Abstract
Intracellular free Ca2+ ions regulate many cellular functions, and in turn, the cell devotes many genes/proteins to keep tight control of the level of intracellular free Ca2+. Here, we review recent work on Ca2+-dependent mechanisms and effectors that regulate the transcription of genes encoding proteins involved in the maintenance of the homeostasis of Ca2+ in the cell.
Keywords: Calcineurin, Calcium, Calcium ATPase, Calcium Channels, Transcriptional Regulation, Transcription Repressor DREAM
Introduction
Control of intracellular free calcium is a delicate balance between mechanisms that provide the ion, including extracellular membrane calcium channels (voltage-, ligand-, and store-operated types) and intracellular calcium release channels (ryanodine (RyR)2 and inositol 1,4,5-trisphosphate (IP3R) receptors), and mechanisms that clear the ion from the cytosol, including calcium pumps and exchangers that are localized both in extra- and intracellular membranes (1, 2). Consolidated evidence, as well as recent evidence, indicates that this group of proteins, with the mission to keep tight control of free Ca2+ concentration, is in turn subjected to regulation by several mechanisms controlled by changes in free Ca2+ concentration. In some cases, these self-regulatory processes involve parallel compensatory changes in several Ca2+ regulatory proteins so that increases or decreases in intracellular stores and cytosolic Ca2+ levels slowly adjust the concentrations of key signaling pathway components (3). In other cases, components of the homeostasis machinery are coordinately modified to respond to chronic pathological conditions as in end stages of heart failure (4, 5) or in neurodegenerative processes. Thus, in Huntington disease striatal neurons accumulate changes in the expression of most, if not all, genes related to Ca2+ homeostasis (6). Also, in Alzheimer disease (AD), changes in the expression of RyRs and STIM (stromal interacting molecule) have been observed in the hippocampus of presymptomatic AD mouse models (7) and post-mortem human brain samples (8) and in B lymphocytes from patients with familial AD mutations in the presenilin-1 gene (9), respectively. Of the different control mechanisms, here we will review those that control Ca2+ homeostasis at the transcriptional level and that are directly regulated by Ca2+. A review of the transcriptional control of calcium homeostasis, with particular emphasis on the roles of members of the Egr (early growth response) family of zinc finger immediate-early transcription factors and the closely related protein WT1 (Wilms tumor suppressor 1), has been published recently (10).
Control of the activity of specific transcriptional networks by Ca2+ is regulated by cytosolic and nuclear mechanisms that decode the calcium signal specificity in terms of frequency and spatial properties. Thus, the Ca2+ entry site (synaptic versus extrasynaptic) or its intracellular source (mitochondria, endoplasmic reticulum (ER), or Golgi apparatus) makes the Ca2+ ions face different microdomains that are composed of specific sets of proteins and determines the biological outcome of the Ca2+ signal by inducing temporal and spatial changes in specific nuclear interactomes. For recent reviews on the formation of Ca2+ microdomains by differential assembly of key Ca2+ signaling proteins within domains, see Refs. 11 and 12.
Work over the last decade has helped to outline three broad mechanisms downstream from the detection of changes in intracellular free Ca2+ concentration by Ca2+ sensors. The first mechanism involves the activation of signaling cascades led by Ca2+-dependent kinases and phosphatases that either modify the trans-activating activity or nuclear localization of transcription factors or modulate cofactors that will change nucleosomal properties, thus changing the accessibility of the transcription initiation complex to specific genes. The second mechanism is based on Ca2+-dependent protein-protein interactions between the calcium sensor and transcription factors or, generally, proteins involved in transcription as parts of the enhanceosome. As a result of this interaction, binding to DNA or recruitment of certain cofactors is modified, and transcription is adjusted. Finally, the third mechanism involves a change in the properties of binding of the calcium sensor to specific sites in the DNA as a result of binding to Ca2+.
Regulation of Voltage-gated Calcium Channels
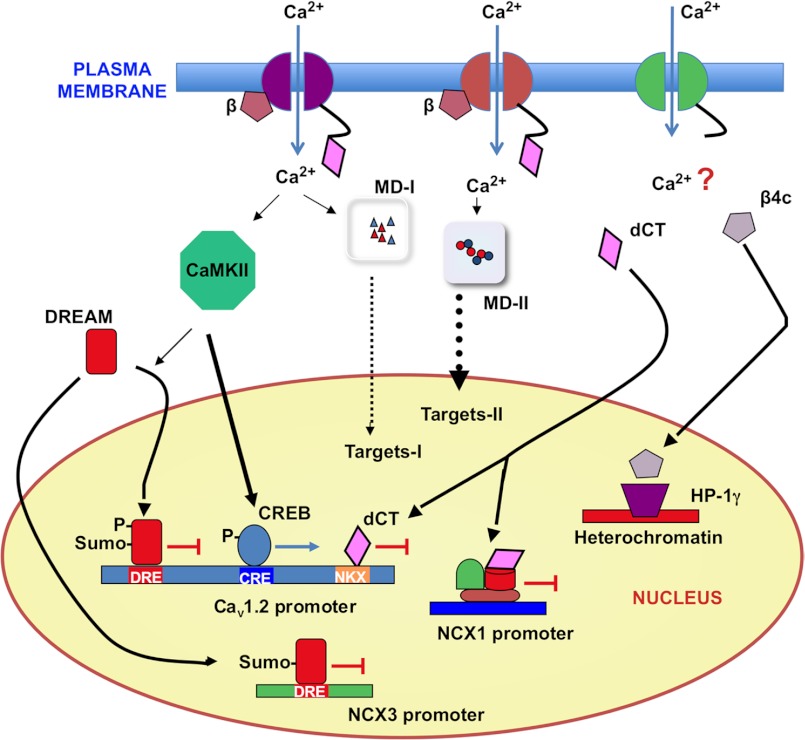
Voltage-gated calcium channels (VGCCs) are multiheteromeric membrane entities composed of a main pore-forming α subunit and several auxiliary subunits, including β, α2δ, and γ forms. In neurons and cardiac myocytes, VGCCs are the main source of extracellular calcium, coupling changes in membrane potential to muscle contraction and changes in gene expression, including their own expression as first shown in cardiac myocytes (13–15). Several mechanisms have been proposed to explain excitation-transcription coupling: (i) the classic signaling through calmodulin/CaMK/CREB phosphorylation, (ii) signaling by VGCC-mediated Ca2+ entry secondary to differential microdomain assembly due to heterogeneous distribution of VGCCs in the membrane, (iii) signaling by nuclear translocation of fragments or subunits of the VGCCs, and (iv) signaling by nuclear translocation of DREAM (downstream responsive element antagonist modulator). Because the two first mechanisms have been extensively reviewed (16–19), here, we focus on recent evidence supporting auto-transcriptional control by the distal C-terminal (dCT) fragment of the L-type channel and by the transcriptional repressor DREAM, as well as the new unexpected nuclear function of specific β subunits. These different mechanisms are schematically shown in Fig. 1.
FIGURE 1.
L-type Ca2+ channels and Ca2+-dependent transcriptional regulation. L-type channels regulate their own expression through different Ca2+-dependent transcriptional mechanisms. Differential clustering of L-type channels at the membrane or different subunit composition determines their involvement in distinct microdomains (MD-I or MD-II), different calcium signaling, and final effect on specific sets of target genes. Phospho-CREB-dependent activation through cAMP-responsive element (CRE) sites and repression mediated by sumoylated DREAM through DRE sites and/or the dCT fragment through NKX2.5/MEF/C/EBP and CRM1 sites are also indicated. Additional transcriptional effects of the dCT fragment and the β4c channel subunit through the interaction with several nucleoproteins are shown.
The dCT fragment corresponds to the fragment spanning from the consensus calpain cleavage site to the C-terminal end of the Cav1.2 channel protein (α subunit). Like other α subunit C-terminal fragments, the dCT fragment was originally associated with the regulation of channel gating (20, 21). The first clue of a nuclear role for the dCT fragment came with the observation of its nuclear presence in neurons (22) and, more recently, in cardiomyocytes (21). In neurons, the nuclear presence of the dCT fragment, also known as the calcium channel-associated transcriptional regulator CCAT, correlated with up-regulation of connexin-31.1 and down-regulation of NCX1 (Na+/Ca2+ exchanger 1) (22), another important regulator of calcium homeostasis. In cardiomyocytes, chromatin immunoprecipitation assays established the interaction between the dCT fragment and an area of the Cav1.2 promoter containing binding sites for NKX2.5/MEF, C/EBP, and CRM1, suggesting an autoregulatory loop in which the dCT fragment is part of the sensing mechanism as well as part of the executing downstream mechanism repressing Cav1.2 expression (23). Regulated dCT nuclear localization has been shown in neurons in response to high extracellular K+ and in cardiomyocytes after serum exposure (22, 23). However, to complete a self-regulatory signal transduction loop, there is missing evidence for either a Ca2+ or calpain requirement for dCT fragment release. Phosphorylation of the dCT fragment by PKA and PKC and dCT fragment interactions with calcineurin and A-kinase anchoring protein are processes that need to be further explored to fully understand the function of the dCT fragment (24).
Acute changes in VGCC expression occur following channel opening, CaMKII activation, and CREB phosphorylation. Interestingly, it has been recently shown that L-type Ca2+ channel current density in ventricular myocytes from CaMKIIδ knock-out mice is increased due to increased expression of the pore-forming Cav1.2 subunit (25), whereas overexpression of either cytosolic (δC) or nuclear (δB) CaMKII isoforms selectively down-regulates the expression of Cav1.2 (26). The effect of CaMKII is related to increased Ca2+-dependent nuclear translocation of the transcriptional repressor DREAM and its binding to a downstream responsive element (DRE) site at position −511 in the Cav1.2 promoter, repressing its transcription (26). It was proposes that L-type Ca2+ channel down-regulation through the Ca2+/CaMKII/DREAM cascade constitutes a physiological feedback mechanism enabling cardiomyocytes to adjust the calcium intrusion through L-type channels to the amount of intracellular calcium detected by CaMKII. However, this study did not specify where CaMKII phosphorylates DREAM and did not analyze which other post-translational modifications in the DREAM protein may be also present in cardiomyocytes. In this regard, we have recently shown that sumoylation regulates the nuclear localization of DREAM in differentiated neurons (27). Single Lys-to-Arg mutations at Lys-26 and Lys-90 reduce DREAM nuclear localization and transcriptional activity, although sumoylation mutants retain the ability to bind to the DRE sequence in vitro (27). Interestingly, it has been reported that the sumoylation process can be enhanced by phosphorylation of specific residues near the sumoylation site (28). No CaMKII consensus phosphorylation sites are located near the two known sumoylation sites in DREAM; however, sumoylation at additional sites cannot be discarded. Nevertheless, through CaMKII-induced increases in sumoylation and nuclear translocation of DREAM and/or through yet unknown mechanisms, our experiments using transgenic mice overexpressing a Ca2+-insensitive dominant active DREAM mutant (daDREAM) have shown a significant reduction of Cav1.2 mRNA levels in the heart as well as in the cerebral cortex (Fig. 2), thus supporting the idea of DREAM regulation of L-type channel expression.
FIGURE 2.
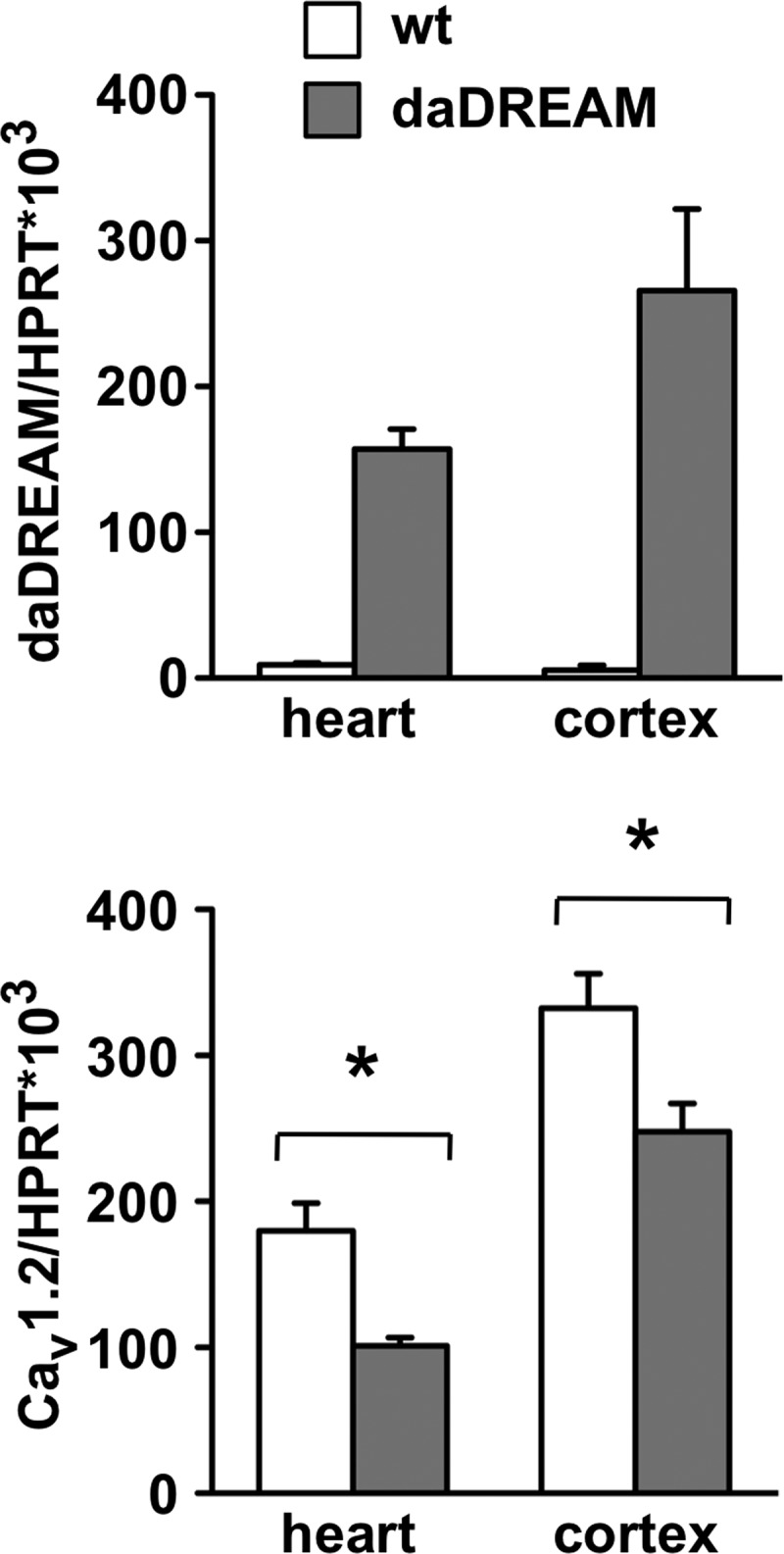
Expression of the L-type channel α subunit is reduced in the heart and cerebral cortex from mice expressing daDREAM. Shown are the results from comparative real-time quantitative PCR analysis of Cav1.2 mRNA levels in wild-type and DREAM-overexpressing transgenic mice. Normalization was done with respect to hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression. *, p < 0.05 (Student's t test, n = 6–7).
Importantly, although not related to transcriptional regulation, two recent reports further implicated DREAM and related KChIPs in the regulation of L-type and also T-type channels at the level of channel gating. One report demonstrated that DREAM forms a signaling complex with Kv4 and voltage-dependent T-type calcium (Cav3) channels in cerebellar stellate cells (29). Electrophysiological measurements propose that T-type channels efficiently couple calcium influx with DREAM/KChIP3 to modulate Kv4 function, establishing DREAM as a physiological calcium sensor for the Kv4 channel in cerebellar stellate neurons. A second work using KChIP2−/− cardiac myocytes has shown that KChIP2, a closely related member of the DREAM/KChIP family of proteins, directly interacts with voltage-dependent L-type calcium channels (Cav1.2), augmenting their current amplitude (30). Whether other KChIPs can also interact with Ca2+ channels and the physiological significance in different tissues remain to be investigated. These reports collectively point to DREAM and other KChIPs as key modulators of membrane conductance through the regulation of several cationic channels at multiple levels.
VGCC β subunits are best known for their roles in regulating surface expression and gating of voltage-gated Ca2+ channel α1 subunits. However, yeast two-hybrid and biochemical assays revealed that the β4c subunit interacts directly with the chromoshadow domain of chromobox protein HP1γ (heterochromatin protein 1γ), a nuclear protein involved in long-term gene silencing (31). As a result of the interaction, the β4c subunit blocks HP1γ and turns on a set of genes (not yet fully characterized) that might include targets related to calcium homeostasis. The effect is specific for the truncated short splice variant β4c subunit that is distinctly expressed in the vestibular and deep cerebellar nuclei. Interestingly, β4a or β4b isoforms widely expressed in the brain do not show this interaction. Site-directed mutagenesis revealed that the primary chromoshadow domain interaction occurs through a β4c C-terminal PXVXL consensus motif, adding the β4c channel subunit to a growing PXVXL protein family with epigenetic responsibilities, including transcriptional regulation (TIF1α) and nucleosome assembly (CAF1) (32). The β4c subunit is therefore a multifunctional protein that is part of the Ca2+ channel and also regulates gene transcription. Further studies should clarify the Ca2+ dependence of this process and its importance in Ca2+ homeostasis.
Regulation of Receptors for IP3 and Ryanodine
IP3R1–3 and RyR1–3 are specialized intracellular Ca2+ release channels located at the sarco/endoplasmic reticulum membrane that respond to IP3 or to Ca2+ and the pyridine nucleotide cyclic ADP-ribose, respectively, releasing calcium from the ER to the cytosol. In a seminal study performed in cerebellar granules, Carafoli and co-workers (33) found that expression of IP3R1 was induced after membrane depolarization with potassium, whereas RyR2 levels remained unchanged. The induction of IP3R1 was absolutely dependent on Ca2+ influx through L-type VGCCs and was abolished by treatment with inhibitors of the Ca2+/calmodulin-dependent protein phosphatase calcineurin, FK506 and cyclosporin A (33). An effect of calcineurin on IP3R1 transcription has been observed also in skeletal muscle (34), although the mechanism is still unresolved because no NFAT-binding sites have been identified in the IP3R1 promoter (35), suggesting that the IP3R1 gene might be an indirect target of activity-dependent gene transcription through the calcineurin/NFAT pathway.
More recently, expression of RyR2 in the hippocampus has been shown to be dependent on the LMO4 (LIM domain only 4) transcription cofactor because it is greatly reduced in mice carrying a forebrain-specific deletion of LMO4 (36). The LMO4 protein was identified as a calcium-responsive transactivator that activates gene expression in an activity-dependent manner (37), indicating that, in the hippocampus, RyR2 expression is Ca2+-dependent and suggesting that LMO4 regulates calcium-induced calcium release in hippocampal neurons (36). In addition, it has been shown that, in response to extracellular stimuli, LMO4 translocates from the cytoplasm to the nucleus (38), where it serves as a cofactor of many transcription factors (37, 39, 40), and also interacts with transmembrane receptors to modulate their signaling (41, 42). Whether LMO4 couples signals from membrane receptors to changes in the expression of RyRs or additional genes related to Ca2+ homeostasis is presently not known. However, it has been recently shown that increased Ca2+ levels after dopamine D1 receptor activation result in a significant increase in RyR1 and RyR2 mRNA and protein levels in midbrain and cerebral cortical neurons in primary culture (43, 44), suggesting an effect at the transcriptional level. Interestingly, a parallel increase in the α2δ subunit of the VGCC was observed in these cultures after dopamine D1 receptor stimulation (45). Increased expression of RyR2 in the midbrain and cortex has also been described after nicotine administration (46). Nicotine-induced RyR2 up-regulation was mediated by CREB phosphorylation and caused a long-lasting reinforcement of Ca2+ signaling via the process of Ca2+-induced Ca2+ release. Because RyR2 up-regulation was itself required for long-term phosphorylation of CREB, this mechanism sets a positive feedback signaling loop with perhaps functionally important implications for the process of addiction to nicotine (46). Supporting this idea, inhibition of RyR activation in vivo abolishes sensitization to nicotine-induced habituated locomotion, a well characterized behavioral index for onset of drug dependence (46).
Regulation of Sodium/Calcium Exchangers and Calcium Pumps
NCX1–3 are the best studied members of the Ca2+/cation antiporter superfamily of proteins (reviewed in Ref. 47). NCX1–3 are integral plasma membrane proteins that mediate Ca2+ and Na+ fluxes across the neuronal membrane, depending on the intracellular concentration of Ca2+ and Na+. Thus, in the “forward mode” NCX will extrude Ca2+ and enter Na+, whereas in the “reverse mode,” NCX will mediate the extrusion of Na+ and the entrance of Ca2+ ions (48, 49). NCX1, the most widely expressed member, is developmentally regulated in the heart, where its expression coincides with the switch of cardiomyocyte Ca2+ handling from the plasma membrane to the sarcoplasmic reticulum (50) and is increased during contractile dysfunction of the heart. Analysis of the NCX1 promoter did not provide clues of a direct Ca2+-dependent regulation of NCX1 (51), although recent work (52) in neonatal rat cardiac myocytes has shown that a rise in cytosolic Ca2+ by exposure to very low concentration of thapsigargin activates the calcineurin/NFAT pathway. As a result, transcription/expression of NCX1 is increased, but also SERCA2 (sarco/endoplasmic reticulum Ca2+-ATPase 2) and phospholamban are induced, providing a thorough homeostatic mechanism for long-term control of cytosolic Ca2+ by Ca2+ ions. Expression of NCX2 and NCX3 is differentially regulated by potassium depolarization in cultured granular neurons via a Ca2+/calcineurin-dependent mechanism that repress NCX2 and activates NCX3 expression (53). In addition, Ca2+-dependent induction of NCX3 is controlled by the Ca2+-dependent unbinding of the transcriptional repressor DREAM from the NCX3 promoter as shown in the hippocampus and the cerebellum in vivo and in cultured cerebellar neurons from daDREAM transgenic mice (54). No effect on NCX1 or NCX2 expression was observed in DREAM-overexpressing mice, suggesting a specific regulation of NCX3 (54).
The plasma membrane Ca2+-ATPases PMCA1–4, together with SERCA1–3 and the secretory pathway Ca2+-ATPases SPCA1 and SPCA2, represent the major transport systems to extrude free calcium from the cytosol, counteracting transient increases that occur during Ca2+ signaling. Early work using cerebellar neurons in culture showed that expression of the four PMCA genes is dependent on calcium, although in a different manner (55–57). Although PMCA4 expression is rapidly down-regulated after Ca2+ entry through NMDA receptors or through VGCCs following exposure to NMDA or to high extracellular potassium, the expression levels of PMCA1–3 are slowly up-regulated, reaching a plateau 48 h after exposure to the same depolarizing agents (56). Furthermore, inhibition of calcineurin blocks the down-regulation of PMCA4 and does not modify the increase in the other three pumps (57). Opposite regulation of PMCA2 and PMCA4 was also reported in sensory neurons from dorsal root ganglia after calcium entry following bradykinin or ATP bath application (58), although this study refers to pump activity, and the levels of the different mRNAs or proteins were not directly assessed. In a more recent study, it was shown that expression of PMCA2 in CA3 pyramidal neurons is reduced following excitatory synapse inactivity induced by AP5/6-cyano-7-nitroquinoxaline-2,3-dione exposure. The effect was specific for PMCA2, with no change in the levels of other pumps, and was rapidly reversed by calcium influx through NMDA receptors (59). Finally, PMCA1 and PMCA4 protein levels are increased in T cells upon T cell receptor activation in parallel with an up-regulation of STIM1 and STIM2 expression (60). The molecular mechanisms directing Ca2+-dependent PMCA induction in these different systems are presently unknown. Induction of the ER calcium sensors STIM1 and STIM2 is mediated by Erg1-binding sites present in their promoters and follows calcium entry and CREB phosphorylation (61). Because STIM proteins and the Ca2+-selective Orai1 channel form the molecular substrate for store-operated calcium entry, their induction upon T cell activation represents another example of Ca2+-dependent transcriptional regulation of calcium homeostasis.
Alternative splicing of the three genes encoding sarco/endoplasmic reticulum Ca2+-ATPases generates up to 10 different isoforms, of which SERCA2b is ubiquitously expressed at varying levels in all cell types (62). Expression of the SERCA2 gene has been widely studied and shown to be regulated by several stimuli in different cell types. Calcium up-regulates SERCA2 expression in cardiac myocytes through the calcineurin/NFAT pathway (52, 63), an effect that is blocked by induction of glycogen synthase kinase-3, a negative regulator of NFAT nuclear translocation (64). Furthermore, Ca2+ oscillations regulate SERCA2 expression in astrocytes after growth factor receptor stimulation (65) and in human lens epithelial cells (66), although there is no evidence of NFAT binding to the SERCA2 promoter. In addition, MAPK- and PKC-dependent induction of Erg1 results in down-regulation of the SERCA2 gene, although, again, the effect is probably indirect because no functional Erg1-binding sites are found in the SERCA2 promoter (10, 67, 68).
Other Ca2+-generated Transcriptional Effectors
The “conventional” PKCα isoform is regulated by diacylglycerol, which binds the C1 domain, and by Ca2+, which binds the C2 domain. Recently, it has been shown that calpain-mediated proteolytic processing of PKCα in ischemic myocardium generates a persistent and constitutively active free catalytic fragment, PKCα-CT (69), which constitutively localizes in the nucleus and is a potent inducer of nucleocytoplasmic shuttling of HDAC5 following phosphorylation. As a result, MEF-dependent inflammatory pathway genes are de-repressed, inducing a cell-autonomous transcriptional inflammatory response as shown by genome-wide analysis and deep RNA sequencing (70). As pointed out, because calpain-mediated processing of PKC isoforms could occur in many tissues wherein calcium is increased by stress or injury, the identification of PKCα-CT as a constitutively active transcriptional regulator might have broad ramifications for understanding and preventing the pathological transcriptional stress response. Furthermore, in a closely related study, Olson and co-workers identified a novel mechanism (71) that could prevent MEF-dependent cardiac hypertrophy after CaMKIIδ-induced nucleocytoplasmic shuttling of HDAC4 (72, 73). The mechanism is triggered by β-adrenergic receptor stimulation and regulates cardiac transcription through regulated proteolysis of HDAC4 (71). Interestingly, the N-terminal HDAC4 cleavage product, HDAC4-NT, selectively inhibits the activity of MEF2 but not the serum response factor, thereby antagonizing the prohypertrophic actions of CaMKII signaling without affecting cardiomyocyte survival. Thus, HDAC4 functions as a molecular nexus for the antagonistic actions of the CaMKII and PKA pathways (71).
Concluding Remarks
Calcium ions control Ca2+ homeostasis through several overlapping mechanisms, acting at different levels and often in opposite directions. As a result, the cell reaches a balance to adjust intracellular free and intraorganellar calcium concentrations under physiological and pathological conditions. The evidence reviewed here clearly supports the idea of a self-regulatory loop by which Ca2+ ions regulate Ca2+ homeostasis. The picture is far from complete, and future studies should explore new mechanisms, such as the potential Ca2+-dependent translational control of Ca2+ homeostasis by microRNAs, an emerging subfield with important physiological implications as shown by their role in L-type calcium channel regulation during neuropathic pain (74) and in NCX1 regulation in ischemic heart injury (75).
Acknowledgment
We thank Dr. Brini for suggestions.
This work was supported by Dirección General de Investigación Científica y Técnica (DGICYT) Grants SAF2008-03469 and SAF2010-21784, CIBERNED, Fundación Reina Sofía Grant PI 006/09, and European Union 6th Framework Program (NeuroNE) Grant LSHG-CT-2006-037627. This article is part of the Thematic Minireview Series on Calcium Function and Disease.
- RyR
- ryanodine receptor
- IP3R
- inositol 1,4,5-trisphosphate receptor
- AD
- Alzheimer disease
- ER
- endoplasmic reticulum
- VGCC
- voltage-gated calcium channel
- CaMK
- Ca2+/calmodulin-dependent protein kinase
- CREB
- cAMP-responsive element-binding protein
- dCT
- distal C-terminal
- MEF
- myocyte enhancer factor
- C/EBP
- CCAAT/enhancer-binding protein
- DRE
- downstream responsive element
- daDREAM
- dominant active DREAM
- KChIP
- Kv channel-interacting protein.
REFERENCES
- 1. Berridge M. J., Lipp P., Bootman M. D. (2000) The versatility and universality of calcium signaling. Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 2. Carafoli E. (2002) Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. U.S.A. 99, 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abell E., Ahrends R., Bandara S., Park B. O., Teruel M. N. (2011) Parallel adaptive feedback enhances reliability of the Ca2+ signaling system. Proc. Natl. Acad. Sci. U.S.A. 108, 14485–14490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minamino T., Kitakaze M. (2010) ER stress in cardiovascular disease. J. Mol. Cell. Cardiol. 48, 1105–1110 [DOI] [PubMed] [Google Scholar]
- 5. Cartwright E. J., Oceandy D., Austin C., Neyses L. (2011) Ca2+ signaling in cardiovascular disease: the role of the plasma membrane calcium pumps. Sci. China Life Sci. 54, 691–698 [DOI] [PubMed] [Google Scholar]
- 6. Hodges A., Strand A. D., Aragaki A. K., Kuhn A., Sengstag T., Hughes G., Elliston L. A., Hartog C., Goldstein D. R., Thu D., Hollingsworth Z. R., Collin F., Synek B., Holmans P. A., Young A. B., Wexler N. S., Delorenzi M., Kooperberg C., Augood S. J., Faull R. L., Olson J. M., Jones L., Luthi-Carter R. (2006) Regional and cellular gene expression changes in human Huntington disease brain. Hum. Mol. Genet. 15, 965–977 [DOI] [PubMed] [Google Scholar]
- 7. Chakroborty S., Goussakov I., Miller M. B., Stutzmann G. E. (2009) Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3×Tg-AD mice. J. Neurosci. 29, 9458–9470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruno A. M., Huang J. Y., Bennett D. A., Marr R. A., Hastings M. L., Stutzmann G. E. (2012) Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer disease. Neurobiol. Aging 33, 1001.e1–1001.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bojarski L., Pomorski P., Szybinska A., Drab M., Skibinska-Kijek A., Gruszczynska-Biegala J., Kuznicki J. (2009) Presenilin-dependent expression of STIM proteins and dysregulation of capacitative Ca2+ entry in familial Alzheimer disease. Biochim. Biophys. Acta 1793, 1050–1057 [DOI] [PubMed] [Google Scholar]
- 10. Ritchie M. F., Zhou Y., Soboloff J. (2011) Transcriptional mechanisms regulating Ca2+ homeostasis. Cell Calcium 49, 314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holton M. L., Wang W., Emerson M., Neyses L., Armesilla A. L. (2010) Plasma membrane calcium ATPase proteins as novel regulators of signal transduction pathways. World J. Biol. Chem. 1, 201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ong H. L., Ambudkar I. S. (2011) The dynamic complexity of the TRPC1 channelosome. Channels 5, 424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davidoff A. J., Maki T. M., Ellingsen O., Marsh J. D. (1997) Expression of calcium channels in adult cardiac myocytes is regulated by calcium. J. Mol. Cell. Cardiol. 29, 1791–1803 [DOI] [PubMed] [Google Scholar]
- 14. Crump S. M., Correll R. N., Schroder E. A., Lester W. C., Finlin B. S., Andres D. A., Satin J. (2006) L-type calcium channel α subunit and protein kinase inhibitors modulate Rem-mediated regulation of current. Am. J. Physiol. Heart Circ. Physiol. 291, H1959–H1971 [DOI] [PubMed] [Google Scholar]
- 15. Schroder E., Magyar J., Burgess D., Andres D., Satin J. (2007) Chronic verapamil treatment remodels ICa,L in mouse ventricle. Am. J. Physiol. Heart Circ. Physiol. 292, H1906–H1916 [DOI] [PubMed] [Google Scholar]
- 16. Greer P. L., Greenberg M. E. (2008) From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 59, 846–860 [DOI] [PubMed] [Google Scholar]
- 17. Mellström B., Savignac M., Gomez-Villafuertes R., Naranjo J. R. (2008) Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol. Rev. 88, 421–449 [DOI] [PubMed] [Google Scholar]
- 18. Cohen S., Greenberg M. E. (2008) Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu. Rev. Cell Dev. Biol. 24, 183–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosen M. R., Cohen I. S. (2006) Cardiac memory: new insights into molecular mechanisms. J. Physiol. 570, 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hulme J. T., Konoki K., Lin T. W., Gritsenko M. A., Camp D. G., 2nd, Bigelow D. J., Catterall W. A. (2005) Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of Cav1.1 channels in skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 102, 5274–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Satin J., Schroder E. A., Crump S. M. (2011) L-type calcium channel autoregulation of transcription. Cell Calcium 49, 306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomez-Ospina N., Tsuruta F., Barreto-Chang O., Hu L., Dolmetsch R. (2006) The C terminus of the L-type voltage-gated calcium channel Cav1.2 encodes a transcription factor. Cell 127, 591–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schroder E., Byse M., Satin J. (2009) L-type calcium channel C terminus autoregulates transcription. Circ. Res. 104, 1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fuller M. D., Emrick M. A., Sadilek M., Scheuer T., Catterall W. A. (2010) Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci. Signal. 3, ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu L., Lai D., Cheng J., Lim H. J., Keskanokwong T., Backs J., Olson E. N., Wang Y. (2010) Alterations of L-type calcium current and cardiac function in CaMKIIδ knock-out mice. Circ. Res. 107, 398–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ronkainen J. J., Hänninen S. L., Korhonen T., Koivumäki J. T., Skoumal R., Rautio S., Ronkainen V. P., Tavi P. (2011) Ca2+-calmodulin-dependent protein kinase II represses cardiac transcription of the L-type calcium channel α1C subunit gene (Cacna1c) by DREAM translocation. J. Physiol. 589, 2669–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palczewska M., Casafont I., Ghimire K., Rojas A. M., Valencia A., Lafarga M., Mellström B., Naranjo J. R. (2011) Sumoylation regulates nuclear localization of repressor DREAM. Biochim. Biophys. Acta 1813, 1050–1058 [DOI] [PubMed] [Google Scholar]
- 28. Yang X. J., Grégoire S. (2006) A recurrent phospho-sumoyl switch in transcriptional repression and beyond. Mol. Cell 23, 779–786 [DOI] [PubMed] [Google Scholar]
- 29. Anderson D., Mehaffey W. H., Iftinca M., Rehak R., Engbers J. D., Hameed S., Zamponi G. W., Turner R. W. (2010) Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat. Neurosci. 13, 333–337 [DOI] [PubMed] [Google Scholar]
- 30. Thomsen M. B., Wang C., Ozgen N., Wang H. G., Rosen M. R., Pitt G. S. (2009) Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ. Res. 104, 1382–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hibino H., Pironkova R., Onwumere O., Rousset M., Charnet P., Hudspeth A. J., Lesage F. (2003) Direct interaction with a nuclear protein and regulation of gene silencing by a variant of the Ca2+ channel β4 subunit. Proc. Natl. Acad. Sci. U.S.A. 100, 307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu X., Lee Y. J., Holm J. B., Terry M. D., Oswald R. E., Horne W. A. (2011) The Ca2+ channel β4c subunit interacts with heterochromatin protein 1 via a PXVXL binding motif. J. Biol. Chem. 286, 9677–9687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Genazzani A. A., Carafoli E., Guerini D. (1999) Calcineurin controls inositol 1,4,5-trisphosphate type 1 receptor expression in neurons. Proc. Natl. Acad. Sci. U.S.A. 96, 5797–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mondin L., Balghi H., Constantin B., Cognard C., Sebille S. (2009) Negative modulation of inositol 1,4,5-trisphosphate type 1 receptor expression prevents dystrophin-deficient muscle cells death. Am. J. Physiol. Cell Physiol. 297, C1133–C1145 [DOI] [PubMed] [Google Scholar]
- 35. Furutama D., Shimoda K., Yoshikawa S., Miyawaki A., Furuichi T., Mikoshiba K. (1996) Functional expression of the type 1 inositol 1,4,5-trisphosphate receptor promoter-lacZ fusion genes in transgenic mice. J. Neurochem. 66, 1793–1801 [DOI] [PubMed] [Google Scholar]
- 36. Qin Z., Zhou X., Gomez-Smith M., Pandey N. R., Lee K. F., Lagace D. C., Béïque J. C., Chen H. H. (2012) LIM domain only 4 (LMO4) regulates calcium-induced calcium release and synaptic plasticity in the hippocampus. J. Neurosci. 32, 4271–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kashani A. H., Qiu Z., Jurata L., Lee S. K., Pfaff S., Goebbels S., Nave K. A., Ghosh A. (2006) Calcium activation of the LMO4 transcription complex and its role in the patterning of thalamocortical connections. J. Neurosci. 26, 8398–8408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen H. H., Xu J., Safarpour F., Stewart A. F. (2007) LMO4 mRNA stability is regulated by extracellular ATP in F11 cells. Biochem. Biophys. Res. Commun. 357, 56–61 [DOI] [PubMed] [Google Scholar]
- 39. Manetopoulos C., Hansson A., Karlsson J., Jönsson J. I., Axelson H. (2003) The LIM-only protein LMO4 modulates the transcriptional activity of HEN1. Biochem. Biophys. Res. Commun. 307, 891–899 [DOI] [PubMed] [Google Scholar]
- 40. Schock S. C., Xu J., Duquette P. M., Qin Z., Lewandowski A. J., Rai P. S., Thompson C. S., Seifert E. L., Harper M. E., Chen H. H. (2008) Rescue of neurons from ischemic injury by peroxisome proliferator-activated receptor-γrequires a novel essential cofactor LMO4. J. Neurosci. 28, 12433–12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Novotny-Diermayr V., Lin B., Gu L., Cao X. (2005) Modulation of the interleukin-6 receptor subunit glycoprotein 130 complex and its signaling by LMO4 interaction. J. Biol. Chem. 280, 12747–12757 [DOI] [PubMed] [Google Scholar]
- 42. Bong Y. S., Lee H. S., Carim-Todd L., Mood K., Nishanian T. G., Tessarollo L., Daar I. O. (2007) ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc. Natl. Acad. Sci. U.S.A. 104, 17305–17310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kurokawa K., Mizuno K., Shibasaki M., Ohkuma S. (2010) Regulation of ryanodine receptors by dopamine D1 receptors during methamphetamine-induced place conditioning. J. Neurochem. 115, 1206–1214 [DOI] [PubMed] [Google Scholar]
- 44. Kurokawa K., Mizuno K., Kiyokage E., Shibasaki M., Toida K., Ohkuma S. (2011) Dopamine D1 receptor signaling system regulates ryanodine receptor expression after intermittent exposure to methamphetamine in primary cultures of midbrain and cerebral cortical neurons. J. Neurochem. 118, 773–783 [DOI] [PubMed] [Google Scholar]
- 45. Kurokawa K., Shibasaki M., Ohkuma S. (2010) Methamphetamine-induced up-regulation of α2/δ subunit of voltage-gated calcium channels is regulated by DA receptors. Synapse 64, 822–828 [DOI] [PubMed] [Google Scholar]
- 46. Ziviani E., Lippi G., Bano D., Munarriz E., Guiducci S., Zoli M., Young K. W., Nicotera P. (2011) Ryanodine receptor-2 up-regulation and nicotine-mediated plasticity. EMBO J. 30, 194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lytton J. (2007) Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem. J. 406, 365–382 [DOI] [PubMed] [Google Scholar]
- 48. Blaustein M. P., Lederer W. J. (1999) Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79, 763–854 [DOI] [PubMed] [Google Scholar]
- 49. Philipson K. D., Nicoll D. A. (2000) Sodium/calcium exchange: a molecular perspective. Annu. Rev. Physiol. 62, 111–133 [DOI] [PubMed] [Google Scholar]
- 50. Tibbits G. F., Xu L., Sedarat F. (2002) Ontogeny of excitation-contraction coupling in the mammalian heart. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 132, 691–698 [DOI] [PubMed] [Google Scholar]
- 51. Xu L., Renaud L., Müller J. G., Baicu C. F., Bonnema D. D., Zhou H., Kappler C. S., Kubalak S. W., Zile M. R., Conway S. J., Menick D. R. (2006) Regulation of Ncx1 expression. Identification of regulatory elements mediating cardiac-specific expression and up-regulation. J. Biol. Chem. 281, 34430–34440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prasad A. M., Inesi G. (2011) Silencing calcineurin A subunit reduces SERCA2 expression in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 300, H173–H180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li L., Guerini D., Carafoli E. (2000) Calcineurin controls the transcription of Na+/Ca2+ exchanger isoforms in developing cerebellar neurons. J. Biol. Chem. 275, 20903–20910 [DOI] [PubMed] [Google Scholar]
- 54. Gomez-Villafuertes R., Torres B., Barrio J., Savignac M., Gabellini N., Rizzato F., Pintado B., Gutierrez-Adan A., Mellström B., Carafoli E., Naranjo J. R. (2005) Downstream regulatory element antagonist modulator regulates Ca2+ homeostasis and viability in cerebellar neurons. J. Neurosci. 25, 10822–10830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carafoli E., Genazzani A., Guerini D. (1999) Calcium controls the transcription of its own transporters and channels in developing neurons. Biochem. Biophys. Res. Commun. 266, 624–632 [DOI] [PubMed] [Google Scholar]
- 56. Guerini D., García-Martin E., Gerber A., Volbracht C., Leist M., Merino C. G., Carafoli E. (1999) The expression of plasma membrane Ca2+ pump isoforms in cerebellar granule neurons is modulated by Ca2+. J. Biol. Chem. 274, 1667–1676 [DOI] [PubMed] [Google Scholar]
- 57. Guerini D., Wang X., Li L., Genazzani A., Carafoli E. (2000) Calcineurin controls the expression of isoform 4CII of the plasma membrane Ca2+ pump in neurons. J. Biol. Chem. 275, 3706–3712 [DOI] [PubMed] [Google Scholar]
- 58. Usachev Y. M., DeMarco S. J., Campbell C., Strehler E. E., Thayer S. A. (2002) Bradykinin and ATP accelerate Ca2+ efflux from rat sensory neurons via protein kinase C and the plasma membrane Ca2+ pump isoform 4. Neuron 33, 113–122 [DOI] [PubMed] [Google Scholar]
- 59. Jensen T. P., Buckby L. E., Empson R. M. (2009) Reduced expression of the “fast” calcium transporter PMCA2a during homeostatic plasticity. Mol. Cell. Neurosci. 41, 364–372 [DOI] [PubMed] [Google Scholar]
- 60. Ritchie M. F., Samakai E., Soboloff J. (2012) STIM1 is required for attenuation of PMCA-mediated Ca2+ clearance during T cell activation. EMBO J. 31, 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ritchie M. F., Yue C., Zhou Y., Houghton P. J., Soboloff J. (2010) Wilms tumor suppressor 1 (WT1) and early growth response 1 (EGR1) are regulators of STIM1 expression. J. Biol. Chem. 285, 10591–10596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Periasamy M., Kalyanasundaram A. (2007) SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 35, 430–442 [DOI] [PubMed] [Google Scholar]
- 63. Vlasblom R., Muller A., Musters R. J., Zuidwijk M. J., Van Hardeveld C., Paulus W. J., Simonides W. S. (2004) Contractile arrest reveals calcium-dependent stimulation of SERCA2a mRNA expression in cultured ventricular cardiomyocytes. Cardiovasc. Res. 63, 537–544 [DOI] [PubMed] [Google Scholar]
- 64. Michael A., Haq S., Chen X., Hsich E., Cui L., Walters B., Shao Z., Bhattacharya K., Kilter H., Huggins G., Andreucci M., Periasamy M., Solomon R. N., Liao R., Patten R., Molkentin J. D., Force T. (2004) Glycogen synthase kinase-3β regulates growth, calcium homeostasis, and diastolic function in the heart. J. Biol. Chem. 279, 21383–21393 [DOI] [PubMed] [Google Scholar]
- 65. Morita M., Kudo Y. (2010) Growth factors up-regulate astrocyte [Ca2+]i oscillation by increasing SERCA2b expression. Glia 58, 1988–1995 [DOI] [PubMed] [Google Scholar]
- 66. Marian M. J., Mukhopadhyay P., Borchman D., Tang D., Paterson C. A. (2008) The effect of hydrogen peroxide on sarco/endoplasmic and plasma membrane calcium ATPase gene expression in cultured human lens epithelial cells. Open Ophthalmol. J. 2, 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arai M., Yoguchi A., Takizawa T., Yokoyama T., Kanda T., Kurabayashi M., Nagai R. (2000) Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca2+-ATPase gene transcription. Circ. Res. 86, 8–14 [DOI] [PubMed] [Google Scholar]
- 68. Brady M., Koban M. U., Dellow K. A., Yacoub M., Boheler K. R., Fuller S. J. (2003) Sp1 and Sp3 transcription factors are required for transactivation of the human SERCA2 promoter in cardiomyocytes. Cardiovasc Res. 60, 347–354 [DOI] [PubMed] [Google Scholar]
- 69. Kang M. Y., Zhang Y., Matkovich S. J., Diwan A., Chishti A. H., Dorn G. W., 2nd. (2010) Receptor-independent cardiac protein kinase Cα activation by calpain-mediated truncation of regulatory domains. Circ. Res. 107, 903–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang Y., Matkovich S. J., Duan X., Diwan A., Kang M. Y., Dorn G. W., 2nd (2011) Receptor-independent protein kinase Cα (PKCα) signaling by calpain-generated free catalytic domains induces HDAC5 nuclear export and regulates cardiac transcription. J. Biol. Chem. 286, 26943–26951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Backs J., Worst B. C., Lehmann L. H., Patrick D. M., Jebessa Z., Kreusser M. M., Sun Q., Chen L., Heft C., Katus H. A., Olson E. N. (2011) Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J. Cell Biol. 195, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Little G. H., Bai Y., Williams T., Poizat C. (2007) Nuclear calcium/calmodulin-dependent protein kinase IIδ preferentially transmits signals to histone deacetylase 4 in cardiac cells. J. Biol. Chem. 282, 7219–7231 [DOI] [PubMed] [Google Scholar]
- 73. Zhang T., Kohlhaas M., Backs J., Mishra S., Phillips W., Dybkova N., Chang S., Ling H., Bers D. M., Maier L. S., Olson E. N., Brown J. H. (2007) CaMKIIδ isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J. Biol. Chem. 282, 35078–35087 [DOI] [PubMed] [Google Scholar]
- 74. Favereaux A., Thoumine O., Bouali-Benazzouz R., Roques V., Papon M. A., Salam S. A., Drutel G., Léger C., Calas A., Nagy F., Landry M. (2011) Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: role in pain. EMBO J. 30, 3830–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aurora A. B., Mahmoud A. I., Luo X., Johnson B. A., van Rooij E., Matsuzaki S., Humphries K. M., Hill J. A., Bassel-Duby R., Sadek H. A., Olson E. N. (2012) MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J. Clin. Invest. 122, 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]