SUMMARY
The time course of the requirement for local protein synthesis in the stabilization of learning-related synaptic growth and the persistence of long-term memory was examined using Aplysia bifurcated sensory neuron-motor neuron cultures. We find that following repeated pulses of serotonin (5-HT) the local perfusion of emetine, an inhibitor of protein synthesis, or a TAT-AS oligonucleotide directed against ApCPEB blocks long-term facilitation (LTF) at either 24 hr or 48 hr and leads to a selective retraction of newly formed sensory neuron varicosities induced by 5-HT. By contrast, later inhibition of local protein synthesis, at 72 hr after 5-HT, has no effect on either synaptic growth or LTF. These results define a specific stabilization phase for the storage of long-term memory during which newly formed varicosities are labile and require sustained CPEB-dependent local protein synthesis to acquire the more stable properties of mature varicosities required for the persistence of LTF.
INTRODUCTION
Studies of a variety of memory systems, ranging in complexity from elementary forms of implicit memory in invertebrates and mammals to more complex forms of hippocampal-based explicit memory, suggest that activity-dependent modulation of both the strength and structure of specific synaptic connections is a key mechanism by which these diverse forms of memory are encoded, processed and stored in the brain (Kandel, 2001; Bailey et al., 2004). Whereas short-term memory, lasting minutes to hours, involves alterations in the effectiveness of preexisting synaptic connections resulting from covalent modifications of preexisting proteins, long-term memory is switched on by a central program of gene expression leading to the synthesis of new proteins and the growth of new synaptic connections (Dash et al., 1990; Alberini et al., 1994; Bartsch et al., 1995, 2000). For both implicit and explicit forms of memory the synaptic growth is thought to represent an enduring cellular change that stabilizes the long-term process (Bailey and Kandel, 1993).
The cellular and molecular mechanisms that contribute to implicit memory storage have been studied most extensively in the monosynaptic connections between identified sensory and motor neurons of the gill-withdrawal reflex of Aplysia both in the intact animal and in dissociated cell culture. Repeated tail shocks to the intact animal induce long-term facilitation (LTF) of the sensory to motor neuron connection that lasts for days and this enduring change in synaptic strength is accompanied by an increase in the number of sensory neuron varicosities that parallels the behavioral duration of the memory (Frost et al., 1985; Bailey and Chen, 1983, 1988a, 1988b, 1989). Both long-term facilitation and the associated synaptic growth can be reconstituted in dissociated sensory-motor neuron culture by repeated presentations of brief pulses of 5-HT, a modulatory neurotransmitter normally released by sensitizing stimuli in the intact animal (Montarolo et al., 1986; Glanzman et al., 1990; Bailey et al., 1992). Time-lapse imaging of cultured Aplysia sensory to motor neuron synapses labeled with activity-sensitive fluorescent proteins has further delineated that long-term facilitation is accompanied by two temporally and morphologically distinct classes of presynaptic structural change: the rapid remodeling and activation of silent preexisting varicosities and the slower growth of new functional varicosities (Kim et al., 2003).
Studies of synapse-specific long-term plasticity in Aplysia first suggested that the molecular mechanisms underlying the initiation of LTF and synaptic growth are likely to differ from those required for their long-term maintenance (Martin et al., 1997; Casadio et al. 1999; Si et al. 2003). Induction of the changes in synaptic function and structure, measured 24 hr after 5-HT treatment, requires only nuclear transcription and central translation whereas the persistence of these synaptic modifications, measured at 72 hr, requires, in addition, local protein synthesis at the synapse (Casadio et al. 1999). However, the time course of local protein synthesis required for the stabilization of learning-induced synaptic growth and the persistence of memory storage has not been clearly demonstrated. For example, for how long is local protein synthesis required to stabilize the population of learning-induced synaptic varicosities? Does local translation of proteins at the activated synapses confer persistence to memory storage by stabilizing the learning-related structural changes? If so, are the learning (5-HT) - induced newly formed varicosities more labile than preexisting varicosities?
The most direct way to address these questions is to examine in parallel both LTF and learning-induced synaptic growth and to determine the effects on both of these processes of locally applying inhibitors of protein synthesis at various time points. Toward that end, we have used the modified Aplysia culture system developed by Martin et al. (1997) consisting of a single bifurcated sensory neuron contacting two spatially separated motor neurons. To determine the role of local protein synthesis in the stabilization of learning-related synaptic growth and the persistence of LTF, we first extended to approximately one week the time window for monitoring the 5-HT-induced long-term functional and structural changes at the sensory to motor neuron synapse. We then applied emetine, an inhibitor of protein synthesis, locally to one set of sensory-motor neuron synapses at various time points. Since the 5-HT-induced synaptic growth in culture does not appear until approximately 12–18 hrs after 5-HT training (Kim et al., 2003; Udo et al., 2005), we applied emetine at 24 hr, 48 hr or 72 hr after giving 5 pulses of 5-HT. We found that local inhibition of protein synthesis blocked LTF when given at either 24 hr or 48 hr after 5-HT treatment but had no effect when applied at 72 hr after 5-HT.
The inhibition of local protein synthesis at 24 hr led to a selective retraction of newly formed varicosities induced by 5-HT when compared to preexisting varicosities. We also observed a selective pruning of learning-induced synaptic growth after local perfusion of the inhibitory transmitter, FMRFamide, when it was applied 24 hr after 5-HT treatment. This late phase of local protein synthesis is importantly regulated by the Aplysia homolog of cytoplasmic polyadenylation element binding protein (ApCPEB), which promotes translational activation (Si et al., 2003). Local application of a specific antisense oligonucleotide to ApCPEB 24 hr after repeated pulses of 5-HT blocked the stable maintenance of both LTF and the learning–induced synaptic growth.
Combined, these results define a temporally distinct and local phase of stabilization at the sensory to motor neuron synapse for LTF that extends to approximately 72 hr. During this time period the 5-HT-induced newly formed sensory neuron varicosities are labile and sensitive to disruption. These labile varicosities are stabilized by sustained CPEB-dependent local protein synthesis and contribute to the persistence of memory storage.
RESULTS
Local protein synthesis is required for the persistence of LTF
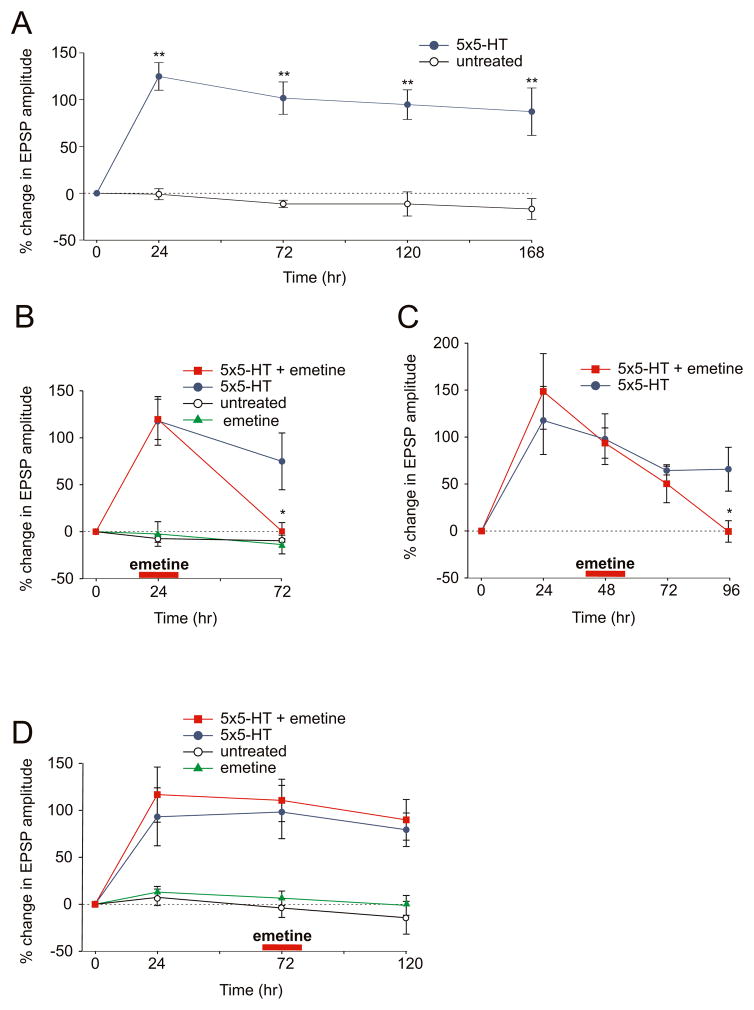
To explore the time course of long lasting synaptic plasticity in the Aplysia dissociated cell culture system, we have monitored the 5-HT-induced increase in synaptic effectiveness at the sensory-motor neuron synapse over a period of approximately one week. Bath application of five pulses of 10 μM 5-HT produces a 124.8% (±14.7%, n = 11) increase in the amplitude of the evoked sensory to motor neuron synaptic potential (EPSP) at 24 hr that persists for at least 72 hr (+101.6% ± 17.3%) and is only slightly reduced when measured at 120 hr (+94.7% ± 15.8%) and 168 hr (+87.2% ± 25.3%; Fig. 1A). The ability to follow the 5-HT-induced long-term functional changes at the sensory to motor neuron synapse in culture over such an extended time period has allowed us to examine directly the time course for the requirement of local protein synthesis in the maintenance of synaptic changes during the later phases of LTF.
Figure 1. A Late Phase of Sustained Local Protein Synthesis is Required for the Persistence of LTF.
(A) Five pulses of 5-HT produce a significant increase in the amplitude of the sensory to motor neuron EPSP at 24 hr that persists for at least one week. In untreated cultures the percent change in EPSP amplitude did not change significantly over the same time period (**, p <0.01, % change in EPSP amplitude in 5-HT treated versus untreated cultures). (B) Local application of 100 μM emetine to one sensory-motor neuron branch at 24 hr after 5-HT-treatment blocks LTF at 72 hr but has no effect on the opposite branch that did not receive emetine (*, p <0.05, % change in EPSP amplitude at 72 hr in the branch treated with 5-HT at time 0 + emetine at 24 hr versus 5-HT alone treated branch). (C) Local application of emetine to one branch 48 hr after 5-HT treatment blocks LTF at 96 hr but has no effect on the opposite branch. (*, p <0.05, change in EPSP amplitude at 96 hr in the branch treated with 5-HT at time 0 + emetine at 48 hr versus 5-HT alone treated branch). (D) Local perfusion of emetine 72 hr after 5-HT treatment did not reduce LTF for a period up to 120 hr. (p = n.s. change in EPSP amplitude at 120 hr in the branch treated with 5-HT at time 0 + emetine at 72 hr versus 5-HT alone treated branch).
As a first step in examining this issue, we locally applied emetine (100 μM), an inhibitor of protein synthesis, for 30 min to one branch of the bifurcated sensory neuron-motor neuron culture. Independent 35S labeling experiments using isolated pleural ganglia as a surrogate preparation indicate that this emetine protocol is by and large reversible over a 24 hr time window (Fig. S1). Local application of emetine was given at three different time intervals −24 hr, 48 hr or 72 hr - after bath application of five pulses of 5-HT. Selective perfusion of emetine to one set of sensory-motor neuron synapses 24 hr after 5-HT treatment blocked LTF at 72 hr (+0.5% ± 9.1%, n = 7; Fig. 1B). We similarly found that local application of emetine 48 hr after 5-HT produced a decrease in LTF at 72 hr and this returned to baseline at 96 hr (% EPSP at 48 hr +93.6 ± 16.1, at 72 hr +50.3 ± 20.2, at 96 hr −0.5 ± 11.4, n = 6; Fig. 1C). However, when emetine was applied at 72 hr after 5-HT training it no longer interfered with the maintenance of LTF at 120 hr (% EPSP at 72 hr +110.6 ± 22.5, at 120 hr +89.9 ± 21.6, n = 7; Fig. 1D). Application of emetine alone at either 24 hr or 72 hr did not induce any significant changes in the baseline synaptic transmission. Taken together, these results indicate that the persistence of LTF in culture still requires local protein synthesis at the synapse 24 hr and 48 hr after 5-HT training. These later time points may correspond to a specific phase of LTF during which the 5-HT-induced modifications at the sensory to motor neuron synapse require production of a new set of proteins locally at the activated synapses.
At 72 hr after its induction, LTF becomes less dependent on local protein synthesis since local application of emetine at this time point does not affect the persistence of synaptic facilitation, at least up to 120 hr. This suggests that either this late phase of local protein synthesis is only required for approximately 48 hr or that after 72 hr the local protein synthesis apparatus assumes a state which is more difficult to perturb. Indeed, when we increased the time for the local application of emetine from 30 min to one hour in a set of cells where LTF was induced 72 hr before; LTF was still unaffected at 120 hr (% EPSP at 72 hr +78.1 ± 7.5, at 120 hr +73.6 ± 12.9, n = 3) even with this longer exposure to emetine. This suggests that high levels of local protein synthesis play a time dependent role in the stabilization of LTF. We cannot exclude the possibility that local protein synthesis may be required for even longer periods but at a lower level.
Local protein synthesis is required for the persistence of 5-HT-induced synaptic growth
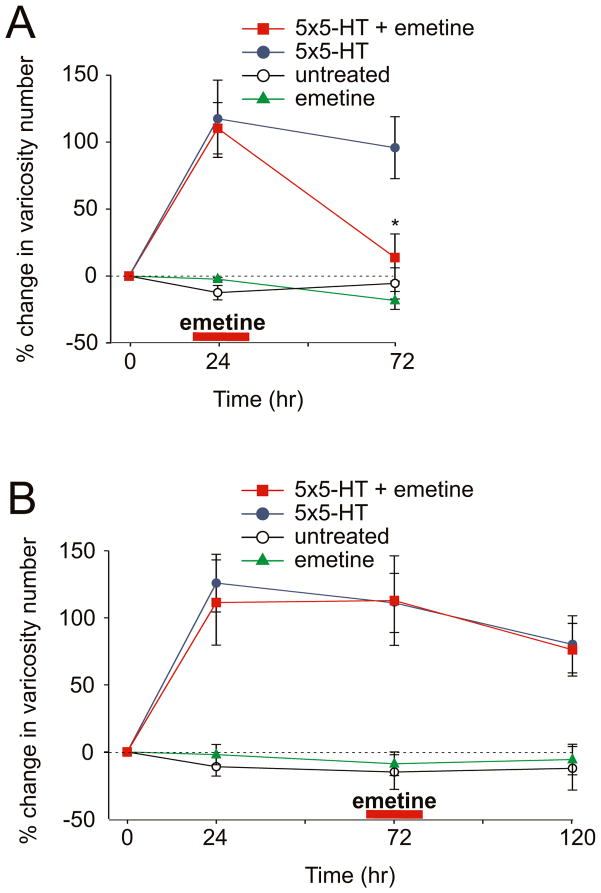
To determine the degree to which the time course of learning-related synaptic growth parallels that of LTF in culture, we labeled sensory neurons with the enhanced green fluorescent protein (eGFP) and imaged the entire population of sensory neuron varicosities in contact with the major processes of the postsynaptic gill-motor neuron L7 (Kim et al., 2003; Udo et al., 2005). We found that 5 pulses of 5-HT resulted in a 125.9% (± 21.5%) increase in the total number of varicosities 24 hr later. This increase persisted for 72 hr (+111.1% ± 22.0) and was only partially reduced at 120 hr (+80.2% ± 21.3%, n = 6 cells, 122 varicosities; Fig. 2B). These results indicate that in the Aplysia dissociated cell culture preparation the 5-HT-induced increase in the total number of sensory neuron varicosities parallels the duration of LTF for a period of at least 5 days.
Figure 2. A Late Phase of Sustained Local Protein Synthesis Is Required for The Persistence of 5-HT-Induced Synaptic Growth.
(A) Local application of emetine 24 hr after 5-HT treatment produced a significant reduction in the total number of sensory neuron varicosities at the treated branch (*, p <0.05, change in varicosity number at 72 hr in the branch treated with 5-HT at time 0 + emetine at 24 hr versus 5-HT alone treated branch). In the control experiment, perfusion of emetine alone at 24 hr had no effect on varicosity number at 72 hr. (B) Selective perfusion of emetine to one branch 72 hr after 5-HT treatment had no significant effect on varicosity number at 120 hr (p = n.s. change in varicosity number at 120 hr in the branch treated with 5-HT at time 0 + emetine at 72 hr versus 5-HT alone treated branch). In both graphs the percent change in the total number of varicosities at 24 hr, 72 hr and 120 hr was obtained by comparing the total number of varicosities measured at each of these different time points to the total number of varicosities measured at time 0.
We next examined whether inhibiting local protein synthesis during these later stages of LTF interferes with the persistence of 5-HT-induced synaptic growth. Emetine was locally applied to one branch of the bifurcated culture system at two different time points after 5-HT treatment: 24 hr or 72 hr. As described above, 5 pulses of 5-HT produced an increase in the total number of sensory neuron varicosities at 24 hr that persisted for at least 120 hr. Application of emetine to one set of sensory-motor neuron synapses 24 hr after 5-HT resulted in a significant decrease in the number of varicosities at 72 hr (+110% ± 19.2% at 24 hr vs. +13.8% ± 17.6% at 72 hr, n = 7 cells, 156 varicosities; Fig. 2A). When emetine was locally applied 72 hr after 5-HT treatment, the increase in varicosity number was not significantly affected at 120 hr (+112.9% ± 33.4% at 72 hr vs. +76.2% ± 19.6% at 120 hr, n = 6 cells, 78 varicosities; Fig. 2B). The similar sensitivity of the 5-HT-induced synaptic growth and LTF to local application of emetine, for a time period extending to approximately 72 hr, raises an intriguing question: Is this sustained phase of local protein synthesis specifically required to stabilize only the 5-HT-induced newly formed varicosities? Or are both the preexisting varicosities as well as the newly formed varicosities equally susceptible to inhibition of local protein synthesis during this time window?
5-HT-induced newly formed varicosities are labile and require local protein synthesis for their stabilization
To explore the labile nature of the 5-HT-induced synaptic growth during this later stabilization phase of LTF, we refined our quantitative analysis and examined the selective effects of emetine at different time points on individual identified sensory neuron varicosities that comprised two morphologically-defined and temporally-distinct populations: 1) preexisting varicosities - varicosities that were present from time 0 and 2) 5-HT-induced newly formed varicosities - varicosities that were only present 24 hr after 5-HT and not at time 0.
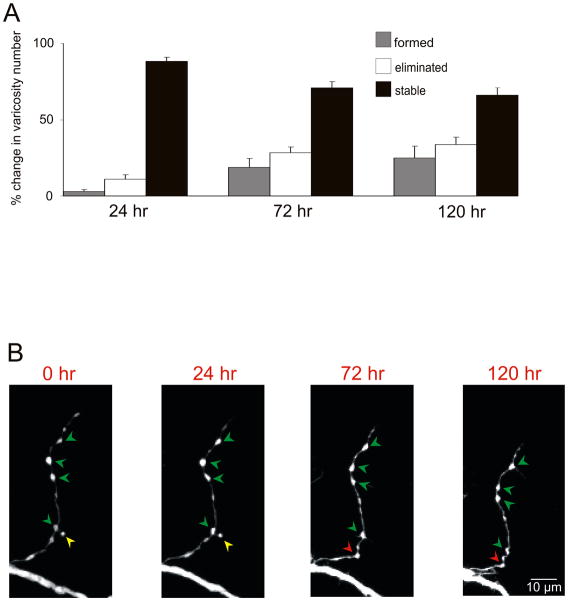
We began this analysis by characterizing the life span of the preexisting sensory neuron varicosities in the untreated group of cells (not exposed to 5-HT) over a period of 6 days. We found that 71.1% (± 4.0%) of the preexisting varicosities present at time 0 were maintained for 72 hr and 66.1% (± 4.8%) persisted for at least 120 hr (n = 10 cells, 179 varicosities). By contrast, only 24.8% (± 7.9%) of the varicosity population was newly formed (varicosities that were formed spontaneously after time 0 in the absence of 5-HT) and 33.8% (± 4.8%) were lost at 120 hr (Figs. 3A and 3B). Local perfusion of emetine by itself did not affect the lifespan of preexisting varicosities as compared to the control group. Indeed, when emetine was applied locally at 24 hr we observed that 79.8% (± 7.1%, n = 5 cells, 96 varicosities) of the preexisting varicosities were maintained up to 72 hr. Similarly when emetine was applied at 72 hr we found that 60.4% (± 7.3%, n = 4 cells, 62 varicosities) of the preexisting varicosities were maintained up to 120 hr.
Figure 3. Life Span of Sensory Neuron Varicosities in Untreated Sensory-Motor Neuron Co-Cultures.
(A) Percentage of varicosities that are stable, new and eliminated (number of varicosities added or eliminated at either 24 hr, 72 hr or 120 hr as a percentage of the number of pre-existing varicosities at time 0) over a period of 6 days in untreated cultures. (B) Repeated imaging of the axonal arbor of a sensory neuron in contact with the motor neuron under untreated conditions (no 5-HT) reveals that the majority of presynaptic varicosities are stable (green arrowheads) for at least 120 hr. The yellow arrowhead indicates a sensory neuron varicosity that is going to be eliminated, while the red arrowhead indicates a newly formed varicosity that appears at 72 hr and is still present at 120 hr.
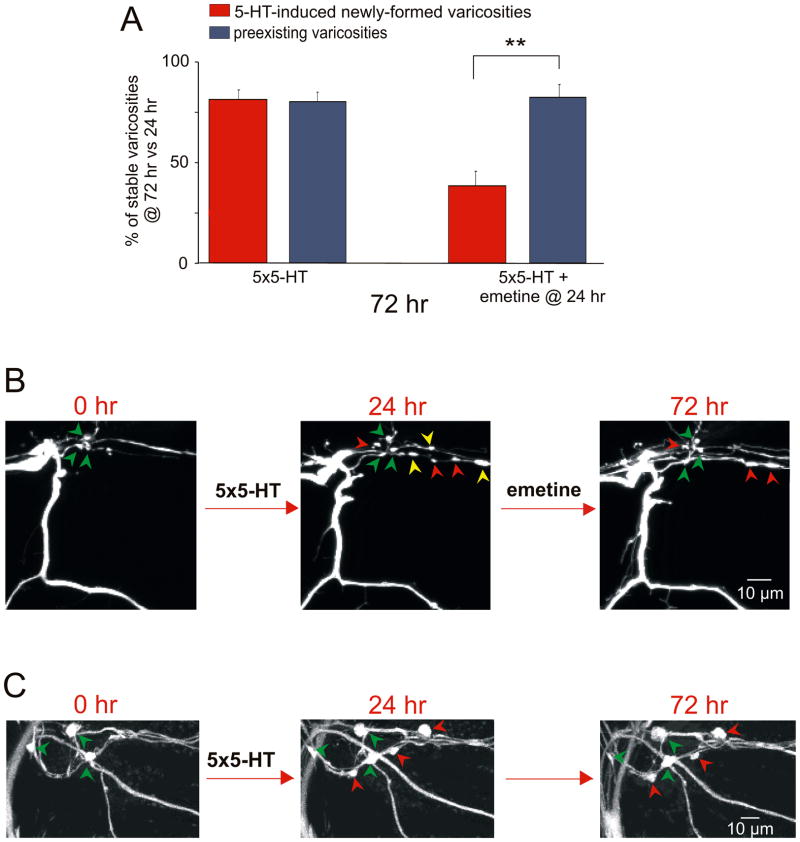
To assess the dynamic properties of the 5-HT-induced newly formed varicosities, we compared their stability under two different experimental conditions: 5-HT and 5-HT+emetine. Culture dishes containing the bifurcated sensory neuron-motor neuron preparation were treated with 5 pulses of 5-HT at time 0 and 24 hr later one of the two branches was perfused locally with emetine. We identified each individual 5-HT-induced newly formed and preexisting varicosity at 24 hr and then re-examined the target field to determine the presence or absence of the same individual varicosities at 72 hr. The number of 5-HT-induced newly formed varicosities and the number of preexisting varicosities that were present at 72 hr were compared to the number of varicosities in the same respective class observed at 24 hr. We found that at the branch that only received 5-HT, 81.3% (± 4.7%, n = 11 cells, 82 varicosities) of the 5-HT-induced newly formed varicosities and 80.3% (± 8.8%, 88 varicosities) of the preexisting varicosities were maintained at 72 hr when compared to 24 hr (Figs. 4A and 4B). By contrast, at the branch that received emetine 24 hr after 5-HT treatment, only 38.1% (± 7.0%, n = 11 cells, 136 varicosities) of the 5-HT-induced newly formed varicosities were maintained at 72 hr versus 81.63% (± 6.2%, 90 varicosities) of the preexisting varicosities. The selective retraction of 5-HT-induced newly formed varicosities induced by local application of emetine demonstrates that during the stabilization phase this population of varicosities is significantly more labile and sensitive to disruption than the population of preexisting sensory neuron varicosities.
Figure 4. Local Perfusion of Emetine at 24 hr Leads to a Selective Retraction of 5-HT-Induced Newly Formed Sensory Neuron Varicosities.
(A) Each histogram illustrates the mean percentage (± SEM) of identified varicosities maintained at 72 hr compared to 24 hr. At the sensory to motor neuron branch that received emetine 24 hr after 5-HT, the percentage of 5-HT-induced newly formed varicosities maintained at 72 hr was significantly less than that of preexisting varicosities (**, p <0.01, percentage of 5-HT-induced newly formed varicosity maintained at 72 hr versus the percentage of preexisting varicosities). At the 5-HT-treated branch that did not receive emetine, most 5-HT-induced newly formed varicosities were maintained similar to the preexisting population of varicosities. In both cases, the 5-HT-induced new varicosities represent varicosities that formed between 0 and 24 hr and remained stable at 72 hr. (B) Confocal images of GFP-labeled sensory neuron varicosities in contact with the initial segment of the motor neuron L7. 24 hr after 5 pulses of 5-HT, several newly formed varicosities are observed (red and yellow arrowheads) in addition to the group of preexisting varicosities (present from time 0; green arrowheads). Local perfusion of emetine at 24 hr induces a selective pruning of three of the 5-HT-induced newly formed varicosities (yellow arrowheads) at 72 hr without affecting most of the preexisting varicosities. (C) After treatment with 5-HT alone, three newly formed varicosities (red arrowheads) appear at 24 hr in addition to the group of preexisting varicosities (green arrowheads). All of the 5-HT-induced newly formed and preexisting varicosities are maintained at 72 hr.
Why are some of the newly formed varicosities, induced by 5-HT, labile and sensitive to disruption by inhibitors of local protein synthesis? To address this question, we examined the functional state of individual 5-HT- induced newly formed varicosities at 24 hr by combining the whole cell marker dsRed-monomer fluorescent protein - to fully delineate the presynaptic compartment - with the activity-sensitive protein ecliptic synapto-PHluorin (synPH). SynPH is a highly pH-sensitive presynaptic marker and its fluorescence increases during exocytosis due to the externalization of the synPH- labeled synaptic vesicles to a more basic exterior medium (Miesenbock et al., 1998). Therefore, depolarization by bath application of KCl (200 mM) allowed us to identify as active varicosities (competent for evoked transmitter release) all of the sensory neuron varicosities undergoing a significant increase in the fluorescence intensity of synPH. After local application of emetine at 24 hr, we reimaged the same synaptic area at 72 hr and found that 63.9% (± 11.7%; n = 10 cells, 52 varicosities) of the 5-HT-induced newly formed varicosities that were synPH-active at 24 hr remained stable and, therefore, were present at 72 hr versus only 29.1% (± 9.1%) of the synPH-inactive varicosities (Fig. S2). These results suggest that because the 5-HT-induced synPH-active newly formed varicosities already have a full complement of synaptic components for transmitter release and structural integrity, they do not require further recruitment of necessary proteins and consequently are more stable and resistant to inhibitors of protein synthesis during the stabilization time window than the population of synPH-inactive newly formed varicosities. Therefore, one reason the remaining 5-HT-induced synPH-inactive newly formed varicosities are labile may reflect, at least in part, the undifferentiated nature of their presynaptic compartment.
Recent in vivo imaging studies in mammals have shown that an important morphological determinant of synapse stability is the size of the postsynaptic compartment. For example, stable dendritic spines are on average significantly larger than transient spines (Trachtenberg et al., 2002). On the other hand, initially smaller, presumably less effective synapses are more likely to be eliminated after LTD induction (Bastrikova, 2008). Therefore, we also compared the size of 5-HT-induced newly formed varicosities at 24 hr that were later observed to disappear following local perfusion of the protein synthesis inhibitor with the size of the 5-HT-induced newly formed varicosities that were maintained at 72 hr. We found that the mean size (cross-sectional area) of the stable newly formed varicosities (13.6 μm2 ± 0.8, n = 10 cells, 125 varicosities) was significantly larger than the mean size of the labile newly formed varicosities (9.6 μm2 ± 0.7, p<0.01). By contrast, we did not observe any significant difference between the size of the stable 5-HT-induced newly formed varicosities and the population of preexisting varicosities (13.5 μm2 ± 1.1, n = 10 cells, 137 varicosities; Fig. S3). Interestingly, the 5-HT-induced synPH-active newly formed varicosities that were stable were also significantly larger (15.5 μm2 ± 2.5) than the labile 5-HT-induced synPH-inactive varicosities (9.6 μm2 ± 0.9, p<0.05; n = 10 cells; 52 varicosities). Taken together, our data indicate that the size, degree of differentiation and functional competency of the presynaptic compartment are all likely to be contributing morphological factors in the stable maintenance of 5-HT-induced synaptic growth.
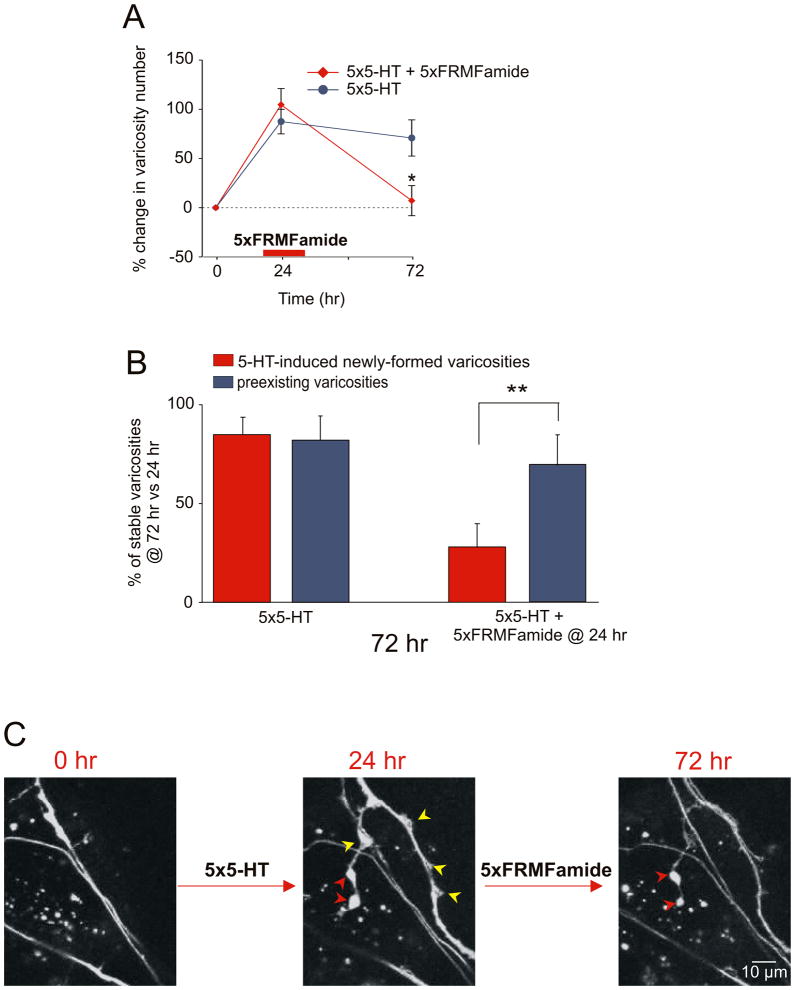
Application of the inhibitory peptide FMRFamide at 24 hr also induces the selective retraction of 5-HT-induced newly formed varicosities
To explore further the extent to which the 5-HT-induced newly formed varicosities are labile and susceptible to disruption during the stabilization phase, we examined the effect of the inhibitory neuromodulator, FMRFamide. Previous studies have found that five repeated applications of FMRFamide at time 0 produce by itself long-term depression and a corresponding reduction in the number of varicosities (Montarolo et al., 1986, 1988). When we locally perfused one branch of the bifurcated culture with repeated pulses of FMRFamide 24 hr after 5-HT training, we observed a significant reduction in the total number of sensory neuron varicosities at 72 hr (+104.6% ± 16.4% at 24 hr vs. +7.1% ± 15.3% at 72 hr, n = 5 cells, 69 varicosities; Fig. 5A). As was the case with the local application of emetine at 24 hr, the FMRFamide-induced reduction in varicosity number reflects, in large part, the selective retraction of the 5-HT-induced newly formed varicosities. Thus, only 27.6% (± 11.8%, n = 5 cells) of the newly formed varicosities were maintained at 72 hr compared to 68.7% (± 15.2) of the preexisting varicosities (Figs. 5B and 5C). These results provide independent support for the idea that learning-induced synaptic growth is labile and susceptible to disruption within a limited and temporally defined period corresponding to the stabilization phase.
Figure 5. Local Application of FMRFamide at 24 hr Mimics the Selective Pruning of 5-HT-Induced Newly Formed Varicosities Induced by Emetine.
Local perfusion of five pulses of FMRFamide 24 hr after five pulses of 5-HT leads to a significant reduction in the total number of sensory neuron varicosities compared with the control branch (*, p <0.05, change in varicosity number at 72 hr in the branch treated with 5-HT at time 0 + FMRFamide at 24 hr versus 5-HT alone treated branch). The percentage change in the total number of varicosities at 24 hr and 72 hr was obtained by comparing the total number of varicosities measured at these different time points to the total number of varicosities measured at time 0. (B) The effect of FMRFamide was assessed at 72 hr on two population of varicosities, 5-HT-induced newly formed and preexisting, independently and used 24 hr as the reference time point. Local application of FMRFamide at 24 hr induces a selective loss of the 5-HT-induced newly formed varicosities compared to preexisting varicosities (**, p <0.01, percentage of 5-HT-induced newly formed varicosity maintained at 72 hr versus the percentage of preexisting varicosities). In the control branch that received no emetine, the percentage of 5-HT-induced varicosities maintained at 72 hr is similar to that of preexisting varicosities. In both cases, the 5-HT-induced new varicosities represent varicosities that formed between 0 and 24 hr and remained stable at 72 hr. (C) Six newly formed sensory neuron varicosities appear 24 hr after 5-HT treatment (red and yellow arrowheads). Local application of FMRFamide at 24 hr after 5-HT leads to the apparent degeneration and retraction of four newly formed varicosities (yellow arrowheads). Only two of the six 5-HT-induced newly formed varicosities (red arrowheads) are maintained at 72 hr.
Local activity of ApCPEB is also required for the stable maintenance of LTF and 5-HT-induced synaptic growth
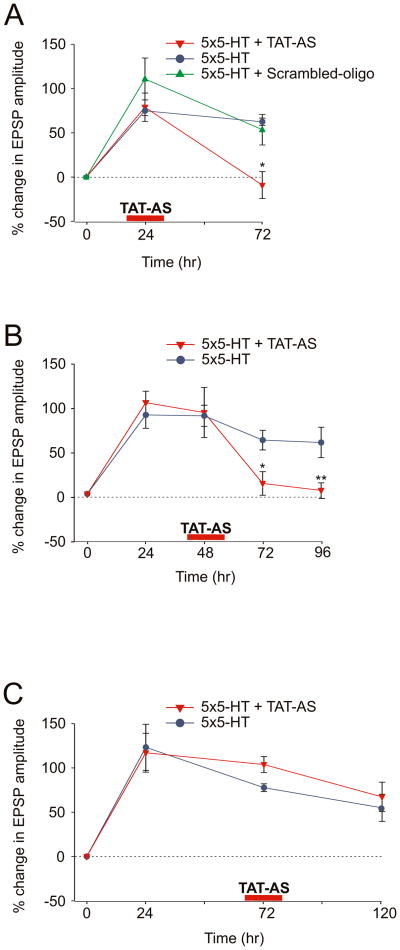
What regulates this local protein synthesis? Si et al. (2003) have found that Aplysia expresses a neuron-specific isoform of CPEB, an RNA binding protein that promotes polyadenylation and translational activation, and that ApCPEB plays an important role in the persistence of LTF. Indeed, the depletion of ApCPEB locally at the activated synapse during 5-HT training (time 0) blocks the maintenance but not the initiation of the 5-HT-induced long-term changes in the strength of the sensory to motor neuron connection. To determine the time window for the local requirement of CPEB during LTF and to compare it to the time window for local protein synthesis, as defined by our emetine studies, we locally perfused a specific ApCPEB antisense oligonucleotide covalently coupled to an 11 amino acid peptide derived from the HIV-TAT protein (TAT-AS) to one branch of the bifurcated sensory-motor neuron culture preparation for 30 min at different time intervals with respect to 5-HT treatment. This antisense oligo has previously been shown to lead to the depletion of ApCPEB mRNA and to a selective decrease in the level of CPEB protein (Si et al. 2003). Local application of the TAT-AS (100 μM) to one branch 24 hr after 5-HT, blocked LTF at 72 hr (% EPSP at 24 hr +78.9 ± 16.0, at 72 hr –9.1 ± 15.3, n = 4; Fig. 6A). Similarly, we found that local application of TAT-AS at 48 hr after 5-HT produced a significant reduction of LTF as tested 72 hr and 96 hr later (%EPSP at 48 hr +91.6 ± 28.1, at 72 hr +11.8 ± 13.3, at 96 hr +4 ± 8.6, n = 6; Fig. 6B). By contrast, local perfusion of the TAT-AS at 72 hr did not interfere with the persistence of 5-HT-induced LTF for a period up to 120 hr (% EPSP at 24 hr +117.1 ± 21.9, at 72 hr +103.8 ± 9.0, at 120 hr +67.3 ± 16.8, n = 6; Fig. 6C).
Figure 6. The Time Window for the Local Requirement of ApCPEB is Similar to the Time Window for Local Protein Synthesis.
(A) Local application of TAT-AS 24 hr after 5-HT blocks LTF (*, p <0.05, change in EPSP amplitude at 72 hr in the branch treated with 5-HT at time 0 + TAT-AS at 24 hr versus 5-HT alone treated branch). (B) Local perfusion of TAT-AS at 48 hr produces a significant reduction of LTF starting from 72 hr (*, p <0.05, change in EPSP amplitude at 72 hr in the branch treated with 5-HT at time 0 + TAT-AS at 48 hr versus 5-HT alone treated branch; **, p <0.01, change in EPSP amplitude at 96 hr in the branch treated with 5-HT at time 0 + TAT-AS at 48 hr versus 5-HT alone treated branch). (C) Local application of TAT-AS at 72 hr did not affect LTF up to 120 hr (p = n.s. change in EPSP amplitude at 120 hr in the branch treated with 5-HT at time 0 + TAT-AS at 72 hr versus 5-HT alone treated branch).
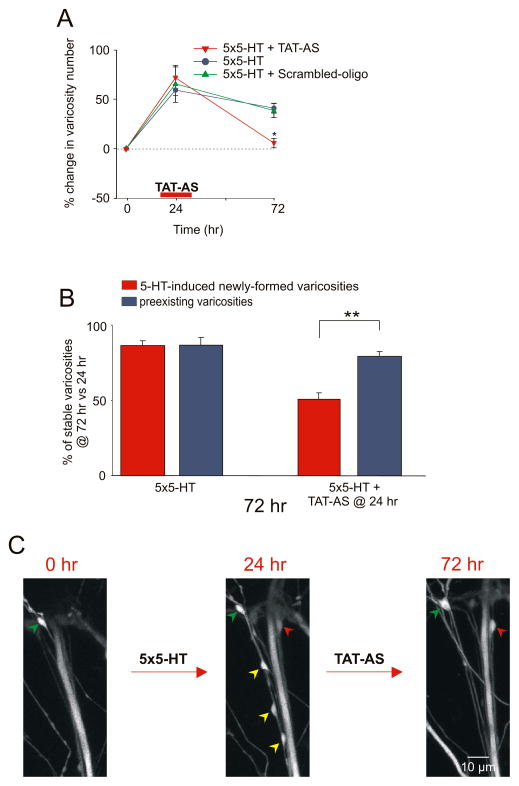
Is the local synthesis of ApCPEB also required for the persistence of 5-HT-induced synaptic growth? Structural analyses of sensory neurons labeled with eGFP revealed that at 72 hr the total number of presynaptic varicosities was significantly reduced in the branch perfused with the TAT-AS 24 hr after 5-HT (+71.8% ± 11.1% at 24 hr vs. +5.3% ± 4.8% at 72 hr, n = 11 cells, 302 varicosities; Fig. 7A) when compared to the control branch (+59.2% ± 5.5% at 24 hr vs. +40.9% ± 5.1% at 72 hr, 250 varicosities). By contrast, local perfusion of the scrambled oligonucleotide-TAT fusion protein 24 hr after 5-HT treatment did not interfere with either the maintenance of LTF (%EPSP +110.8 ± 23.7 at 24 hr, +53.5 ± 17.3 at 72 hr, n = 3; Fig. 6A) or the persistence of the 5-HT-induced increase in varicosity number (+65.6% ± 18.9% at 24 hr vs. +38.4% ± 12.3% at 72 hr, n = 3 cells, 118 varicosities; Fig. 7A). As illustrated in Figures 7B and 7C, local perfusion of the TAT-AS selectively reduced the number of 5-HT-induced newly formed varicosities maintained at 72 hr (50.7% ± 4.3%) compared to preexisting varicosities (79.4% ± 3%), similar to what we had observed with the local perfusion of emetine or FMRFamide. Taken together, these results suggest that local synthesis of ApCPEB is required for 24 hours after 5-HT to stabilize learning-related synaptic growth for the persistence of LTF.
Figure 7. Local Requirement of ApCPEB for the Stabilization of 5-HT-Induced Synaptic Growth.
(A) Local perfusion of TAT-AS 24 hr after 5-HT leads to a significant reduction in the total number of sensory neuron varicosities compared with the control branch (*, p <0.05, change in varicosity number at 72 hr in the branch treated with 5-HT at time 0 + TAT-AS at 24 hr versus 5-HT alone treated branch). The percentage change in the total number of varicosities at 24 hr and 72 hr was obtained by comparing the total number of varicosities measured at these different time points to the total number of varicosities measured at time 0. (B) The effect of TAT-AS was assessed at 72 hr on two population of varicosities, 5-HT-induced newly formed and preexisting, independently and used 24 hr as the reference time point. Local application of TAT-AS at 24 hr induces a selective loss of the 5-HT-induced newly formed sensory neuron varicosities compared to preexisting varicosities (**, p <0.01, percentage of 5-HT-induced newly formed varicosity maintained at 72 hr versus the percentage of preexisting varicosities). In both cases, the 5-HT-induced new varicosities represent varicosities that formed between 0 and 24 hr and remained stable at 72 hr. (C) 24 hr after repeated applications of 5-HT, four newly formed varicosities (red and yellow arrowheads) are present. The perfusion of TAT-AS at 24 hr induces the retraction of three newly formed varicosities (yellow arrowheads) without affecting the group of preexisting varicosities (green arrowheads). The red arrowhead represents the only 5-HT-induced newly formed varicosity in this field that is maintained at 72 hr.
DISCUSSION
Although a number of studies have now suggested an association of new macromolecular synthesis and structural changes with various forms of long-term memory, little is known about the temporal requirement of local protein synthesis for the stable maintenance of learning-related synaptic growth on the one hand and the role of this new growth for the persistence of the changes in synaptic effectiveness that accompany memory storage on the other (Bailey and Kandel, 1993; Kandel, 2001; Bailey et al., 2004). To address these issues, we have extended to one week the time window for analyzing the 5-HT-induced changes in function and structure of Aplysia sensory to motor neuron synapses in culture. We find that repeated applications of 5-HT produce LTF and an increase in the total number of sensory neuron varicosities that persist in parallel for approximately 7 days. The ability to follow these enduring changes in functional and structural plasticity at a defined synapse over an extended time window has allowed us to examine directly the role of ongoing local protein synthesis during the later phases of LTF. This in turn has made it possible to determine the specific contribution of local translation to the stabilization of learning-related synaptic growth and to the persistence of memory storage.
Persistence of LTF and 5-HT-induced synaptic growth requires a late phase of sustained local protein synthesis
Local translation of mRNAs in both the pre- and postsynaptic compartment has been proposed as an important mechanism used by neurons to obtain synapse-specific modifications during long-lasting forms of plasticity (Frey and Morris, 1997; Kang and Schuman, 1996; Martin et al., 1997; Sutton and Schuman, 2006). For example, studies of synapse-specific long-term plasticity in Aplysia culture have provided evidence for local translation at the presynaptic site. Repeated applications of 5-HT to one branch of the bifurcated sensory neuron-motor neuron culture preparation produce a synapse-specific form of LTF that requires local protein synthesis for the generation of a retrograde signal to the nucleus and for the maintenance of the growth of new synaptic connections (Martin et al., 1997; Casadio et al., 1999).
In Aplysia, local protein synthesis at the postsynaptic site has also been found to play a critical role in the induction of a LTF evoked by an asymmetric pattern of 5-HT exposure consisting of 5 minutes of 5-HT at the synapse coincident with 25 minutes of 5-HT at the sensory neuron soma (Sherff and Carew, 2004). In hippocampal slices, postsynaptic protein synthesis has been found to be critical for induction of long-term plasticity, including synaptic facilitation produced by applications of BDNF (Kang and Schuman, 1996), long-term potentiation (LTP) induced by strong tetanic trains (Bradshaw et al., 2003) as well as synaptic depression elicited by the activation of metabotropic receptors (Huber et al., 2001). Beyond its role during synaptic plasticity, local translation in the dendritic compartment may also contribute to long-lasting changes in behavior. Mice that lack the dendritic targeting element (3′UTR) of CAMKIIα mRNAs have a reduction of CAMKIIα in the postsynaptic density that is manifest as an impairment in spatial memory and contextual conditioning as well as an inhibition of late-phase LTP (Miller et al., 2002). A similar dependence on local translation for long-term memory has also been found in Drosophila. Using a Drosophila mutant expressing a fluorescent reporter of CAMKII 3′UTR to specifically monitor changes in dendritic protein synthesis, Ashraf et al. (2006) demonstrated that the induction of long-term memory following olfactory conditioning was associated with a significant increase in fluorescent CAMKII at the glomerular synapses of the antennal lobe.
In both higher invertebrates and mammals, most investigations of the requirement of local protein synthesis during long-term memory have sampled only a single or very few and early time points, typically either during training or for the first few hours following the training protocol. Consequently, the temporal evolution of these translation-dependent phases is not well understood and, in particular, their contribution to the later stages of long-term memory is not known. For example, most studies in Aplysia have examined induction and, therefore, have applied inhibitors of protein synthesis during a specific time window that encompasses the 5-HT training protocol, a point at which neither LTF nor the growth of new sensory neuron varicosities have yet been established. By applying inhibitors of protein synthesis to the synapse at much later time points e.g. 24 hr, 48 hr and 72 hr after 5-HT-training, we have been able to examine directly the role of local protein synthesis in the stabilization, as opposed to the induction, of the long-term synaptic changes that accompany LTF. We find that local and synapse-specific application of emetine at 24 hr blocks LTF and the 5-HT-induced increase in varicosity number but when given at 72 hr inhibiting local protein synthesis has no effect on either. These results suggest the existence of a sustained phase of local protein synthesis that extends from approximately 24 hr to 72 hr after 5-HT training in culture. This later period for ongoing protein synthesis at the synapse may correspond to a specific and local “stabilization phase” of long-term memory required to maintain the 5-HT-induced newly formed sensory neuron varicosities for the persistence of LTF. Consistent with this idea of a late phase of protein synthesis that is specifically required for the stabilization of long-term memory, Bekinschtein et al. (2007) have shown that inhibition of protein synthesis in the rat hippocampus 12 hr after the acquisition of a one-trial associative learning task does not lead to any immediate deficit in the persistence of memory when rats were tested 2 days after training but it does impair memory retention when rats were tested at 7 days.
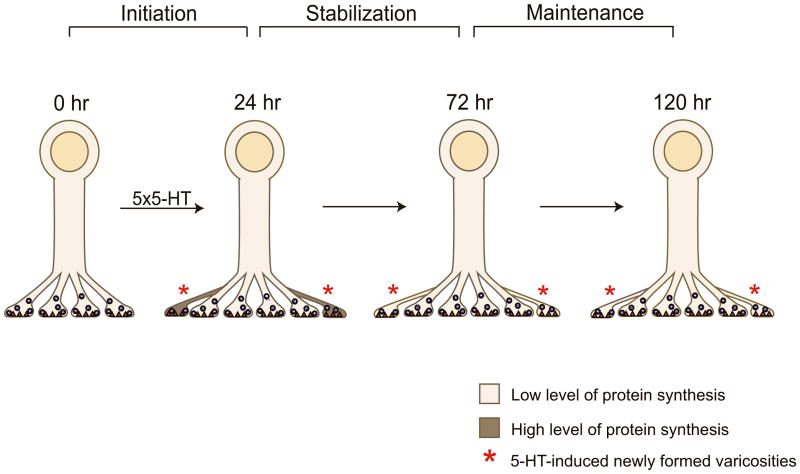
To explore how this local protein synthesis is regulated, we examined the role of a translational regulator, CPEB. We found that the stabilization of 5-HT-induced synaptic growth and the persistence of LTF rely upon the local synthesis of ApCPEB as well as protein synthesis. These results reinforce the previous finding by Si et al. (2003) that ApCPEB plays a critical role in the long-term maintenance of LTF and suggest that the activity of ApCPEB as a translational regulator is required during a specific temporal window when the 5-HT-induced synaptic changes are labile and must be stabilized to persist. Interestingly, CPEB appears to be more active and therefore more capable of regulating the synthesis of other mRNAs when it is in the prion-like state (Si et al, 2003). Since the conversion of CPEB molecules to prion-like states requires high levels of the proteins, as observed following 5-HT stimulation, it is likely that several peak periods of protein synthesis occur during this stabilization phase. It is also possible that CPEB stimulates synthesis of itself thus creating a positive feedback loop. Such sustained synthesis of CPEB might be necessary to compensate the short half-life of the protein as demonstrated in the pleural ganglia of Aplysia where the induced level of CPEB following 5-HT treatment returns almost to the basal levels within 5 to 6 hours (Si et al., 2003). After this stabilization phase is over, ApCPEB as well as local protein synthesis are either not required or may still be necessary, but perhaps at lower levels of activity, which then could serve to maintain the total population of sensory neuron varicosities i.e. both preexisting and newly formed, for the duration of the memory (Fig. 8).
Figure 8. A Late Phase of Sustained Local Protein Synthesis is Required to Stabilize Learning-Related Synaptic Growth.
Five pulses of 5-HT lead to the formation of new sensory neuron varicosities at 24 hr (initiation). The population of 5-HT-induced newly formed varicosities is significantly more labile than the population of preexisting varicosities at this time point and selectively requires higher levels of local protein synthesis for a period of approximately 2 days (24 hr-72 hr) in order to be stabilized. Once this stabilization phase is over, the level of local protein synthesis that is required for the long-term maintenance of the newly formed varicosities is now much lower and presumably similar to that required by the population of preexisting varicosities.
The existence of this later, critical period for local translation at the sensory to motor neuron synapse during LTF is consistent with the idea that the requirement of protein synthesis during long-term memory is likely to consist of multiple time-dependent phases, perhaps each with their own molecular requirements, that encompass the initiation, expression and maintenance of memory storage (Kandel, 2001). The relatively brief time window of these protein synthesis-dependent phases compared to the duration of the memory further suggests that high levels of translation are not continuously required over the entire life span of the memory and raises the question of how the molecular memory at the synapse is maintained. Our findings that local protein synthesis selectively stabilizes 5-HT-induced synaptic growth suggest that activity-dependent regulation of the proteome at the synapse is one attractive candidate for the molecular mechanisms that underlie the persistence of memory storage.
5-HT- induced newly formed sensory neuron varicosities are labile and sensitive to disruption during the stabilization phase
Once the 5-HT-induced new sensory neuron varicosities are formed they are labile and highly sensitive to disruption for a limited period that we have referred to as the stabilization phase of LTF. By quantitatively analyzing the consequences of inhibiting either local protein synthesis or the local activation of a specific translational regulator at 24 hr on the fate of individual identified varicosities that comprise two morphologically-defined and temporally-distinct populations of sensory neuron presynaptic compartments – 5-HT-induced newly formed varicosities and preexisting varicosities – we have found a preferential retraction of the learning-induced synaptic growth at 72 hr. The selective pruning of 5-HT-induced varicosities can be mimicked with local perfusion of the inhibitory neuromodulator FMRFamide 24 hr after 5-HT. Since the population of preexisting varicosities is essentially unaffected by local application of emetine, TAT-AS for ApCPEB, or FMRFamide in the 72 hr vs. 24 hr comparisons, our findings also suggest that LTF at these later time points may be entirely maintained by the newly formed varicosities that are induced by 5-HT.
Why are the learning-induced newly formed sensory neuron varicosities labile and so sensitive to disruption during this stabilization phase? The results of our study indicate that the stable maintenance of the 5-HT-induced newly formed varicosities is likely correlated with the size and state of morphological differentiation of their presynaptic compartment. Stable 5-HT-induced newly formed varicosities are on average significantly larger and functionally more effective than the labile newly formed varicosities. By contrast, 5-HT-induced newly formed varicosities that are synPH-inactive and on average smaller are more likely to disappear after local perfusion of protein synthesis inhibitors. This would also suggest that among the mRNAs that are locally translated to stabilize the 5-HT-induced newly formed varicosities some are likely to encode molecular components that are important for the assembly, maturation and maintenance of a fully differentiated and functionally competent presynaptic release site.
These results on the presynaptic compartment in Aplysia are consistent with findings on the postsynaptic compartment in mammals where a recent study in hippocampal slice culture has found that the new spines formed upon LTP induction are initially very unstable and that 15–19 hours are required for these postsynaptic sites to become functional synapses (De Roo et al., 2008). The fact that not all of the synPH-active varicosities at 24 hr were resistant to inhibitors of local protein synthesis – 36.1% disappeared at 72 hr – may be accounted for by other factors involved in the stabilization process. These could include the insertion of cell adhesion molecules, differentiation of the postsynaptic receptive and signaling apparatus and the functional integrity and maturation of the synaptic cytoskeleton.
The stabilization phase of LTF requires not only local protein synthesis but also the local activity of ApCEPB. How might ApCPEB stabilize the 5-HT-induced newly formed sensory neuron varicosities? Construction of cDNA libraries from the isolated axonal neurites of Aplysia sensory neurons has facilitated identification of mRNAs that encode structural proteins such as α1-tubulin and N-actin as well as translational elements including CPEB, the elongation factor eEF1α and several ribosomal proteins (Moccia et al., 2003, Moroz et al., 2006). One of the mechanisms proposed for the local activation of these mRNAs is polyadenylation (McGrew et al., 1989). The observations in Aplysia that N-actin mRNA contains a cytoplasmic polyadenylation element (CPE) sequence which binds to CPEB and that the increase of the poly(A) tail of N-actin mRNA parallels the induction of CPEB in response to 5-HT support the hypothesis that CPEB may be one of the key molecules involved in the regulation of local protein synthesis through a mechanism of polyadenylation (DesGroseillers et al., 1994; Liu and Schwartz, 2003; Si et al., 2003). Thus, CPEB might contribute to the stabilization of learning-related synaptic growth by controlling the local synthesis of both the cytoskeletal components of the presynaptic compartment as well as regulatory molecules important for synaptic maturation.
Stabilization of learning-induced synaptic growth represents a central issue in the study of long-term memory since it provides a structural platform for understanding the molecular changes that contribute to the persistence of memory storage. Recent in vivo imaging studies of genetically engineered mice have begun to examine the stability of synaptic connections in the mammalian brain. Using two-photon microscopy, Holtmaat et al. (2005) found that during development 65% of new postsynaptic spines in the somatosensory cortex were transient since they appeared and disappeared over a period of few days. However, when the somatosensory cortex and visual cortex were examined in adult mice the rate of spine turnover was much lower and most spines (73%) were stable for a period of several weeks. These results suggest that during development, when the brain is presumably more plastic, most synaptic connections are unstable. As the animals mature, progressively more of these synapses may be stabilized leading to the persistence of experience-induced changes. By specifically monitoring the 5-HT-induced newly formed presynaptic varicosities during the later stages of LTF in Aplysia, we have found that only the learning-related changes in synaptic structure are labile for a period of approximately 2 days. During this critical period the newly formed varicosities require a sustained increase in the local synthesis of proteins to acquire the more stable properties of “mature” varicosities and this stabilization of learning-induced synaptic growth leads to the persistence of LTF.
The results of the present study provide the most direct evidence to date that the stable maintenance of long-term synaptic plasticity requires the growth of new synaptic connections. Moreover, our finding that 5-HT-induced newly formed sensory neuron varicosities are sensitive to disruption by local application of inhibitors of protein synthesis for a critical period lasting several days indicates, for the first time, that new synapses formed after a memory-inducing stimulus are initially labile and require subsequent events involving ongoing local translation to be maintained. This suggests a potential difference in the molecular characteristics of the population of synapses that is induced by learning as compared to the population of preexisting synapses and, in turn, raises several questions central to an understanding of the cellular and molecular mechanisms that contribute to the persistence of memory storage. For example, does this stabilization process require coordinated trans-synaptic signaling between the pre- and postsynaptic neurons? What are the specific proteins produced during this late phase of ongoing local translation and how do they stabilize learning-induced synaptic growth? Once stabilized do these newly formed synapses exhibit a repertoire of functional and structural plasticity in response to activity-dependent stimuli that is similar to that of preexisting synapses or does one of the two populations of synapses now serve as a “stem” population to be selectively recruited for future learning-induced modifications? The ability to follow the same individual identified presynaptic varicosities for extended periods of time in the Aplysia sensory to motor neuron culture preparation now allow these questions to be addressed directly and should increase our understanding of the molecular events and signaling pathways that underlie the stabilization of learning-related synaptic remodeling and synaptic growth and their contribution to the persistence of long-term memory.
EXPERIMENTAL PROCEDURES
Aplysia Culture and Electrophysiology
Bifurcated sensory-motor neuron cultures were prepared and electrophysiology was done as described by Martin et al. (1997). To produce LTF, five bath applications of 10 μM 5-HT (100 μM; Sigma) were given at 15 min intervals and EPSPs were recorded before and 24 hr after 5-HT treatment (Montarolo et al., 1986). The percentage of change in EPSP amplitude was obtained by comparing the EPSPs measured at the indicated time points to the EPSP value measured at time 0. In experiments using emetine (100 μM; Sigma), ApCPEB antisense oligonucleotide (100 μM; Alta Bioscience, Birmingham, United Kingdom) or FMRFamide (1 μM; Calbiochem), the drugs were diluted in L15 containing 0.05% fast green and locally applied to the indicated synapse using a perfusion microelectrode connected to a picospritzer (Martin et al., 1997; Si et al., 2003). Emetine and ApCPEB antisense oligonucleotide were continuously delivered for 30 min using very low pressure (<1 lb/in2) whereas for the experiments with FMRFamide, five separated episodes of FMRFamide were applied at 10 min intervals.
DNA Injection and Cell Imaging
DNA constructs of e-GFP, monomeric DsRed and synapto-PHluorin were subcloned into the Aplysia expression vector pNEX3 and microinjected into the nucleus of 3-day-old bifurcated sensory neurons co-cultured with L7 gill-motor neurons, as previously described (Kaang 1996; Kim et al. 2003). Two days after the microinjection, the bifurcated sensory neurons expressing the injected DNA construct were identified and fluorescent images were acquired by using LSM Pascal (Zeiss, NY) laser confocal scanning microscope with a 40 X, NA 1.3 objective (Kim et al., 2003). A serial set of image stacks was collected along the sensory neuron axonal neurites contacting the cell body, initial segment and proximal processes of the motor cell. The same area was then imaged over the following days and the fluorescence levels were kept constant by adjusting the excitation intensity. The imaging sessions always followed the electrophysiological recordings.
ApCPEB TAT-Oligo
ApCPEB antisense oligonucleotides consisted of the oligo conjugated to a TAT protein (derived from HIV) which greatly increases the cell permeability of the oligo. The oligo was prepared as described by Si et al. (2003).
Quantification of Structural Changes
Serial images of the fluorescently-labeled sensory neuron varicosities and their three-dimensional reconstruction were analyzed using Metamorph software (Universal Imaging Corp, Philadelphia, PA, USA). For each sensory neuron we counted the total number of varicosities that were identified according to criteria previously established in vivo (Bailey at al 1979; Bailey and Chen, 1983). We therefore considered as varicosities all the labeled sensory neuron axonal swellings with a long diameter of >3 μm in contact with the initial segment, cell body and proximal processes of the motor neuron since it has been shown that only these varicosities have active zones (Bailey at al 1979; Glanzman et al., 1989; Kim et al., 2003). Quantification of total varicosity number was done blind with regard to experimental condition. In addition, in some experiments using 5-HT we defined two different populations of sensory neuron varicosities based on their past stimulus histories: (1) preexisting varicosities were identified as varicosities present from the first day of imaging and (2) 5-HT-induced newly formed varicosities were identified as varicosities that only appeared 24 hr after 5-HT treatment. To identify the same individual varicosity at each time point, we utilized a number of different fiduciary points throughout both the image stack as well as in individual confocal slices of the image stack. These landmarks included major branches and branch points of the sensory neuron, the relationship of the individual varicosity being followed with neighbouring varicosities on the same axon as well as varicosities on adjacent axons, etc. The percentage of change in varicosity number was obtained by comparing the total number of sensory neuron varicosities (5-HT-induced newly formed + preexisting) measured at the indicated time points to the total number of varicosities measured at time 0 (baseline). For the two specific populations of sensory neuron varicosities – preexisting and 5-HT-induced newly formed – we identified and followed the same individual varicosities at each time point and then compared the number of varicosities in each of the two classes at 72 hr to the number of varicosities in the same respective class observed at 24 hr. Cross-sectional area of the sensory neuron varicosities was computed by using the Metamorph software. A region of interest (ROI) was drawn around the perimeter of each varicosity in the reconstructed Z-series images and its area was calculated in μm2.
Statistical Analysis
All the data are presented as the mean ± SEM. Either an unpaired or paired Student’s t test was used to determine the statistical significance between two data sets. The statistical significance was indicated by *p < 0.05, **p < 0.01.
Supplementary Material
Acknowledgments
We thank Luana Fioriti for her critical comments on an earlier version of this manuscript and Changki Min (POSTECH) for preparation of the DsRed/pNEX expression vector. This work was supported by the Howard Hughes Medical Institute (to E.R.K), National Institutes of Health grant MH37134 (to C.H.B.), a fellowship from the Italian Academy for Advanced Studies in America (to M.C.M.), a Brain Research Center Frontier Program Grant M103KV010008-06K2201-00810 from Korea Ministry of Science and Technology (to J-H. Kim) and the Kavli Institute for Brain Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983;220:91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc Natl Acad Sci USA. 1988a;85:2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Long-term sensitization in Aplysia increases the number of presynaptic contacts onto the identified gill motor neuron L7. Proc Natl Acad Sci USA. 1988b;85:9356–9359. doi: 10.1073/pnas.85.23.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Time course of structural changes at identified sensory neuron synapses during long-term in Aplysia. J Neurosci. 1989;9:1774–1780. doi: 10.1523/JNEUROSCI.09-05-01774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Montarolo PG, Chen M, Kandel ER, Schacher S. Inhibitors of protein and RNA synthesis block the structural changes that accompany long-term heterosynaptic plasticity in the sensory neurons of Aplysia. Neuron. 1992;9:749–758. doi: 10.1016/0896-6273(92)90037-e. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Thompson EB, Castellucci VF, Kandel ER. Ultrastructure of the synapses of sensory neurons that mediate the gill-withdrawal reflex in Aplysia. J Neurocytol. 1979;8:415–444. doi: 10.1007/BF01214801. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen A, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural changes. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Ghirardi M, Casadio A, Giustetto M, Karl KA, Zhu H, Kandel ER. Enhancement of memory-related long-term facilitation by ApAF, a novel transcription factor that acts downstream from both CREB1 and CREB2. Cell. 2000;103:595–608. doi: 10.1016/s0092-8674(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. PNAS. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis-and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Bradshaw KD, Emptage NJ, Bliss TV. A role for protein synthesis in hippocampal late LTP. Eur J Neurosci. 2003;18:3150–3152. doi: 10.1111/j.1460-9568.2003.03054.x. [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Mendez P, Poglia L, Muller D. Activity-dependent PSD formation and stabilization of newly formed spines in hippocampal slice cultures. Cereb Cortex. 2008;18:151–161. doi: 10.1093/cercor/bhm041. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L, Auclair D, Wickham L, Maalouf M. A novel actin cDNA is expressed in the neurons of Aplysia Californica. Biochim Biophys Acta. 1994;1217:322–324. doi: 10.1016/0167-4781(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frost WN, Castellucci VF, Hawkins RD, Kandel ER. Monosynaptic connections made by the sensory neurons of the gill- and siphon-withdrawal reflex in Aplysia participate in the storage of long-term memory for sensitization. Proc Natl Acad Sci USA. 1985;82:8266–8269. doi: 10.1073/pnas.82.23.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman DL, Kandel ER, Schacher S. Identified target motor neuron regulates neurite outgrowth and synapse formation of Aplysia sensory neurons in vitro. Neuron. 1989;3:441–450. doi: 10.1016/0896-6273(89)90203-1. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Kandel ER, Schacher S. Target-dependent structural changes accompanying long-term synaptic facilitation in Aplysia neurons. Science. 1990;249:779–802. doi: 10.1126/science.2389145. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Tractenberg JT, Wilbrecht L, Shepherd GM, Zang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Kaang BK. Parameters influencing ectopic gene expression in Aplysia neurons. Neurosci Lett. 1996;221:29–32. doi: 10.1016/s0304-3940(96)13279-1. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kim JH, Udo H, Li HL, Youn TY, Chen M, Kandel ER, Bailey CH. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron. 2003;40:151–165. doi: 10.1016/s0896-6273(03)00595-6. [DOI] [PubMed] [Google Scholar]
- Liu J, Schwartz JH. The cytoplasmic polyadenylation element binding protein and polyadenylation of messenger RNA in Aplysia neurons. Brain Res. 2003;959:68–76. doi: 10.1016/s0006-8993(02)03729-0. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose J, Chen M, Bailey CH, Kandel ER. Synapse-specific long-term facilitation of Aplysia sensory somatic synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- McGrew LL, Dworkin-Rastl E, Dworkin MB, Richter JD. Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev. 1989;3:803–815. doi: 10.1101/gad.3.6.803. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1988;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Moccia R, Chen D, Lyles V, Kapuya E, EY, Kalachikov S, Spahn CM, Frank J, Kandel ER, Barad M, Martin KC. An unbiased cDNA library prepared from isolated Aplysia sensory neuron processes is enriched for cytoskeletal and translational mRNAs. J Neurosci. 2003;23:9409–9417. doi: 10.1523/JNEUROSCI.23-28-09409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Kandel ER, Schacher S. Long-term heterosynaptic inhibition in Aplysia. Nature. 1988;333:171–174. doi: 10.1038/333171a0. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, Jezzini S, Lovell P, Iannucculli W, Chen M, Nguyen T, Sheng H, Shaw R, Kalachikov S, Panchin YV, Farmerie W, Russo JJ, Ju J, Kandel ER. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ. Parallel somatic and synaptic processing in the induction of intermediate-term and long-term synaptic facilitation in Aplysia. Proc Natl Acad Sci USA. 2004;101:7463–7468. doi: 10.1073/pnas.0402163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in Aplysia. Cell. 2003;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;6917:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Udo H, Jin I, Kim JH, Li HL, Youn T, Hawkins RD, Kandel ER, Bailey CH. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires CdC42, N-WASP and PAK in Aplysia sensory neurons. Neuron. 2005;45:887–901. doi: 10.1016/j.neuron.2005.01.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.