Genetic inactivation of the transcription factor GATA-6 in the embryoid body induces massive apoptosis at the early stage of ES cell differentiation. Evidence is provided that BMP-2 is a direct transcription target of GATA-6 and mediates GATA-6-dependent cell survival in concert with endoderm-derived basement membrane.
Abstract
GATA-6 is a zinc-finger transcription factor essential for early embryogenesis. Ablation of GATA-6 in mice impairs endoderm differentiation and causes apoptosis of epiblast cells. The endoderm defects have been attributed to the loss of HNF4, disabled-2, and GATA-4. However, the mechanisms underlying epiblast apoptosis are unclear. In this study we used mouse embryonic stem cell–derived embryoid bodies (EBs) as a model for peri-implantation development and found that ablation of GATA-6 causes massive apoptosis during EB differentiation. Endoderm grafting experiments and ectopic basement membrane (BM) assembly suggest that both BM and non-BM factors contribute to cell survival. Furthermore, the increased cell death in mutant EBs is accompanied by reduced expression of bone morphogenetic protein 2 (BMP-2). Chromatin immunoprecipitation reveals direct binding of GATA-6 to the Bmp2 promoter. Treatment of the mutant EBs with BMP-2 markedly suppresses apoptosis, whereas stable overexpression of the BMP antagonist noggin or a dominant-negative BMP receptor in normal EBs leads to increased apoptosis. Last, activation of SMAD1/5 by phosphorylation is significantly inhibited in the absence of GATA-6, and this is reversed by exogenous BMP-2. Treatment of normal EBs with SMAD phosphorylation inhibitor increases apoptosis. Collectively these results suggest that GATA-6 promotes cell survival by regulating endoderm expression of BMP-2 and BM during embryonic epithelial morphogenesis.
INTRODUCTION
During peri-implantation development, the inner cell mass of the blastocyst-stage embryo differentiates into two cell lineages—the epiblast, which gives rise to the embryo proper, and the primitive endoderm, which further produces two extraembryonic cell types, the parietal and visceral endoderm. The parietal endoderm migrates on the inner surface of the trophectoderm and secretes large amounts of laminins, type IV collagen, and other basement membrane (BM) components that assemble into a BM (Reichert's membrane in mice). The visceral endoderm is associated with the epiblast and is active in transport of nutrients and fluids. It also secretes matrix proteins required for the formation of the embryonic BM, which separates the visceral endoderm from the epiblast and induces epiblast polarization and cavitation (Li et al., 2003; Rossant, 2004). Moreover, the anterior endoderm serves as a guidance cue for gastrulation as the embryo develops further (Tam et al., 2006).
GATA-binding proteins are zinc-finger transcription factors that recognize the consensus DNA sequence (T/A)GATA(A/G) and are essential for cellular differentiation during embryonic development. GATA transcription factors can be divided into two subfamilies based upon sequence homologies and their tissue distribution. The GATA-1/2/3 subfamily is expressed predominantly in blood cells, and targeted deletion of their genes in mice impairs hematopoiesis (Pevny et al., 1991; Tsai et al., 1994; Pandolfi et al., 1995). GATA-4/5/6 are primarily expressed in, and required for, the development of endoderm lineages and the cardiovascular system (Kuo et al., 1997; Molkentin et al., 1997; Morrisey et al., 1998; Laforest et al., 2011). GATA-6–expressing cells can be detected as early as the eight-cell stage in mouse embryos and gradually segregate from the Nanog-expressing epiblast in blastocyst-stage embryos to form the primitive endoderm on the surface of the epiblast (Plusa et al., 2008). During the course of endoderm specification and differentiation, GATA-6–positive cells begin to express other endoderm markers, such as platelet-derived growth factor receptor α, GATA-4, α-fetoprotein, and disabled-2. Forced expression of GATA-6 in embryonic stem (ES) cells induces endoderm differentiation, whereas targeted deletion of the Gata6 gene in mice blocks endoderm differentiation and leads to embryonic death between E5.5 and E7.5 (Morrisey et al., 1998; Koutsourakis et al., 1999; Fujikura et al., 2002; Cai et al., 2008). An initial analysis of the GATA-6–null phenotype based on in situ hybridization genotyping showed that the visceral endoderm formed but was unable to mature (Morrisey et al., 1998). Later, a study using immunohistochemistry for genotyping revealed that the primitive, visceral, and parietal endoderm all failed to develop in GATA-6–null embryos, which is consistent with the findings on embryoid body (EB) differentiation (Morrisey et al., 1998; Capo-Chichi et al., 2005; Cai et al., 2008). Nonetheless, these studies established an essential role for GATA-6 in differentiation of extraembryonic endoderm in early embryogenesis.
Analysis of GATA-6–null embryos also demonstrated increased epiblast apoptosis. However, the underlying molecular mechanisms are unknown (Morrisey et al., 1998). Chimeric analysis suggests that death of the epiblast is likely due to a lack of or a defect in supporting visceral endoderm (Morrisey et al., 1998; Koutsourakis et al., 1999). In this study, we investigated the mechanisms of GATA-6–dependent survival using ES cell–differentiated EBs, an in vitro model for peri-implantation development (Coucouvanis and Martin, 1995; Li and Yurchenco, 2006). Our results suggest that GATA-6–dependent expression of bone morphogenetic protein 2 (BMP-2) and select components of the BM provides important survival signals for ES cells during their differentiation into primitive germ layers.
RESULTS
Ablation of GATA-6 causes extensive apoptosis at the early stage of EB differentiation
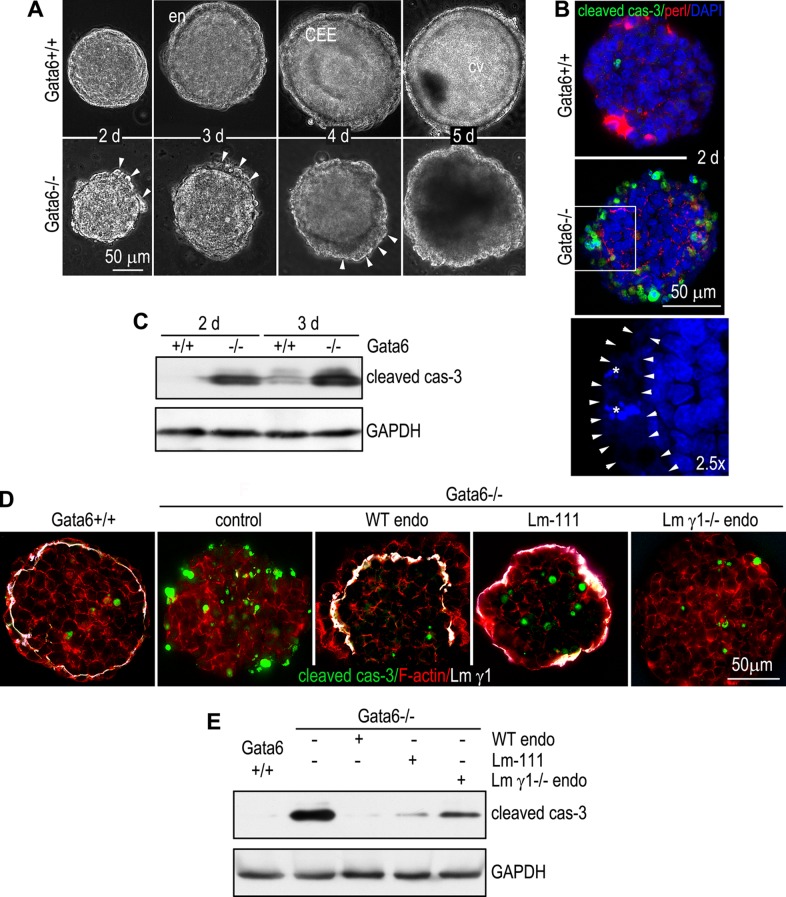
Targeted deletion of the Gata6 gene in mice blocks endodermal differentiation and leads to early embryonic lethality (Morrisey et al., 1998; Koutsourakis et al., 1999; Cai et al., 2008). Terminal deoxynucleotidyl transferase dUTP nick end labeling of the mutant embryos revealed increased apoptosis of epiblast cells (Morrisey et al., 1998). To determine the underlying mechanisms, we used ES cell-differentiated EBs (Coucouvanis and Martin, 1995; Li et al., 2003). Microarray analysis of 2-, 3- and 5-d EBs for GATA transcription factors revealed that GATA-4 and GATA-6 were significantly up-regulated on day 3, when endoderm began to form on the EB surface, and further increased on day 5, when EBs developed into two-germ-layer (endoderm and a columnar epiblast epithelium [CEE]) epithelial cysts (Supplemental Figure S1A). In 2-d EBs, GATA-6 was expressed in the nucleus of a small number of cells at the EB periphery (Supplemental Figure S1B). Sparse GATA-6–positive cells could also be detected in the EB interior. A linear BM as evidenced by perlecan immunofluorescence had not formed in the majority of the EBs at this stage of differentiation. On day 3, most of GATA-6–positive cells appeared on the EB surface, and a distinct outer layer of endoderm was observed by live phase microscopy (Figure 1A). Between 3 and 5 d, the epiblast cells in contact with the BM polarized to become the CEE with a characteristic apical actin belt (Supplemental Figure S1B), whereas the centrally located cells not in contact with the BM died from apoptosis, creating a proamniotic-like cavity (Li et al., 2003). In contrast to this normal differentiation process, GATA-6–null EBs failed to develop endoderm or assemble an underlying BM (Supplemental Figure S1C). The expression of the endoderm markers GATA-4 and α-fetoprotein was reduced (Supplemental Figure S1D). The laminin α1 chain, nidogen, and collagen IV—the major BM components—were also significantly decreased, whereas the expression of the laminin β1/γ1 chains was largely unchanged. Perlecan—the major proteoglycan in BMs—was increased in the absence of GATA-6. These results suggest that the expression of the laminin α1 chain, nidogen, and collagen IV is selectively regulated by GATA-6. Beginning on day 2, cells at the periphery of the mutant EBs underwent apoptosis (Figure 1A). Apoptotic debris gradually accumulated on the EB surface and formed a granulative layer by day 4. Apoptosis was confirmed by immunofluorescence of cleaved caspase-3 and 4′,6-diamidino-2-phenylindole (DAPI) staining of condensed and fragmented nuclei, which were often embedded in a layer of amorphous nuclear materials (Figure 1B). Immunoblot analysis revealed increased caspase-3 activation in 2- and 3-d mutant EBs before central apoptosis occurred (Figure 1C). Collectively these results demonstrate that ablation of GATA-6 in EBs leads to apoptosis of peripheral, presumptive endoderm and epiblast cells.
FIGURE 1:
Inactivation of GATA-6 induces massive apoptosis of peripheral cells during EB differentiation. (A) Live phase micrographs show the formation of endoderm (en), a columnar epiblast epithelium (CEE), and a proamniotic-like central cavity (cv) during normal EB differentiation. Ablation of GATA-6 blocked endoderm and CEE differentiation, prevented cavitation, and induced massive apoptosis. Arrowheads indicate apoptotic cells on the EB surface. (B) Gata6+/+ and Gata6−/− EBs were cultured for 2 d and immunostained for cleaved caspase-3 (cas-3) and BM perlecan. Nuclei were counterstained with DAPI. Caspase-3 activation was markedly increased in Gata6−/− EBs. Bottom, a 2.5× magnification of the boxed region of the middle showing only DAPI staining. Arrowheads define amorphous nuclear staining at the EB periphery. Asterisks indicate fragmented nuclei. (C) Immunoblot analysis confirmed increased caspase-3 activation in Gata6−/− EBs cultured for 2 and 3 d. (D) Gata6−/− EBs were treated with 100 μg/ml laminin (Lm)-111 or grafted with wild-type (WT) or Lm γ1–null endoderm (endo) cells and cultured for 2 d. Gata6+/+ and untreated Gata6−/− EBs were used as controls. EBs were immunostained for cleaved caspase-3 (cas-3) and Lm γ1. F-actin was stained with rhodamine-phalloidin. Grafting normal endoderm cells onto GATA-6–null EBs prevented apoptosis of peripheral cells, whereas grafting Lm γ1–null endoderm cells or Lm-111 treatment partially inhibited apoptosis. (E) Immunoblot analysis for cleaved caspase-3 confirmed the immunostaining observations.
Contributions of endoderm-derived factors to epiblast polarization and survival
If the increased apoptosis and impaired epiblast polarity and cavitation after GATA-6 ablation are caused by a blockade of endoderm differentiation, grafting normal endoderm cells onto GATA-6–null EBs should rescue the defects. To test this hypothesis, we isolated endoderm cells from 5-d control EBs and cultured them with 1-d GATA-6–null EBs in hanging drops for 24 h. The chimeric EBs were then cultured in suspension for 3 d. Phase microscopy of cryosections revealed the formation of an endoderm layer on the EB surface, which induced epiblast polarization and cavitation (Supplemental Figure S2). Immunostaining for α-fetoprotein and the laminin γ1 chain confirmed the formation of endoderm and an underlying BM. Analysis of the chimeric EBs cultured for 2 d showed that the abnormal, peripheral apoptosis of the mutant EBs was prevented by the grafted endoderm cells (Figure 1, D and E). These results suggest that apoptosis in GATA-6–null EBs is caused by failure of endoderm differentiation. When normal endoderm is provided, epiblast polarity and cavitation are completely rescued.
In addition to transporting nutrients and fluids, the visceral endoderm also secretes BM proteins and growth factors. The latter function is believed to play an inductive role in epiblast polarization and cavitation (Coucouvanis and Martin, 1995, 1999; Murray and Edgar, 2000; Li et al., 2002, 2003). To determine the BM-dependent and BM-independent functions of the endoderm in epiblast polarization and survival, we incubated 1-d GATA-6–null EBs with 100 μg/ml laminin-111 for 4 d. Laminin treatment led to the assembly of an ectopic BM on the EB surface, which induced epiblast polarization and cavitation (Supplemental Figure S2). This is consistent with a previous report that exogenous laminin can rescue the differentiation of laminin γ1-null EBs (Murray and Edgar, 2000; Li et al., 2002). Furthermore, immunofluorescence and immunoblot analysis revealed that treatment of GATA-6–null EBs with laminin for 24 h inhibited apoptosis of the peripheral cells, although the inhibitory effect was slightly less than that of grafting with normal endoderm cells (Figure 1, D and E). To test whether endoderm provides survival signals besides BM, we grafted endoderm cells isolated from laminin γ1-null EBs, which neither secrete trimeric laminins nor assemble a BM (Smyth et al., 1999; Murray and Edgar, 2000; Li et al., 2002). Immunostaining for α-fetoprotein and laminin γ1 confirmed the formation of endoderm but not BM on the surface of GATA-6–null EBs (Supplemental Figure S2). Laminin γ1–null endoderm failed to convert the GATA-6–null EBs into polarized epithelial cysts. However, apoptosis of the peripheral cells was significantly reduced after endoderm grafting (Figure 1, D and E). These results suggest that GATA-6–dependent endoderm differentiation protects the adjacent cells from apoptosis in a non-cell-autonomous manner. Both BM and BM-independent factors contribute to epiblast survival.
BMP-2 is significantly up-regulated by GATA-6 in endoderm during EB differentiation
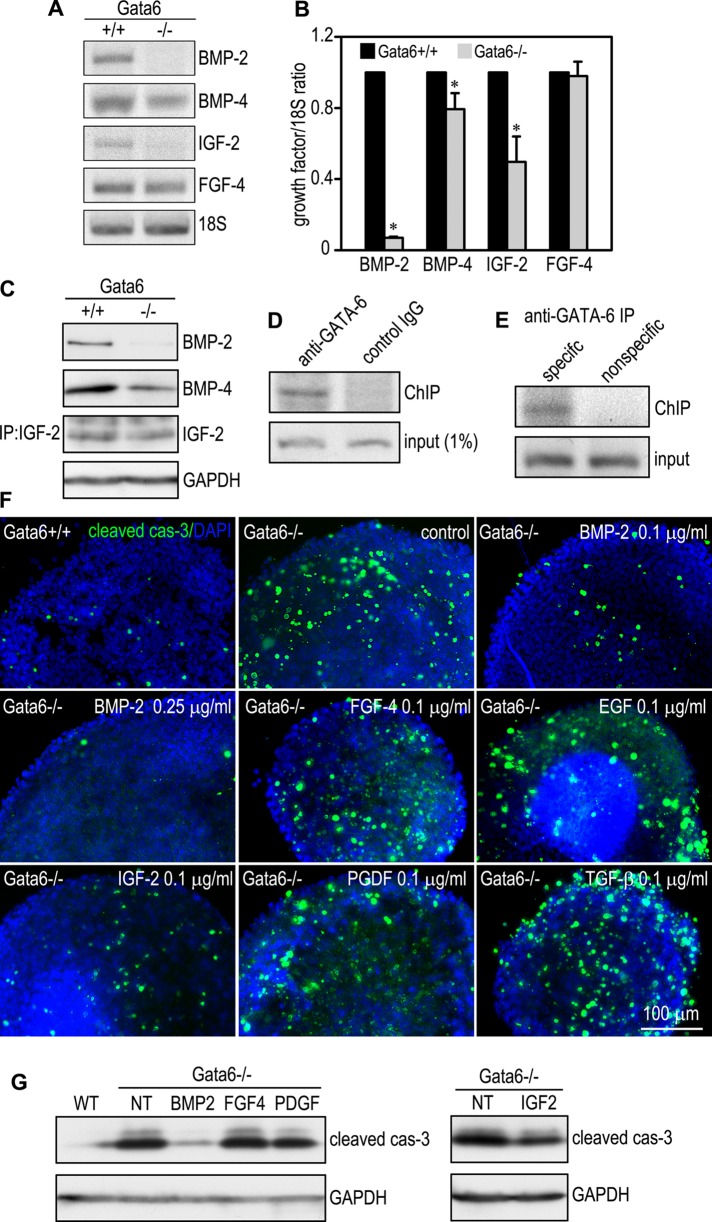
BMP-2 and -4, fibroblast growth factors (FGFs), and platelet-derived growth factors (PDGFs) have been suggested to regulate endoderm differentiation (Arman et al., 1998; Coucouvanis and Martin, 1999; Li et al., 2001; Artus et al., 2010). Our microarray analysis demonstrated that the expression of BMP-2, insulin-like growth factor 2 (IGF-2), transforming growth factor β (TGF-β), and vascular endothelial growth factor A (VEGF-A) was increased, whereas BMP-4, FGF-4, PDGF-A, PDGF-C, and VEGF-C were reduced, during EB differentiation (Supplemental Figure S3A). Of note, the mRNA for BMP-2 was increased 127-fold in 3-d EBs and 361-fold in 5-d EBs compared with 2-d EBs. To determine whether the expression of these growth factors might be altered in GATA-6–null EBs, we performed reverse transcription (RT)-PCR and immunoblot analysis on 2-d EBs. The mRNAs for BMP-4 and IGF-2 were reduced, whereas that for FGF-4 was unchanged, in the absence of GATA-6 (Figure 2, A and B). Of importance, BMP-2 mRNA was readily detectable in 2-d normal EBs and was nearly absent from GATA-6–null EBs. The reduction of BMP-2, BMP-4, and IGF-2 was confirmed by immunoblotting at the protein level (Figure 2C). As anticipated, immunostaining showed that BMP-2 was expressed mainly by endoderm (Supplemental Figure S3B), which is in agreement with the in situ hybridization study in mouse embryos (Coucouvanis and Martin, 1999). Of interest, we observed an enrichment of BMP-2 in the BM underlying the endoderm. These results suggest that altered expression of growth factors in GATA-6–null EBs may also contribute to apoptosis of the peripheral cells.
FIGURE 2:
BMP-2 is downstream of GATA-6 to inhibit apoptosis during EB differentiation. (A) RT-PCR analysis on 2-d EBs demonstrated that the mRNAs for BMP-2, BMP-4, and IGF-2 were reduced, whereas the FGF-4 mRNA was largely unchanged in the absence of GATA-6. (B) Ethidium bromide–stained gels were analyzed by densitometry. The ratio of the growth factors to 18S RNA was plotted (*p < 0.01, n = 4). (C) Two-day EBs were subjected to immunoblotting for BMP-2 and BMP-4. IGF-2 in the conditioned medium was immunoprecipitated using anti-IGF-2 antibody, followed by immunoblotting using the same antibody. GAPDH serves as a loading control. (D) A GATA-6–binding sequence in the BMP-2 promoter was detected by chromatin immunoprecipitation using anti–GATA-6 antibody but not control IgG. (E) BMP-2 immunoprecipitates were amplified using either primers specific for the GATA-6 consensus sequence (specific) or nonspecific primers (nonspecific). The correct PCR product was only obtained using the specific primers. (F) One-day EBs were treated with or without the growth factors for 24 h, and whole-mount staining was carried out for cleaved caspase-3 (cas-3). Nuclei were counterstained with DAPI. Treatment of Gata6−/− EBs with BMP-2 dose dependently inhibited apoptosis. IGF treatment had a partial effect. (G) Lysates of EBs treated as described were analyzed by immunoblotting for cleaved caspase-3. GAPDH serves as a loading control. BMP-2 treatment significantly inhibited caspase-3 activation in Gata6−/− EBs, whereas IGF-2 had a mild effect.
GATA-6 directly binds to the Bmp2 promoter
Because both BMP-2 and GATA-6 are expressed by endoderm cells and ablation of GATA-6 inhibits BMP-2 expression, we reasoned that BMP-2 might be a direct transcription target of GATA-6. Sequence analysis revealed that the promoter of the mouse Bmp2 gene contains two GATA-6 consensus binding sequences at −1121 and −1201. To test whether GATA-6 binds to the promoter of Bmp2, we performed chromatin immunoprecipitation (ChIP) on 2-d wild-type EBs. PCR analysis demonstrated that anti–GATA-6 antibody, but not the control immunoglobulin G (IgG), immunoprecipitated the GATA-6–binding sequences in the Bmp2 promoter (Figure 2D). In addition, the PCR product containing the GATA-6 consensus sequence was not detected in the BMP-2 immunoprecipitates using a nonspecific primer set (Figure 2E). This result suggests that Bmp2 is a target gene of GATA-6 in differentiating EBs.
Treatment of GATA-6-null EBs with BMP-2 inhibits apoptosis of the peripheral cells
If the observed reduction of BMP-2/4 and IGF-2 causes increased apoptosis of the peripheral cells in GATA-6–null EBs, supplementation of the growth factors to the culture medium should rescue the apoptotic cell death. To test this hypothesis, we treated 1-d GATA-6–null EBs with or without 0.1 μg/ml BMP-2, FGF-4, epidermal growth factor (EGF), IGF-2, PDGF, and TGF-β1, respectively, for 24 h. Apoptosis was analyzed by whole-mount immunostaining for cleaved caspase-3 since it occurred mostly in the peripheral cells of the mutant EBs. BMP-2 treatment markedly inhibited the activation of caspase-3, whereas FGF-4, EGF and PDGF had no effect (Figure 2F). Increasing the BMP-2 concentration to 0.25 μg/ml led to further reduction of apoptosis. Treatment of the mutant EBs with IGF-2 also moderately reduced caspase-3 activation, whereas TGF-β showed the opposite effect. Immunoblot analysis confirmed that exogenous BMP-2 significantly reduced apoptosis of GATA-6–null EBs, whereas IGF-2 had a mild effect (Figure 2G). Because BMP-4 binds to the same receptor as BMP-2 (Koenig et al., 1994; Coucouvanis and Martin, 1999; Miyazono et al., 2010), our results suggest that the reduction of BMP-2/4 expression in GATA-6–null EBs also contributes to increased apoptosis of the peripheral cells.
Overexpression of noggin or dominant-negative bone morphogenetic protein receptor induces apoptosis in normal EBs
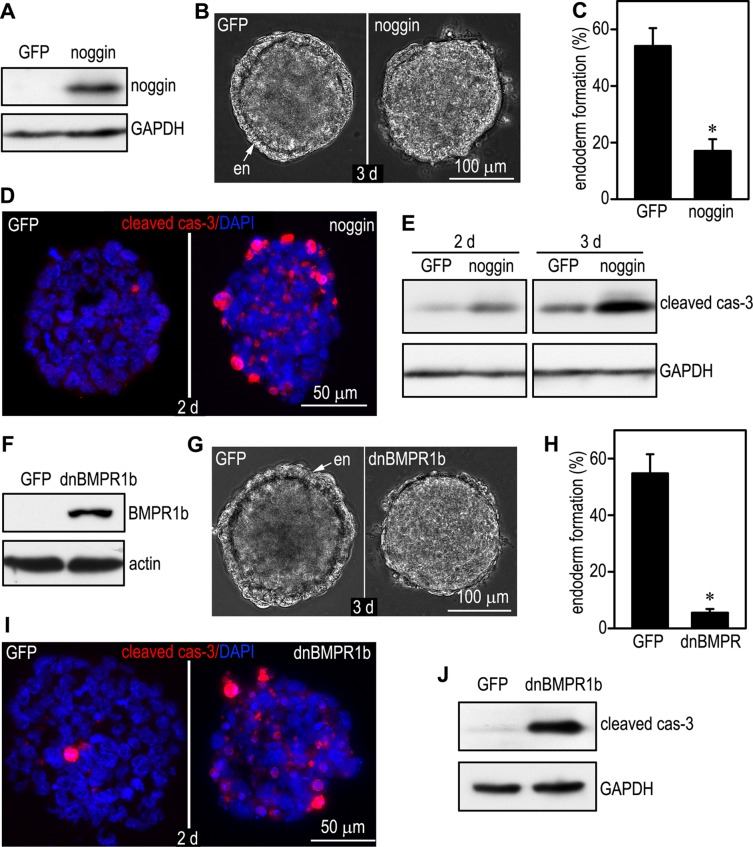
If GATA-6–regulated expression of BMP-2/4 contributes to the survival of the peripheral cells during EB differentiation, overexpression of the BMP antagonist noggin, which binds to BMPs and prevent their interactions with BMP receptors (Re'em-Kalma et al., 1995), or a dominant-negative BMP receptor in normal EBs would be expected to induce apoptosis of those cells. To test this hypothesis, we stably expressed noggin in normal EBs. Noggin expression was barely detected by immunoblotting in 2-d EBs transfected with pCXN2-IRES-GFP alone (Figure 3A). Overexpression of noggin in normal EBs inhibited endoderm formation. Live phase microscopy showed that 54% of 3-d control EBs developed a distinct outer endoderm layer, whereas only 17% of the noggin-overexpressing EBs had endoderm on their surface (Figure 3, B and C). To determine the effect of overexpression of noggin on apoptosis, we first examined caspase-3 activation by immunostaining of 2-d EBs. Overexpression of noggin significantly increased the number of cleaved caspase-3–positive cells at the EB periphery (Figure 3D). Of interest, caspase-3 activation was also increased in the centrally located cells. Immunoblot analysis confirmed increased cleaved caspase-3 in both 2- and 3-d EBs overexpressing noggin (Figure 3E). To further examine the role of BMP signaling in cell survival, we established stable ES cell clones that expressed dominant-negative (dn), kinase-dead BMP receptor 1b (K231R, dn BMPR1b), which has been shown to block BMP signaling and inhibit endoderm formation in teratocarcinoma cell–derived EBs (Zou and Niswander, 1996; Coucouvanis and Martin, 1999). The control EBs expressed very little, if any, BMPR1b, which is consistent with the finding that BMPR1b is not expressed in pregastrulation embryos (Figure 3F; Dewulf et al., 1995). Overexpression of dn BMPR1b, which can compete with BMPR1a for dimerization with BMPR2 but cannot phosphorylate receptor-activated SMAD (R-SMAD; Zou and Niswander, 1996), markedly inhibited endoderm differentiation. The efficiency of endoderm formation dropped from 55% in 3-d control EBs to 6% in dn BMPR1b EBs (Figure 3, G and H). Expression of dn BMPR1b also significantly increased apoptosis of 2-d EBs, similar to the effect of noggin overexpression (Figure 3, I and J). Collectively these results suggest that activation of the BMP signaling pathway by GATA-6 promotes cell survival during EB differentiation.
FIGURE 3:
Overexpression of noggin or dominant-negative BMPR induces apoptosis in wild-type EBs. (A) Immunoblots show noggin overexpression in 2-d EBs stably transfected with pCXN2-noggin-IRES-GFP (noggin) compared with EBs transfected with the vector alone (GFP). GAPDH serves as a loading control. (B) Phase micrographs of 3-d live EBs show the formation of a continuous layer of endoderm (en) on the surface of a GFP EB but with only a short stretch of endoderm cells on a noggin EB. (C) EBs were cultured for 3 d, and endoderm formation was examined by live phase microscopy. EBs with an outer endoderm layer were counted and plotted as a percentage of total EBs examined. n= 10 experiments with a total of 633–648 EBs for each group. *p < 0.01. Overexpression of noggin inhibited endoderm formation. (D) Two-day EBs were immunostained for cleaved caspase-3 (cas-3). Nuclei were counterstained with DAPI. Overexpression of noggin increased apoptosis compared with EBs expressing GFP alone. (E) EBs were cultured for 2 and 3 d and analyzed by immunoblotting for cleaved caspase-3. Overexpression of noggin increased apoptosis of both 2- and 3-d EBs. GAPDH serves as a loading control. (F) Immunoblots show overexpression of dn BMPR1b in 2-d normal EBs. (G) Phase contrast micrographs of 3-d live EBs show impaired endoderm (en) formation in dnBMPR1b EBs. (H) Three-day EBs with an endoderm layer were counted by live phase microscopy and plotted as a percentage of total EBs examined. n = 10 experiments with a total of 656–694 EBs for each group. *p < 0.01. Ectopic expression of dn BMPR1b markedly inhibited endoderm formation. (I) Two-day EBs were immunostained for cleaved caspase-3. Nuclei were counterstained with DAPI. Expression of dn BMPR1b increased apoptosis. (J) Immunoblot analysis of 2-d EBs confirmed that the expression of dn BMPR1b increased apoptosis compared with the control GFP EBs.
BMP-dependent SMAD activation is selectively inhibited in GATA-6–null EBs
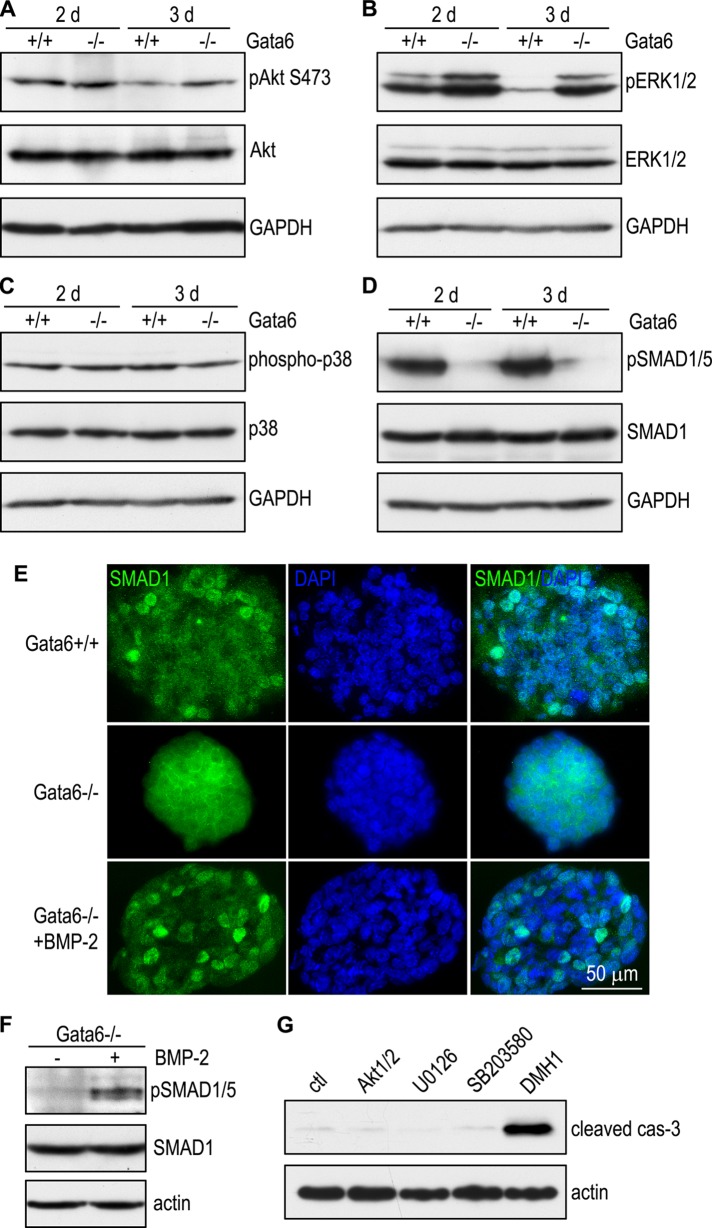
BMP-2 and 4 bind to heteromultimers of type I and type II BMPRs and activate the canonical SMAD signaling pathway and/or alternative mitogen-activated protein (MAP) kinase signaling pathways, such as the extracellular signal-regulated kinase (ERK) and p38 MAP kinase (Derynck and Zhang, 2003). In addition, BMP-2 has been shown to stimulate the phosphatidylinositol 3-kinase (PI3K)–Akt pathway (Ghosh-Choudhury et al., 2002). To delineate the downstream effectors for BMP-mediated survival, we examined activation-related phosphorylation of Akt, ERK1/2, p38 MAP kinase, and SMAD1/5 (R-SMAD downstream of BMPRs). Immunoblot analysis using phosphorylation-specific antibodies revealed that Akt phosphorylation at S473 was reduced during normal EB differentiation and negatively correlated with expression of phosphatase and tensin homologue, which antagonizes the PI3K-Akt pathway by dephosphorylating phosphatidylinositol (3,4,5)-trisphosphate (Figure 4A and unpublished observations). Ablation of GATA-6 did not significantly alter Akt phosphorylation. By contrast, ERK1/2 phosphorylation at T202/Y204 in the activation loop was markedly reduced during EB differentiation, and this was prevented by inactivation of GATA-6 (Figure 4B). Phosphorylation of p38 MAP kinase was not significantly changed either during EB differentiation or in the absence of GATA-6 (Figure 4C). Remarkably, phosphorylation of SMAD1/5 at S463/465 was nearly abolished in GATA–6-null EBs compared with wild-type controls (Figure 4D). We did not observe significant changes in expression levels of Akt, ERK1/2, p38, and SMAD1. SMAD1 was localized predominantly to the nucleus of the peripheral cells of 2-d normal EBs, whereas it was localized in the cytoplasm in GATA-6–null EBs (Figure 4E). To test whether reduced activation of SMAD1/5 after GATA-6 ablation results from decreased BMP-2 expression, we incubated 2-d GATA-6–null EBs with or without BMP-2 (0.1 μg/ml) for 16 h. BMP-2 treatment restored SMAD1/5 phosphorylation in GATA-6–null EBs (Figure 4F). In addition, SMAD1 was detected in the nucleus after BMP-2 treatment (Figure 4E). To further examine whether the activation of the SMAD pathway provides a survival signal, we cultured 1-d wild-type EBs for 24 h with 5 μM of Akt1/2 inhibitor, the MAPK kinase 1/2 inhibitor U0126, the p38 inhibitor SB203580, or the R-SMAD phosphorylation inhibitor DMH1. Among these inhibitors, only DMH1 markedly induced apoptosis, as evidenced by caspase-3 activation (Figure 4G). Collectively these results suggest that the canonical SMAD signaling pathway is selectively activated by BMP-2 in the peripheral cells of differentiating EBs in a GATA-6–dependent manner and likely mediates BMP-dependent cell survival.
FIGURE 4:
BMP-2 activates the SMAD pathway to inhibit apoptosis during EB differentiation. (A) EBs cultured for 2 and 3 d were analyzed by immunoblotting for phospho-Akt S473 (pAkt473), Akt, and GAPDH. Akt phosphorylation was slightly reduced in both Gata6+/+ and Gata6−/− EBs after 3 d in culture. (B) Immunoblots show reduced ERK1/2 phosphorylation at T202/Y204 (pERK1/2) in 3-d normal EBs compared with 2-d EBs. By contrast, the pERK1/2 level was higher in 2-d Gata6−/− EBs and only slightly reduced after 3 d. Total ERK1/2 was unchanged in both 2- and 3-d Gata6+/+ and Gata6−/− EBs. (C) Immunoblot analysis of 2- and 3-d EBs showed that p38 MAP kinase phosphorylation at T180/Y182 (phospho-p38) was slightly reduced in 3-d Gata6−/− EBs, whereas the total p38 remained unchanged. (D) EBs were cultured for 2 and 3 d and analyzed by immunoblotting for phospho-SMAD1/5 (S463/465) and SMAD1. Ablation of GATA-6 nearly abolished SMAD1/5 phosphorylation in both 2- and 3-d EBs. (E) immunofluorescence micrographs show that SMAD1 was localized in the nucleus of peripheral cells in 2-d normal EBs, whereas it was mainly in the cytoplasm in Gata6−/− EBs. Treatment of the mutant EBs with BMP-2 (0.1 μg/ml) for 16 h induced translocation of SMAD1 to the nucleus. Nuclei were counterstained with DAPI. (F) Two-day Gata6−/− EBs were treated with 0.1 μg/ml BMP-2 for 16 h and harvested for immunoblot analysis. BMP-2 treatment increased SMAD1/5 phosphorylation but had no effect on total SMAD1 expression. Actin serves as a loading control. (G) One-day normal EBs were treated for 24 h with 5 μM of Akt1/2 inhibitor, the MAPK kinase inhibitor U0126, the p38 inhibitor SB203580, or the R-SMAD inhibitor DMH1. EB lysates were analyzed by immunoblotting for cleaved caspase-3. Actin serves as a loading control. DMH1 treatment selectively induced caspase-3 activation.
DISCUSSION
GATA-6 has been shown to play an essential role in endoderm development in both early embryos and differentiating EBs. In this study, we observed increased apoptosis of GATA-6–null EBs before the occurrence of cavitation-related cell death. Grafting of normal endoderm cells onto the mutant EBs completely prevented apoptosis and rescued epiblast polarization and cavitation. Laminin-induced assembly of an ectopic BM on the mutant EBs or grafting with laminin γ1–null endoderm cells, which do not secrete trimeric laminins and thus cannot make BMs, partially inhibited apoptosis, suggesting that both endoderm-derived BM and non-BM factors contribute to GATA-6–dependent cell survival. Furthermore, we showed that BMP-2 is a transcriptional target of GATA-6 and is expressed predominantly by endoderm cells and accumulated in the BM. Its expression is significantly down-regulated in GATA-6–null EBs. Treatment of the mutant EBs with BMP-2 selectively inhibits the observed apoptosis, whereas overexpression of noggin or a dominant-negative BMP receptor in control EBs induces apoptosis. The survival signal of BMP-2 is likely transmitted by the canonical SMAD pathway. Taken together, these results suggest a molecular model in which GATA-6 promotes cell survival by regulating endoderm expression of BMP-2 and BM proteins during embryonic epithelial morphogenesis (Figure 5).
FIGURE 5:
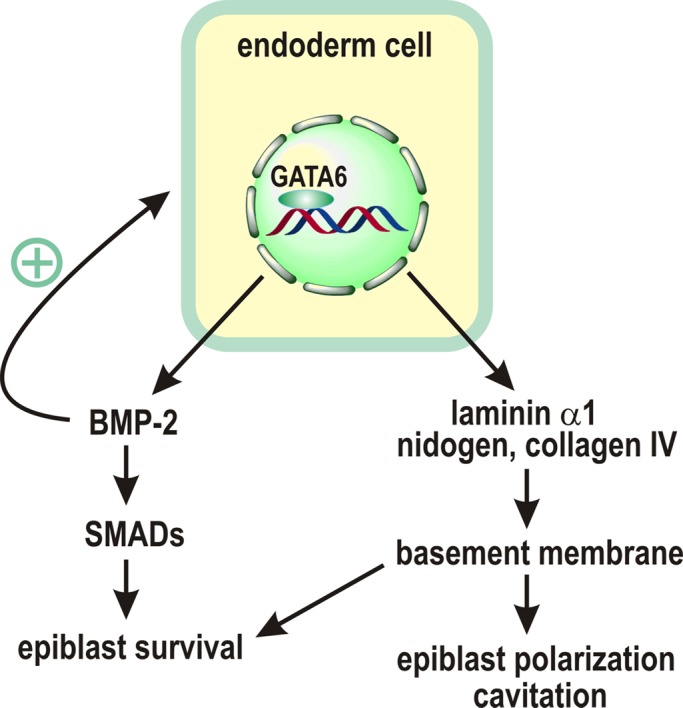
A model for GATA-6 regulation of epiblast polarization and survival. GATA-6 regulates endoderm differentiation and the expression of laminin α1, nidogen, and collagen IV, which assemble into a BM. The BM in turn induces epiblast polarization and cavitation. GATA-6 also directly controls the expression of BMP-2, which acts in concert with the BM to protect the epiblast from apoptosis. In addition, BMP-2 serves as a positive feedback signal for endoderm differentiation.
Endoderm-derived BM has been suggested to mediate epiblast survival during EB differentiation (Coucouvanis and Martin, 1995, 1999; Murray and Edgar, 2000; Li et al., 2002, 2003). Our previous studies showed that targeted inactivation of the BM receptors integrin β1 and dystroglycan leads to increased apoptosis of the epiblast (Li et al., 2002). The Rho GTPase Rac1 acts downstream of integrins to transduce the antiapoptotic signal (He et al., 2010). However, it is unclear whether growth factors are also involved in epiblast survival. In this study, we used GATA-6–null EBs that do not form endoderm and provide evidence for a role of endoderm-derived BMP-2 in epiblast survival. First, BMP-2 is significantly up-regulated during normal EB differentiation and is expressed by endoderm. GATA-6 directly binds to the Bmp2 promoter, and GATA-6 ablation reduces BMP-2 expression at both mRNA and protein levels. Second, treatment of GATA-6-null EBs with recombinant active BMP-2 dose dependently inhibits apoptosis. Last, overexpression of the BMP antagonist noggin or a dominant-negative BMP receptor in normal EBs induces apoptosis. There is a caveat that overexpression of noggin or the dominant-negative BMPR also inhibits the formation of an endoderm layer. Impaired endoderm formation can trigger apoptosis through reduced BM and BMP-2 expression. However, our immunostaining results show that GATA-6–positive cells still exist but seem unable to migrate to the surface in 2-d noggin-overexpressing EBs, although apoptosis is already evident at this stage of differentiation (unpublished data). Our data suggest a prosurvival role for BMP signaling in the differentiation of primitive germ layers.
BMP signaling is able to induce or inhibit apoptosis during embryonic development depending on organism type, developmental stage, and tissue context (Yokouchi et al., 1996; Zou and Niswander, 1996; Yang et al., 1999; Ashique et al., 2002; Belecky-Adams et al., 2002; Liu et al., 2005; Eblaghie et al., 2006; Pajni-Underwood et al., 2007; Shu et al., 2011). The proapoptotic activity of BMPs has been well documented in chicken limb development. BMP-2, -4, and -7 are expressed abundantly in interdigital mesenchymal cells, which undergo apoptosis during the separation of digits. Overexpression of a dominant BMP receptor inhibits interdigital apoptosis and results in webfeet (Yokouchi et al., 1996; Zou and Niswander, 1996). In mice, targeted deletion of the BMPR1a gene in the limb bud causes up-regulation of FGF-4 and -8, which inhibit interdigital mesenchymal apoptosis (Pajni-Underwood et al., 2007). BMPs have also been shown to support cell survival during tissue morphogenesis, especially in epithelial formation. During chicken eye development, phosphorylated and activated SMAD1, a downstream effector of BMP receptors, is localized to the nucleus of lens fiber cells (Belecky-Adams et al., 2002). Overexpression of noggin increases programmed cell death in the lens epithelium. During mouse lung branching morphogenesis, ablation of BMPR1a leads to decreased phosphorylation of SMAD1/5/8 and extensive apoptosis of epithelial cells (Eblaghie et al., 2006). In this study, we observed a reduction in BMP-2/4 expression and SMAD1/5 phosphorylation in GATA-6–null EBs. The reduced SMAD phosphorylation is reversed by exogenous BMP-2. Treatment of the mutant EBs with the R-SMAD inhibitor DMH1, but not inhibitors of MAP kinases and Akt, induces apoptosis of the presumptive endoderm and epiblast cells. These results suggest that BMP signaling promotes embryonic epithelial cell survival through the canonical SMAD pathway.
During mouse peri-implantation development, BMP-2 is expressed by the endoderm, whereas BMP-4 is mainly detected in the inner cell mass and is down-regulated in polarized epiblast (Coucouvanis and Martin, 1999). Treatment of teratocarcinoma cell–derived EBs with high concentrations of BMP-2 or 4 promoted the differentiation of the visceral endoderm and cavitation. These findings are in agreement with our microarray and immunofluorescence data, showing down-regulation of BMP-4 and up-regulation of BMP-2 during EB differentiation. We also observed impaired endoderm differentiation in EBs overexpressing noggin or dominant-negative BMPR1b, suggesting that BMP signaling regulates the formation of the visceral endoderm. Therefore, GATA-6-regulated BMP-2 expression may constitute an autoregulatory positive feedback loop that promotes endoderm differentiation/maturation. FGF-4 and PDGF are also required for the differentiation of the extraembryonic endoderm (Arman et al., 1998; Li et al., 2001; Artus et al., 2010). However, treatment of GATA-6–null EBs with FGF4 or PDGF neither promotes endoderm differentiation nor inhibits apoptosis. For PDGF, this is likely because its receptor is a transcription target of GATA-6 and may be down-regulated in the absence of GATA-6 (Artus et al., 2010). IGF-2 is expressed by trophectoderm and visceral endoderm during peri-implantation development (Lee et al., 1990). Treatment with IGF-2 slightly reduces apoptosis of GATA-6–null EBs, suggesting that IGF-2 may also act as an endoderm-derived factor to protect the epiblast from apoptosis.
In summary, our results suggest a model in which GATA-6 regulates endoderm differentiation and the expression of laminin α1, nidogen, and collagen IV, which assemble into an underlying BM. The BM in turn induces epiblast polarization and cavitation. GATA-6 also directly activates the expression of BMP-2, which acts in concert with the BM to protect the epiblast from apoptosis. In addition, BMP-2 promotes endoderm differentiation in an autocrine manner.
MATERIALS AND METHODS
Culturing of ES cell and embryoid bodies
The ES cell lines used for this study were wild-type R1 and RW.4, GATA-6–null, and laminin γ1–null ES cells (Morrisey et al., 1998; Smyth et al., 1999). All the ES cells were cultured on mitomycin C–treated STO cells. EB differentiation was initiated from ES cell aggregates in suspension culture as described previously (Li and Yurchenco, 2006).
Antibodies, growth factors, and cDNA constructs
Rabbit monoclonal antibodies (mAbs) to GATA-6, cleaved caspase-3, phospho-Akt S473, Akt, phospho-ERK1/2 T202/Y204, ERK1/2, phospho-p38 T180/Y182, p38, phospho-SMAD1/5 S463/465, and SMAD1 were from Cell Signaling (Danvers, MA). Rabbit polyclonal antibodies (pAbs) to GATA-6 and GATA-4 and rat mAb to perlecan were from Santa Cruz Biotechnology (Santa Cruz, CA). α-Fetoprotein pAb was from ICN (Irvine, CA). Rabbit pAbs to BMP-2 and BMP-4 were from FabGennix (Frisco, TX). Rabbit anti-noggin pAb was from Abcam (Cambridge, MA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)and laminin γ1 mAbs were from Millipore (Billerica, MA). Actin pAb was from Sigma-Aldrich (St. Louis, MO). Laminin-111 isolated from ESH tumors and rabbit pAbs to nidogen, laminin α1, and laminin-111 were kindly provided by Peter Yurchenco of Robert Wood Johnson Medical School (Piscataway, NJ). Recombinant human BMP-2, recombinant mouse FGF-4 and EGF, human platelet PDGF, recombinant human TGF-β1 and IGF-2, rabbit anti-mouse IGF-2 pAb, rat anti-noggin mAb, and mouse anti-BMPR1b mAb were from R&D Systems (Minneapolis, MN). The Cy3-, Cy5-, and horseradish peroxidase–conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). Rhodamine-phalloidin and Alexa 488–conjugated secondary antibodies were from Molecular Probes (Eugene, OR).
Full-length mouse noggin and BMPR1b cDNAs were purchased from Open Biosystems (Lafayette, CO). The cDNAs were subcloned into pCXN2-IRES-GFP with the N-terminal FLAG tag removed. Dominant-negative, kinase-dead BMPR1b K231R was created using a QuikChange Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA). The fidelity of the constructs was verified by DNA sequencing.
Stable transfection of ES cells
For stable expression of noggin and dn BMPR1b, wild-type ES cells were transfected with the cDNA constructs using Lipofectamine 2000 reagent (Invitrogen, Grand Island, NY). Stable ES cell clones were selected based on G418 (500 μg/ml) resistance and green fluorescent protein (GFP) fluorescence. ES cells stably transfected with pCXN2-ITES-GFP were established as controls.
Gene expression profiling and RT-PCR
Total RNA was isolated from 2-, 3-, and 5-d normal EBs with TRIzol reagent (Invitrogen) and was reverse transcribed to cRNA. Fragmented cRNA was hybridized to Affymetrix mouse genome 430 2.0 microarray chips (Affymetrix, Santa Clara, CA). Hybridization data were analyzed by MAS 5.0 software (Affymetrix). For RT-PCR analysis, total RNA isolated from 2-d EBs was reverse transcribed to cDNA. The PCR primers used are as follows: BMP-2, 5′-CAGATCTTCCGGGAACAGATAC (forward) and 5′-GCTGTTTGTGTTTGGCTTGAC (reverse); BMP-4, 5′-CCCAGAGAATGAGGTGATCTC (forward) and 5′-CACAATCCAATCATTCCAGC (reverse); IGF-2, 5′-GGAAGTCGATGTTGGTGCTTC (forward) and 5′-ACGATGACGTTTGGCCTCTC (reverse); FGF-4, 5′-CAACGTGGGCATCGGATTC (forward) and 5′-CTTGGTCCGCCCGTTCTTAC (reverse); PDGF-A, 5′-GGCTGGCTCGAAGTCAGATC (forward) and 5′-TTCAGGTTGGAGGTCGCAC (reverse); PDGF-C, 5′-TGCCAGGAAAGCAGACTTC (forward) and 5′-GAGTGACTTATGCAATCCCTTG (reverse); 18S RNA, 5′-TCAAGAACGAAAGTCGGAGG (forward) and 5′-GGACATCTAAGGGCATCACA (reverse).
Immunofluorescence
EBs were fixed with 3% paraformaldehyde, embedded in OCT compound, and sectioned on a Leica cryostat (Leica, Wetzlar, Germany). EB processing and immunostaining were performed as described previously (Li and Yurchenco, 2006). Nuclei were counterstained with DAPI. For whole-mount staining, 2-d EBs were fixed and permeabilized in 0.5% Triton X-100 for 45 min. EBs were stained in Eppendorf tubes and mounted on glass slides without spacers. Slides were examined with an Eclipse TE2000 inverted fluorescence microscope (Nikon, Melville, NY), and digital images were acquired with an Orca-03 cooled charge-coupled device camera (Hamamatsu, Hamamatsu, Japan) controlled by IP Lab software (Scanalytics, Rockville, MD).
Immunoblotting and immunoprecipitation
EBs were collected by sedimentation under gravity, washed once in phosphate-buffered saline (PBS), and lysed in radioimmunoprecipitation assay buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% sodium deoxycholate) or SDS lysis buffer (50 mM Tris, pH 7.4, 1% SDS) containing protease and phosphatase inhibitor cocktails. Immunoblotting were performed as described previously (Li et al., 2002). For immunoprecipitation, EBs were cultured for 2 d, and conditioned media were collected and precleared using protein A/G beads. The volume of conditioned media used for immunoprecipitation was adjusted according to the protein amount of the EBs. Rabbit anti-mouse IGF-2 antibody was used for both immunoprecipitation and immunoblotting.
Endoderm grafting
Wild-type and laminin γ1–null EBs were cultured for 5 d and collected by sedimentation by gravity. Endoderm cells on the EB surface were isolated as described previously (Liu et al., 2009). Briefly, EBs were washed with PBS and digested with 0.05% trypsin and 0.2 mM EDTA for 2 min. The loosely adhering endoderm cells on the EB surface were detached by pippeting, and the endoderm-free EBs were removed by sedimentation. The purity of endoderm cells was >90% based on immunostaining for laminin-111 and α-fetoprotein. To graft the endoderm cells onto the surface of GATA-6–null EBs, we mixed the mutant EBs cultured for 1 d with isolated endoderm cells at a ratio of 1:100 in hanging drops for 24 h. The EBs were either collected or cultured in suspension for additional 4 d for immunostaining and immunoblot analysis.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assay was carried out using a Millipore ChIP assay kit as per manufacturer's instructions. The PCR primers for the GATA-6 binding sequence in the BMP-2 promoter are 5′-AGAAATCCATGCACCTTCC (forward) and 5′-CATTGCTGA CCAGTAAGTAC (reverse).
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01GM081674) to S.L.
Abbreviations used:
- BM
basement membrane
- BMP
bone morphogenetic protein
- EB
embryoid body
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-04-0313) on August 1, 2012.
*These authors contributed equally to this work.
†Present address: Plastic and Aesthetic Surgery, The First Hospital, Jilin University, Changchun, China 130021.
REFERENCES
- Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA. 1998;95:5082–5087. doi: 10.1073/pnas.95.9.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artus J, Panthier JJ, Hadjantonakis AK. A role for PDGF signaling in expansion of the extra-embryonic endoderm lineage of the mouse blastocyst. Development. 2010;137:3361–3372. doi: 10.1242/dev.050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Cai KQ, Capo-Chichi CD, Rula ME, Yang DH, Xu XX. Dynamic GATA6 expression in primitive endoderm formation and maturation in early mouse embryogenesis. Dev Dyn. 2008;237:2820–2829. doi: 10.1002/dvdy.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capo-Chichi CD, Rula ME, Smedberg JL, Vanderveer L, Parmacek MS, Morrisey EE, Godwin AK, Xu XX. Perception of differentiation cues by GATA factors in primitive endoderm lineage determination of mouse embryonic stem cells. Dev Biol. 2005;286:574–586. doi: 10.1016/j.ydbio.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126:535–546. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki Ji J, Niwa H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277:33361–33368. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- He X, et al. Rac1 is essential for basement membrane-dependent epiblast survival. Mol Cell Biol. 2010;30:3569–3581. doi: 10.1128/MCB.01366-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig BB, et al. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol. 1994;14:5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Laforest B, Andelfinger G, Nemer M. Loss of Gata5 in mice leads to bicuspid aortic valve. J Clin Invest. 2011;121:2876–2887. doi: 10.1172/JCI44555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Pintar J, Efstratiadis A. Pattern of the insulin-like growth factor II gene expression during early mouse embryogenesis. Development. 1990;110:151–159. doi: 10.1242/dev.110.1.151. [DOI] [PubMed] [Google Scholar]
- Li S, Yurchenco PD. Matrix assembly, cell polarization, and cell survival: analysis of peri-implantation development with cultured embryonic stem cells. Methods Mol Biol. 2006;329:113–125. doi: 10.1385/1-59745-037-5:113. [DOI] [PubMed] [Google Scholar]
- Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003;4:613–624. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fassler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J Cell Biol. 2002;157:1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen Y, Scheele S, Arman E, Haffner-Krausz R, Ekblom P, Lonai P. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J Cell Biol. 2001;153:811–822. doi: 10.1083/jcb.153.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, He X, Corbett SA, Lowry SF, Graham AM, Fassler R, Li S. Integrins are required for the differentiation of visceral endoderm. J Cell Sci. 2009;122:233–242. doi: 10.1242/jcs.037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005;283:282–293. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P, Edgar D. Regulation of programmed cell death by basement membranes in embryonic development. J Cell Biol. 2000;150:1215–1221. doi: 10.1083/jcb.150.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajni-Underwood S, Wilson CP, Elder C, Mishina Y, Lewandoski M. BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development. 2007;134:2359–2368. doi: 10.1242/dev.001677. [DOI] [PubMed] [Google Scholar]
- Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V, Orkin SH, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re'em-Kalma Y, Lamb T, Frank D. Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development. Proc Natl Acad Sci USA. 1995;92:12141–12145. doi: 10.1073/pnas.92.26.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J. Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Semin Cell Dev Biol. 2004;15:573–581. doi: 10.1016/j.semcdb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Shu B, et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Loebel DA, Tanaka SS. Building the mouse gastrula: signals, asymmetry and lineages. Curr Opin Genet Dev. 2006;16:419–425. doi: 10.1016/j.gde.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–1580. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Sakiyama J, Kameda T, Iba H, Suzuki A, Ueno N, Kuroiwa A. BMP-2/-4 mediate programmed cell death in chicken limb buds. Development. 1996;122:3725–3734. doi: 10.1242/dev.122.12.3725. [DOI] [PubMed] [Google Scholar]
- Zou H, Niswander L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science. 1996;272:738–741. doi: 10.1126/science.272.5262.738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.