Abstract
The mitochondrial membrane potential that powers the generation of ATP also facilitates mitochondrial Ca2+ shuttling. This process is fundamental to a wide range of cellular activities, as it regulates ATP production, shapes cytosolic and endoplasmic recticulum Ca2+ signaling, and determines cell fate. Mitochondrial Ca2+ transport is mediated primarily by two major transporters: a Ca2+ uniporter that mediates Ca2+ uptake and a Na+/Ca2+ exchanger that subsequently extrudes mitochondrial Ca2+. In this minireview, we focus on the specific role of the mitochondrial Na+/Ca2+ exchanger and describe its ion exchange mechanism, regulation by ions, and putative partner proteins. We discuss the recent molecular identification of the mitochondrial exchanger and how its activity is linked to physiological and pathophysiological processes.
Keywords: Calcium Transport, Mitochondria, Mitochondrial Transport, Sodium/Calcium Exchange, Sodium Transport, CGP-37157, NCLX
Introduction
The movement of calcium ions in and out of mitochondria is mediated through two membranes. The first is the outer membrane, which is relatively permeable to ions and small molecules; via this membrane, Ca2+ transport is facilitated by the non-selective voltage-dependent anion channel (1). The second is the inner mitochondrial membrane, where Ca2+ transport is tightly regulated and ion translocation depends on the activity of specialized calcium transporters. Inward calcium flux across this membrane is mediated primarily via a highly selective pathway termed the mitochondrial Ca2+ uniporter (MCU).3 The MCU displays the electrophysiological characteristics of a gated inwardly rectifying Ca2+ channel with low affinity and high Ca2+ selectivity (2). Conversely, outward calcium flux is mediated primarily by a mitochondrial Na+/Ca2+ exchanger (NCX) and also by a H+/Ca2+ exchanger. The steep membrane potential (mitochondrial Δψ) of ∼180 mV (negative inside) across the mitochondrial inner membrane is the main driving force for mitochondrial Ca2+ entry. It allows mitochondria to rapidly take up Ca2+ from strategically located microdomains of high Ca2+ near the plasma membrane and endoplasmic reticulum (ER), generated during Ca2+-signaling events. As Ca2+ enters the mitochondria, it is partially buffered through the formation of calcium phosphate complexes. The ability to take up substantial amounts of Ca2+ underlines two principal roles of mitochondria; it enables mitochondria to shape the spatiotemporal profile of cytosolic Ca2+ signals and to simultaneously transmit these events into the mitochondrial matrix. In the matrix, Ca2+ up-regulates the activity of Ca2+-sensitive dehydrogenases of the Krebs cycle and the F0F1-ATP synthase (3), thereby controlling the rate of ATP production. A pathological aspect of this process is linked to mitochondrial Ca2+ overload that may trigger the activation of the mitochondrial permeability transmission pore, which in turn releases apoptotic and necrotic signal factors, leading to cell death (4). To restore resting mitochondrial Ca2+ levels, massive amounts of accumulated Ca2+ are extruded from the mitochondria mainly through the activity of the mitochondrial NCX. This process of delayed mitochondrial Ca2+ release plays a dual role in refilling the ER Ca2+ stores and in shaping the amplitude and duration of the cytosolic Ca2+ signal. Because of its strict Na+ dependence, the mitochondrial NCX links between cytosolic Na+ elevations and mitochondrial Ca2+ release. Despite the well recognized importance of mitochondrial Ca2+ shuttling in cellular biology, documented for almost half a century, the molecular identities of the major mitochondrial Ca2+-shuttling transporters, i.e. the Ca2+ uniporter and the NCX, remained elusive. In the last 2 years, promising candidates of the NCX, the Na+/Ca2+/Li+ exchanger (NCLX) (5) and the MCU (6, 7), were discovered. Here, we briefly describe several functional, molecular, kinetic, and regulatory aspects of the mitochondrial NCX, review its molecular identification, and discuss its physiological and pathophysiological roles. The interested reader is also referred to excellent reviews describing in more detail the physiological implications of mitochondrial Ca2+ shuttling (8, 9) and the role of the mitochondrial NCX (10, 11).
Mitochondrial Na+/Ca2+ Exchange, Discovery, Ionic Selectivity, and Inhibitors
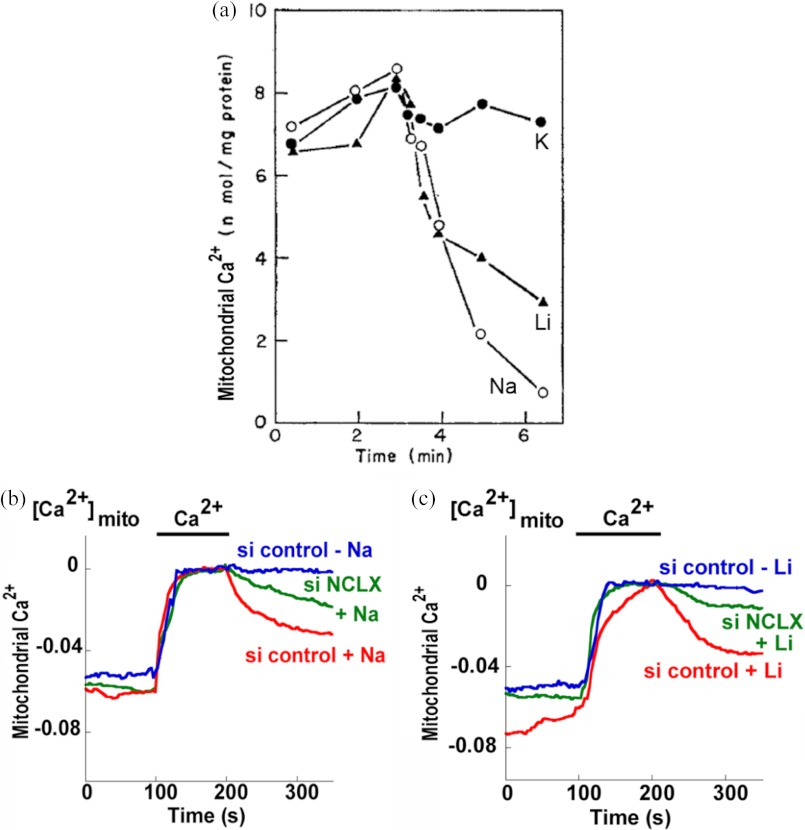
Carafoli et al. (12) were the first to discover that Na+ induces the release of Ca2+ from heart mitochondria (Fig. 1). Mitochondrial Na+/Ca2+ exchange was found to be rather selective toward Ca2+, as Mg2+ (12) and Mn2+ (13) were not transported by the exchanger. On the other hand, the exchanger is only moderately selective for Na+, as Li+ can substitute for this ion (Fig. 1) to evoke mitochondrial Ca2+ release, whereas Cs+, K+, and Rb+ fail to do so (12, 14). Importantly, this functional fingerprint differs from the Li+ inert transport mediated by plasma membrane NCX transporters and was later used to identify the mitochondrial NCX. Mitochondrial Na+/Ca2+ exchange is regulated by various ions and molecules. Micromolar concentrations of cytosolic Ca2+ induced a partial (∼70%) inhibition of mitochondrial Na+/Ca2+ exchange (15), and the cooperative inhibitory profile indicates that Ca2+ interacts with several cytoplasm-facing sites of the exchanger. NCLX does not share the hallmark cytosolic Ca2+ regulatory site of plasma membrane NCX proteins.4 Calcium-binding sites are, however, often elusive, and further molecular analysis is required to determine whether they are present on NCLX. Mitochondrial Na+/Ca2+ exchange is also regulated by a narrow range of pH 7.5–7.6, but whether this type of regulation is mediated by a pH sensor on the exchanger or by an interplay with other mitochondrial ion transporters such as the mitochondrial Na+/H+ exchanger is unclear (16). Potentiation of NCX activity is induced by K+ ions (17, 18), by BSA (18), and by short chain alkanols (Fig. 2) (19). In contrast, inhibition of Na+/Ca2+ exchange is induced by several ions such as Zn2+, Co2+, Sr2+, Ni2+, Mg2+, Ba2+, and La3+ (15, 20–23). Several compounds inhibit mitochondrial Na+/Ca2+ exchange. Among them are CGP-37157 (24, 25), tetraphenylphosphonium (21), trifluoperazine (15), diltiazem (24), verapamil (26), clonazepam (24), amiloride (27), and cyclosporin A (28). The most selective and effective inhibitor of mitochondrial Na+/Ca2+ exchange is the CGP-37157 compound (24, 25). The affinity of CGP-37157 is at least 10-fold higher than that of any other blocker, and when tested in intact cells, CGP-37157 did not interfere with contraction and did not affect Ca2+ fluxes through L-type Ca2+ channels or the activity of the ER Ca2+ pump sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), in isolated mitochondria and cardiomyocytes (29, 30). Furthermore, an at least 10-fold higher concentration of CGP-37157 is required to inhibit the plasma membrane NCX isoforms (31). Due to these properties, most subsequent studies employed CGP-37157 at 10 μm or below. Recent studies suggest, however, that even low concentrations of CGP-37157 may modulate other Ca2+ transporters. For example, when the effect of CGP-37157 was determined in cardiac sarcoplasmic reticulum (SR) microsomes reconstituted into lipid bilayers, it augmented Ca2+ flux via the ryanodine receptors and blocked the SR Ca2+ pump SERCA (32). This finding suggested that CGP-37157 displays, as do other benzothiazepines, a complex pattern of interference with transporters involved in Ca2+ signaling. A noteworthy example is the interaction of this compound with the L-type Ca2+ channel, which led to conflicting findings on the role of the exchanger in regulating insulin secretion (33, 34). Thus, studies aiming to elucidate the physiological role of the exchanger should consider employing additional and independent functional criteria, for example, monitoring Na+ dependence of the exchanger or using molecular tools for modulating the expression or activity of NCLX.
FIGURE 1.
Functional and molecular identification of the mitochondrial NCX. a, the first evidence for Na+- or Li+-dependent mitochondrial Ca2+ exchange. Original data were taken from Ref. 12. Li+ (▴), Na+ (○), or K+ (●) was added to isolated mitochondria while monitoring Ca2+ efflux. b and c, note that Na+ promoted the mitochondrial efflux of Ca2+, demonstrating a Na+/Ca2+ exchange pathway in this organelle. Unlike the plasma membrane NCX family members, the mitochondrial exchanger also mediated Li+/Ca2+ exchange, indicating that it has distinct functional properties. NCLX was identified as the mitochondrial exchanger. Studies were conducted on cells expressing the mitochondrial Ca2+ sensor mito-pericam. Knocking down the expression of mitochondrial NCLX using NCLX siRNA (si NCLX) abolished either Na+-dependent (b) or Li+-dependent (c) mitochondrial Ca2+ efflux. Thus, NCLX shares similar functional properties with the mitochondrial NCX. Original data were taken from Ref. 5. si control, control siRNA.
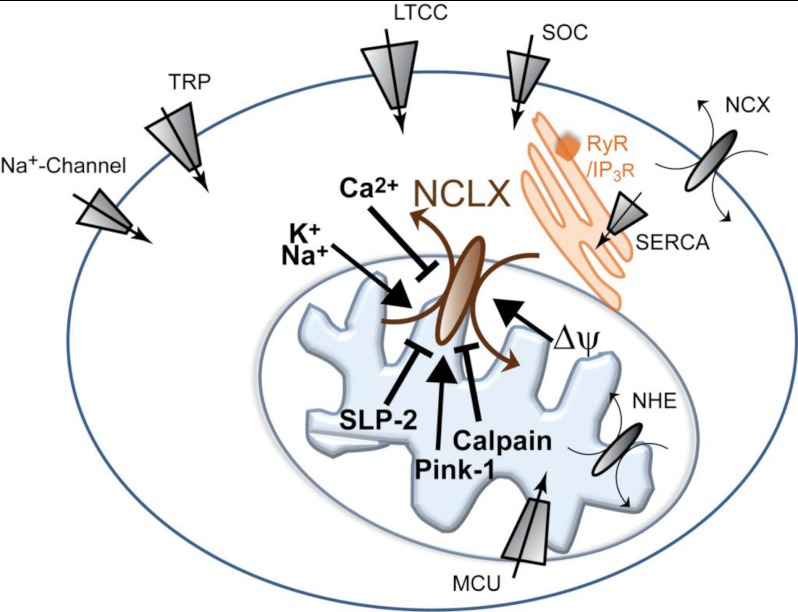
FIGURE 2.
Schematic representation of ions, transporters, and kinases that interact or modulate the activity of the mitochondrial NCX. Functional cross-talk between the ER and plasma membrane fluxes of Na+ and Ca2+ regulates the activity of the mitochondrial NCX, which in turn controls the Ca2+ content in the ER or plasma membrane microdomain and Ca2+ signaling in the cytosol. NHE, Na+/H+ exchanger; RyR, ryanodine receptor; IP3R, inositol trisphosphate receptor; SOC, store-operated calcium channel; LTCC, L-type Ca2+ channel; TRP, transient receptor potential channel.
Molecular Identity of the Mitochondrial NCX
In 1992, Garlid and co-workers (35) reported the successful purification of an ∼110-kDa mitochondrial polypeptide that, upon reconstitution into proteoliposomes, exhibited NCX and Li+/Na+ exchanger activity. Antibodies made against this protein blocked the reconstituted Na+/Ca2+ activity and identified the ∼110-kDa fragment along with an additional non-active ∼60-kDa fragment. However, although these promising findings were corroborated by subsequent work (18), they were not followed up at the molecular level, and 2 decades would pass before the exchanger gene was finally identified. The failure to identify the exchanger led to the hypothesis that plasma membrane NCX isoforms are targeted to and contribute to Ca2+ transport in the mitochondria. This hypothesis was supported by the presence of NCX1–3 isoforms in isolated mitochondrial fractions and in situ (36, 37). There were, however, several lines of evidence against this theory. The localization of NCX family members to the mitochondria was controversial (38), antibodies against the mitochondrial NCX did not react with polypeptides from cardiac sarcolemma vesicles (35), and enhanced plasma membrane NCX expression did not correlate with mitochondrial exchange activity (39). Perhaps most importantly, the cation selectivity and inhibitor sensitivity of the mitochondrial NCX were clearly distinct from those of all plasma membrane NCX family members (18).
The molecular identification of the mitochondrial NCX came about when we sought to identify a gene mediating Na+/Zn2+ exchange activity, monitored primarily on neuronal cell membranes (40). On the basis of this mechanism, we reasoned that it belongs to the NCX superfamily and cloned a putative member termed FLJ22233 (41). Phylogenetic analysis of this gene indicated that it did not belong to either one of the major mammalian Na+/Ca2+ subfamilies, NCX and the Na+/Ca2+/K+ exchanger (SLC8 and SLC24), and that it diverged earlier in evolution to form a distinct subfamily with a single mammalian member (42, 43). The FLJ22233 gene product mediated Na+/Ca2+ but not Na+/Zn2+ exchange. Unexpectedly and in contrast to other NCXs, this transporter mediated both Li+- and Na+-dependent Ca2+ exchange activity and was therefore named NCLX (5). The Li+/Ca2+ exchange of NCLX prompted us to ask whether it is the mediator of this activity in mitochondria. Immunoblot analysis and immunoelectron microscopy analyses in cultured cells and native tissue revealed NCLX localization in the inner membranes of mitochondria, and increased expression of NCLX enhanced mitochondrial Ca2+ efflux (5). Furthermore, NCLX-mediated mitochondrial Ca2+ efflux was fully blocked by CGP-37157, by NCLX siRNAs, or by a catalytic mutant that had strong dominant-negative effect on mitochondrial Na+/Ca2+ exchange. Finally, Ca2+ efflux mediated by mitochondrial NCLX was Li+- or Na+-dependent (Fig. 1). Altogether, the localization and functional analyses indicate that NCLX is the long-sought mitochondrial NCX (5). These findings gained independent support by a recent study showing that NCLX was localized to mitochondria in B lymphocytes and that mitochondrial Na+-dependent Ca2+ exchange in these cells was abolished by genetic knock-out or by siRNA-mediated knockdown of NCLX expression (44).
Regulation of the Mitochondrial Exchanger by Protein Interactions
Several studies suggested that interaction of the mitochondrial NCX with other proteins (18, 35) regulated its activity (Fig. 2) (45–49). At least two protein kinases (PKC and PINK1), a member of the stomatin family (SLP-2), and the anti-apoptotic protein Bcl-2 modulate the activity of the mitochondrial NCX. A report by Yang et al. (49) showed that tetanic-induced synaptic potentiation (TISP) is dependent on the activity of the mitochondrial NCX, which is in turn regulated by PKC. Surprisingly, although TISP is Ca2+-dependent, it occurs in the absence of extracellular Ca2+. To clarify the functional basis for this type of synaptic potentiation, Yang et al. showed that an increase in Na+ concentration at nerve terminals triggers Ca2+ release from mitochondria and that inhibition of the mitochondrial NCX or PKC activity abolishes TISP. Direct interaction between PKC and the mitochondrial exchanger appears plausible, as the mitochondrial localization of PKC, as well as its physical interaction with transport proteins on both the outer and inner mitochondrial membranes, has been reported (47). Gandhi et al. (46) showed that the loss of PINK1 function in neurons leads to profound inactivation of mitochondrial Na+/Ca2+ exchange, triggering mitochondrial Ca2+ overload and neuronal death in a model of Parkinson disease. Similarly, Zhu et al. (39) found that Na+/Ca2+ exchange activity is inhibited in isolated mitochondria from hearts of transgenic mice overexpressing Bcl-2. A similar inhibitory effect was recently found for the mitochondrial protein SLP-2 (48). A study by Da Cruz et al. (48) showed that enhancing SLP-2 expression causes inhibition, whereas depleting SLP-2 expression results in augmentation of mitochondrial Na+/Ca2+ exchange activity. Finally, the calcium-dependent μ-calpain mediates cleavage of the mitochondrial NCX in isolated mitochondria (45). Analyses of NCLX sequence have not show a clear PINK1 or Bcl-2 phosphorylation motif,4 suggesting that the interaction of these proteins with the exchanger may be indirect. The molecular identification of the exchanger as described below invites future studies to elucidate the molecular basis of these important regulatory mechanisms.
Stoichiometry of the Mitochondrial NCX
Whether the mitochondrial NCX action is electrogenic is a key issue, as it determines the transmitochondrial Ca2+ and Na+ gradients. Early studies indicated that the velocity of Ca2+ efflux is a function of [Na+]4 and that it is stimulated by energy-linked respiration (14, 26, 50), suggesting an electrogenic process in which three Na+ ions are exchanged for one Ca2+ per cycle. Subsequent studies further indicated that the thermodynamic requirements for the Ca2+ gradient established by the mitochondrial NCX require that it mediates electrogenic transport (51, 52). In contrast, several studies presented evidence against electrogenicity of the exchanger, as its activity does not produce a change in mitochondrial Δψ (53), additional evaluation of the Na+/Ca2+ kinetics suggested that the velocity of Ca2+ efflux is in fact a function of [Na+] (27, 54),4 and mitochondrial Na/Ca2+ exchange activity was not affected by an electroneutral H+/Ca2+ uncoupler (A23187), thereby favoring the electroneutral model (55). A recent analysis by Kim and Matsuoka (56) successfully measured changes in mitochondrial Δψ induced by mitochondrial Na+/Ca2+ exchange following blockage of the respiratory chain. They found that, under these conditions, activation of the mitochondrial exchanger resulted in hyperpolarization when the exchanger acted in reverse mode (Ca2+in/Na+out) and in depolarization when the exchanger was active in direct Ca2+ forward mode (Na+in/Ca2+out). These findings are consistent with a stoichiometry of at least three Na+ ions/one Ca2+ ion per transport cycle and agree with a recent theoretical model also favoring a similar exchange mode (56, 57). Interestingly, when expressed in HEK293 cells, a fraction of NCLX reaches the plasma membrane and allows direct measurement of a Ca2+-dependent outward current, which is consistent with a stoichiometry of three to four Na+ ions/one Ca2+ ion (41, 58). Taken together, a growing number of studies now support an electrogenic mode of three to four Na+ ions/one Ca2+ ion per transport cycle mediated by the mitochondrial exchanger, and electrophysiological analysis of its activity may provide another important tool for studying its function and regulation.
Cytosolic Na+ and Mitochondrial NCX Activity
The mitochondrial NCX is also the major pathway for Na+ influx into mitochondria. Mitochondrial Na+ is subsequently balanced by a Na+/H+ exchanger, an important mitochondrial transporter whose molecular identity remains to be elucidated. The Km of the mitochondrial exchanger for Na+ is ∼7–10 mm, remarkably tuned to the resting cytosolic Na+ concentration. This implies that even a small change in cytosolic Na+ will strongly modulate its activity. Such effects were indeed documented in several cell types and were linked to various physiological processes, including synaptic activity (49), energy balance (59, 60), and phagocytosis (61). Another interesting demonstration of Na+-induced mitochondrial Ca2+ release was recently reported in a study showing that, in smooth muscle cells, activation of TRPC6 following ER Ca2+ store depletion leads to a marked increase in cytosolic Na+, followed by subsequent release of mitochondrial Ca2+ (62). This Ca2+ release may contribute to salt-sensitive hypertension. Whether this phenomenon is extended to other cell types or other TRPC family members is an intriguing possibility with broad and important implications in cellular physiology. In cardiac myocytes, elevated cytosolic Na+ is followed by strong acceleration of mitochondrial Ca2+ efflux, which inhibits the mitochondrial metabolic rate and formation of reactive oxygen species (59, 63). In melanoma cells, a rise in cytosolic Na+ mediated by a splice variant of the Na+ channel Nav1.6 promotes mitochondrial calcium release, which accelerates the invasiveness of these tumor cells (60). Activation of mitochondrial Na+/Ca2+ exchange by an increase in intracellular Na+ is also a prime suspect in several cellular pathologies such as heart failure, cancer, and hypertension. Collectively, these studies highlight a role of cytosolic Na+ as a dynamic and important regulator of the mitochondrial NCX.
Role of the Mitochondrial NCX in the Regulation of ER Ca2+
Over recent years, many studies (reviewed in Refs. 64–67) have revealed a dynamic and structural association between mitochondria and ER compartments where specialized and restricted calcium shuttling occurs (Fig. 2). The release of Ca2+ through the mitochondrial NCX supports a major pathway by which mitochondria feed Ca2+ into the ER. Mitochondria act both to recycle Ca2+ leaving the ER, preventing the depletion of neighboring ER regions (68), and to facilitate the shuttling of Ca2+ from plasma membrane store-operated Ca2+ channels en route to the ER and cytosol (69–71). The physiological significance of the mitochondrial NCX in keeping Ca2+ shuttling between the ER and mitochondria was demonstrated by various studies showing, for example, that it is critical for maintaining and controlling the frequency of oscillatory Ca2+ waves (72–75) or for proper protein folding (76). Recently, this activity was addressed for the first time using both pharmacological and genetic manipulation tools, demonstrating that, in B lymphocytes, the mitochondrial Na+/Ca2+ exchanger NCLX maintains Ca2+ recycling between mitochondria and ER, thereby playing a pivotal role in B cell responses to antigen (56). Finally, in vivo measurements of mitochondrial Ca2+ recycling in mouse skeletal muscle during contraction also revealed an important role for the NCX in Ca2+ shuttling between the mitochondria and SR (77).
Mitochondrial Na+/Ca2+ Exchange in Brain and Secretory Systems
The dominant role of the exchanger is to counterbalance mitochondrial Ca2+ uptake following transient cytosolic elevations by subsequent release to maintain resting mitochondrial Ca2+ at the required physiological levels. The released mitochondrial Ca2+ plays a major role in modulating the cytosolic Ca2+ response, as it generates a “shoulder”-like extension that shapes the magnitude and duration of the cytosolic Ca2+ signal (78–83). The high density of mitochondria in synaptic regions and the importance of Ca2+ signaling in these sites prompted many studies to focus on the role of the mitochondrial exchanger in synaptic transmission. Indeed, in various neuronal preparations, synaptosomal neurotransmitter release was modulated by CGP-37157. These effects were reproduced by inducing changes in intracellular Na+, thus linking the mitochondrial exchanger to several forms of synaptic plasticity (49, 81, 84, 85) and arguing against a non-selective effect of CGP-37157. Similarly, other forms of secretion are also modulated by the activity of the mitochondrial exchanger such as exocytosis of gonadotropins from rat gonadotrophs (75) and secretion of glutamate by cortical astrocytes (86).
There is a large body of literature describing the connection between brain disorders and abnormalities in mitochondrial Ca2+ handling, and only few examples are briefly described here. Cytosolic and mitochondrial Ca2+ overloads are principal pathophysiological events during brain ischemia. The majority of studies suggest a neuroprotective role for blocking the mitochondrial exchanger activity (11). A harmful effect of mitochondrial Na+/Ca2+ exchange activity following ischemic-like conditions was shown in rat hippocampal slices, where a prolonged neuronal cytosolic Ca2+ rise triggered by toxic levels of NMDA was augmented by elevation of cytosolic Na+ and blocked by CGP-37157 (87). Similarly, in hypoxic slices, increased release of free fatty acids (a marker for tissue damage) was reduced by CGP-37157 (88). The increase in mitochondrial Ca2+ by CGP-37157 in immature neurons during NMDA insult also induces a neuroprotective release of mitochondrial NO (89). In contrast, however, Scanlon et al. (90) reported that application of CGP-37157 does not profoundly alter glutamate-mediated changes in mitochondrial function following toxic NMDA insults. Also, mitochondrial NCX activity is required for the neuroprotective effect of cannabinoids during neuronal Ca2+ overload (91). Thus, whether mitochondrial Na/Ca2+ exchange activity is harmful or neuroprotective during ischemia requires additional investigation. A molecular control for the exchanger expression or activity employing, for example, NCLX knock-out mice may help in clarifying some of the uncertainties related to the role of the mitochondrial exchanger in these syndromes.
Dysfunction of neuronal metabolism is a major factor in many forms of seizure activity. Seizure-like events are accompanied by frequent stimulation of sodium channels that are likely to contribute to intracellular Na+ loads (92) and have been shown to be translated into mitochondrial Ca2+ transients conveyed by the MCU and NCX (93). These findings indicate that, through modulation of mitochondrial Ca2+, epileptic activity results in changes in mitochondrial function that may contribute to subsequent neuronal injury. Impairments in Ca2+ homeostasis and mitochondrial function are also hallmark events in Parkinson (PD), Huntington (HD), and Alzheimer (AD) diseases. Although an elegant study showed that striatal neurons expressing the HD disease-related mutant huntingtin exhibit impaired mitochondrial Ca2+ shuttling following mitochondrial Ca2+ loads, the specific role of the mitochondrial exchanger remained unclear (94). Chin et al. (95) found a possible link between neuronal damage and mitochondrial Ca2+ shuttling in AD, as application of β-amyloid, the main component of senile plaques found in the brains of AD patients, to rat basal forebrain neurons triggered a cytosolic Ca2+ overload that could be partially rescued by CGP-37157. In PD, the genetic mutated markers Parkin and PINK1 are targeted to the mitochondria and regulate diverse aspects of mitochondrial function, including membrane potential and calcium homeostasis. PINK1-deficient neurons show excessive mitochondrial Ca2+ overload linked to profound inhibition of the mitochondrial NCX; however, how this kinase regulates the exchanger is still unresolved (46). Further studies are required to determine whether changes in the activity or expression pattern of the mitochondrial NCX are part of AD, PD, and HD etiology.
Mitochondrial Na+/Ca2+ Exchange in Heart
In cardiomyocytes, the ability of the mitochondria to modulate cytosolic Ca2+ transients is not fully resolved. An intriguing and controversial issue is whether the mitochondrial Ca2+ shuttling can manage to follow a beat-to-beat rate (reviewed in Refs. 96–98). Some of this controversy is probably related to differences in species and developmental stages of the cardiac preparations or the limited accessibility and specificity of the MCU and the NCX inhibitors in intact cardiomyocytes. In addition, ATP production during normal cardiac pacing does not seem to be strongly affected by mitochondrial Ca2+, consistent with multiple pathways involved in this process (99). Mitochondrial Ca2+ does seem to play a metabolic role during accelerated pacing or following ischemia. The latter syndrome may lead to a rise in cytosolic Na+ that triggers mitochondrial Ca2+ efflux, which in turn depletes mitochondrial Ca2+ content and causes oxidative phosphorylation inhibition and ATP shortage (59). Conversely, according to another study, hypoxia may lead to mitochondrial Ca2+ overload due to reversal of the mitochondrial exchanger (100). The molecular identification of both MCU and NCLX now provides potentially more selective genetic tools to assess the physiological and pathophysiological roles of mitochondrial Ca2+ shuttling in intact cardiac preparations.
Conclusions
Forty years of research led to the recognition of the major role played by the mitochondrial NCX in cellular biology. Numerous studies showed that, by releasing mitochondrial Ca2+, the mitochondrial NCX regulates the cells metabolic rate, shapes intracellular Ca2+ signaling, and contributes to cell death in normal or disease states. Other studies demonstrated that the mitochondrial NCX is regulated by the cellular ionic milieu or by various proteins. A principal limitation in understanding the physiological roles of this exchanger was that its molecular identity was unknown, and studies had to rely on inhibitors (primarily CGP-37157) that could also modulate the activity of other Ca2+ transporters. Identification of the mitochondrial NCX NCLX can facilitate molecular based biochemical and biophysical analysis aimed to identify its regulatory sites and partner proteins. NCLX knock-out or knockdown models may be used to study the role of the mitochondrial exchanger in vivo and under tight genetic control to allow a tissue-specific analysis of the exchanger's role in heart, brain, and endocrine pancreas and to determine whether a change in its expression pattern in animals and humans is linked to neuronal, cardiac, or endocrine disorders.
This article is part of the Thematic Minireview Series on Ins and Outs of Calcium Transport.
I. Sekler, personal communication.
- MCU
- mitochondrial Ca2+ uniporter
- NCX
- Na+/Ca2+ exchanger
- NCLX
- Na+/Ca2+/Li+ exchanger
- ER
- endoplasmic reticulum
- SR
- sarcoplasmic reticulum
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- TISP
- tetanic-induced synaptic potentiation
- PD
- Parkinson disease
- HD
- Huntington disease
- AD
- Alzheimer disease.
REFERENCES
- 1. Rapizzi E., Pinton P., Szabadkai G., Wieckowski M. R., Vandecasteele G., Baird G., Tuft R. A., Fogarty K. E., Rizzuto R. (2002) Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 159, 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirichok Y., Krapivinsky G., Clapham D. E. (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 [DOI] [PubMed] [Google Scholar]
- 3. Das A. M. (2003) Regulation of the mitochondrial ATP synthase in health and disease. Mol. Genet. Metab. 79, 71–82 [DOI] [PubMed] [Google Scholar]
- 4. Giacomello M., Drago I., Pizzo P., Pozzan T. (2007) Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 14, 1267–1274 [DOI] [PubMed] [Google Scholar]
- 5. Palty R., Silverman W. F., Hershfinkel M., Caporale T., Sensi S. L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., Khananshvili D., Sekler I. (2010) NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. U.S.A. 107, 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baughman J. M., Perocchi F., Girgis H. S., Plovanich M., Belcher-Timme C. A., Sancak Y., Bao X. R., Strittmatter L., Goldberger O., Bogorad R. L., Koteliansky V., Mootha V. K. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gunter T. E., Buntinas L., Sparagna G., Eliseev R., Gunter K. (2000) Mitochondrial calcium transport: mechanisms and functions. Cell Calcium 28, 285–296 [DOI] [PubMed] [Google Scholar]
- 9. Szabadkai G., Duchen M. R. (2008) Mitochondria: the hub of cellular Ca2+ signaling. Physiology 23, 84–94 [DOI] [PubMed] [Google Scholar]
- 10. Gunter T. E., Sheu S. S. (2009) Characteristics and possible functions of mitochondrial Ca2+ transport mechanisms. Biochim. Biophys. Acta 1787, 1291–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castaldo P., Cataldi M., Magi S., Lariccia V., Arcangeli S., Amoroso S. (2009) Role of the mitochondrial sodium/calcium exchanger in neuronal physiology and in the pathogenesis of neurological diseases. Prog. Neurobiol. 87, 58–79 [DOI] [PubMed] [Google Scholar]
- 12. Carafoli E., Tiozzo R., Lugli G., Crovetti F., Kratzing C. (1974) The release of calcium from heart mitochondria by sodium. J. Mol. Cell. Cardiol. 6, 361–371 [DOI] [PubMed] [Google Scholar]
- 13. Gavin C. E., Gunter K. K., Gunter T. E. (1990) Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem. J. 266, 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crompton M., Capano M., Carafoli E. (1976) Respiration-dependent efflux of magnesium ions from heart mitochondria. Biochem. J. 154, 735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayat L. H., Crompton M. (1985) Ca2+-dependent inhibition by trifluoperazine of the Na+/Ca2+ carrier in mitoplasts derived from heart mitochondria. FEBS Lett. 182, 281–286 [DOI] [PubMed] [Google Scholar]
- 16. Baysal K., Brierley G. P., Novgorodov S., Jung D. W. (1991) Regulation of the mitochondrial Na+/Ca2+ antiport by matrix pH. Arch. Biochem. Biophys. 291, 383–389 [DOI] [PubMed] [Google Scholar]
- 17. Crompton M., Heid I., Carafoli E. (1980) The activation by potassium of the sodium/calcium carrier of cardiac mitochondria. FEBS Lett. 115, 257–259 [DOI] [PubMed] [Google Scholar]
- 18. Paucek P., Jaburek M. (2004) Kinetics and ion specificity of Na+/Ca2+ exchange mediated by the reconstituted beef heart mitochondrial Na+/Ca2+ antiporter. Biochim. Biophys. Acta 1659, 83–91 [DOI] [PubMed] [Google Scholar]
- 19. Rottenberg H., Marbach M. (1992) The effect of alkanols on Ca2+ transport in brain mitochondria. Cell Calcium 13, 41–47 [DOI] [PubMed] [Google Scholar]
- 20. Wingrove D. E., Gunter T. E. (1986) Kinetics of mitochondrial calcium transport. I. Characteristics of the sodium-independent calcium efflux mechanism of liver mitochondria. J. Biol. Chem. 261, 15159–15165 [PubMed] [Google Scholar]
- 21. Wingrove D. E., Gunter T. E. (1986) Kinetics of mitochondrial calcium transport. II. A kinetic description of the sodium-dependent calcium efflux mechanism of liver mitochondria and inhibition by ruthenium red and by tetraphenylphosphonium. J. Biol. Chem. 261, 15166–15171 [PubMed] [Google Scholar]
- 22. Ligeti E., Bodnar J., Karoly E., Lindner E. (1981) Ni2+, a new inhibitor of mitochondrial calcium transport. Biochim. Biophys. Acta 656, 177–182 [DOI] [PubMed] [Google Scholar]
- 23. Lukács G. L., Fonyó A. (1986) The Ba2+ sensitivity of the Na+-induced Ca2+ efflux in heart mitochondria: the site of inhibitory action. Biochim. Biophys. Acta 858, 125–134 [DOI] [PubMed] [Google Scholar]
- 24. Matlib M. A., Lee S. W., Depover A., Schwartz A. (1983) A specific inhibitory action of certain benzothiazepines and benzodiazepines on the sodium/calcium exchange process of heart and brain mitochondria. Eur. J. Pharmacol. 89, 327–328 [DOI] [PubMed] [Google Scholar]
- 25. Chiesi M., Schwaller R., Eichenberger K. (1988) Structural dependence of the inhibitory action of benzodiazepines and related compounds on the mitochondrial Na+/Ca2+ exchanger. Biochem. Pharmacol. 37, 4399–4403 [DOI] [PubMed] [Google Scholar]
- 26. Wolkowicz P. E., Michael L. H., Lewis R. M., McMillin-Wood J. (1983) Sodium/calcium exchange in dog heart mitochondria: effects of ischemia and verapamil. Am. J. Physiol. 244, H644–H651 [DOI] [PubMed] [Google Scholar]
- 27. Jurkowitz M. S., Altschuld R. A., Brierley G. P., Cragoe E. J., Jr. (1983) Inhibition of Na+-dependent Ca2+ efflux from heart mitochondria by amiloride analogs. FEBS Lett. 162, 262–265 [DOI] [PubMed] [Google Scholar]
- 28. Wei A. C., Liu T., Cortassa S., Winslow R. L., O'Rourke B. (2011) Mitochondrial Ca2+ influx and efflux rates in guinea pig cardiac mitochondria: low and high affinity effects of cyclosporin A. Biochim. Biophys. Acta 1813, 1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cox D. A., Matlib M. A. (1993) A role for the mitochondrial Na+/Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J. Biol. Chem. 268, 938–947 [PubMed] [Google Scholar]
- 30. Cox D. A., Conforti L., Sperelakis N., Matlib M. A. (1993) Selectivity of inhibition of Na+/Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J. Cardiovasc. Pharmacol. 21, 595–599 [DOI] [PubMed] [Google Scholar]
- 31. Czyz A., Kiedrowski L. (2003) Inhibition of plasmalemmal Na+/Ca2+ exchange by mitochondrial Na+/Ca2+ exchange inhibitor 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP-37157) in cerebellar granule cells. Biochem. Pharmacol. 66, 2409–2411 [DOI] [PubMed] [Google Scholar]
- 32. Neumann J. T., Diaz-Sylvester P. L., Fleischer S., Copello J. A. (2011) CGP-37157 inhibits the sarcoplasmic reticulum Ca2+-ATPase and activates ryanodine receptor channels in striated muscle. Mol. Pharmacol. 79, 141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee B., Miles P. D., Vargas L., Luan P., Glasco S., Kushnareva Y., Kornbrust E. S., Grako K. A., Wollheim C. B., Maechler P., Olefsky J. M., Anderson C. M. (2003) Inhibition of mitochondrial Na+/Ca2+ exchanger increases mitochondrial metabolism and potentiates glucose-stimulated insulin secretion in rat pancreatic islets. Diabetes 52, 965–973 [DOI] [PubMed] [Google Scholar]
- 34. Luciani D. S., Ao P., Hu X., Warnock G. L., Johnson J. D. (2007) Voltage-gated Ca2+ influx and insulin secretion in human and mouse β-cells are impaired by the mitochondrial Na+/Ca2+ exchange inhibitor CGP-37157. Eur. J. Pharmacol. 576, 18–25 [DOI] [PubMed] [Google Scholar]
- 35. Li W., Shariat-Madar Z., Powers M., Sun X., Lane R. D., Garlid K. D. (1992) Reconstitution, identification, purification, and immunological characterization of the 110-kDa Na+/Ca2+ antiporter from beef heart mitochondria. J. Biol. Chem. 267, 17983–17989 [PubMed] [Google Scholar]
- 36. Gobbi P., Castaldo P., Minelli A., Salucci S., Magi S., Corcione E., Amoroso S. (2007) Mitochondrial localization of Na+/Ca2+ exchangers NCX1–3 in neurons and astrocytes of adult rat brain in situ. Pharmacol. Res. 56, 556–565 [DOI] [PubMed] [Google Scholar]
- 37. Minelli A., Castaldo P., Gobbi P., Salucci S., Magi S., Amoroso S. (2007) Cellular and subcellular localization of Na+/Ca2+ exchanger protein isoforms NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium 41, 221–234 [DOI] [PubMed] [Google Scholar]
- 38. Papa M., Canitano A., Boscia F., Castaldo P., Sellitti S., Porzig H., Taglialatela M., Annunziato L. (2003) Differential expression of the Na+/Ca2+ exchanger transcripts and proteins in rat brain regions. J. Comp. Neurol. 461, 31–48 [DOI] [PubMed] [Google Scholar]
- 39. Zhu L., Yu Y., Chua B. H., Ho Y. S., Kuo T. H. (2001) Regulation of sodium/calcium exchange and mitochondrial energetics by Bcl-2 in the heart of transgenic mice. J. Mol. Cell. Cardiol. 33, 2135–2144 [DOI] [PubMed] [Google Scholar]
- 40. Ohana E., Segal D., Palty R., Ton-That D., Moran A., Sensi S. L., Weiss J. H., Hershfinkel M., Sekler I. (2004) A sodium/zinc exchange mechanism is mediating extrusion of zinc in mammalian cells. J. Biol. Chem. 279, 4278–4284 [DOI] [PubMed] [Google Scholar]
- 41. Palty R., Ohana E., Hershfinkel M., Volokita M., Elgazar V., Beharier O., Silverman W. F., Argaman M., Sekler I. (2004) Lithium/calcium exchange is mediated by a distinct potassium-independent sodium/calcium exchanger. J. Biol. Chem. 279, 25234–25240 [DOI] [PubMed] [Google Scholar]
- 42. Cai X., Lytton J. (2004) The cation/Ca2+ exchanger superfamily: phylogenetic analysis and structural implications. Mol. Biol. Evol. 21, 1692–1703 [DOI] [PubMed] [Google Scholar]
- 43. Cai X., Lytton J. (2004) Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J. Biol. Chem. 279, 5867–5876 [DOI] [PubMed] [Google Scholar]
- 44. Kim B., Takeuchi A., Koga O., Hikida M., Matsuoka S. (2012) Pivotal role of mitochondrial Na+/Ca2+ exchange in antigen receptor-mediated Ca2+ signaling in DT40 and A20 B lymphocytes. J. Physiol. 590, 459–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kar P., Chakraborti T., Samanta K., Chakraborti S. (2009) μ-Calpain-mediated cleavage of the Na+/Ca2+ exchanger in isolated mitochondria under A23187-induced Ca2+ stimulation. Arch. Biochem. Biophys. 482, 66–76 [DOI] [PubMed] [Google Scholar]
- 46. Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E., Klupsch K., Downward J., Latchman D. S., Tabrizi S. J., Wood N. W., Duchen M. R., Abramov A. Y. (2009) PINK1-associated Parkinson disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell 33, 627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baines C. P., Song C. X., Zheng Y. T., Wang G. W., Zhang J., Wang O. L., Guo Y., Bolli R., Cardwell E. M., Ping P. (2003) Protein kinase Cϵ interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 92, 873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Da Cruz S., De Marchi U., Frieden M., Parone P. A., Martinou J. C., Demaurex N. (2010) SLP-2 negatively modulates mitochondrial sodium/calcium exchange. Cell Calcium 47, 11–18 [DOI] [PubMed] [Google Scholar]
- 49. Yang F., He X. P., Russell J., Lu B. (2003) Ca2+ influx-independent synaptic potentiation mediated by mitochondrial Na+/Ca2+ exchanger and protein kinase C. J. Cell Biol. 163, 511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Crompton M., Künzi M., Carafoli E. (1977) The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium/calcium carrier. Eur. J. Biochem. 79, 549–558 [DOI] [PubMed] [Google Scholar]
- 51. Baysal K., Jung D. W., Gunter K. K., Gunter T. E., Brierley G. P. (1994) Na+-dependent Ca2+ efflux mechanism of heart mitochondria is not a passive Ca2+/2Na+ exchanger. Am. J. Physiol. 266, C800–C808 [DOI] [PubMed] [Google Scholar]
- 52. Jung D. W., Baysal K., Brierley G. P. (1995) The sodium/calcium antiport of heart mitochondria is not electroneutral. J. Biol. Chem. 270, 672–678 [DOI] [PubMed] [Google Scholar]
- 53. Affolter H., Carafoli E. (1980) The Na+/Ca2+ antiporter of heart mitochondria operates electroneutrally. Biochem. Biophys. Res. Commun. 95, 193–196 [DOI] [PubMed] [Google Scholar]
- 54. Crompton M., Heid I. (1978) The cycling of calcium, sodium, and protons across the inner membrane of cardiac mitochondria. Eur. J. Biochem. 91, 599–608 [DOI] [PubMed] [Google Scholar]
- 55. Brand M. D. (1985) The stoichiometry of the exchange catalyzed by the mitochondrial sodium/calcium antiporter. Biochem. J. 229, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim B., Matsuoka S. (2008) Cytoplasmic Na+-dependent modulation of mitochondrial Ca2+ via electrogenic mitochondrial Na+/Ca2+ exchange. J. Physiol. 586, 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dash R. K., Beard D. A. (2008) Analysis of cardiac mitochondrial Na+/Ca2+ exchanger kinetics with a biophysical model of mitochondrial Ca2+ handling suggests a 3:1 stoichiometry. J. Physiol. 586, 3267–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Palty R., Hershfinkel M., Yagev O., Saar D., Barkalifa R., Khananshvili D., Peretz A., Grossman Y., Sekler I. (2006) Single α-domain constructs of the Na+/Ca2+ exchanger, NCLX, oligomerize to form a functional exchanger. Biochemistry 45, 11856–11866 [DOI] [PubMed] [Google Scholar]
- 59. Maack C., Cortassa S., Aon M. A., Ganesan A. N., Liu T., O'Rourke B. (2006) Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ. Res. 99, 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carrithers M. D., Chatterjee G., Carrithers L. M., Offoha R., Iheagwara U., Rahner C., Graham M., Waxman S. G. (2009) Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J. Biol. Chem. 284, 8114–8126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carrithers L. M., Hulseberg P., Sandor M., Carrithers M. D. (2011) The human macrophage sodium channel NaV1.5 regulates mycobacteria processing through organelle polarization and localized calcium oscillations. FEMS Immunol. Med. Microbiol. 63, 319–327 [DOI] [PubMed] [Google Scholar]
- 62. Poburko D., Liao C. H., Lemos V. S., Lin E., Maruyama Y., Cole W. C., van Breemen C. (2007) Transient receptor potential channel 6-mediated, localized cytosolic [Na+] transients drive Na+/Ca2+ exchanger-mediated Ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ. Res. 101, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 63. Kohlhaas M., Liu T., Knopp A., Zeller T., Ong M. F., Böhm M., O'Rourke B., Maack C. (2010) Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation 121, 1606–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Patergnani S., Suski J. M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Giorgi C., Marchi S., Missiroli S., Poletti F., Rimessi A., Duszynski J., Wieckowski M. R., Pinton P. (2011) Calcium signaling around mitochondria-associated membranes (MAMs). Cell Commun. Signal. 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Olson M. L., Chalmers S., McCarron J. G. (2012) Mitochondrial organization and Ca2+ uptake. Biochem. Soc. Trans. 40, 158–167 [DOI] [PubMed] [Google Scholar]
- 66. Rizzuto R., Pozzan T. (2006) Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369–408 [DOI] [PubMed] [Google Scholar]
- 67. Jeyaraju D. V., Cisbani G., Pellegrini L. (2009) Calcium regulation of mitochondrial motility and morphology. Biochim. Biophys. Acta 1787, 1363–1373 [DOI] [PubMed] [Google Scholar]
- 68. Arnaudeau S., Kelley W. L., Walsh J. V., Jr., Demaurex N. (2001) Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 276, 29430–29439 [DOI] [PubMed] [Google Scholar]
- 69. Malli R., Frieden M., Osibow K., Zoratti C., Mayer M., Demaurex N., Graier W. F. (2003) Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, sore-operated Ca2+ entry, and Ca2+ store refilling. J. Biol. Chem. 278, 44769–44779 [DOI] [PubMed] [Google Scholar]
- 70. Malli R., Frieden M., Trenker M., Graier W. F. (2005) The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J. Biol. Chem. 280, 12114–12122 [DOI] [PubMed] [Google Scholar]
- 71. Poburko D., Liao C. H., van Breemen C., Demaurex N. (2009) Mitochondrial regulation of sarcoplasmic reticulum Ca2+ content in vascular smooth muscle cells. Circ. Res. 104, 104–112 [DOI] [PubMed] [Google Scholar]
- 72. Hashiatni H., Lang R. J., Suzuki H. (2010) Role of perinuclear mitochondria in the spatiotemporal dynamics of spontaneous Ca2+ waves in interstitial cells of Cajal-like cells of the rabbit urethra. Br. J. Pharmacol. 161, 680–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim B. J., Jun J. Y., So I., Kim K. W. (2006) Involvement of mitochondrial Na+/Ca2+ exchange in intestinal pacemaking activity. World J. Gastroenterol. 12, 796–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hernández-SanMiguel E., Vay L., Santo-Domingo J., Lobatón C. D., Moreno A., Montero M., Alvarez J. (2006) The mitochondrial Na+/Ca2+ exchanger plays a key role in the control of cytosolic Ca2+ oscillations. Cell Calcium 40, 53–61 [DOI] [PubMed] [Google Scholar]
- 75. Kaftan E. J., Xu T., Abercrombie R. F., Hille B. (2000) Mitochondria shape hormonally induced cytoplasmic calcium oscillations and modulate exocytosis. J. Biol. Chem. 275, 25465–25470 [DOI] [PubMed] [Google Scholar]
- 76. Osibow K., Frank S., Malli R., Zechner R., Graier W. F. (2006) Mitochondria maintain maturation and secretion of lipoprotein lipase in the endoplasmic reticulum. Biochem. J. 396, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rudolf R., Mongillo M., Magalhães P. J., Pozzan T. (2004) In vivo monitoring of Ca2+ uptake into mitochondria of mouse skeletal muscle during contraction. J. Cell Biol. 166, 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. White R. J., Reynolds I. J. (1996) Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J. Neurosci. 16, 5688–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Simpson P. B., Russell J. T. (1998) Mitochondrial Ca2+ uptake and release influence metabotropic and ionotropic cytosolic Ca2+ responses in rat oligodendrocyte progenitors. J. Physiol. 508, 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hoyt K. R., Stout A. K., Cardman J. M., Reynolds I. J. (1998) The role of intracellular Na+ and mitochondria in buffering of kainate-induced intracellular free Ca2+ changes in rat forebrain neurons. J. Physiol. 509, 103–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tang Y., Zucker R. S. (1997) Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 18, 483–491 [DOI] [PubMed] [Google Scholar]
- 82. Brandenburger Y., Kennedy E. D., Python C. P., Rossier M. F., Vallotton M. B., Wollheim C. B., Capponi A. M. (1996) Possible role for mitochondrial calcium in angiotensin II- and potassium-stimulated steroidogenesis in bovine adrenal glomerulosa cells. Endocrinology 137, 5544–5551 [DOI] [PubMed] [Google Scholar]
- 83. Babcock D. F., Herrington J., Goodwin P. C., Park Y. B., Hille B. (1997) Mitochondrial participation in the intracellular Ca2+ network. J. Cell Biol. 136, 833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee D., Lee K. H., Ho W. K., Lee S. H. (2007) Target cell-specific involvement of presynaptic mitochondria in post-tetanic potentiation at hippocampal mossy fiber synapses. J. Neurosci. 27, 13603–13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. García-Chacón L. E., Nguyen K. T., David G., Barrett E. F. (2006) Extrusion of Ca2+ from mouse motor terminal mitochondria via a Na+/Ca2+ exchanger increases post-tetanic evoked release. J. Physiol. 574, 663–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Reyes R. C., Parpura V. (2008) Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J. Neurosci. 28, 9682–9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang Y., Lipton P. (1999) Cytosolic Ca2+ changes during in vitro ischemia in rat hippocampal slices: major roles for glutamate and Na+-dependent Ca2+ release from mitochondria. J. Neurosci. 19, 3307–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pilitsis J. G., Diaz F. G., O'Regan M. H., Phillis J. W. (2002) Inhibition of mitochondrial Na+/Ca2+ exchange by 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one attenuates free fatty acid efflux in rat cerebral cortex during ischemia-reperfusion injury. Neurosci. Lett. 321, 1–4 [DOI] [PubMed] [Google Scholar]
- 89. Marks J. D., Boriboun C., Wang J. (2005) Mitochondrial nitric oxide mediates decreased vulnerability of hippocampal neurons from immature animals to NMDA. J. Neurosci. 25, 6561–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Scanlon J. M., Brocard J. B., Stout A. K., Reynolds I. J. (2000) Pharmacological investigation of mitochondrial Ca2+ transport in central neurons: studies with CGP-37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger. Cell Calcium 28, 317–327 [DOI] [PubMed] [Google Scholar]
- 91. Ryan D., Drysdale A. J., Lafourcade C., Pertwee R. G., Platt B. (2009) Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J. Neurosci. 29, 2053–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fekete A., Franklin L., Ikemoto T., Rózsa B., Lendvai B., Sylvester Vizi E., Zelles T. (2009) Mechanism of the persistent sodium current activator veratridine-evoked calcium elevation: implication for epilepsy. J. Neurochem. 111, 745–756 [DOI] [PubMed] [Google Scholar]
- 93. Kovács R., Kardos J., Heinemann U., Kann O. (2005) Mitochondrial calcium ion and membrane potential transients follow the pattern of epileptiform discharges in hippocampal slice cultures. J. Neurosci. 25, 4260–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lim D., Fedrizzi L., Tartari M., Zuccato C., Cattaneo E., Brini M., Carafoli E. (2008) Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. J. Biol. Chem. 283, 5780–5789 [DOI] [PubMed] [Google Scholar]
- 95. Chin J. H., Tse F. W., Harris K., Jhamandas J. H. (2006) β-Amyloid enhances intracellular calcium rises mediated by repeated activation of intracellular calcium stores and nicotinic receptors in acutely dissociated rat basal forebrain neurons. Brain Cell Biol. 35, 173–186 [DOI] [PubMed] [Google Scholar]
- 96. Griffiths E. J., Rutter G. A. (2009) Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim. Biophys. Acta 1787, 1324–1333 [DOI] [PubMed] [Google Scholar]
- 97. Griffiths E. J., Balaska D., Cheng W. H. (2010) The ups and downs of mitochondrial calcium signaling in the heart. Biochim. Biophys. Acta 1797, 856–864 [DOI] [PubMed] [Google Scholar]
- 98. Dedkova E. N., Blatter L. A. (2008) Mitochondrial Ca2+ and the heart. Cell Calcium 44, 77–91 [DOI] [PubMed] [Google Scholar]
- 99. Balaban R. S. (2009) The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta 1787, 1334–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Griffiths E. J. (1999) Reversal of mitochondrial sodium/calcium exchange during metabolic inhibition in rat cardiomyocytes. FEBS Lett. 453, 400–404 [DOI] [PubMed] [Google Scholar]