Abstract
Purpose:
To compare the effects of preoperative use of topical anti-inflammatory prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery.
Design:
Randomized clinical trial.
Materials and Methods:
This single-center, masked, randomized clinical study comprised 140 patients scheduled for cataract surgery. Patients (35 in each group) were randomized to receive placebo, prednisolone acetate, ketorolac tromethamine 0.4% or nepafenac. These eye drops were administered three times daily for the two days prior to surgery. The pupillary diameters were measured by the surgeon using a compass prior to the corneal section and at the end of surgery. The primary outcome was the number of patients with pupil ≥ 6mm at the end of the surgery; the secondary outcome was the number of patients with pupil ≥ 6mm at the beginning of the surgery.
Results:
All the patients achieved pupil ≥ 6mm at the beginning of the surgery. The number of patients in the prednisolone (29/35), nepafenac (31/35) and ketorolac (30/35) groups with pupil ≥ 6mm was greater than in the placebo group in the maintenance of intraoperative mydriasis (19/35 – P =0.003). There was no statistical difference among the prednisolone, nepafenac and ketorolac groups in the maintenance of intraoperative mydriasis (P =.791). There were no complications during surgery or related to the preoperative use of the eye drops.
Conclusion:
Preoperative use of ketorolac, prednisolone and nepafenac was effective in maintaining intraoperative mydriasis when compared with placebo.
Keywords: Cataract, inflammation, mydriasis, prevention and control, surgery
Cataract extraction increases the concentration of prostaglandins (PGs) E and PGs F in aqueous humor, resulting in hyperemia, miosis and breakdown of the blood-aqueous barrier.[1]
Topical anti-inflammatory drugs are commonly used in the management of ocular inflammation and cystoid macular edema related to cataract surgery.[2–4] It has been suggested the use of anti-inflammatory drugs before surgery, to achieve better intraoperative mydriasis. The miosis that occurs during cataract surgery is in part mediated by PGs.[5] Preoperative treatment using nonsteroidal antiinflammatory drugs (NSAIDs) has been shown to be effective in maintaining mydriasis during cataract surgery.[2] The mechanism of their action is dependent on their ability to cause cyclooxygenase inhibition and thereby inhibit the production of PGs in response to surgical trauma.[6] Steroids block the release of arachidonic acid, which is also a precursor for PGs synthesis. Unlike nonsteroidal agents, steroid eye drops have not been extensively studied for their antimiotic effect.[7–9]
Cataract surgery complications increase when miosis occurs. It was reported that, when mydriasis is greater than 6mm, the incidence of posterior capsule rupture is reduced by half.[10] In addition, the increasing number of toric and multifocal intraocular lenses draws attention to the importance of maintaining well dilated pupils (> 6mm) during cataract surgery.
Topical steroids are the most prescribed medication for postoperative periods. It comprises the steroid group derivative of cholesterol. Prednisolone is one of the most commonly used. Although prolonged use of steroids may result in cataract formation, and secondary ocular infections, there are no previous reports showing that its use for a short period before surgery has side effects superior to the use of nonsteroidal anti-inflammatory drugs (NSAIDs). Furthermore, steroid-induced IOP elevation almost never occurs in less than five days.[11,12]
Nonsteroidal anti-inflammatory drugs do not comprise the steroid group derivative of cholesterol. The main options for eye drops are ketorolac tromethamine, diclofenac, flurbiprofen, indomethacin and nepafenac. Less influence on intraocular pressure control is one of the main advantages of these medications when used for a long period. Nepafenac, a new prodrug, which is hydrolyzed by intraocular tissues to amfenac has demonstrated superior intraocular penetration when compared with other anti-inflammatory drugs in both anterior segment and retinal tissue following topical ocular administration.[13,14]
The objective of the original study was to compare the effect of preoperative use of topical anti-inflammatory prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, in the maintenance of intraoperative mydriasis in cataract surgery.
Materials and Methods
This single-center, masked, randomized clinical study comprised 140 patients with cataract [Fig. 1]. The study took place in Campinas, Brazil. Eligible participants were recruited from March 2009 to March 2010.
Figure 1.
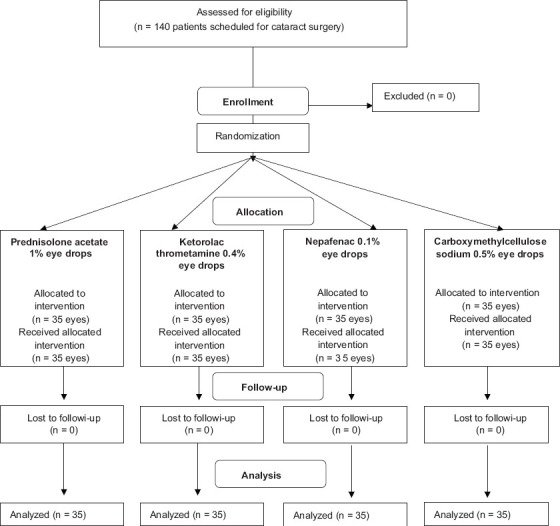
CONSORT flow diagram: Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, in the maintenance of intraoperative mydriasis during cataract surgery
The inclusion criteria were as follows: patients with nuclear cataract density of 2 and 3 by LOCS II (> 50 years old), with indication for cataract surgery with intraocular lens implant, under local anesthesia.
The exclusion criteria were as follows: diabetes, hypertension, patients using nonsteroidal anti-inflammatory, alphablocker, topical eye drops (including antiglaucoma drugs), history of uveitis, macular disease, pseudoexfoliation syndrome, congenital ocular abnormalities, cataract density of 1 and 4 by LOCS II and previous intraocular surgery.[15]
Patients with cataract were randomized to receive either placebo carboxymethylcellulose sodium 0.5%, prednisolone acetate 1%, ketorolac tromethamine 0.4% or nepafenac 0.1%. These eye drops were administered 48 hours before surgery by mask fashion, three times daily for two days prior to surgery.
The randomization was done in a blocking manner. Each of the four intervention groups received 35 different numbers from a random number table. These numbers were transferred to small individual envelopes and also fixed at one of the opaque eye drop bottles. It helped not only to randomize the patients but also to mask the treatment groups until data analysis. Four small envelopes, one of each intervention group, were sealed and placed into a larger envelope, totalizing 35 large envelopes containing four small individual envelopes in each one. This comprises the block length of four to assure that every four patients have received all four interventions. When a patient was included in the study, a pharmacist took for him a small individual envelope and after discovering the random number, she took the respective eye drop bottle. The surgeon and the ophthalmologist who collected the data did not know the randomized groups.
The eye drop group was revealed to the researchers once recruitment, data collection, and statistical analyses were complete. All study participants were masked to treatment assignment.
Gatifloxacin was prescribed four times daily for two days prior to surgery, with interval time of at least 15 minutes between two eye drops. Preoperative mydriasis was accomplished with tropicamide 0.5% and phenylephrine 5% eye drops, one drop instilled into the patient's eyes 60, 45 and 30 minutes before surgery (three doses). Peribulbar anesthesia was performed with lidocaine 2% (3ml) in the inferior-temporal quadrant, associated with an oral dose of diazepam 5 mg, 30 minutes before surgery, without additional sedation. Lidocaine was not used intraocularly. The phacoemulsification (Infiniti®, Alcon Inc., Hünenberg, Switzerland) was performed by a single surgeon.
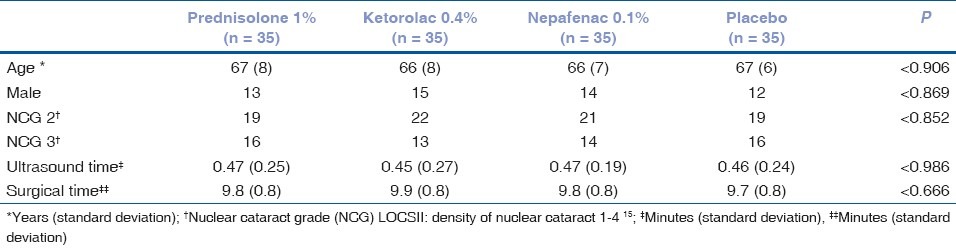
The surgeon used the same standardized small-incision phacoemulsification technique in all patients. In short, 1.0mm and 3.0mm clear corneal incisions were made and a capsulorhexis 4.0mm in diameter was created. A stop-chop phacoemulsification technique was used and foldable intraocular lenses were implanted in the capsular bag. The phacoemulsification parameters were established prior to all surgeries and were the same for all patients. Cataract surgery was conducted using the Legacy® Series 2000 Machine (Alcon Laboratories, Inc.). The parameters used were as follows: balanced saline solution, with the height of the bottle set at 100 cm, 40 ml/minute aspiration flow rate, 450mmHg vacuum and phaco power 50%. Intracameral adrenaline was not used in the irrigation solution. Ultrasound time and surgical times were registered at the end of each surgery [Table 1].
Table 1.
Baseline demographic and clinical characteristics. Effect of preoperative use of anti-inflammatory topical agents on the maintenance of intraoperative mydriasis during cataract surgery
The eye drop was revealed to the researchers once recruitment, data collection, and statistical analyses were complete. All study participants were masked to the treatment assignment.
To ensure the standardization of illumination during pupillary measurement, the surgeon used the same microscope (Leica M820, Germany) and the illumination was kept constant in all cases. The horizontal and vertical diameters of the pupil were measured in millimeters using a compass under the microscope (directly on the eye) at the following stages: before surgery (prior to the corneal incision) and at the conclusion of surgery. The preset standard magnification of the operating microscope was ensured at each of the two time points.
The primary outcome was the number of patients with pupil ≥ 6mm (vertical and horizontal diameters) at the end of the surgery to measure the efficacy of each medication in the maintenance of intraoperative mydriasis during cataract surgery. The secondary outcome was the number of patients with pupil ≥ 6mm (vertical and horizontal diameters) at the beginning of the surgery (prior to the corneal section) to measure the efficacy of each medication to achieve preoperative mydriasis.
Ethics committee approval was obtained and all participants gave informed consent (Conep - National Research Ethics Committee – Brazil – 0816.0.146.000-10). Also the study was registered at Clinical Trials protocol NCT00865540.
A sample size of 140 patients (35 per group) was planned to compare groups for primary outcome (pupil > 6mm at the end of the surgery). With an assumption of a 50% rate of pupil ≥ 6mm in the placebo group, this sample size provided a 80% probability of detecting a difference as small as 35% in the other groups. Results of these analyses were considered as statistically significant when the P-values were < 0.05. Measures of central tendency and dispersion were determined by median, mean and standard deviation. Categorical variables were analyzed using the chi-square (Yates) test and, for continuous variables, one-way analysis of variance (ANOVA) tables were used.
Results
No patient loss was registered from the day of inclusion in the trial to the end of surgeries.
Baseline demographic and clinical characteristics were similar in all groups. There were no differences regarding ages (P = 0.930), neither in age-related cataract density (P = 0.852), nor in gender distribution (P = 0.896), ultrasound time (P = 0.986) and surgical time (P = 0.666) [Table 1].
All patients achieved pupil ≥ 6mm at the beginning of the surgery. The number of patients in prednisolone (29/35), nepafenac (31/35) and ketorolac (30/35) groups with pupil ≥ 6mm was greater than the placebo group in the maintenance of intraoperative mydriasis at the conclusion of surgery (19/35 – P = 0.003 – Tables 2 and 3). There were no complications during surgery or related to the preoperative use of the eye drops.
Table 2.
Effect of preoperative use of topical antiinflammatory agent on the maintenance of intraoperative mydriasis during cataract surgery. Distribution of patients according to pupil size at the end of the surgery
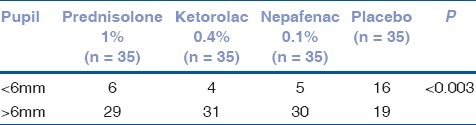
Table 3.
Effect of preoperative use of topical antiinflammatory agent on the maintenance of intraoperative mydriasis during cataract surgery. Distribution of patients according to pupil diameter at the beginning and at the end of the surgery
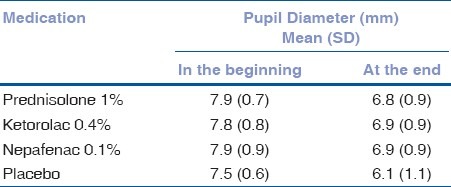
There was no statistical difference among the prednisolone, nepafenac and ketorolac groups in the maintenance of intraoperative mydriasis (P = 0.791).
Discussion
In the present study, the use of ketorolac 0.4%, prednisolone 1% and nepafenac 0.1% three times daily for two days preoperatively demonstrated a statistically significant difference in the maintenance of intraoperative mydriasis when compared with the placebo group.
Nepafenac 0.1% was superior to placebo in the inhibition of intraoperative miosis. These results were similar to those of Cervantes-Coste et al., who also found that the prophylactic use of nepafenac 0.1% was safe and effective in maintaining mydriasis during cataract surgery.[16]
The use of topical prednisolone 1% to maintain intraoperative mydriasis was superior to placebo. Shaikh et al., analyzed the antimiotic effect of topical prednisolone and flurbiprofen.[8] In the analysis of the study, there were no significant differences in maintaining mydriasis at any stage of surgery in the prednisolone and flurbiprofen groups when comparing with the placebo group. Unfortunately, the comparison of the prednisolone and flurbiprofen groups with the placebo group (sodium chloride 0.9%) was not ideal, since the author used epinephrine 1:106, a potent direct-acting mydriatic agent, in all groups. Because of this confounding bias, there was a failure when assessing the anti-inflammatory effect of eye drops.
Epinephrine is one of the alternatives to improve intraoperative mydriasis that were not analyzed in this study. Although the potential for systemic absorption of epinephrine can lead to sympathomimetic effects (such as excessive sweating, pallor, faintness, occipital headaches, hypertension, palpitations, tachycardia, and cardiac arrhythmias, particularly in patients with preexisting cardiac disease), its use has additive benefit for inhibiting intraoperative miosis, regardless of whether antiprostaglandins were used.[17–21] However, in undiluted and weakly diluted solutions, the bisulfite preservative included in most epinephrine preparations is shown to cause corneal endothelial damage and subsequent corneal haziness.[22,23]
Our results with ketorolac were similar to those of Stewart et al., who demonstrated that ketorolac 0.5% used before surgery provided effective and well-tolerated inhibition of surgically induced miosis during cataract surgery when compared with placebo.[24]
An interesting finding from this study (with economic impact) was the fact that, since there been no significant differences among ketorolac, prednisolone and nepafenac in the maintenance of intraoperative mydriasis during cataract surgery, these medicines can be used in surgical practice with similar efficacy. Considering that prednisolone has a lower cost than ketorolac or nepafenac and, moreover, during the post-operative period, steroid use is mandatory, while the use of non-steroidal anti-inflammatory drugs is optional,[4] it becomes an option for a single drug as a mydriatic adjuvant at preoperative care and as an anti-inflammatory agent at postoperative period of cataract surgery. In spite of its topical use, preoperative steroids theoretically reduce immunity with increased risk of opportunist infection (herpes and fungi); in this study, it did not increase the risk of surgical complications. In addition the ocular surface precipitation of prednisolone did not interfere with surgical view.
The disadvantages of this study are excluding diabetics, eyes with hard cataracts or pseudoexfoliation limits the applicability of the results considered for these specific patients.
We are unaware of previous clinical trial reports about the maintenance of intraoperative mydriasis during cataract surgery, simultaneously including, a relatively new NSAID eye drop (nepafenac), the most prescribed NSAID eye drop for that purpose (ketorolac), as well as the steroid eye drop already used routinely during the postoperative period, (prednisolone) and we could find no reference to it in a computerized search at PubMed. Additional studies are warranted to confirm our findings.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Camras CB, Miranda OC. The putative role of prostaglandins in surgical miosis. Prog Clin Biol Res. 1989;312:197–210. [PubMed] [Google Scholar]
- 2.Nichols J, Snyder RW. Topical nonsteroidal anti-inflammatory agents in ophthalmology. Curr Opin Ophthalmol. 1998;9:40–4. doi: 10.1097/00055735-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Schalnus R. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica. 2003;217:89–98. doi: 10.1159/000068563. [DOI] [PubMed] [Google Scholar]
- 4.Kim A, Stark WJ. Are topical NSAIDs needed for routine cataract surgery? Am J Ophthalmol. 2008;146:483–5. doi: 10.1016/j.ajo.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Duffin RM, Camras CB, Gardner SK, Pettit TH. Inhibitors of surgically induced miosis. Ophthalmology. 1982;89:966–79. doi: 10.1016/s0161-6420(82)34693-x. [DOI] [PubMed] [Google Scholar]
- 6.Podos SM. Prostaglandins, nonsteroidal anti-inflammatory agents and eye disease. Trans Am Ophthalmol Soc. 1976;74:637–60. [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni PS. Steroids in ocular therapy. In: Zimmerman TJ, editor. Textbook of Ocular Pharmacology. Philadelphia, PA: Lippincott-Raven; 1997. pp. 63–7. [Google Scholar]
- 8.Shaikh MY, Mars JS, Heaven CJ. Prednisolone and flurbiprofen drops to maintain mydriasis during phacoemulsification cataract surgery. J Cataract Refract Surg. 2003;29:2372–7. doi: 10.1016/s0886-3350(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 9.Dubé P, Boisjoly HM, Bazin R, Chamberland G, Laughrea PA, Dubé I. Comparison of prednisolone acetate and indomethacin for maintaining mydriasis during cataract surgery. Can J Ophthalmol. 1990;25:234–8. [PubMed] [Google Scholar]
- 10.Guzek JP, Holm M, Cotter JB, Cameron JA, Rademaker WJ, Wissinger DH, et al. Risk factors for intraoperative complications in 1000 extracapsular cataract cases. Ophthalmology. 1987;94:461–6. doi: 10.1016/s0161-6420(87)33424-4. [DOI] [PubMed] [Google Scholar]
- 11.François J. Corticosteroid glaucoma. Ann Ophthalmol. 1977;9:1075–80. [PubMed] [Google Scholar]
- 12.Cantor LB, editor. American Academy of Ophthalmology - Fundamentals and Principles of Ophthalmology. 2007-2008 ed. San Francisco, CA: American Academy of Ophthalmology; 2007. Ocular Pharmacotherapeutics; pp. 419–20. [Google Scholar]
- 13.Ke TL, Graff G, Spellman JM, Yanni JM. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation. 2000;24:371–84. doi: 10.1023/a:1007001131987. [DOI] [PubMed] [Google Scholar]
- 14.Lindstrom R, Kim T. Ocular permeation and inhibition of retinal inflammation: An examination of data and expert opinion on the clinical utility of nepafenac. Curr Med Res Opin. 2006;22:397–404. doi: 10.1185/030079906X89775. [DOI] [PubMed] [Google Scholar]
- 15.Chylack LT, Leske MC, McCarthy D, Khu P, Kashiwagi T, Sperduto R. Lens opacities classification system II (LOCS II) Arch Ophthalmol. 1989;107:991–7. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 16.Cervantes-Coste G, Sánchez-Castro YG, Orozco-Carroll M, Mendoza-Schuster E, Velasco-Barona C. Inhibition of surgically induced miosis and prevention of postoperative macular edema with nepafenac. Clin Ophthalmol. 2009;3:219–26. doi: 10.2147/opth.s4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liou SW, Chen CC. Maintenance of mydriasis with one bolus of epinephrine injection during phacoemulsification. J Ocul Pharmacol Ther. 2001;17:249–53. doi: 10.1089/108076801750295281. [DOI] [PubMed] [Google Scholar]
- 18.Gimbel HV. The effect of treatment with topical nonsteroidal anti-inflammatory drugs with and without intraoperative epinephrine on the maintenance of mydriasis during cataract surgery. Ophthalmology. 1989;96:585–8. doi: 10.1016/s0161-6420(89)32845-4. [DOI] [PubMed] [Google Scholar]
- 19.Bhallil S, Andalloussi IB, El Abdouni O, Mahjoubi I, Tahri H. Is there a perioperative circulatory side effect of intracameral epinephrine in hypertensive patients undergoing phacoemulsification? Oman J Ophthalmol. 2010;3:161–2. doi: 10.4103/0974-620X.71914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundberg B, Behndig A. Intracameral mydriatics in phacoemulsification surgery obviate the need for epinephrine irrigation. Acta Ophthalmol Scand. 2007;85:546–50. doi: 10.1111/j.1600-0420.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- 21.Greenbaum S. Anesthesia for Eye Surgery. In: Tasman WS, Jaeger EP, editors. Duane's Clinical Ophthalmology. 2007 ed. Philadelphia: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 22.Duffin RM, Pettit TH, Straatsma BR. Maintenance of mydriasis with epinephrine during cataract surgery. Ophthalmic Surg. 1983;14:41–5. [PubMed] [Google Scholar]
- 23.Slack JW, Edelhauser HF, Helenek MJ. A bisulfite-free intraocular epinephrine solution. Am J Ophthalmol. 1990;110:77–82. doi: 10.1016/s0002-9394(14)76942-9. [DOI] [PubMed] [Google Scholar]
- 24.Stewart R, Grosserode R, Cheetham JK, Rosenthal A. Efficacy and safety profile of ketorolac 0.5% ophthalmic solution in the prevention of surgically induced miosis during cataract surgery. Clin Ther. 1999;21:723–32. doi: 10.1016/S0149-2918(00)88323-X. [DOI] [PubMed] [Google Scholar]


