Abstract
Problem
Malawi’s national guidelines recommend that infants exposed to the human immunodeficiency virus (HIV) be tested at 6 weeks of age. Rollout of services for early infant diagnosis has been limited and has resulted in the initiation of antiretroviral therapy (ART) in very few infants.
Approach
An early infant diagnosis programme was launched. It included education of pregnant women on infant testing, community sensitization, free infant testing at 6 weeks of age, active tracing of HIV-positive infants and referral for treatment and care.
Local setting
The programme was established in two primary care facilities in Blantyre, Malawi.
Relevant changes
Of 1214 HIV-exposed infants, 71.6% presented for early diagnosis, and 14.5% of those who presented tested positive for HIV. Further testing of 103 of these 126 apparently HIV-positive infants confirmed infection in 88; the other 15 results were false positives. The initial polymerase chain reaction testing of dried blood spots had a positive predictive value (PPV) of 85.4%. Despite active tracing, only 87.3% (110/126) of the mothers of infants who initially tested positive were told their infants’ test results. ART was initiated in 58% of the infants with confirmed HIV infection.
Lessons learnt
Early infant diagnosis of HIV infection at the primary care level in a resource-poor setting is challenging. Many children in the HIV diagnosis and treatment programme were lost to follow-up at various stages. Diagnostic tools with higher PPV and point-of-care capacity and better infrastructures for administering ART are needed to improve the management of HIV-exposed and HIV-infected infants.
Résumé
Problème
Les lignes directrices nationales du Malawi recommandent que les nourrissons exposés au virus de l'immunodéficience humaine (VIH) soient testés à l'âge de 6 semaines. Le déploiement des services pour le diagnostic précoce chez les nourrissons a été limité et n'a donné lieu au lancement de la thérapie antirétrovirale (TAR) que chez très peu de nourrissons.
Approche
Un programme de diagnostic infantile précoce a été lancé. Il comprenait l'éducation des femmes enceintes quant aux tests des nourrissons, la sensibilisation des communautés, le test gratuit des nourrissons âgés de 6 semaines, le traçage actif des nourrissons séropositifs et l'aiguillage pour le traitement et les soins.
Environnement local
Le programme a été établi dans deux établissements de soins de santé primaires à Blantyre, au Malawi.
Changements significatifs
Sur 1 214 nourrissons exposés au VIH, 71,6% bénéficiaient d’un diagnostic précoce, et 14,5% des nourrissons présentés étaient séropositifs. Des tests supplémentaires effectués sur 103 de ces 126 nourrissons apparemment séropositifs ont confirmé l'infection de 88 d'entre eux; les 15 autres résultats étant faussement positifs. Les tests initiaux de réaction en chaîne par polymérase sur des taches de sang séché avaient une valeur prédictive positive (VPP) de 85,4%. Malgré le traçage actif, seules 87,3% (110/126) des mères de nourrissons initialement testés positifs ont été informées des résultats des tests de leurs nourrissons. Un TAR a été initié chez 58% des nourrissons dont l'infection par le VIH a été confirmée.
Leçons tirées
Le diagnostic précoce de l'infection par le VIH des nourrissons est un défi au niveau des soins primaires dans un contexte de ressources limitées. Le suivi de nombreux enfants, dans le programme de diagnostic et de traitement du VIH, s'est interrompu à divers stades. Les outils de diagnostic avec une VPP et une capacité de lieu de soins plus élevées, et de meilleures infrastructures pour l'administration du TAR, sont nécessaires pour améliorer la prise en charge des nourrissons exposés et infectés par le VIH.
Resumen
Situación
Las directrices nacionales de Malawi recomiendan que se realice la prueba del virus de la inmunodeficiencia humana (VIH) a los lactantes expuestos al mismo cuando cumplan las 6 semanas de edad. Se ha restringido el desarrollo de los servicios de diagnóstico temprano de lactantes, lo que ha dado como resultado que muy pocos lactantes hayan comenzado con una terapia antirretroviral (TAR).
Enfoque
Se lanzó un programa de diagnóstico temprano de lactantes que incluyó la formación de mujeres embarazadas acerca de las pruebas para lactantes, la sensibilización de la comunidad, pruebas gratuitas para lactantes de 6 semanas de edad, el seguimiento activo de lactantes seropositivos y la derivación para su tratamiento y atención sanitaria.
Marco regional
El programa se estableció en dos centros de atención sanitaria primaria en Blantye, Malawi.
Cambios importantes
De los 1214 lactantes expuestos, el 71,6% acudió al diagnóstico temprano y de ellos, el 14,5% dio positivo para el VIH. Otras pruebas realizadas a 103 de los, al parecer, 126 lactantes seropositivos confirmaron la infección en 88 lactantes, los otros 15 resultados fueron falsos positivos. La reacción en cadena de la polimerasa inicial de muestras de sangre seca tuvo un valor predictivo positivo (VPP) del 85,4%. A pesar del seguimiento activo, sólo se comunicó el resultado de las pruebas al 87,3% (110/126) de las madres de lactantes que en un principio dieron positivo. La TAR se comenzó en el 58% de los lactantes con una infección por VIH confirmada.
Lecciones aprendidas
El diagnóstico temprano para la infección por VIH en lactantes en el ámbito de la atención primaria en entornos con pocos recursos requiere un gran esfuerzo. En las distintas etapas se perdió el rastro de muchos niños del programa de diagnóstico y tratamiento del VIH. Las herramientas de diagnóstico con un VPP alto y la capacidad de los puntos de atención, así como la mejora de las infraestructuras para la administración de la TAR son necesarias para mejorar la gestión de los lactantes expuestos al VIH e infectados por él.
ملخص
المشكلة
توصي المبادئ التوجيهية الوطنية في ملاوي بفحص الرضع المعرضين للإصابة بفيروس العوز المناعي البشري (HIV) عند سن 6 أسابيع. وكان نشر الخدمات المعنية بالتشخيص لدى الرضع في مرحلة مبكرة محدوداً ونتج عنه بدء العلاج بمضادات الفيروسات القهقرية (ART) لدى عدد قليل للغاية من الأطفال.
الأسلوب
تم إطلاق برنامج تشخيص الرضع في مرحلة مبكرة. وتضمن تثقيف السيدات الحوامل بشأن فحوص الرضع وتوعية المجتمع المحلي وفحوص الرضع المجانية عند سن 6 أسابيع واقتفاء الأثر النشط للرضع الإيجابيين لفيروس العوز المناعي البشري والإحالة للعلاج والرعاية.
المواقع المحلية
تم إنشاء البرنامج في مرفقي رعاية أولية في بلانتاير، بملاوي.
التغيّرات ذات الصلة
خضعت نسبة 71.6 % من إجمالي 1214 رضيعاً معرضين للإصابة بفيروس العوز المناعي البشري إلى تشخيص مبكر وكانت نتيجة فحص 14.5 % من الذين خضعوا للتشخيص المبكر إيجابية لفيروس العوز المناعي البشري. وأكدت الفحوص الإضافية التي خضع لها 103 من 126 رضيعاً إيجابيين لفيروس العوز المناعي البشري بشكل واضح على العدوى في 88 حالة؛ غير أن النتائج الخمس عشرة الأخرى كانت إيجابية على نحو زائف. وكان لاختبار تفاعل البوليميراز المتسلسل الأولي لبقع الدم الجافة قيمة تنبؤية إيجابية (PPV) بنسبة 85.4 %. وعلى الرغم من اقتفاء الأثر النشط، لم يتم إخبار سوى 87.3 % (110/126) من أمهات الرضع الذين كانت النتيجة الأولية لفحوصهم إيجابية بنتائج أطفالهم الرضع . وتم بدء العلاج بمضادات الفيروسات القهقرية لدى 58 % من الرضع الذين تأكد إصابتهم بفيروس العوز المناعي البشري.
الدروس المستفادة
يمثل تشخيص عدوى فيروس العوز المناعي البشري لدى الرضع في مرحلة مبكرة على مستوى الرعاية الأولية في المواقع فقيرة الموارد تحدياً. وقد افتقد العديد من الأطفال في برنامج تشخيص فيروس العوز المناعي البشري وعلاجه إلى المتابعة في مراحل متعددة. وينبغي توافر وسائل تشخيص ذات قيمة تنبؤية إيجابية وقدرة نقاط رعاية أعلى وتحسين البنية الأساسية لإدارة العلاج بمضادات الفيروسات القهقرية بغية تحسين التدبير العلاجي للرضع المعرضين للإصابة بفيروس العوز المناعي البشري والمصابين به.
摘要
问题
根据马拉维的国家指导方针的建议,暴露于艾滋病病毒(HIV)的婴儿要在6 周龄时接受检测。婴儿早期诊断服务的推广受到了限制,导致接受抗逆转录病毒疗法(ART)的婴儿人数极少。
方法
推出婴儿早期诊断计划。它包括对孕妇进行婴儿检测教育、社区宣传、婴儿在 6 周龄的免费检测以及艾滋病毒呈阳性的婴儿的积极跟踪和转诊治疗与护理。
当地状况
该计划在马拉维的布兰太尔两个初级保健医院中展开。
相关变化
暴露于艾滋病毒的1214 名婴儿中,71.6%接受早期诊断,其中14.5%检测为HIV阳性。对这些明显艾滋病毒呈阳性的126 名婴儿当中的103 名进行进一步检测,确认感染88 例;其他15 个结果为假阳性。初步的干血点聚合酶链反应检测阳性预测值(PPV)为85.4%。尽管积极跟踪,只有87.3%(110/126)的初步检测呈阳性的婴儿的母亲被告知其婴儿的检测结果。证实感染艾滋病毒的婴儿中有58% 开始接受ART治疗。
经验教训
在资源贫乏的环境中开展初级保健等级的感染艾滋病毒的婴儿早期诊断充满挑战。艾滋病毒诊断和治疗方案中有许多儿童在后续的各个阶段无法进行跟踪。需要具有更高PPV和即时能力的诊断工具以及更好的管理ART的基础设施,以改善对暴露于艾滋病毒和艾滋病毒感染的婴儿的管理。
Резюме
Проблема
Согласно национальным нормативам Малави, тестирование младенцев, подверженных вирусу иммунодефицита человека (ВИЧ), рекомендуется проводить в шестинедельном возрасте. Внедрение услуг по ранней диагностике инфекции у младенцев было ограничено, что привело к проведению антиретровирусной терапии (АРТ) у очень малого числа младенцев.
Подход
Произведен запуск программы по ранней диагностике инфекции у младенцев. Программа включала в себя информирование беременных женщин о тестировании младенцев, проведение разъяснительной работы среди местного населения, бесплатное тестирование младенцев шестинедельного возраста, активное наблюдение за ВИЧ-положительными младенцами и направление их на лечение и уход.
Местные условия
Программа реализовывалась в двух учреждениях первой медицинской помощи в г. Блантайр, Малави.
Соответствующие изменения
Из 1214 младенцев, подверженных риску заражения ВИЧ, 71,6% направлялись на раннюю диагностику, и у 14,5% из числа направленных, результат на ВИЧ оказался положительным. Дальнейшее тестирование 103 из 126 вероятно ВИЧ-положительных младенцев подтвердило наличие инфекции у 88 детей; остальные 15 результатов были ошибочно-положительными. Положительное предсказательное значение (ППЗ) первоначального тестирования на основе полимеразной цепной реакции сухих капель крови составляло 85,4%. Несмотря на активное отслеживание, только 87,3% (110/126) матерям, чьи младенцы изначально имели положительные результаты, были сообщены результаты теста. АРТ проводилась у 58% младенцев с подтвержденной ВИЧ-инфекцией.
Извлеченные уроки
Проведение ранней диагностики ВИЧ-инфекции у младенцев на уровне первичной медицинской помощи в условиях нехватки ресурсов является затруднительным. Многие младенцы, участвовавшие в программе лечения и диагностики ВИЧ, выбывали из наблюдения на различных стадиях. Средства диагностики с высоким ППЗ и вместимость центров предоставления медицинских услуг и лучшая инфраструктура для назначения АРТ необходимы для улучшения ведения ВИЧ-инфицированных младенцев и младенцев, подверженных риску заражения ВИЧ.
Background
According to the recommendations of the World Health Organization (WHO), infants known to have been exposed to the human immunodeficiency virus (HIV) should undergo a virological test for infection at 4 to 6 weeks of age.1,2 Antiretroviral therapy (ART) should be initiated upon diagnosis of HIV infection in children aged less than 24 months.3 However, implementing programmes for such early infant diagnosis and treatment has proved challenging.4–7
In Malawi, 13.8% of the children born to HIV-positive mothers in 2009 were themselves HIV-positive as infants,8 but only 29% of those in need of ART received such treatment.9 National guidelines in Malawi recommend that infants exposed to HIV be tested by polymerase chain reaction (PCR) for the detection of viral deoxyribonucleic acid (DNA) at 6 weeks of age wherever the facilities and resources for these assays are available.10 In Malawi, as in several other countries, most early infant diagnosis is hospital-based and few infants receive ART after testing.8,11
We report here the experiences and challenges encountered during implementation of early infant diagnosis in two community health centres in Malawi.
Setting and approach
As part of recruitment procedures for a study assessing the impact of HIV infection on child neurodevelopmental processes, early infant diagnosis services were established at two health-care facilities in Blantyre. One of these facilities, the Zingwangwa Health Centre, is an urban primary care centre that does not initiate or administer ART but that refers those in need of ART to a hospital. The other study facility, the Mlambe Mission Hospital, is located in a semi-rural area and serves as a primary and secondary care centre with on-site ART services. Both study facilities run programmes for the prevention of mother-to-child transmission (PMTCT) of HIV and both recommend breastfeeding and cotrimoxazole prophylaxis for all HIV-exposed infants.
As pregnant women and mothers with infants attended the study centres for PMTCT and postnatal visits, study staff explained to them the importance of early infant HIV diagnosis and the benefits of early ART for infants. To further increase awareness of early infant diagnosis, posters and brochures were distributed and community sensitization was performed. Each HIV-positive mother was given an appointment card. At 6 weeks of age (or at the earliest visit thereafter), each infant of an HIV-positive mother was referred for cotrimoxazole prophylaxis and tested for HIV DNA. Permission for home visits was requested from the infant’s mother, who was asked to return with the infant, for a follow-up, 4 weeks later.
A sample of blood was collected from each HIV-exposed infant via a heel prick. These samples were transferred to Protein Saver 903® cards (Whatman Ltd, Piscataway, United States of America), which were then dried and individually packaged with desiccant sachets before being transported to the Malawi–Liverpool–Wellcome Trust research laboratory in Blantyre. At the laboratory, the dried blood spots on the cards were tested for HIV DNA using version 1.5 of the Amplicor® HIV-1 DNA test kit (Roche, Basel, Switzerland), which is based on a PCR. The laboratory included internal quality control procedures for each 4-weekly testing batch and participated in an external quality control programme run by the United States Centers for Disease Control and Prevention.
At follow-up, mothers of the children found to be PCR-negative for HIV DNA were counselled on how to minimize transmission risk and the importance of repeat testing while breastfeeding. The mothers of the PCR-positive infants were counselled on the importance of early infant ART and their infants were referred to the nearest ART clinic. Community health staff, who were reimbursed by study funds, were asked to trace the mothers of PCR-positive infants who did not return for the scheduled follow-up.
Attempts were made to retest each child who was initially found PCR-positive. In most cases, the same PCR-based assay as used initially was employed to test a second blood spot on the child’s 6-week sample card. However, if the relevant sample card could not be located, the HIV ribonucleic acid (RNA) in a second blood sample collected from the child was quantified, at the University of North Carolina’s project laboratory in Lilongwe, using version 1.5 of the Amplicor® HIV-1 Monitor (Roche). If the retest sample gave a positive result, the child was considered truly positive. If the retest sample appeared negative, another blood sample was collected from the child and either tested for HIV DNA or assayed for HIV RNA. If this sample was found negative, the child’s result was considered a false positive. The final result for any child who was found first positive and then negative and then was unavailable for a second retest was recorded as inconclusive.
Both the University of Malawi College of Medicine Research and Ethics Committee and the University of North Carolina at Chapel Hill Institutional Review Board approved the study protocol.
Results
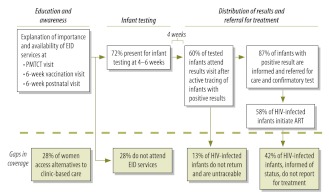
Between January 2008 and June 2010, 7234 women, of whom 1214 (16.8%; 95% confidence interval, CI: 15.9%–17.6%) were HIV-infected, participated in PMTCT activities at either of the two study centres. Although 920 (75.8%) of the infants of the HIV-positive mothers presented for early infant diagnosis, consent for the necessary testing was only obtained from the mothers of 869 (94.5%; 95% CI: 93.0%–95.9%) of these children. In consequence, only 71.6% (95% CI: 69.0%–74.1%) of the HIV-exposed infants seen at the two study centres during the study period were tested for HIV DNA. Although all but one of the 50 women who declined to give consent for the infant testing said that they wanted to get permission from their spouses, none of these women ever returned for early infant diagnosis. Self-reported PMTCT coverage, for both mothers and infants at the two study sites, was 92.4%.
Overall, 126 (14.5%) of the 869 infants tested for HIV DNA gave a positive initial result. The original sample cards for 61 of the PCR-positive children were relocated and another blood spot from each of these cards was checked for HIV DNA in the PCR-based assay. Of the 61 children who were retested in this way, 41 gave a positive result on retesting and 20 gave a negative result. Although fresh blood samples were collected from 17 of the 20 children found PCR-negative on retesting, only two of these 17 fresh samples gave a positive result when checked for HIV DNA (5 samples) or assayed for HIV RNA (12 samples). The original sample cards for 65 of the children who were initially found positive for HIV DNA had been thrown away after the initial testing. Fresh blood samples were obtained from 45 of these 65 children and all 45 of these samples gave a positive result when assayed for HIV RNA. Confirmatory testing was not possible for the 20 children who were lost to follow-up. Among the 106 infants with any confirmatory testing, 88 were confirmed as HIV-infected and 15 were considered HIV-negative; the results for the remaining three children, who could not be retested fully, were inconclusive. Overall, 14.6% of the 103 children who were retested fully were found to have been falsely positive in the initial round of testing. The positive predictive value of the assay used in this initial round, which was based on the detection, by PCR, of HIV DNA in dried spots of blood from infants aged about 6 weeks, was therefore 85.4%.
Only 521 (60%) of the mothers of tested infants returned to the study centres to receive the results of the initial testing. However, compared with the other mothers of tested infants, a mother of a child found HIV-positive when first tested by PCR was significantly more likely to have received the results of her infant’s test (87.3% versus 55.3%; P < 0.0001). The initiation of ART was recorded for 51 children, who represented 58% of all of the children with confirmed HIV infection.
Discussion
Important lessons can be drawn from our experience (Box 1). Routine early infant diagnosis at the primary care level in a resource-poor setting is feasible but challenging, even when well supported by research funds. A strength of our approach was the integration of the early infant diagnosis programme into the existing PMTCT services, which facilitated delivery of messages about the importance and availability of early infant diagnosis and early ART to the target population of HIV-infected pregnant women. The scheduling of appointments for early infant diagnosis so that they coincided with routine visits for the vaccination of infants at 6 weeks of age eliminated the need for additional visits for the sole purpose of testing infants for early diagnosis. These approaches resulted in the HIV testing of more than two thirds of the HIV-exposed infants seen at the study centres.
Box 1. Summary of main lessons learnt.
Integration of early infant diagnosis services with existing PMTCT services is feasible and facilitates delivery of messages about the importance of early infant diagnosis to HIV-infected women.
Despite active tracing of HIV-infected children, there are high rates of loss to follow-up at every stage of the early infant diagnosis programme.
Improved diagnostic tools with point-of-care capacity are necessary to allow for more streamlined testing programmes with the potential for better linkage to infant antiretroviral treatment.
HIV, human immunodeficiency virus; PMTCT, prevention of mother-to-child transmission.
Our experiences with the early infant diagnosis programme also highlighted many challenges. Unfortunately, since HIV exposure was not documented on the standard infant health passports and there was limited privacy in the clinics, it proved impossible to integrate the services for early infant diagnosis with vaccination clinics. The high percentage of false positive results in the initial round of HIV tests (14.6%) was unexpected, given the previously reported high specificity of the PCR-based assay that was used.12,13 Further investigations indicated that laboratory contamination, resulting from the manual manipulation of the sample cards, was the most likely cause of the low specificity. This observation underscores the need for stringent quality control and confirmatory testing. The long delay between the collection of the initial blood samples and the availability of the results of confirmatory testing complicated the communication of an initial positive result and the management of the children with such a result. Knowing the overwhelming benefits of early ART, we did not withhold such treatment until the confirmatory results were available. We carefully counselled the mothers of infants who were initially found PCR-positive while realizing that a positive result on an infant’s HIV test is devastating news and that the occurrence of false positive results can undermine community trust in early infant diagnosis.
The use of dried blood spots, which can be collected at any clinic and transported without refrigeration to a laboratory equipped for PCR-based analysis,12 has been an important step towards universal access to early infant diagnosis. However, by eliminating the need to transport samples or to return to a clinic to retrieve the test results, a rapid point-of-care test for infant HIV diagnosis14 could still greatly enhance the operational feasibility of early infant diagnosis programmes in resource-poor settings.
A unique aspect of our approach was the active tracing of HIV-positive infants, which resulted in the receipt of the infants’ HIV test results by the families of 87% of these infants. Tracing is unlikely to be possible without dedicated funds. Sustainable strategies to improve the communication of test results to the caregivers of infants in routine settings need to be explored. Infant treatment programmes also need to be scaled up to maximize the number of HIV-infected infants who initiate ART – the ultimate goal of early infant diagnosis programmes.
While promising, the 71.6% coverage with early infant diagnostic services that was recorded in the present study indicates that one in four HIV-exposed infants did not access such services. Additionally, the coverage recorded here may be an underestimate of the true value, since the 28% of women in Malawi who deliver at home, with the help of traditional birth attendants,15 may never be offered early infant diagnostic testing (Fig. 1). The involvement of traditional birth attendants in early infant diagnosis programmes and the integration of early infant diagnosis into vaccination clinics with community outreach could complement any clinic-based activities. The routine seeking of permission for infant testing from both the mother and her partner should be explored, as permission from the partner was the primary reason that women who presented for early infant diagnosis gave for not proceeding with the testing.
Fig. 1.
Opportunities to improve early infant diagnosis of HIV infection, based on experiences at two primary care centres, Malawi, 2008–2010
ART, antiretroviral therapy; EID, early infant diagnosis; HIV, human immunodeficiency virus; PMTCT, prevention of mother-to-child transmission.
Although careful documentation and a large sample size were important strengths of this evaluation, the research setting limits the generalizability of our findings. The substantial human resources and financial support that were available in the present study, through linkage with the research team, probably created a “best-case scenario” for a setting that is usually resource-poor. Even under these conditions, however, the implementation of early infant diagnosis at the primary-care level was challenging, with the dropouts that occurred at every step diminishing the number of HIV-infected children who gained access to ART. Our experience suggests that, to maximize the benefits of early infant HIV diagnosis programmes, a simple, affordable and highly specific point-of-care test for infant HIV diagnosis and better linkage to care are both needed.
Acknowledgements
The Malawi–Liverpool–Wellcome Trust Research Programme receives funding from the Wellcome Trust. The authors are grateful to all study and clinical staff at Mlambe Mission Hospital, Zingwangwa Health Centre and Queen Elizabeth Central Hospital, and to all participating women and children.
Funding:
Funding was provided by the United States National Institutes of Health/National Institute of Child Health and Human Development, via grant R01 HD053216.
Competing interests:
None declared.
References
- 1.WHO antiretroviral therapy for infants and children. Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group meeting Geneva: World Health Organization; 2008. Available from: http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport 2008.pdf [accessed 15 May 2012].
- 2.Creek TL, Sherman GG, Nkengasong J, Lu L, Finkbeiner T, Fowler MG, et al. Infant human immunodeficiency virus diagnosis in resource-limited settings: issues, technologies, and country experiences. Am J Obstet Gynecol. 2007;197:S64–71. doi: 10.1016/j.ajog.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Antiretroviral therapy for HIV infection in infants and children: towards universal access. Recommendations for a public health approach Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 4.Cook RE, Ciampa PJ, Sidat M, Blevins M, Burlison J, Davidson MA, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambezia, Mozambique. J Acquir Immune Defic Syndr. 2011;56:e104–9. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braitstein P, Songok J, Vreeman R, Wools-Kaloustian K, Koskei P, Walusuna L, et al. ‘Wamepotea’ (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya. J Acquir Immune Defic Syndr. 2011;57:e40–6. doi: 10.1097/QAI.0b013e3182167f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciampa PJ, Burlison JR, Blevins M, Sidat M, Moon TD, Rothman RL, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J Acquir Immune Defic Syndr. 2011;58:115–9. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- 7.Nuwagaba-Biribonwoha H, Werq-Semo B, Abdallah A, Cunningham A, Gamaliel JG, Mtunga S, et al. Introducing a multi-site program for early diagnosis of HIV infection among HIV-exposed infants in Tanzania. BMC Pediatr. 2010;10:44. doi: 10.1186/1471-2431-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malawi HIV and AIDS monitoring and evaluation report: 2008–2009. UNGASS country progress report Geneva: Joint United Nations Programme on HIV/AIDS, 2010. Available from: http://www.unaids.org/en/dataanalysis/monitoringcountryprogress/progressreports/2010countries/malawi_2010_country_progress_report_en.pdf [accessed 15 May 2012].
- 9.UNAIDS report on the global AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS, 2010. Available from: http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf [accessed 12 October 2011].
- 10.Guidelines for paediatric HIV testing and counselling Lilongwe: Malawi Ministry of Health; 2007. [Google Scholar]
- 11.Chatterjee A, Tripathi S, Gass R, Hamunime N, Panha S, Kiyaga C, et al. Implementing services for early infant diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health. 2011;11:553. doi: 10.1186/1471-2458-11-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherman GG, Cooper PA, Coovadia AH, Puren AJ, Jones SA, Mokhachane M, et al. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J. 2005;24:993–7. doi: 10.1097/01.inf.0000187036.73539.8d. [DOI] [PubMed] [Google Scholar]
- 13.Driver GA, Patton JC, Moloi J, Stevens WS, Sherman GG. Low risk of contamination with automated and manual excision of dried blood spots for HIV DNA PCR testing in the routine laboratory. J Virol Methods. 2007;146:397–400. doi: 10.1016/j.jviromet.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DA, Crowe SM, Garcia M. Point-of-care testing. Curr HIV/AIDS Rep. 2011;8:31–7. doi: 10.1007/s11904-010-0067-z. [DOI] [PubMed] [Google Scholar]
- 15.Malawi demographic and health survey (2010 MDHS) report Zomba: National Statistical Office of Malawi; 2010. Available from: http://www.nso.malawi.net/index.php?option=com_content&view=article&id=175&Itemid=46 [accessed 15 May 2012].