Abstract
Purpose:
To compare the safety, efficacy, and dosing regimen of intravitreal ranibizumab as an adjunct to laser therapy for the treatment of macular edema secondary to branch retinal vein occlusion (BRVO).
Materials and Methods:
Thirty eyes of 30 patients of BRVO of at least 6 weeks duration were randomized into three groups: Group 1 received grid laser treatment alone, Group 2 received a single dose of intravitreal injection of ranibizumab (0.5 mg / 0.05 ml) followed by grid laser treatment on 7th day following injection, while Group 3 received three loading doses of intravitreal ranibizumab at monthly interval (i.e. 0, 1, & 2 months) + standard laser treatment 7 days after the 1st injection. Outcome measure noted at 6 months follow-up were the improvement in best-corrected visual acuity (BCVA) and central macular thickness (CMT).
Results:
At 6 months follow-up, there was an average gain of 12 letters (P=0.05), 17.5 letters (P=0.05) and 19 letters (P=0.05) in groups 1, 2, and 3, respectively, with the decrease in CMT being 208.7 μm (P=0.05), 312.9 μm (P= 0.05) and 326.8 μm (P=0.05), respectively, in these groups. Gain in BCVA of more than 3 lines was noted in 1/10 patients in Group 1(10%) as compared to 3/10 (30%) and 4/10 (40%) patients in groups 2 and 3, respectively.
Conclusion:
The gain in BCVA and reduction in CMT were better with combination therapy (single- and triple- dose regimen) compared to grid laser alone. Single dose of intravitreal ranibizumab with grid laser seems to be an effective therapy.
Keywords: Branch retinal vein occlusion, laser, Lucentis, macular edema
Retinal vein occlusion disease is estimated to be the second most common cause of retinal vascular disease.[1] Macular edema is a frequent cause of visual acuity loss from branch retinal vein occlusion (BRVO). The Branch Vein Occlusion study demonstrated that argon laser photocoagulation improved the visual outcome significantly in eyes with perfused BRVO of 3-18 months duration and reduced visual acuity of 20/40 to 20/200 due to macular edema. As the disease was seen to resolve spontaneously in one-third of the patients, treatment was delayed for at least 3 months to permit maximum resorption of intra-retinal blood and edema.
During the last decade, anti-vascular endothelial growth factor (anti- VEGF) therapy evolved as a major treatment modality. The BRAVO study found intravitreal ranibizumab to be effective in the treatment of macular edema secondary to BRVO.[2] However, no study has been done comparing the effectiveness of combination therapy of laser with ranibizumab with standard grid laser treatment alone in persistent macular edema secondary to BRVO. We believe that unlike with Age-related macular degeneration (AMD) and Diabetic Retinopathy treatment, retinal vein occlusion (RVO) is an inner retinal disease, and a passive edema in the inner retina does not result in photoreceptor damage as rapidly as in AMD or DR, and so there is lesser demand for frequent intravitreal injections. Moreover, as RVO is a result of acute process, unlike AMD and DR which are the result of chronic disease process, the treatment required will be less aggressive.
Hence, with the hypothesis in background that an injection of anti-VEGF further decreases the macular edema, allowing effective laser uptake at a lower power, a small, prospective, randomized, controlled trial was carried out to compare the safety and efficacy of intra-vitreal ranibizumab (0.5 mg/0.05 ml) as an adjunct to laser treatment with standard laser treatment in patients with visual impairment due to macular edema secondary to BRVO.
Materials and Methods
This prospective, comparative study adhered to the tenets of Declaration of Helsinki. The patients included had BRVO of at least 6 weeks duration, perfused as confirmed on fluorescein angiography, with central macular thickness (CMT) of ≥ 250 μm, and baseline visual acuity of 20/40 or worse. Perfused BRVO was defined as lacking evidence of neovascularization in the retina or iris, with no obvious macular ischemia. The exclusion criteria were previous treatment for BRVO, such as intravitreal injection, subtenon injection, or laser photocoagulation, since the time of onset of BRVO, a history of glaucoma, macular edema secondary to other causes, such as age-related macular degeneration and diabetic retinopathy.
After obtaining an informed consent and explaining the treatment outcomes, the patients were randomized into three groups. The baseline characteristics of the patients in three groups were comparable as shown in Table 1. Group 1 received standard grid laser treatment alone. Group 2 received a single intravitreal injection of ranibizumab (Lucentis; Genentech, San Francisco, CA, USA ) (0.5 mg / 0.05 ml) on Day 0 followed by grid laser treatment on Day 7, while Group 3 received three doses of intravitreal ranibizumab at monthly interval (i.e. 0, 1, and 2 months) with grid laser treatment on the 7th day following the first injection. At baseline, all the patients unde rwent a thorough ophthalmological examination, including best-corrected visual acuity (BCVA) measurement with a Snellen chart and Early Treatment Diabetic Retinopathy (ETDRS) chart, applanation, tonometry, ophthalmoscopy, slit- lamp examination with 90D, fluorescein angiography, and optical coherence tomography (OCT ; Model 3000; Carl Zeiss Meditec Inc., Dublin, CA, USA). For grid laser therapy, the guidelines followed were:
Table 1.
Baseline characteristics of patients in three groups
Spot size: 50 - 100 μm
Exposure: 0.05 - 0.1 second
Burn intensity: Mild
Number: As per areas of diffuse retinal thickening
Placement: 1 - 2 burn-widths apart (500 - 3000 μm from center of fovea)
Wavelength: Green
Eyes that were randomized into groups 2 and 3 received intravitreal ranibizumab ( 0.5 mg/ 0.05 ml) under sterile conditions. After the injection, a topical antibiotic was applied and the patients were monitored for potential injection-related complications. The main parameters evaluated were BCVA and CMT on OCT at 1, 3, and 6 months after the initial injection. Fluorescein angiography was performed at baseline and at each monthly visit for 6 months. Blood pressure was measured at baseline and at each monthly visit.
Statistical analysis was performed using a commercially available statistical software package (SPSS for Windows, version 16.0; SPSS, Chicago, IL, USA). Visual acuity was converted into the logarithm of the minimum angle of resolution (logMAR) and decimal system for statistical calculations. Univariate categorical analysis was performed using the two-paired t-test, Chi-square test, Mann- Whitney U test, or Fisher's exact test, as appropriate. The data were analyzed via repeated-measures analysis of variance with a Bonferroni correction. The level of statistical significance was set at 0.05 (two-sided) in all statistical tests.
Results
Visual acuity outcomes
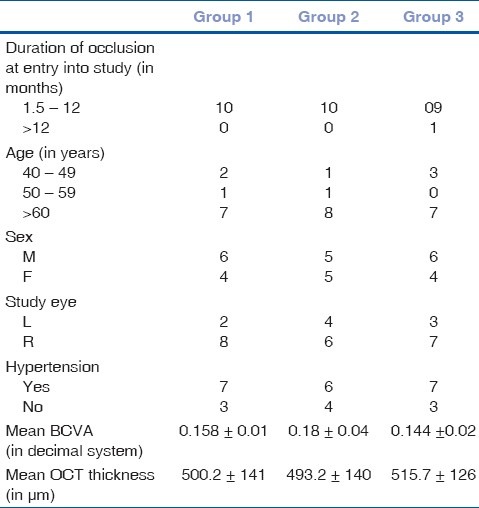
In Group 1, mean BCVA improved from 0.158± 0.01 at baseline to 0.162±0.02 at 1 month, 0.192± 0.01 at 3 months, and 0.289 at 6 months, i.e., there was a BCVA improvement of 11± 3 letters at 1 month to 11.5± 5 letters at 3 months and 12± 5 letters at the end of 6 months (P= 0.05). In Group 2, the response was rapid after the intravitreal injection, with a mean BCVA improvement of 16± 4 letters at 1 month from baseline (from 0.18± 0.04 at baseline to 0.433± 0.02 at 1 month). After 3 months, the mean BCVA improved by 17± 5 letters (0.439± 0.02), and at 6 months the gain increased to 17.5± 5 letters (0.459± 0.02) (P= 0.05).In Group 3, there was an average gain of 15.8± 2 letters at the end of 1 month (from 0.144± 0.02 at baseline to 0.306± 0.02 at 1 month), which increased to 17.7± 3 letters at the end of 3 months (0.338± 0.02) and was sustained at 18± 4 letters (0.432± 0.02) at the end of 6 months (P= 0.05). Intergroup comparison for BCVA at months 1, 3, and 6 was not statically significant, but in Group 1, the mean improvement in BCVA of more than 3 lines was noted in only 10% of the patients as compared to 40% in Group 2 and 30% in Group 3. A comparison of outcomes between the three groups is depicted in Fig. 1.
Figure 1.
Comparison of visual outcomes between Group 1 (laser alone), Group 2 (single loading dose with laser), and Group 3 (triple loading dose with laser) over a period of 6 month
Imaging outcomes
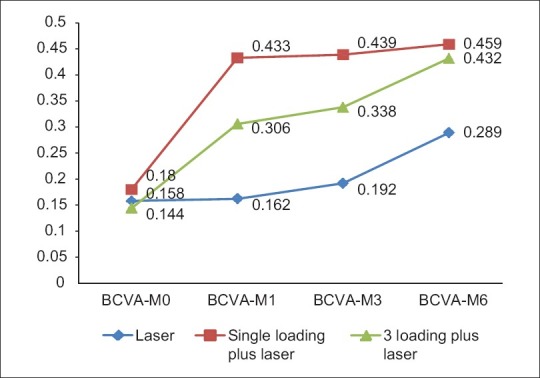
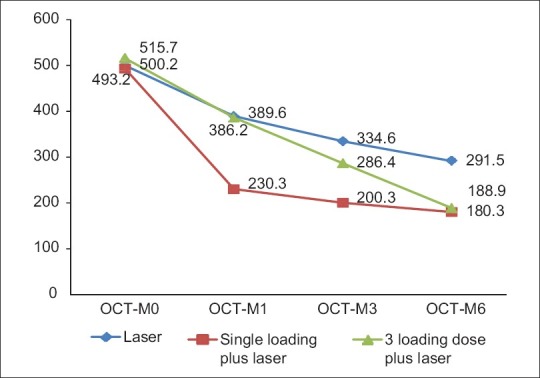
Paralleling the improvement in BCVA, ranibizumab treatment led to a rapid reduction in the (CMT). Similar responses were observed in single and triple- dose regimens. In Group 1, center point thickness decreased from a mean of 500.2± 141μm at baseline to 389.6± 120μm at 1 month, 334.6± 117 μm at 3 months and 291.5± 109μm at 6 months (P= 0.05). In Group 2 (single-dose regimen), there was a rapid decrease in mean CRT from 493.2± 140μm at baseline to 230.3± 96 μm at 1 month, 200.3± 92 μm at 3 months that further decreased to 180.3± 78 μm at 6 months (P= 0.05). In Group 3, (triple-dose regimen) mean CMT decreased from 515.7± 126 μm at baseline to 386.2± 97 μm at 1 month, 286.4± 87 μm at 3 months, and was sustained at 188.9± 76 μm at 6 months (P= 0.05). Though intergroup comparison results were not statically significant, at the end of 6 months, Group 1 showed a decrease in CMT of 208.7 μm as compared to 312.9 and 326.8 μm in groups 2 and 3, respectively. The changes in the mean OCT thickness in the three groups have been illustrated in Fig. 2. The changes in the fundus, fluorescein imaging, and OCT at 1, 3, and 6 months in groups 1, 2 and 3 have been depicted in Figs. 3–5.
Figure 2.
Changes in the mean OCT thickness in the three groups over 6 months following treatment
Figure 3.
Changes in the fundus, FFA, and OCT in Group 1 at 1, 3, and 6 months follow- up
Figure 5.
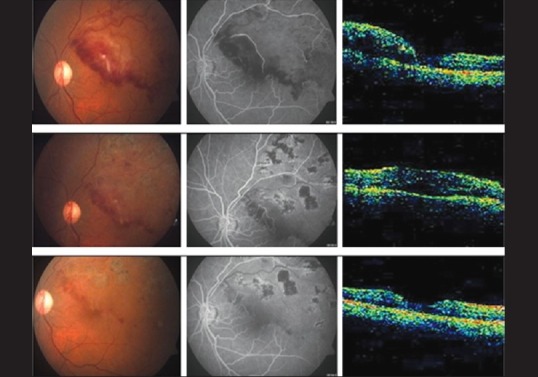
Changes in the fundus, FFA, and OCT in Group 3 at 1, 3, and 6 months follow- up
Figure 4.
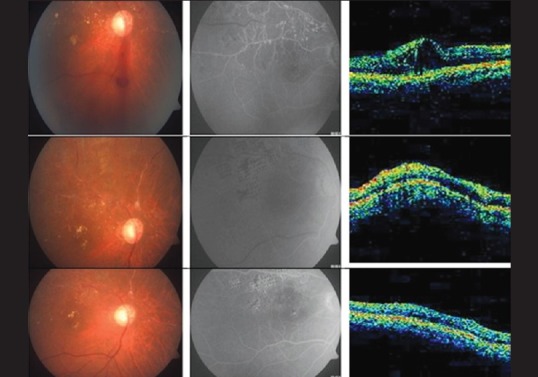
Changes in the fundus, FFA, and OCT in Group 2 at 1, 3, and 6 months follow- up
Discussion
The natural history of macular edema secondary to BRVO was delineated in the Branch Vein Occlusion Study (BVOS).[1] BVOS also demonstrated a benefit with grid photocoagulation in eyes with BRVO of 3- 18 months duration and visual acuity of 20/40 to 20/200. Treated eyes were more likely to gain 2 lines of visual acuity (65%) compared with the untreated eyes (37%). Furthermore, treated eyes were more likely to have 20/40 or better vision at 3 years follow-up (60% vs. 34% untreated), with a mean visual acuity improvement of 1.3 lines ETDRS versus 0.2 lines in the untreated group.
The rationale for the use of anti -VEGF to treat macular edema secondary to BRVO follows from the observation that the increase in retinal capillary permeability that results in macular edema may be caused by a breakdown of the blood- retina barrier, mediated in part by VEGF,[3] a 45-kDa glycoprotein. Therefore, attenuation of the effects of VEGF may reduce macular edema associated with BRVO. Anti -VEGF has been demonstrated to bind and neutralize all the biologically active forms of VEGF, and therefore may be an effective therapy for macular edema.
The BRAVO trial (a phase 3, multicenter, randomized, sham injection-controlled study of the efficacy and safety of ranibizumab injection compared with sham in patients with macular edema secondary to BRVO) assessed the safety and efficacy of ranibizumab in patients with BRVO.[3] Patients included in the study had macular edema involving the foveal center secondary to BRVO, central subfield macular thickness of 250 μm or greater on OCT, and BCVA of 20/40 to 20/400. Patients were randomly assigned to six monthly injections of ranibizumab, either 0.3 mg or 0.5 mg, or to sham injection. In 397 patients randomized, the mean gain from baseline at month 6 was 16.6 letters in patients receiving 0.3 mg of ranibizumab, 18.3 letters in those receiving 0.5 mg, and 7.3 in those receiving sham injections. By month 6, most patients in the two ranibizumab groups gained at least 3 lines of BCVA (55.2% in the 0.3 mg group and 61.1% in the 0.5 mg group), while most of those in the sham group did not (28.8%). This trial, however, enrolled all comers, irrespective of the duration of their disease.
We believe that as the disease was seen to resolve spontaneously in one-third of the patients in the BVOS study, treatment can be delayed for at least 6 weeks to permit maximum resorption of intra-retinal blood and edema. In this small, randomized, controlled study, intravitreal ranibizumab at 4 weeks interval along with grid laser provided rapid and sustained improvement of BCVA in subjects with BRVO for 6 months period. 40% of the subjects gained atleast 3 lines of vision in 24 weeks. The rapid improvement in vision was paralleled by reductions in macular thickness. Almost similar improvements were observed in the single- and the triple- dose groups.
It is our belief that the endpoint gain in BCVA would be greater if an anti-VEGF is used prior to laser therapy. Anti-VEGF would decrease the macular thickness, allowing effective laser uptake at a lower power. The results in Groups 2 and 3 of our study illustrate this point (as 70% of the treated eyes gained and maintained 2 or more lines of BCVA from baseline).
In BRAVO study, after six doses of intravitreal ranibizumab at the end of 6 months, there was a gain of 16.6 letters and 18.3 letters and the mean changes in CMT were 337.2 and 345.2 μm in 0.3 and 0.5 mg groups, respectively. Although a direct comparison cannot be made because of difference in the study design, it is worthwhile noting that in our study the mean improvement in BCVA was 17.5 letters in Group 2 and 18 letters in Group 3 at the end of 6 months. Similarly, a decrease in CRT of 312.9 and 326.8 μm in groups 2 and 3, respectively was noted.
So, in our study design, though no significant difference for a gain in BCVA was noted in the three treatment groups, the fact that gain in BCVA of more than 3 lines was noted in 40% patients of combination therapy compared to 10% patient in standard laser group helped us conclude that ranibizumab may be used as an effective and safe adjunct to laser in the treatment of macular edema secondary to BRVO. Since economics plays a major role in treatment involving anti-VEGF administration, this alternative treatment modality may prove to be a viable option in the developing countries.
Limitation of this study includes the small study population. Despite this limitation, the results of this study suggest that intravitreal ranibizumab is an effective option for the treatment of BRVO and that larger, more definitive, randomized clinical trial are warranted to determine the optimal treatment interval and duration.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.The Branch Vein Occlusion Study Group. Argon Laser photocoagulation for macular edema in Branch vein occlusion. Am J Ophthalmol. 1984;98:271–82. doi: 10.1016/0002-9394(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 2.Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle CA, et al. BRAVO investigators.Ranibizumab for Macular Oedema following BRVO: Six month primary end point results of a phase 3 study. Ophthalmology. 2010;117:1102–12. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Campochiaro PA, Hafiz G, Shah SM, Vguyen QD, Ying H, Do DV, et al. Ranibizumab for macular oedema due to retinal vein occlusions; implications of VEGF as a critical stimulator. Mol Ther. 2008;16:791–9. doi: 10.1038/mt.2008.10. [DOI] [PubMed] [Google Scholar]


