Abstract
Described herein is the efficient synthesis and evaluation of bioactive arginine-glycine-aspartic acid (RGD) functionalized polynorbornene based materials for cell adhesion and spreading. Polynorbornenes containing either linear or cyclic RGD peptides were synthesized by ring-opening metathesis polymerization (ROMP) using the well-defined ruthenium initiator [(H2IMes)(pyr)2(Cl)2Ru=CHPh]. The random copolymerization of three separate norbornene monomers allowed for the incorporation of water-soluble polyethylene glycol (PEG) moieties, RGD cell recognition motifs, and primary amines for post-polymerization cross-linking. Following polymer synthesis, thin-film hydrogels were formed by cross-linking with bis(sulfosuccinimidyl) suberate (BS3), and the ability of these materials to support human umbilical vein endothelial cell (HUVEC) adhesion and spreading was evaluated and quantified. When compared to control polymers containing either no peptide or a scrambled RDG peptide, polymers with linear or cyclic RGD at varying concentrations displayed excellent cell adhesive properties in both serum-supplemented and serum-free media. Polymers with cyclic RGD side chains maintained cell adhesion and exhibited comparable integrin binding at a 100-fold lower concentration than those carrying linear RGD peptides. The precise control of monomer incorporation enabled by ROMP allows for quantification of the impact of RGD structure and concentration on cell adhesion and spreading. The results presented here will serve to guide future efforts for the design of RGD functionalized materials with applications in surgery, tissue engineering, and regenerative medicine.
Keywords: polynorbornenes, ROMP, RGD, thin films, HUVEC, cell adhesion
INTRODUCTION
Synthetic polymers are important as materials for biomedical applications including uses in implants, tissue engineering, and regenerative medicine.1–3 Many of these materials have the desired mechanical properties, stability, and elasticity, while remaining non-toxic. Nevertheless, inadequate in vivo interaction remains a problem, often leading to foreign body responses that prevent the clinical use of these materials.1 Cell adhesion, migration, and intracellular signaling in vivo are mediated by integrins, a class of heterodimeric transmembrane receptors that bind extracellular matrix proteins such as fibronectin.4 One approach to promote biological recognition of synthetic materials is the incorporation of cell adhesive proteins or peptides, thereby promoting cell recognition via interactions with integrins.1–4
The use of small peptides has significant benefits relative to coating synthetic surfaces with matrix proteins such as fibronectin, which suffer from susceptibility to enzymatic degradation, immunogenicity, and high cost.1–3 The RGD sequence is the minimal binding domain of fibronectin necessary to recognize cell surface integrins,5–7 and materials modified with RGD peptides have been shown to facilitate cell adhesion, spreading, and wound healing via enhanced rates of migration of individual cells.8–10 In similar fashion, materials formed from cross-linked artificial extracellular matrix proteins presenting the RGD sequence promote cell spreading and adhesion in a manner that can be modulated by varying the density of the RGD ligand.11–14
Significant work has been reported regarding the interaction of structurally varied RGD peptides with αvβ3 and α5β1 integrin receptors due to their central roles in homeostasis and disease.15–17 In particular, cyclic RGD peptides have been shown to exhibit improved affinity, receptor selectivity, and enzymatic stability relative to linear peptides.18–21 Kessler and coworkers have developed a class of cyclic RGD peptides that have been reported to demonstrate subnanomolar affinity for the αvβ3 receptor and low nanomolar affinity for the α5β1 receptor.16,22,23 The functionalization of surfaces with cyclo(RGDfK) peptides was found to stimulate osteoblast adhesion and proliferation, while the soluble peptide cyclo(RGDf(NMe)V) is currently in phase III trials for treatment of glioblastoma.22,23 Few efficient methods for the incorporation of cyclic RGD peptides in synthetic materials have been described with applications for promoting cell adhesion and spreading.1,23
Synthetic polymers covalently modified with RGD peptides can be formed by blending, co-polymerization or post-polymerization modification, methods that do not ensure maximal incorporation of the bioactive peptide and often provide little control over polymer molecular weights.1,24 Ring-opening metathesis polymerization (ROMP) is an ideal method for polymer synthesis because of its high functional group tolerance, excellent control of molecular weight, and ability to provide narrow PDIs.25–27 Notably, ROMP polymers containing polynorbornene backbones have been demonstrated to be non-toxic in a variety of systems including mammalian cell lines.25,28–30 Methods for the post-polymerization modification of ROMP polymers with cyclo(RGDfK) have recently been described with applications for tumor imaging.31,32 Seminal studies reported ROMP of norbornene monomers covalently modified with bioactive peptides, including the RGD motif, for the synthesis of oligomers as multivalent ligands for inhibition of cell adhesion.33,34,35 These early studies examined only soluble materials and employed a linear variant of the RGD peptide. Since these initial reports, significant improvements in ruthenium-based catalysts for ROMP have been reported allowing for rapid initiation at room temperature and greater catalyst lifetimes. These improved catalysts provide increased stability to the polar functional groups found in peptides, and therefore, they give longer polymer chains, uniform incorporation of peptides, and low PDIs with monomodal distributions.36, 37
We sought to efficiently access a new class of RGD functionalized materials that are amenable to post-polymerization cross-linking and that exhibit cell adhesive properties. To this end, we synthesized linear and cyclic RGD conjugated norbornenes that were copolymerized using ROMP. This work constitutes the first application of a recently reported bis-pyridine ruthenium catalyst for ROMP of complex RGD-conjugated norbornenes, providing copolymers with precise control of monomer incorporation and excellent control of molecular weight and PDIs. The synthesis of RGD-containing polymers described here is a vast improvement from previous reports using ROMP. Following polymer cross-linking, the resulting thin-film hydrogels were quantitatively evaluated to study the impact of RGD structure and content on HUVEC adhesion and spreading. Strikingly, cross-linked polynorbornene thin films containing cyclic RGD were observed to be effective at a 100-fold lower concentration than those containing the linear peptide at supporting cell adhesion and spreading under serum-free conditions.
EXPERIMENTAL SECTION
Polymer Synthesis General Procedure
The random copolymers were synthesized via ROMP with catalyst [(H2IMes)(pyr)2(Cl)2Ru=CHPh] (7) at [M]0/[C]0=99 (Scheme 2). For all polymerizations, the protected amine monomer 2 (Nor-Amine) was held constant at 20 mol%, and the percentage of RGD (3 or 4) or control RDG peptides (5 or 6) was varied from 0 to 10 mol%. The norbornene-PEG monomer 1 (Nor-PEG) was added as needed to keep the ratio of monomers to catalyst constant. The polymerization reactions were run for 35 min, and complete incorporation of monomers was confirmed by 1H NMR. Reactions were terminated upon addition of ethyl vinyl ether38 followed by treatment with tris(hydroxymethyl)phosphine (THMP) for removal of ruthenium.39 The polymers were precipitated into diethyl ether (Et2O), and the side chains were deprotected with a mixture of trifluoroacetic acid (TFA), triisopropylsilane (TIPS), and water (95:2.5:2.5). Precipitation into Et2O, followed by purification via dialysis (MWCO 25000), and lyophilization of the aqueous solutions provided the pure polymers for biological evaluation. Polymers were stored as 10 wt% solutions in Nanopure water at −20 °C. Polymers with a range of molecular weights were synthesized by varying catalyst loadings from 0.5 to 4% while keeping the Nor-cycRGD monomer 4 constant at 1%. The polydispersity indices (PDI) and average molecular weights (Mn) were calculated by gel permeation chromatography (GPC) following polymerization reactions. Complete side-chain deprotection of all polymers was confirmed by 1H NMR. The synthetic details and characterization of monomers and copolymers, including a representative polymerization procedure, are provided in the Supporting Information.
Scheme 2.
Random copolymerizations of norbornene monomers via ROMP.
Kinetic Analysis
A stock solution (1 mL) of the specified monomer at 0.02 M in CD2Cl2/CD3OD (4:1) was added to an oven dried septum screw cap NMR tube under an inert atmosphere. The ruthenium initiator 7 (10 μL of a 0.02 M solution in CD2Cl2) was then added to the NMR tube through the septum cap and inverted quickly to mix the solution. The tube was quickly placed in the NMR spectrometer (Varian Mercury 500 MHz) making note of the time before the first acquisition. Spectra were acquired at 26-second intervals (8 scans) using a 0.5 second interval between scans for proton relaxation. The polymerization was monitored until complete consumption of starting monomers. The percent conversion was calculated from integrations of [polymer]/[polymer + monomer] over time (see Supporting Information).
Preparation of Thin-Film Hydrogels
Samples for biological evaluation were prepared on 12 mm (Deckglaser) cover glasses that were functionalized with (3-aminopropyl)triethoxysilane.40,41 Amine functionalization was performed by soaking coverslips in saturated KOH in EtOH for 5 min. The coverslips were then rinsed with Nanopure water, treated with 6N aq. NaOH for 5 min, rinsed again with water, and allowed to dry under a stream of air. The coverslips were added to a 95% EtOH/H2O solution containing 2% (3-aminopropyl)triethoxysilane and allowed to soak for 2 min. The coverslips were then transferred to a MeOH bath, removed, and allowed to dry in air.
A 2.5 wt% solution of desired polymer in Nanopure water was added to 1 eq. of bis(sulfosuccinimidyl) suberate (BS3) relative to the primary amine in the polymer. An amine functionalized coverslip was placed on the spin coater (Specialty Coating Systems, 6800 Spin Coater Series). Approximately 70 μL of the 2.5 wt% polymer/BS3 solution was carefully added covering the entire glass surface. The sample was then allowed to coat the surface at 2500 rpm for 30 seconds. All coated samples were allowed to dry overnight to provide thin-film hydrogels for subsequent cell adhesion, viability, and spreading studies. AFM analyses of representative thin films are included in the Supporting Information.
Cell Culture
Human umbilical vein endothelial cells (HUVECs) (Lonza, CC-2519) were cultured at 37 °C under a humidified atmosphere of 5% CO2. The cells were grown in endothelial cell growth medium (Cell Applications, 211–500) supplemented with 1% penicillin/streptomycin (Gibco, 105140122). The cells were continuously maintained in the culture medium and sub-cultured every 3–4 days up to 10 passages. Cells from passages 6–10 were used in studies of adhesion, viability, and spreading.
Cell Adhesion Studies
Cells at 70–80% confluence were washed with warm PBS, detached, and resuspended in either regular growth medium or serum-free medium (Cell Applications, 113–500). An aliquot of 5 × 104 cells was seeded per well in a 24-well plate containing polymer-coated coverslips. Cells were allowed to attach for 24 h in the incubator before examination by phase contrast microscopy.
Cell Viability
Cell viability on thin-film hydrogels was assessed using a Live/Dead Viability kit (Invitrogen, L3224). HUVECs were seeded at a density of 5 × 104 cells per well in a 24-well plate containing coated coverslips. Cells were allowed to grow for 24 h in serum-supplemented or serum-free media in an incubator prior to staining according to the manufacturer’s protocol. Cells were then imaged on an inverted epifluorescence microscope (IX71, Olympus) with a humidified chamber and temperature control at 37 °C. Images were taken with identical acquisition settings and processed using ImageJ 1.45s (National Institutes of Health, USA). Cell viability experiments were run in triplicate.
Cell Spreading
HUVECs were seeded at a concentration of 5 × 104 cells per well in a 24-well plate in serum-free medium. Cells were imaged on thin-film hydrogels at 15, 30, 45, 60, 90, 120, 180, 240, and 300 min. Images were taken using a 10× phase contrast objective on a Nikon Eclipse TE300 inverted microscope. Images were manually scored for the number of spread versus nonspread (rounded) cells. Spreading experiments were run in triplicate.
RESULTS AND DISCUSSION
Monomer and Polymer Design and Synthesis
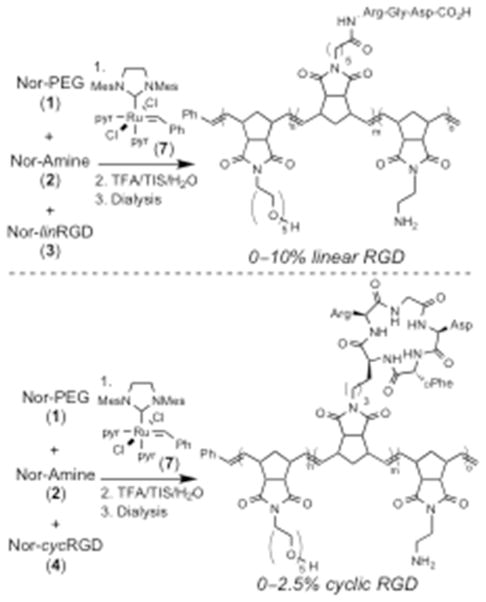
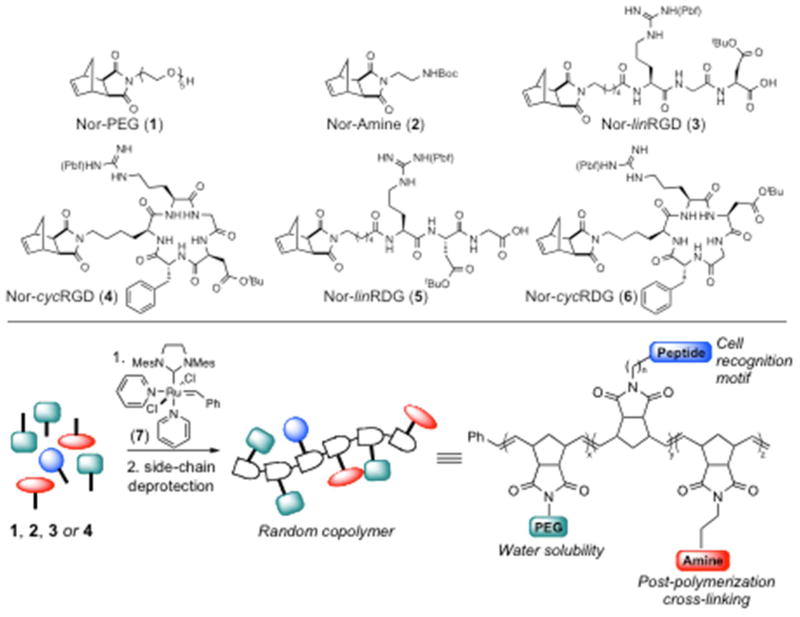
The random copolymers were designed to incorporate three separate monomers: a water-soluble PEG unit (1), the RGD cell recognition motif (3 or 4), and a reactive amine functional group (2) for post-polymerization cross-linking (Figure 1). Monomers 1 and 2 were synthesized readily using previously reported condensation reactions of cis-5-norbornene-exo-2,3-dicarboxylic anhydride with the corresponding amines.37,42 Peptide substituted norbornene monomers (3–6) were synthesized via Fmoc solid phase peptide synthesis (SPPS) on 2-chlorotrityl chloride resin. Notably, monomers 3–6 were designed to incorporate a linker between the highly functionalized peptide and the ROMP reactive norbornene unit, thereby ensuring good reactivity in polymerizations. For monomers Nor-linRGD (3) or Nor-linRDG (5), N-(hexanoic acid)-cis-5-norbornene-exo-dicarboximide was coupled to the amino terminus of the peptides on resin. The monomers were then cleaved from the resin using mild acidic conditions providing side-chain protected, norbornene-conjugated peptides 3 and 5, which were used in polymerization reactions without further purification.
Figure 1.
Ruthenium catalyzed ROMP of norbornene monomers (top) for the synthesis of random copolymers incorporating water soluble PEGs, RGD peptides, and amines for post-polymerization cross-linking (bottom).
The structure of monomer Nor-cycRGD (4) was designed based on the tumor targeting αvβ3 antagonist cyclo(RGDfK) and its more potent dimer.43,44 Therefore, the synthesis of monomer Nor-cycRGD (4) was initiated by SPPS of the side-chain protected linear sequence NH2-Asp(OtBu)-DPhe-Lys(Nor)-Arg(Pbf)-Gly-CO2H (8), where the lysine side chain incorporates the norbornene functionality (Scheme 1). Cyclization of 8 with propylphosphonic anhydride (T3P), following the procedure of Liu et. al.,45 provided Nor-cycRGD (4) in excellent yields (85%). The control monomer Nor-cycRDG (6) was synthesized by a similar method with simple transposition of the Asp(OtBu) and Gly residues during SPPS of the linear peptide prior to cyclization.
Scheme 1.

Synthesis of Nor-cycRGD (4).
Polymers were synthesized readily from norbornene monomers 1–6 by initiation with ruthenium catalyst 7 (Scheme 2). Ruthenium catalysts containing bis-pyridine ligands have been demonstrated to initiate rapidly at room temperature, and therefore, provide good molecular weight control of the resulting polymers.36,46 All copolymerizations were run for 35 min assuring complete reaction of all monomers, and terminated with the addition of ethyl vinyl ether.38 The RGD content in the polymers was controlled by varying the percentage of the desired RGD monomer (3 or 4) in the monomer feed prior to initiation with 7. For all polymerizations, 20% of the Nor-Amine (2) was incorporated with variable amounts of Nor-PEG (1) in order to keep the monomer to catalyst ratio constant. Polymers containing linear RGD peptides were synthesized with a range of 0–10% Nor-linRGD (3) in the monomer feed. Polymers with no peptide or 10% of the scrambled sequence lin-RDG were also made as negative controls for biological evaluation. Polymers with cyclic peptide were synthesized with a range of 0–2.5% Nor-cycRGD (4) in the polymerization reactions. A negative control polymer containing 2.5% scrambled cyc-RDG was prepared for biological studies. Polymers containing cyclic RGD were prepared with lower peptide concentrations due to the enhanced binding affinity of the cyclic peptide for cell surface integrins.15–21
Because of the complexity of peptide substituted norbornene monomers (3–6), we examined if polymerization reactions were characterized by a linear dependence of average molecular weight (Mn) on monomer to catalyst ratio ([M]0/[C]0) thereby indicating a living polymerization.25–27,47 Polymers with 1% cyc-RGD were synthesized at varying monomer to catalyst ratios ([M]0/[C]0=199, 99, 49, and 24, Table 1 entries 7, 3, 8, and 9 respectively), and the molecular weights (Mn) of these polymers were found to increase linearly with increased [M]0/[C]0 (see Supporting Information). Consistent with the living nature of these polymerizations, all protected polymers were isolated in excellent yields (95–100%) and gave narrow PDIs (1.04–1.11). GPC analysis for all polymers showed good control of molecular weight as well as monomodal distributions for all polymers (see Table 1 for representative GPC data). Precise control of molecular weight and low PDI values are important factors for the use of ROMP as a general method for the synthesis of polymers. Additionally, since soluble polymers would inhibit cell adhesion,33,34 narrow PDIs assure consistent cross-linking of polymers. Following polymerization, the side-chain protecting groups were cleaved with TFA and purified by dialysis. The aqueous solutions were lyophilized to provide white foams in good yields (60–78%) and were characterized by 1H NMR to assure complete deprotection of the polymer side chains.
Table 1.
GPC Data for random copolymers incorporating linear or cyclic RGD peptides.
| Entry | Protected Polymer* | [M]0/[C]0 | Mn GPC (kDa) | Mn theo (kDa) | PDI |
|---|---|---|---|---|---|
| 1 | 10% lin-RGD | 99 | 52 | 42 | 1.05 |
| 2 | 10% lin-RDG | 99 | 49 | 42 | 1.06 |
| 3 | 1% cyc-RGD | 99 | 45 | 37 | 1.05 |
| 4 | 0.1% cyc-RGD | 99 | 47 | 36 | 1.07 |
| 5 | 2.5% cyc-RDG | 99 | 48 | 38 | 1.04 |
| 6 | No peptide | 99 | 45 | 36 | 1.04 |
| 7 | 1% cyc-RGD | 199 | 74 | 74 | 1.11 |
| 8 | 1% cyc-RGD | 49 | 25 | 18 | 1.05 |
| 9 | 1% cyc-RGD | 24 | 15 | 8.9 | 1.10 |
Determined by GPC with 0.2M LiBr/DMF solution. GPC data collected post-polymerization with side chains protected. GPC traces and data for all other polymers can be found in the Supporting Information.
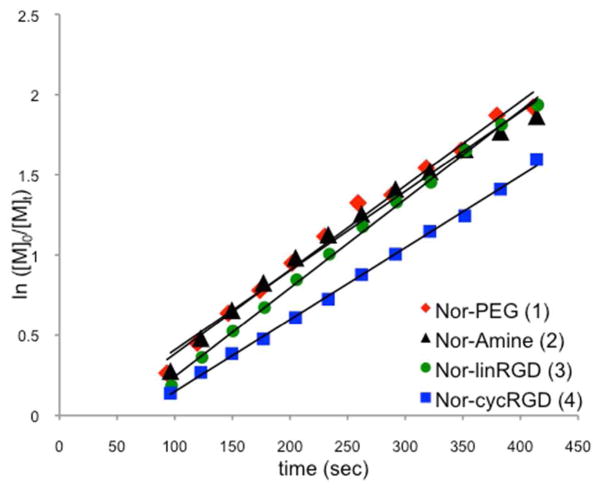
In order to determine if the copolymers described here were true random copolymers or contained gradients in composition, we examined the relative rates of incorporation for norbornene monomers 1–4 in homopolymerization reactions. Previously reported polymerizations with other peptide-conjugated norbornenes have demonstrated kinetic evidence for random copolymer formation.37 Kinetic studies were conducted by observing the disappearance of monomer and appearance of polymer by 1H NMR at monomer to catalyst ratios of 100:1. First order kinetics was observed for all monomers examined with similar rates of incorporation for homopolymer formation (Figure 2). Monomer Nor-cycRGD (4) reacts at a slightly lower rate than monomers 1–3, possibly due to its larger size and increased steric hindrance closer to the reactive norbornene moiety. Nevertheless, all homopolymerization reactions reached full conversion within 12 min, indicating that RGD content in the final polymers can be controlled by the initial monomer feed ratios.
Figure 2.
Log plot of the homopolymerization of monomers 1–4 with [M]0:[C]0=100:1 in 4:1 CD2Cl2/CD3OD. Linear least-square fitting provided the following slopes [kobs (sec−1)]: monomer 1: 0.0052, monomer 2: 0.0049, monomer 3: 0.0054, monomer 4: 0.0045.
Following synthesis and characterization of RGD functionalized polymers and controls, samples for cell adhesion were prepared. A 2.5 wt% aqueous solution of polymer in the presence of BS3 was spin-coated on amine functionalized coverslips. The coverslips were allowed to dry overnight prior to biological evaluation. Representative thin films were examined by AFM and ranged in thickness from 54 to 65 nm as dry films; upon absorption of water, the films swelled to 79–87 nm. The films were homogeneous and smooth.
Cell Adhesion and Viability
Cell adhesion was initially evaluated qualitatively via phase contrast microscopy 24 h post seeding of HUVECs on cross-linked polynorbornene thin films. A range of 1–10% linear RGD containing polymers (Table 2, 11–14) were examined and compared to controls that contained either no peptide (9) or the scrambled polymer with 10% lin-RDG (10). Polymers containing linear RGD required peptide concentrations above 1% in order to promote cell attachment after 24 h. As expected, control polymers 9 and 10 did not promote cell adhesion. Polymer 14, carrying 10% lin-RGD, was used as a comparison with cyclic RGD polymers since thin films of 14 demonstrated good cell attachment.
Table 2.
Cell adhesion on polymer thin films containing linear RGD peptides.
| Polymer | % linear RGD | % linear RDG (control) | Cell Adhesion (+/−) |
|---|---|---|---|
| 9 | 0 | 0 | − |
| 10 | 0 | 10 | − |
| 11 | 1 | 0 | − |
| 12 | 2.5 | 0 | + |
| 13 | 5 | 0 | + |
| 14 | 10 | 0 | + |
Polymers incorporating cyclic RGD at concentrations between 0.01 and 2.5% (Table 3, 16–22) were subsequently examined for their ability to support cell adhesion and survival 24 h post seeding. Consistent with enhanced binding, these cyclic RGD containing thin films supported cell adhesion at concentrations as low as 0.05% (17), a 50-fold lower concentration than the minimum RGD concentration for linear RGD films. Positive cell adhesion of these synthetic polynorbornene thin films corresponds to integrin recognition of RGD concentrations in the low nanomolar range. Cell adhesion with 2.5% cyc-RGD (22) was observed to be comparable to that observed on thin films bearing 1% cyc-RGD (21). From the results of these cell adhesion assays, thin films containing 1% cyc-RGD (21) and 0.1% cyc-RGD (18) were examined quantitatively in cell viability and spreading assays.
Table 3.
Cell adhesion on polymer thin films containing cyclic RGD peptides.
| Polymer | % cyclic RGD | % cyclic RDG (control) | Cell Adhesion (+/−) |
|---|---|---|---|
| 9 | 0 | 0 | − |
| 15 | 0 | 2.5 | − |
| 16 | 0.01 | 0 | − |
| 17 | 0.05 | 0 | + |
| 18 | 0.1 | 0 | + |
| 19 | 0.25 | 0 | + |
| 20 | 0.5 | 0 | + |
| 21 | 1 | 0 | + |
| 22 | 2.5 | 0 | + |
Cell viability was assessed using a Live/Dead Viability assay (fluorescent dyes calcein-AM and ethidium homodimer-1), 24 h after seeding on polymer thin-film samples carrying either linear or cyclic RGD. Fluorescence images were acquired, and the number of live cells (green) versus dead cells (red) was quantified for thin films containing 10% lin-RGD (14), 1% cyc-RGD (21), and 0.1% cyc-RGD (18) (Figure 3). Negative control samples included scrambled sequences 10% lin-RDG (10), 2.5% cyc-RDG (15), and a polymer film with no peptide (9). Under serum-supplemented conditions (Figure 3, A), 87% of cells were alive in samples with 10% lin-RGD, while both cyc-RGD samples provided 95% cell viability. Examination of the fluorescence images illustrates that the negative controls have no attached cells (Figure 3, C–E). While cells exhibit similar characteristics on films with 10% lin-RGD (Figure 3, F) and 0.1% cyc-RGD (Figure 3, H), samples with 1% cyc-RGD (Figure 3, G) show an enhanced ability to support cell attachment. Under more stringent serum-free conditions (Figure 3, B), a significant decrease in cell viability was observed for 10% lin-RGD (59%) and modest decreases were found for samples with 0.1% cyc-RGD (81%) and 1% cyc-RGD (89%). As shown in Figure 3 (B, L and N), both 10% lin-RGD (Figure 3, L) and 0.1% cyc-RGD (Figure 3, N), which differ 100-fold in peptide concentration, demonstrate similar levels of cell survival. Impressively, samples with 1% cyc-RGD (Figure 3, M) show similar adhesion and spreading in serumfree and serum-supplemented conditions. The superior cell viability of thin-film samples containing cyclic RGD peptides is consistent with the enhanced binding affinity of cyclic peptides for integrins, leading to increased cell adhesion and survival on cyc-RGD polynorbornene thin films. Specifically, the binding of cyclic RGD peptides to the αvβ3 integrins, which are overexpressed in HUVECs, has been reported to be subnanomolar in affinity.22,23
Figure 3.
Quantitative and qualitative assessment of HUVEC cell viability on cross-linked polynorbornenes using a fluorescent Live/Dead Viability assay. Green fluorescence (calcein-AM) indicates live cells and red fluorescence (ethidium homodimer-1) indicates dead cells. Cell viability in serum-supplemented media (A) and serum-free media (B) 24 h post seeding. Fluorescence images of HUVEC adhesion after 24 hours in serum media (C–H) of polynorbornene thin films incorporating no peptide (C), 10% lin-RDG peptide (D), 2.5% cyc-RDG (E), 10% lin- RGD (F), 1% cyc-RGD (G), and 0.1% cyc-RGD (H). Fluorescence images of similar samples in serum-free media are shown in I–N. Scale bars = 100 μm.
Cell Spreading
The time frame for cell attachment and spreading of HUVECs on cross-linked polynorbornene thin films was also investigated. HUVECs plated on thin films were examined at intervals of 15, 30, 45, 60, 90, 120, 180, 240, and 300 min using phase contrast microscopy. The more stringent, serum-free conditions were utilized for the comparison of cell spreading on 1% cyc-RGD versus 10% lin-RGD thin films alongside negative controls (no peptide and scrambled sequences; Figure 4). HUVECs on polymers containing both 1% cyc-RGD and 10% lin-RGD showed excellent adhesion and spreading within 300 min. With 1% cyc-RGD, half of the cells were spread as early as 15 min (Figure 4, E), while 10% lin-RGD provided similar results at 90 min (Figure 4, C). This significantly faster rate of adhesion is consistent with the increased affinity of cyclic RGD peptides for cell surface integrins.23 As illustrated in Figure 4 (A), cell spreading and overall adhesion are significantly enhanced for the 1% cyc-RGD polymer compared to 10% lin-RGD. Additionally, cells cultured on the polymer thin films exhibit morphology and spreading similar to positive control samples of HUVECs seeded directly in wells of tissue culture treated plates.
Figure 4.
HUVEC spreading on cross-linked polynorbornene thin films (A). Positive control samples of HUVECs seeded directly in wells of tissue culture treated plates were examined alongside polynorbornene thin films. Phase contrast images show cells cultured on 10% lin-RGD (B, C, D) and 1% cyc-RGD (E, F, G) spreading at 15, 90, and 300 min, respectively. These images can be compared to those on negative controls (no peptide (H), 10% lin-RDG (I), and 2.5% cyc-RDG (J)) each at 300 min. Data represents three separate experiments. Scale bars = 100 μm.
CONCLUSIONS
The results reported here provide an efficient method for the preparation of linear and cyclic RGD-conjugated norbornene monomers 3 and 4. Monomers containing the RGD cell recognition motifs were copolymerized with Nor-PEG (1) and Nor-Amine (2) to provide excellent yields of polynorbornenes with varying concentrations of linear or cyclic RGD peptide side chains. Cross-linked thin films of polymers carrying cyclic RGD were active at a 100-fold lower peptide concentration than linear RGD containing polymers for cell viability of HUVECs. Polymer samples with 1% cyc-RGD were observed to be superior for cell attachment, viability, and spreading under stringent, serum-free conditions. The disclosed copolymer formation with RGD based monomers provides evidence for the broad utility of ROMP as a controlled method for incorporating a range of peptides into polymers. These results have important implications for the synthesis of biomaterials that support cell adhesion, and studies investigating the impact of incorporating multiple cell-recognition motifs and increasing the linker length between the polymer backbone and the RGD moiety are currently ongoing. The evaluation of these materials for supporting stem cell attachment and growth for biomedical applications is also underway.
Supplementary Material
Acknowledgments
We thank Professor Chin-Lin Guo for use of his inverted epifluorescence microscope, and Mr. Jiun-Yann Yu for assistance with the microscope. Ms. Chithra Krishnamurthy is thanked for helpful discussions. This research was supported by the National Institutes of Health (5R01-GM31332, F32 HL091440), the NSF Materials Research Science and Engineering Center at Caltech (DMR 0520565), the Beckman Institute at Caltech (postdoctoral fellowship to R.M.C.), and grants from the California Institute for Regenerative Medicine. Materia, Inc. is thanked for its donation of metathesis catalysts.
Footnotes
The authors declare no competing financial interest.
Supporting Information Available
Additional details for the synthetic preparation of monomers 1–6, preparation and characterization data for all polymers, AFM images of thin films, fluorescence images, and 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 2.Sakiyama-Elbert SE, Hubbell JA. Annu Rev Mater Res. 2001;31:183–201. [Google Scholar]
- 3.Langer R. Acc Chem Res. 2000;33:94–101. doi: 10.1021/ar9800993. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama SK, Olden K, Yamada KM. Cancer Metastasis Rev. 1995;14:173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- 5.Pierschbacher MD, Ruoslahti E. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 6.Massia SP, Hubbell JA. J Cell Biol. 1991;114:1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong J, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 8.Pettit DK, Hoffman AS, Horbett TA. J Biomed Mater Res. 1994;28:685–691. doi: 10.1002/jbm.820280605. [DOI] [PubMed] [Google Scholar]
- 9.Aucoin L, Griffith CM, Pleizier G, Deslandes Y, Sheardown H. J Biomat Sci-Polym E. 2002;13:447–462. doi: 10.1163/156856202320253956. [DOI] [PubMed] [Google Scholar]
- 10.Verrier S, Pallu S, Bareille R, Jonczyk A, Meyer J, Dard M, Amedee J. Biomaterials. 2002;23:585–596. doi: 10.1016/s0142-9612(01)00145-4. [DOI] [PubMed] [Google Scholar]
- 11.Heilshorn SC, Di Zio KA, Welsh ER, Tirrell DA. Biomaterials. 2003;24:4245–4252. doi: 10.1016/s0142-9612(03)00294-1. [DOI] [PubMed] [Google Scholar]
- 12.Liu JC, Heilshorn SC, Tirrell DA. Biomacromolecules. 2004;5:497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 13.Liu JC, Tirrell DA. Biomacromolecules. 2008;9:2984–2988. doi: 10.1021/bm800469j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong E, Tzlil Sh, Tirrell DA. Proc Natl Acad Sci. 2010;107:19302–19307. doi: 10.1073/pnas.1008291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risau W. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 16.Schottelius M, Laufer B, Kessler H, Wester H. Acc Chem Res. 2009;42:969–980. doi: 10.1021/ar800243b. [DOI] [PubMed] [Google Scholar]
- 17.Stepp MA. Exp Eye Res. 2006;83:3–15. doi: 10.1016/j.exer.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Koivunen E, Wang B, Ruoslahti E. Nat Biotechnol. 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 19.Assa-Munt N, Jia X, Laakkonen P, Ruoslahti E. Biochemistry. 2001;40:2373–2378. doi: 10.1021/bi002101f. [DOI] [PubMed] [Google Scholar]
- 20.Burkhart DJ, Kalet BT, Coleman MP, Post GC, Koch TH. Mol Cancer Ther. 2004;3:1593–1604. [PubMed] [Google Scholar]
- 21.Kolhar P, Kotamraju VR, Hikita ST, Clegg DO, Ruoslahti E. J Biotechnol. 2010;146:143–146. doi: 10.1016/j.jbiotec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Haubner R, Gratias R, Diefenbach B, Goodman SL, Jonczyk A, Kessler H. J Am Chem Soc. 1996;118:7461–7472. [Google Scholar]
- 23.Kantlehner M, Schaffner P, Finsinger D, Meyer J, Jonczyk A, Diefenbach B, Nies B, Holzemann G, Goodman SL, Kessler H. ChemBioChem. 2000;1:107–114. doi: 10.1002/1439-7633(20000818)1:2<107::AID-CBIC107>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Komazawa H, Saiki I, Igarashi Y, Azuma I, Kojima M, Orikasa A, Ono M, Itoh I. J Bioact Compat Polym. 1993;8:258–274. [Google Scholar]
- 25.Grubbs RH, editor. Handbook of Metathesis. Vol. 3 Wiley-VCH; Weinheim: 2003. [Google Scholar]
- 26.Trnka TM, Grubbs RH. Acc Chem Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 27.Leitgeb A, Wappel J, Slugovc C. Polymer. 2010;51:2927–2946. [Google Scholar]
- 28.Gestwicki JE, Strong LE, Cairo CW, Boehm FJ, Kiessling LL. Chem Biol. 2002;9:163–169. doi: 10.1016/s1074-5521(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 29.Kolonko EM, Pontrello JK, Mangold SL, Kiessling LL. J Am Chem Soc. 2009;131:7327–7333. doi: 10.1021/ja809284s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lienkamp K, Madkour AE, Musante A, Nelson CF, Nusslein K, Tew GN. J Am Chem Soc. 2008;130:9836–9843. doi: 10.1021/ja801662y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biswas S, Wang X, Morales AR, Ahn H, Belfield KD. Biomacromolecules. 2011;12:441–449. doi: 10.1021/bm1012212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miki K, Kimura A, Oride K, Kuramochi Y, Matsuoka H, Harada H, Hiraoka M, Ohe K. Angew Chem Int Ed. 2011;50:6567–6570. doi: 10.1002/anie.201101005. [DOI] [PubMed] [Google Scholar]
- 33.Maynard HD, Okada SY, Grubbs RH. Macromolecules. 2000;33:6239–6248. [Google Scholar]
- 34.Maynard HD, Okada SY, Grubbs RH. J Am Chem Soc. 2001;123:1275–1279. doi: 10.1021/ja003305m. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen MN, Lebarbe T, Zouani OF, Pichavant L, Durrieu MC. Biomacromolecules. 2012;13:896–904. doi: 10.1021/bm201812u. [DOI] [PubMed] [Google Scholar]
- 36.Love JA, Morgan JP, Trnka TM, Grubbs RH. Angew Chem Int Ed. 2002;41:4035–4037. doi: 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.Conrad RM, Grubbs RH. Angew Chem Int Ed. 2009;48:8328–8330. doi: 10.1002/anie.200903888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwab P, France MB, Ziller JW, Grubbs RH. Angew Chem Int Ed. 1995;34:2039–2041. [Google Scholar]
- 39.Maynard HD, Grubbs RH. Tetrahedron Lett. 1999;40:4137–4140. [Google Scholar]
- 40.Saneinejad S, Shoichet MS. J Biomed Mater Res. 1998;42:13–19. doi: 10.1002/(sici)1097-4636(199810)42:1<13::aid-jbm3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 41.Kleinfeld D, Kahler KH, Hockberger PE. J Neurosci. 1988;8:4098–4120. doi: 10.1523/JNEUROSCI.08-11-04098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matson JB, Grubbs RH. J Am Chem Soc. 2008;130:6731–6733. doi: 10.1021/ja802010d. [DOI] [PubMed] [Google Scholar]
- 43.Janssen M, Oyen WJ, Massuger LF, Frielink C, Dijkgraaf I, Edwards DS, Radjopadhye M, Corstens FH, Boerman OC. Cancer Biother Radiopharm. 2002;17:641–646. doi: 10.1089/108497802320970244. [DOI] [PubMed] [Google Scholar]
- 44.Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, Radjopadhye M, Boonstra H, Corstens FH, Boerman OC. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- 45.Dai X, Su Z, Liu JO. Tetrahedron Lett. 2000;41:6295–6298. [Google Scholar]
- 46.Choi TL, Grubbs RH. Angew Chem Int Ed. 2003;42:1743–1746. doi: 10.1002/anie.200250632. [DOI] [PubMed] [Google Scholar]
- 47.Gilliom LR, Grubbs RH. J Am Chem Soc. 1986;108:733–742. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.