmacology (Berl) 1976;47:217–223. doi: 10.1007/BF00427605. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Muntoni AS, Pistis M, Melis M, Gessa GL. Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J Neurosci. 1999;11:1037–1041. doi: 10.1046/j.1460-9568.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci USA. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni AS, Gessa GL. Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharmacol Exp Ther. 1995;272:781–785. [PubMed] [Google Scholar]
- Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch Intern Med. 1966;118:304–309. [PubMed] [Google Scholar]
- Engelmann JM, Radke AK, Gewirtz JC. Potentiated startle as a measure of the negative affective consequences of repeated exposure to nicotine in rats. Psychopharmacology (Berl) 2009;207:13–25. doi: 10.1007/s00213-009-1632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978;180:509–531. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci. 2001;21:6897–6904. doi: 10.1523/JNEUROSCI.21-17-06897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–252. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- Fride E, Weinstock M. Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci. 1988;42:1059–1065. doi: 10.1016/0024-3205(88)90561-9. [DOI] [PubMed] [Google Scholar]
- Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci. 1999;19:7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Prolonged activation of mesolimbic dopaminergic neurons by morphine withdrawal following clonidine: participation of imidazoline and norepinephrine receptors. Neuropsychopharmacology. 2003;28:1140–1149. doi: 10.1038/sj.npp.1300161. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Habituation of prepulse inhibition of the startle reflex using an auditory prepulse close to background noise. Behav Neurosci. 1995;109:388–395. doi: 10.1037//0735-7044.109.3.388. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Segal DS. Shock-induced aggression: opposite effects of intraventricularly infused dopamine and norepinephrine. Behav Biol. 1974;10:99–104. doi: 10.1016/s0091-6773(74)91704-0. [DOI] [PubMed] [Google Scholar]
- Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Greba Q, Kokkinidis L. Peripheral and intraamygdalar administration of the dopamine D1 receptor antagonist SCH 23390 blocks fear-potentiated startle but not shock reactivity or the shock sensitization of acoustic startle. Behav Neurosci. 2000;114:262–272. doi: 10.1037//0735-7044.114.2.262. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Mansari ME, Blier P. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus coeruleus, and dorsal hippocampus. Mol Pharmacol. 2008;74:1463–1475. doi: 10.1124/mol.108.048033. [DOI] [PubMed] [Google Scholar]
- Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235:322–335. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- Haertzen CA, Hooks NT. Changes in personality and subjective experience associated with the chronic administration and withdrawal of opiates. J Nerv Ment Dis. 1969;148:606–614. doi: 10.1097/00005053-196906000-00004. [DOI] [PubMed] [Google Scholar]
- Harris AC, Atkinson DM, Aase DM, Gewirtz JC. Double dissociation in the neural substrates of acute opiate dependence as measured by withdrawal-potentiated startle. Neuroscience. 2006;139:1201–1210. doi: 10.1016/j.neuroscience.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 2004;171:140–147. doi: 10.1007/s00213-003-1573-0. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Involvement of D2 dopamine receptors in the nucleus accumbens in the opiate withdrawal syndrome. Nature. 1994;371:155–157. doi: 10.1038/371155a0. [DOI] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Herman JP, Guillonneau D, Dantzer R, Scatton B, Semerdjian-Rouquier L, Le Moal M. Differential effects of inescapable footshocks and of stimuli previously paired with inescapable footshocks on dopamine turnover in cortical and limbic areas of the rat. Life Sci. 1982;30:2207–2214. doi: 10.1016/0024-3205(82)90295-8. [DOI] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilstrom B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998;779:214–225. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Hipps PP, Eveland MR, Meyer ER, Sherman WR, Cicero TJ. Mass fragmentography of morphine: relationship between brain levels and analgesic activity. J Pharmacol Exp Ther. 1976;196:642–648. [PubMed] [Google Scholar]
- Hull EM, Bitran D, Pehek EA, Warner RK, Band LC, Holmes GM. Dopaminergic control of male sex behavior in rats: effects of an intracerebrally-infused agonist. Brain Res. 1986;370:73–81. doi: 10.1016/0006-8993(86)91106-6. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Kungel M, Horst H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res. 1993;97:71–82. doi: 10.1007/BF00228818. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann NY Acad Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Nader K, van der Kooy D. Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res. 2002;129:17–29. doi: 10.1016/s0166-4328(01)00327-8. [DOI] [PubMed] [Google Scholar]
- Li L, Fulton JD, Yeomans JS. Effects of bilateral electrical stimulation of the ventral pallidum on acoustic startle. Brain Res. 1999;836:164–172. doi: 10.1016/s0006-8993(99)01651-0. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Jin WQ. Decrease of ventral tegmental area dopamine neuronal activity in nicotine withdrawal rats. NeuroReport. 2004;15:1479–1481. doi: 10.1097/01.wnr.0000126218.25235.b6. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Ghasemzadeh M, Kalivas P. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1997;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Res. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Davis M. Enhancement of the acoustic startle response in rats by the dopamine D1 receptor agonist SKF 82958. Psychopharmacology (Berl) 1999;144:373–380. doi: 10.1007/s002130051020. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Davis M. GABA in the deep layers of the superior colliculus/mesencephalic reticular formation mediates the enhancement of startle by the dopamine D1 receptor agonist SKF 82958 in rats. J Neurosci. 2000;20:5374–5381. doi: 10.1523/JNEUROSCI.20-14-05374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA. Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Van't Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, et al. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, LeDoux JE. Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci. 1999;113:891–901. doi: 10.1037//0735-7044.113.5.891. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, et al. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience. 2005;134:9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB. Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. Eur J Neurosci. 2007;25:3099–3108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res. 1991;566:348–350. doi: 10.1016/0006-8993(91)91724-f. [DOI] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rada P, Johnson DF, Lewis MJ, Hoebel BG. In alcohol-treated rats, naloxone decreases extracellular dopamine and increases acetylcholine in the nucleus accumbens: evidence of opioid withdrawal. Pharmacol Biochem Behav. 2004;79:599–605. doi: 10.1016/j.pbb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Radke AK, Rothwell PE, Gewirtz JC. An anatomical basis for opponent process mechanisms of opiate withdrawal. J Neurosci. 2011;31:7533–7539. doi: 10.1523/JNEUROSCI.0172-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, et al. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayof A, Hosseini S, Zarrindast M. Effects of morphine on rat behaviour in the elevated plus maze: The role of central amygdala dopamine receptors. Behav Brain Res. 2009;202:171–178. doi: 10.1016/j.bbr.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D1 alone for appetitive eating but D1 and D2 together for fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MW, Leslie CA, Bennett JP. Apparent synaptic dopamine deficiency induced by withdrawal from chronic cocaine treatment. Brain Res. 1991;538:337–339. doi: 10.1016/0006-8993(91)90451-z. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Arias M, Pinazo J, Minarro J, Stinus L. Effects of SCH 23390, raclopride, and haloperidol on morphine withdrawal-induced aggression in male mice. Pharmacol Biochem Behav. 1999;64:123–130. doi: 10.1016/s0091-3057(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Melis F, Carboni S, Gessa GL. Marked decrease of extraneuronal dopamine after alcohol withdrawal in rats: reversal by MK-801. Eur J Pharmacol. 1991;200:371–372. doi: 10.1016/0014-2999(91)90600-u. [DOI] [PubMed] [Google Scholar]
- Rothwell PE, Thomas MJ, Gewirtz JC. Distinct profiles of anxiety and dysphoria during spontaneous withdrawal from acute morphine exposure. Neuropsychopharmacology. 2009;34:2285–2295. doi: 10.1038/npp.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD, Meechan SM. Conditioned place aversion produced by dopamine release inhibition. Eur J Pharmacol. 1994;260:133–137. doi: 10.1016/0014-2999(94)90329-8. [DOI] [PubMed] [Google Scholar]
- Shen RY. Ethanol withdrawal reduces the number of spontaneously active ventral tegmental area dopamine neurons in conscious animals. J Pharmacol Exp Ther. 2003;307:566–572. doi: 10.1124/jpet.103.053371. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl) 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- Stinus L, Le Moal M, Koob GF. Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience. 1990;37:767–773. doi: 10.1016/0306-4522(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Suto N, Wise RA. Satiating effects of cocaine are controlled by dopamine actions in the nucleus accumbens core. J Neurosci. 2011;31:17917–17922. doi: 10.1523/JNEUROSCI.1903-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Mogenson GJ, Gerfen CR, Robinson P. Evidence for a projection from the lateral preoptic area and substantia innominata to the mesencephalic locomotor region in the rat. Brain Res. 1984;295:161–178. doi: 10.1016/0006-8993(84)90827-8. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Morphine withdrawal aggression: modification with D1 and D2 receptor agonists. Psychopharmacology (Berl) 1992;108:177–184. doi: 10.1007/BF02245304. [DOI] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting-A Kee R, Walton CH, Hansen DM, Razavi R, Clarke L, et al. Different neural systems mediate morphine reward and its spontaneous withdrawal aversion. Eur J Neurosci. 2009;29:2029–2034. doi: 10.1111/j.1460-9568.2009.06749.x. [DOI] [PubMed] [Google Scholar]
- Walters CL, Aston-Jones G, Druhan JP. Expression of fos-related antigens in the nucleus accumbens during opiate withdrawal and their attenuation by a D2 dopamine receptor agonist. Neuropsychopharmacology. 2000;23:307–315. doi: 10.1016/S0893-133X(00)00113-5. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Muscat R. Apomorphine anorexia: a behavioural and neuropharmacological analysis. Psychopharmacology (Berl) 1985;87:351–356. doi: 10.1007/BF00432720. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, et al. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–628. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]