Background: The BabA adhesin mediates binding of Helicobacter pylori to the gastric epithelium.
Results: Binding of BabA to blood group O and A determinants on type 4 core chains was demonstrated.
Conclusion: The BabA binds to blood group determinants on both type 1 and type 4 core chains.
Significance: Characterization of the binding specificities of BabA is important for understanding the interactions between H. pylori and target cells.
Keywords: Bacterial Adhesion, Glycobiology, Glycolipid Structure, Helicobacter pylori, Mass Spectrometry (MS)
Abstract
Certain Helicobacter pylori strains adhere to the human gastric epithelium using the blood group antigen-binding adhesin (BabA). All BabA-expressing H. pylori strains bind to the blood group O determinants on type 1 core chains, i.e. to the Lewis b antigen (Fucα2Galβ3(Fucα4)GlcNAc; Leb) and the H type 1 determinant (Fucα2Galβ3GlcNAc). Recently, BabA strains have been categorized into those recognizing only Leb and H type 1 determinants (designated specialist strains) and those that also bind to A and B type 1 determinants (designated generalist strains). Here, the structural requirements for carbohydrate recognition by generalist and specialist BabA were further explored by binding of these types of strains to a panel of different glycosphingolipids. Three glycosphingolipids recognized by both specialist and generalist BabA were isolated from the small intestine of a blood group O pig and characterized by mass spectrometry and proton NMR as H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer), Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), and a mixture of three complex glycosphingolipids (Fucα2Galβ4GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer, Fucα2Galβ3GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer, and Fucα2Galβ4(Fucα3)GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer). In addition to the binding of both strains to the Globo H hexaglycosylceramide, i.e. a blood group O determinant on a type 4 core chain, the generalist strain bound to the Globo A heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), i.e. a blood group A determinant on a type 4 core chain. The binding of BabA to the two sets of isoreceptors is due to conformational similarities of the terminal disaccharides of H type 1 and Globo H and of the terminal trisaccharides of A type 1 and Globo A.
Introduction
Attachment of microbes to cell surface receptors on the target tissue is considered an essential step in the initiation, establishment, and maintenance of infection. In recent years, a large number of studies have aimed at the identification of potential microbial host receptors, the majority of which appear to be glycoconjugates (1–3). Glycoconjugates exhibit a characteristic and specific pattern of expression, which is dependent on the animal species, age, individual, and cell type (4). Thus, the recognition of a specific carbohydrate receptor on the host cell surface determines at least in part the host, tissue, and age specificities of microbial infections.
Adherence of the gastric pathogen Helicobacter pylori to human gastric epithelial cells is required for prolonged persistence in the stomach. Initial studies of potential target cell receptors for H. pylori demonstrated the binding of certain strains of this bacterium to the Lewis b blood group antigen (Fucα2Galβ3(Fucα4)GlcNAc;2 Leb)3 (5), and subsequently the H. pylori Leb-binding adhesin, blood group antigen-binding adhesin (BabA) was identified (6). H. pylori strains expressing BabA together with the vacuolating cytotoxin VacA and the cytotoxin-associated antigen CagA (triple positive strains) are associated with severe gastric diseases such as peptic ulcer and gastric adenocarcinoma (7, 8).
Subsequent studies demonstrated that the BabA adhesin has adapted to the fucosylated blood group antigens most prevalent in the local population (9). In Europe and the United States where blood group A, B, and O phenotypes all are common, the H. pylori strains (designated generalist strains) bind to blood group A, B, and O type 1 determinants. However, in populations such as the indigenous South American native population, which only has the blood group O phenotype, the H. pylori strains (designated specialist strains) bind only to the blood group O type 1 determinants (Leb and the H type 1). Thus, the carbohydrate binding site of BabA of generalist strains can accommodate an extension of the blood group O determinant with an α3-linked GalNAc or Gal (creating the blood group A and B determinants, respectively), whereas this extension is not tolerated by the BabA of specialist strains. Consequently, the BabA adhesins from these strains have differences in the architecture of their carbohydrate binding sites.
In the present study, the structural requirements for carbohydrate recognition by BabA of generalist and specialist H. pylori strains were further explored. Radiolabeled H. pylori strains were examined for binding to a panel of different glycosphingolipids from various sources separated on thin-layer plates, and glycosphingolipids recognized by wild type specialist and/or generalist H. pylori, but not by a deletion mutant strain lacking the BabA adhesin, were isolated and characterized by mass spectrometry and proton NMR. Comparative binding studies demonstrated that the BabA adhesin in addition to blood group determinants on type 1 core chains recognizes blood group O and A determinants on type 4 core chains with binding to Globo H (i.e. H type 4) by both strains and Globo A (i.e. A type 4) by the generalist strain. Inspection of minimum energy models revealed topographical similarities in the spatial orientation of the terminal disaccharide (Fucα2Galβ3) of the Globo H and H5 type 1 glycosphingolipids, accounting for the BabA cross-reactivity.
EXPERIMENTAL PROCEDURES
H. pylori Strains, Culture Conditions, and Labeling
The generalist H. pylori strain J99 and the construction of the J99/BabA− mutant babA::cam were described by Mahdavi et al. (10). The specialist H. pylori strain S831 was described (9).
For chromatogram binding experiments, the bacteria were grown in a microaerophilic atmosphere at 37 °C for 48 h on Brucella medium (Difco) containing 10% fetal calf serum (Harlan Sera-Lab, Loughborough, UK) inactivated at 56 °C and BBL IsoVitaleX Enrichment (BD Biosciences). The mutant strain J99/BabA− was cultured on the same medium supplemented with chloramphenicol (20 μg/ml). Bacteria were radiolabeled by the addition of 50 μCi [35S]methionine (Amersham Biosciences) diluted in 0.5 ml of phosphate-buffered saline (PBS), pH 7.3 to the culture plates. After incubation for 12–72 h at 37 °C under microaerophilic conditions, the bacteria were harvested, centrifuged three times, and thereafter suspended to 1 × 108 cfu/ml in PBS. The specific activities of the suspensions were ∼1 cpm/100 H. pylori organisms.
Chromatogram Binding Assays
Reference glycosphingolipids were isolated and characterized by mass spectrometry and proton NMR as described (11).
Thin-layer chromatography was performed on glass- or aluminum-backed silica gel 60 HPTLC plates (Merck). Mixtures of glycosphingolipids (40 μg) or pure compounds (40 ng–4 μg) were separated using chloroform/methanol/water (60:35:8 by volume) as the solvent system. Chemical detection was accomplished by anisaldehyde (12).
Binding of 35S-labeled H. pylori to glycosphingolipids on thin-layer chromatograms was done as reported previously (13). Dried chromatograms were dipped for 1 min in diethyl ether/n-hexane (1:5 by volume) containing 0.5% (w/v) polyisobutylmethacrylate (Aldrich). After drying, the chromatograms were soaked in PBS containing 2% bovine serum albumin (w/v), 0.1% NaN3 (w/v), and 0.1% Tween 20 (by volume) for 2 h at room temperature. The chromatograms were subsequently covered with radiolabeled bacteria diluted in PBS (2–5 × 106 cpm/ml). Incubation was done for 2 h at room temperature followed by repeated washings with PBS. The chromatograms were thereafter exposed to XAR-5 x-ray films (Eastman Kodak Co.) for 12 h.
Chromatogram binding assays with mouse monoclonal antibodies directed against the Globo H determinant (MBr1, Enzo Life Sciences), the Leb determinant (BG-6/T218, Signet/Covance), the H type 1 determinant (17-206, Abcam), and the H type 2 determinant (A583, DakoCytomation Norden A/S) were done as described (13) using 125I-labeled monoclonal anti-mouse antibodies (Z0259, DakoCytomation Norden A/S) for detection.
Isolation of H. pylori-binding Glycosphingolipids
Total acid and non-acid glycosphingolipid fractions were isolated by standard methods (11). Briefly, the material was lyophilized and then extracted in two steps in a Soxhlet apparatus with chloroform and methanol (2:1 and 1:9 by volume, respectively). The material obtained was subjected to mild alkaline hydrolysis and dialysis followed by separation on a silicic acid column. Acid and non-acid glycosphingolipid fractions were obtained by chromatography on a DEAE-cellulose column. To separate the non-acid glycolipids from alkali-stable phospholipids, this fraction was acetylated and separated on a second silicic acid column followed by deacetylation and dialysis. Final purifications were done by chromatographies on DEAE-cellulose and silicic acid columns.
The non-acid glycosphingolipid fractions were separated by repeated silicic acid chromatography, and final separation was achieved by HPLC or by chromatography on Iatrobead (Iatrobeads 6RS-8060, Iatron Laboratories, Tokyo, Japan) columns and elution with chloroform/methanol/water (65:25:4 by volume) followed by chloroform/methanol/water (60:35:8 by volume) and finally chloroform/methanol/water (40:40:12 by volume). Throughout the separation procedures, aliquots of the fractions obtained were analyzed by thin-layer chromatography, and fractions that were colored green by anisaldehyde were tested for binding of H. pylori using the chromatogram binding assay. The fractions were pooled according to the mobility on thin-layer chromatograms and their H. pylori binding activity.
Endoglycoceramidase Digestion and LC-ESI/MS
Endoglycoceramidase II from Rhodococcus spp. (14) (Takara Bio Europe S.A., Gennevilliers, France) was used for hydrolysis of glycosphingolipids. Briefly, 50 mg of glycosphingolipids were suspended in 100 ml of 0.05 m sodium acetate buffer, pH 5.0 containing 120 mg of sodium cholate and sonicated briefly. Thereafter, 1 milliunit of endoglycoceramidase II was added, and the mixture was incubated at 37 °C for 48 h. The reaction was stopped by addition of chloroform/methanol/water to the final proportions 8:4:3 (by volume). The oligosaccharide-containing upper phase thus obtained was separated from detergent on a Sep-Pak QMA cartridge (Waters, Milford, MA). The eluant containing the oligosaccharides was dried under nitrogen and under vacuum.
The glycosphingolipid-derived oligosaccharides were analyzed by LC/MS and MS/MS as described (15). In brief, the oligosaccharides were separated on a column (200 × 0.180 mm) packed in house with 5-mm porous graphite particles (Hypercarb, Thermo Scientific) and eluted with an acetonitrile gradient (A, 10 mm ammonium bicarbonate; B, 10 mm ammonium bicarbonate in 80% acetonitrile). The saccharides were analyzed in the negative ion mode on an LTQ linear quadrupole ion trap mass spectrometer (Thermo Electron, San José, CA).
LC-ESI/MS and ESI/MS/MS of Native Glycosphingolipids
The glycosphingolipids (dissolved in methanol/acetonitrile, 75:25 by volume) were separated on a 200 × 0.150-mm column packed in house with 5-mm polyamine II particles (YMC Europe GmbH, Dinslaken, Germany) and eluted with a water gradient (A, 100% acetonitrile; B, 10 mm ammonium bicarbonate). Samples were analyzed on an LTQ linear quadrupole ion trap mass spectrometer by LC-ESI/MS at −3.5 kV. A full scan (m/z 500–1800; two microscans; maximum time, 100 ms; target value, 30,000) was performed followed by data-dependent MS2 scans (two microscans; maximum time, 100 ms; target value, 10,000) with a normalized collision energy of 35%, an isolation window of 2.5 units, an activation q of 0.25, and an activation time of 30 ms.
Proton NMR Spectroscopy
1H NMR spectra were acquired on a Varian 600-MHz spectrometer at 30 °C. Samples were dissolved in dimethyl sulfoxide/D2O (98:2 by volume) after deuterium exchange. Two-dimensional double quantum-filtered correlated spectroscopy (COSY) spectra were recorded using the standard pulse sequence (16).
Molecular Modeling
Minimum energy models of different glycosphingolipids were constructed using the CHARMm force field within the Discovery Studio molecular modeling package (Accelrys, Inc., San Diego, CA) and literature values as starting points for the glycosidic torsion angles (17, 18).
RESULTS
Binding of H. pylori to Glycosphingolipid Mixtures
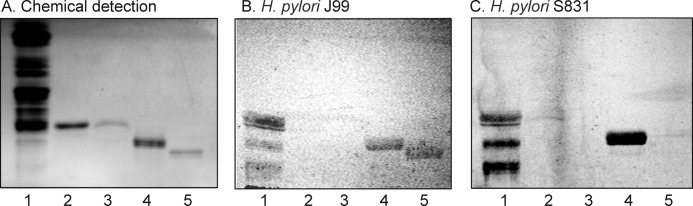
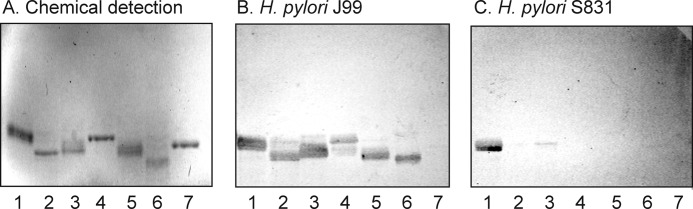
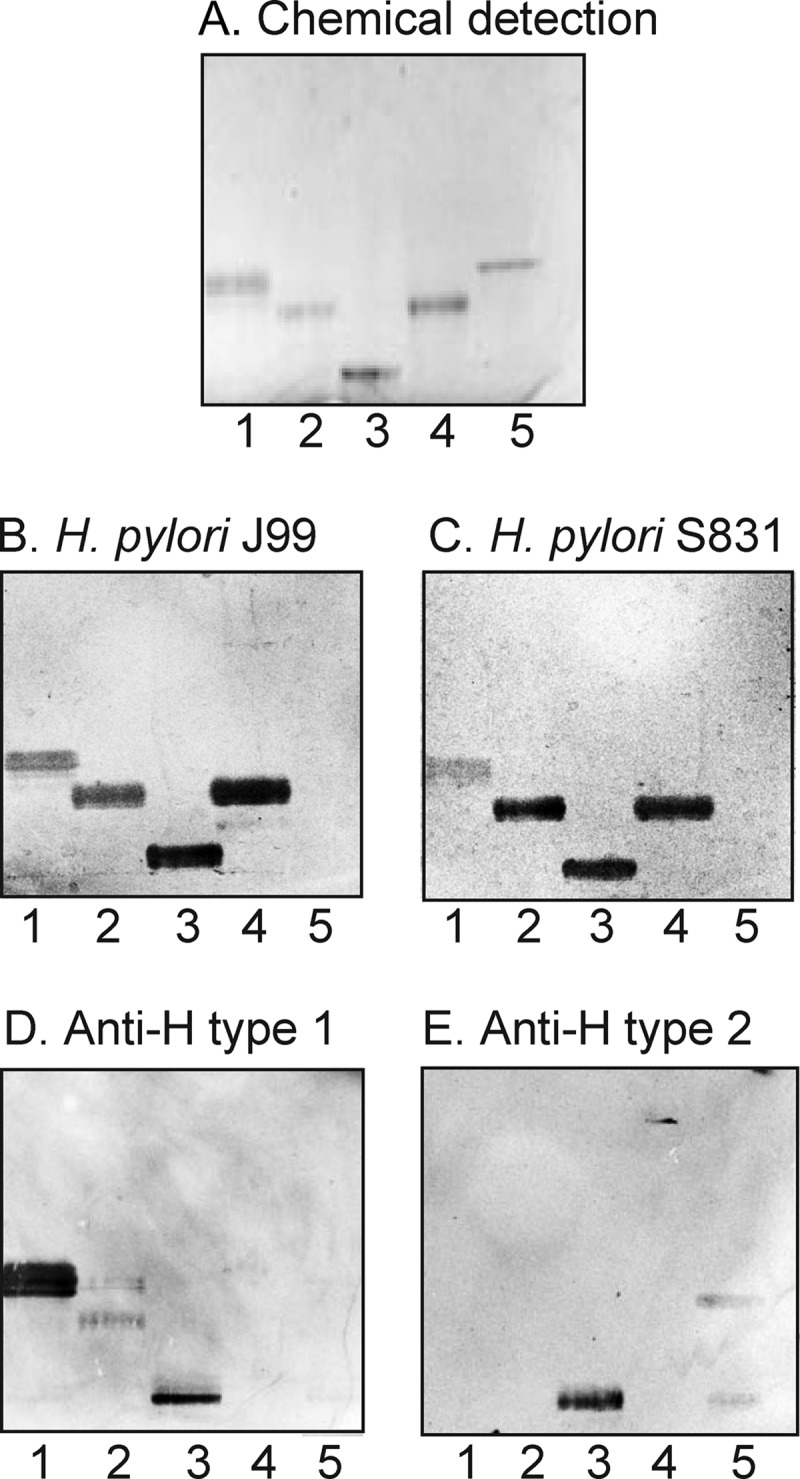
Screening for BabA-mediated binding of H. pylori was done by binding of the generalist H. pylori strain J99, the specialist strain S831, and the deletion mutant strain J99/BabA− to non-acid glycosphingolipid fractions from various sources to expose the bacteria to a large number of potentially binding-active carbohydrate structures. Thus, the binding of the bacteria to non-acid glycosphingolipid mixtures isolated from the small intestine of different species (human, rat, cat, and pig (19–23)), erythrocytes of different species (human, cat, rabbit, dog, horse, chicken, and sheep (24)), human cancers (lung, kidney, colon, liver, and gastric cancers (25)), and human stomach (26) was tested. Thereby, three glycosphingolipids recognized by both the generalist and specialist H. pylori strain were detected in the non-acid glycosphingolipid fraction from the small intestinal epithelium of a blood group O pig (Fig. 1, B and C, lane 1). The binding-active compounds migrated in the penta-, hexa-, and octa-/nonaglycosylceramide regions, respectively. No binding of the deletion mutant strain J99/BabA− to the porcine intestinal glycosphingolipids was obtained (data not shown), indicating that the binding of the wild type bacteria to these compounds was mediated by BabA.
FIGURE 1.
Binding of a generalist and a specialist H. pylori strain to non-acid glycosphingolipids of the small intestinal epithelium of a blood group O pig. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system. The chromatogram in A was stained with anisaldehyde. Duplicate chromatograms were incubated with the 35S-labeled H. pylori generalist strain J99 (B) and the H. pylori specialist strain S831 (C) followed by autoradiography for 12 h as described under “Experimental Procedures.” Lane 1, non-acid glycosphingolipids of the intestinal epithelium of a blood group O pig, 40 μg; lane 2, reference H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer), 4 μg; lane 3, reference Lea pentaglycosylceramide (Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 4, reference Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 4 μg; lane 5, reference B type 1 heptaglycosylceramide (Galα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg.
Isolation of the H. pylori-binding Glycosphingolipids from Porcine Intestine
A total non-acid fraction from blood group O porcine small intestinal epithelium (160 mg) was separated by repeated silica gel chromatography and Iatrobead column chromatography, and the subfractions obtained were tested for H. pylori binding activity. After pooling of binding-active fractions, three subfractions containing H. pylori-binding glycosphingolipids were obtained. One of these fraction (designated fraction P-I (0.2 mg)) migrated in the pentaglycosylceramide region, whereas the fraction designated fraction P-II (0.2 mg) migrated in the hexaglycosylceramide region (Fig. 2, lanes 1 and 2). LC-ESI/MS of the third fraction containing the slowest migrating H. pylori-binding compounds showed that this was a mixture of several glycosphingolipids. This fraction was therefore further separated on an Iatrobead column, and after pooling of the H. pylori-binding fractions, 0.3 mg of the slow migrating H. pylori-binding compound (designated fraction P-III) was obtained (Fig. 2, lane 3).
FIGURE 2.
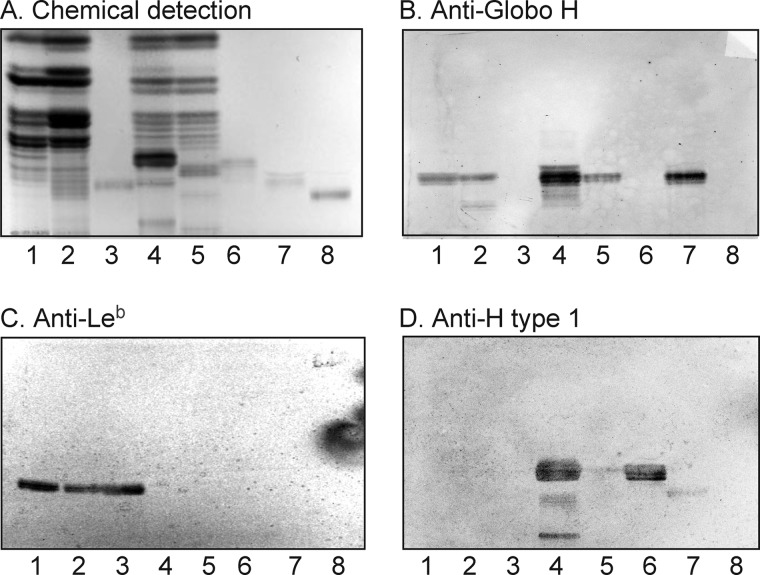
H. pylori-binding glycosphingolipids isolated from the small intestinal epithelium of a blood group O pig. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system. The chromatogram in A was stained with anisaldehyde. Duplicate chromatograms were incubated with the 35S-labeled H. pylori generalist strain J99 (B), the H. pylori specialist strain S831 (C), the monoclonal anti-H type 1 antibody 17-206 (D), and the monoclonal anti-H type 2 antibody 92FR-A2 (E) followed by autoradiography for 12 h as described under “Experimental Procedures.” Lane 1, fraction P-I isolated from pig intestine, 2 μg; lane 2, fraction P-II from pig intestine, 2 μg; lane 3, fraction P-III from pig intestine, 2 μg; lane 4, reference Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 5, reference H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer), 2 μg.
Characterization of the H. pylori-binding Fraction P-I from Porcine Intestine
LC-ESI/MS, proton NMR, and antibody binding demonstrated that fraction P-I was a mixture of the H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer) and the B5 pentaglycosylceramide (Galα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (data not shown).
Characterization of the H. pylori-binding Fraction P-II from Porcine Intestine
Characterization of the BabA binding fraction P-II demonstrated the Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer) as the major compound. This conclusion was based on the following properties. (i) ESI/MS of the native fraction P-II gave a major [M − 2H+]2− ion at m/z 784, corresponding to a molecular ion at m/z 1568, demonstrating a glycosphingolipid with one Fuc, one HexNAc, and four Hex residues and phytosphingosine with hydroxy 16:0 fatty acid (data not shown). The series of C, Y, and Z ions obtained by MS2 of the [M − 2H+]2− ion at m/z 784 demonstrated a Fuc-Hex-HexNAc-Hex-Hex-Hex sequence (supplemental Fig. S1).
(ii) LC-ESI/MS of oligosaccharides gives the resolution of isomeric saccharides, and the carbohydrate sequence can be deduced from series of C type fragment ions obtained by MS2 (15). In addition, diagnostic cross-ring 0,2A type fragment ions are present in MS2 spectra of oligosaccharides with a Hex or HexNAc substituted at C-4 and thus allow differentiation of linkage positions (15, 27, 28).
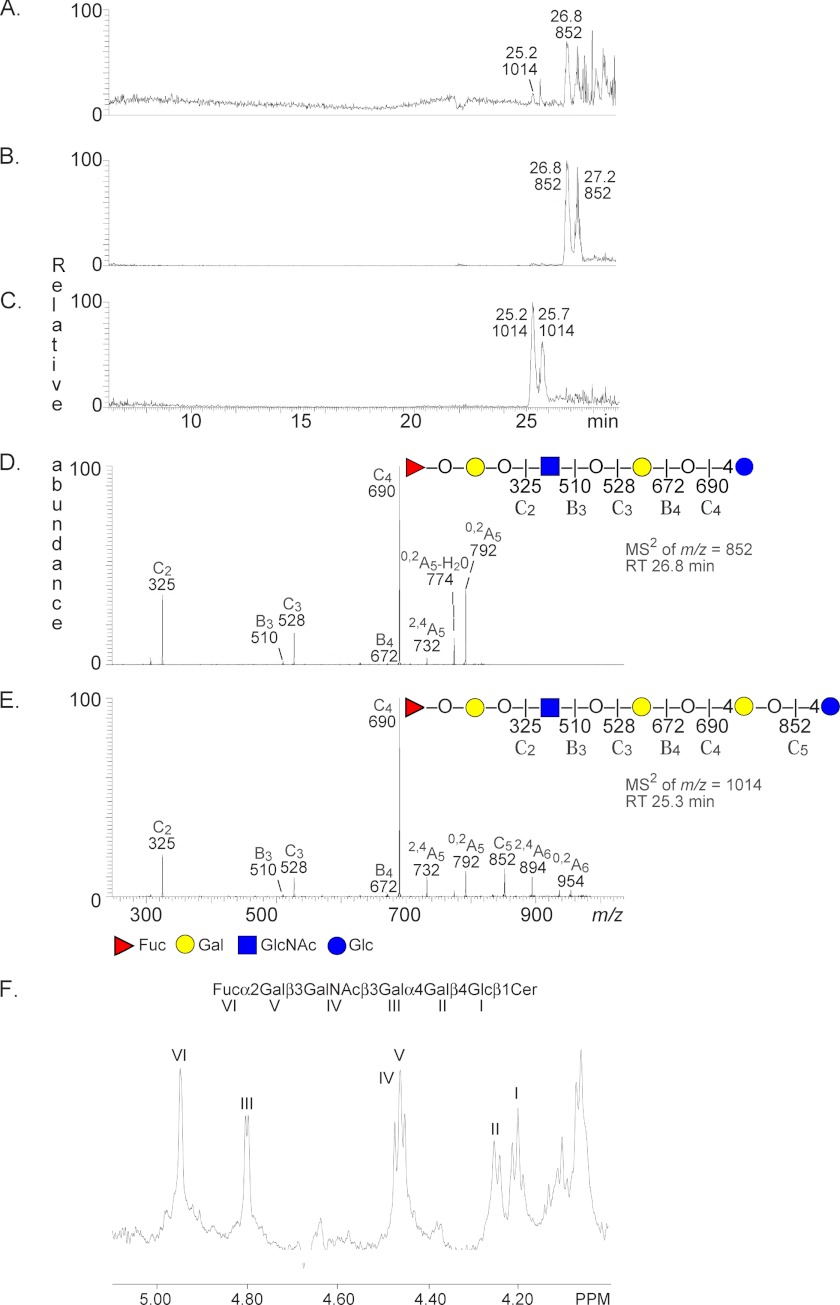
LC-ESI/MS of the oligosaccharides obtained by hydrolysis of fraction P-II with Rhodococcus endoglycoceramidase II gave two late eluting molecular ions (Fig. 3, A–C). These ions were found at m/z 852 (retention time, 26.8–27.2 min) and at m/z 1014 (retention time, 25.2–25.7 min) and demonstrated one oligosaccharide with one Fuc, one HexNAc and three Hex residues and one oligosaccharide with one Fuc, one HexNAc, and four Hex residues, respectively.
FIGURE 3.
Characterization of the H. pylori BabA-binding fraction P-II from the small intestinal epithelium of a blood group O pig. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides obtained by digestion of the H. pylori BabA-binding fraction P-II with Rhodococcus endoglycoceramidase II. B, mass chromatogram of m/z 852. C, mass chromatogram of m/z 1014. D, MS2 spectrum of the [M − H+]− ion at m/z 852 (retention time (RT), 26.8 min). The interpretation formula shows the deduced oligosaccharide sequence. E, MS2 spectrum of the [M − H+]− ion at m/z 1014 (retention time, 25.3 min). The interpretation formula shows the deduced oligosaccharide sequence. F, anomeric region of the 600-MHz proton NMR spectrum of fraction P-II (30 °C). The sample was dissolved in dimethyl sulfoxide/D2O (98:2 by volume) after deuterium exchange.
The MS2 spectrum of the molecular ion at m/z 852 (Fig. 3D) had a C type fragment ion series (C2 at m/z 325, C3 at m/z 528, and C4 at m/z 690), demonstrating a Fuc-Hex-HexNAc-Hex-Hex sequence. The features of this MS2 spectrum were very similar to the MS2 spectrum of reference H type 1 pentaglycosylceramide (15).
MS2 of the molecular ion at m/z 1014 (Fig. 3E) also gave a series of C type fragment ions with C2 at m/z 325, C3 at m/z 528, and C4 at m/z 690 along with a C5 ion at m/z 852, identifying a Fuc-Hex-HexNAc-Hex-Hex-Hex sequence. The 0,2A5 fragment ion at m/z 792 and the 0,2A6 fragment ion at m/z 954 indicated that the two hexoses at the reducing end were substituted at C-4, i.e. a Fuc-Hex-HexNAc-Hex-4Hex-4Hex sequence.
(iii) The anomeric region of the proton NMR spectrum of fraction P-II (Fig. 3F) revealed a single dominating species with six carbohydrate residues that is identical to the previously published Globo H glycosphingolipid (29) as evidenced by signals at 4.949 (Fucα2), 4.802 (Galα4), 4.468 (GalNAcβ3), 4.456 (Galβ3), 4.247 (Galβ4), and 4.208 ppm (Glcβ1), thus yielding the sequence Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer in accordance with the mass spectrometry data above.
Thus, by mass spectrometry and proton NMR, the BabA-binding hexaglycosylceramide of blood group O pig intestine was identified as the Globo H glycosphingolipid. In the base peak chromatogram from LC-ESI/MS of the oligosaccharides obtained by hydrolysis of fraction P-II with Rhodococcus endoglycoceramidase (Fig. 3A), the major molecular ion was found at m/z 852, corresponding to the H type 1 pentaglycosylceramide. Still, proton NMR demonstrated that fraction P-II was a relatively pure Globo H glycosphingolipid. This discrepancy is due to the restricted hydrolytic capacity of the Rhodococcus endoglycoceraminidase II, which has a relative resistance of hydrolysis for globo series glycosphingolipids (14, 30). The ideal enzyme would have been the ceramide glycanase from Macrobdella decora that has a more universal hydrolytic activity toward glycosphingolipids (31). However, the M. decora enzyme is no longer available commercially.
Characterization of the Slow Migrating H. pylori-binding Fraction P-III from Porcine Intestine
Antibody binding, mass spectrometry, and proton NMR demonstrated that fraction P-III was a mixture of two branched decaglycosylceramides with terminal H type 1 epitopes (Fucα2Galβ3GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer and Fucα2Galβ4GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer) and a related undecaglycosylceramide with a Fucα3 substitution of the GlcNAc of the 6-branch, yielding an Ley determinant (Fucα2Galβ4(Fucα3)GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer). This conclusion is based on the following observations. (i) The glycosphingolipid fraction P-III was stained by both the anti-H type 1 antibody and the anti-H type 2 antibody (Fig. 2, D and E, lane 3).
(ii) ESI/MS of the native fraction P-III gave a major [M − 2H+]2− ion at m/z 1132, corresponding to a molecular ion at m/z 2264, indicating a decasaccharide with two Fuc, three HexNAc, and five Hex residues combined with sphingosine and non-hydroxy 16:0 fatty acid (data not shown). In addition, there was an [M − 2H+]2− ion at m/z 1205, corresponding to a molecular ion at m/z 2410, suggesting an undecasaccharide with three Fuc, three HexNAc, and five Hex residues combined with sphingosine and non-hydroxy 16:0 fatty acid.
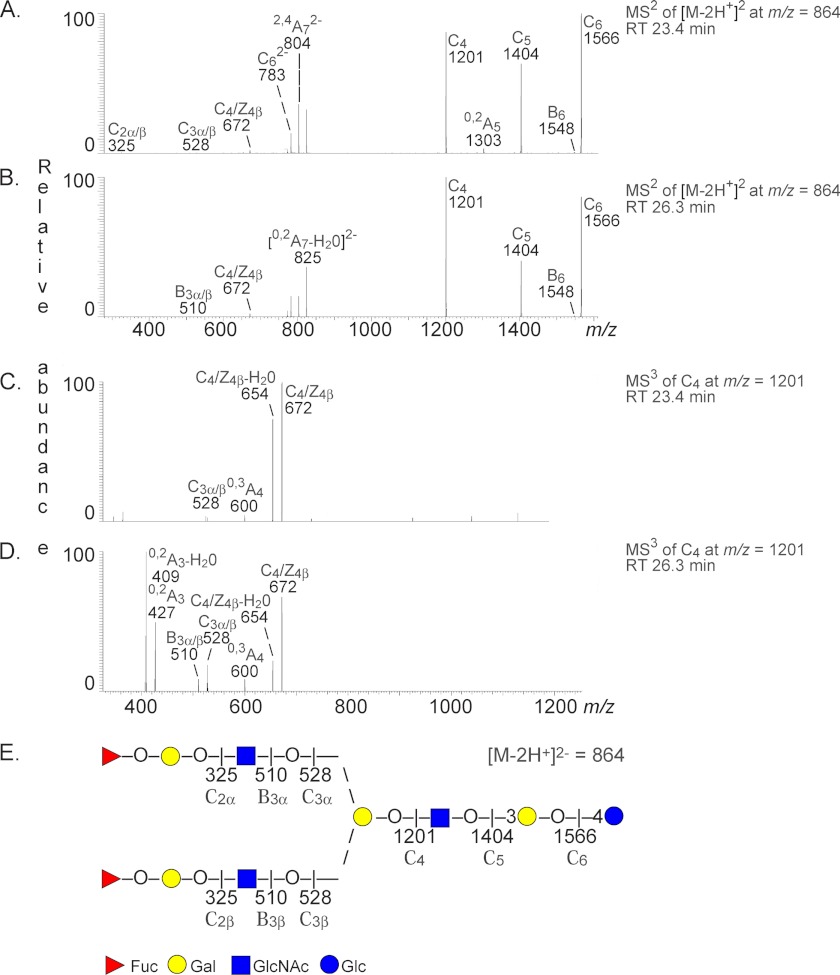
(iii) LC-ESI/MS of the oligosaccharides obtained by hydrolysis of fraction P-III with Rhodococcus endoglycoceramidase II had two [M − 2H+]2− ions at m/z 864, corresponding to molecular ions at m/z 1728, demonstrating two decasaccharides, both with two Fuc, three HexNAc, and five Hex residues (supplemental Fig. S2). The minor [M − 2H+]2− ion eluted at 23.4–24.5 min, and the major [M − 2H+]2− ion eluted at 25.8–26.1 min. The MS2 spectra of the minor and major [M − 2H+]2− ions both had weak lower mass regions, but in both cases, a terminal Fuc-Hex-HexNAc sequence was indicated by C2 ions at m/z 325 and/or C3 ions at m/z 528 or B3 ions at m/z 510 (Fig. 4, A and B). In addition, there were intense C type ions at m/z 1201, 1404, and 1566.
FIGURE 4.
LC-ESI/MS of the decasaccharides obtained by hydrolysis of H. pylori-binding fraction P-III with Rhodococcus endoglycoceramidase II. A, MS2 spectrum of the [M − 2H+]2− ion at m/z 864 (retention time (RT), 23.4 min). B, MS2 spectrum of the [M − 2H+]2− ion at m/z 864 (retention time, 26.3 min). C, MS3 spectrum of the ion at m/z 1201 (retention time, 23.4 min). D, MS3 spectrum of the ion at m/z 1201 (retention time, 26.3 min). E, interpretation formula showing the deduced oligosaccharide sequence.
MS3 of the ion at m/z 1201 at retention time 23.4 min gave a C3 ion at m/z 528, again demonstrating a terminal Fuc-Hex-HexNAc sequence (Fig. 4C). In contrast, the MS3 spectrum of the ion at m/z 1201 at retention time 26.3 min was dominated by an intense 0,2A3 ion at m/z 427 and a 0,2A3 − H2O ion at m/z 409, which together with the C3 ion at m/z 528 identified a terminal Fuc-Hex-HexNAc sequence with 4-substitution of the HexNAc, i.e. a type 2 core chain (Fig. 4D) (15, 27, 28).
Both MS3 spectra (Fig. 4, C and D) had C4/Z4β ions at m/z 672. These ions are obtained by double glycosidic cleavage at the 3-linked bond of the branched Hex residue and thus comprise the 6-linked carbohydrate chain and the core branching Hex residue (32). The 0,3A4 ions at m/z 600 obtained by cross-ring cleavages present in both MS3 spectra further confirm the Fuc-Hex-HexNAc sequence on the 6-branch (32).
Thus, these MS2 and MS3 spectral features suggested that fraction P-III contained two branched decasaccharides, i.e. two Fuc-Hex-HexNAc-(Fuc-Hex-HexNAc-)Hex-HexNAc-Hex-Hex saccharides. The terminal Fuc-Hex-HexNAc sequences of the minor compound had type 1 core chains, whereas the terminal Fuc-Hex-HexNAc sequences of the major compound had type 2 core chain on at least one branch.
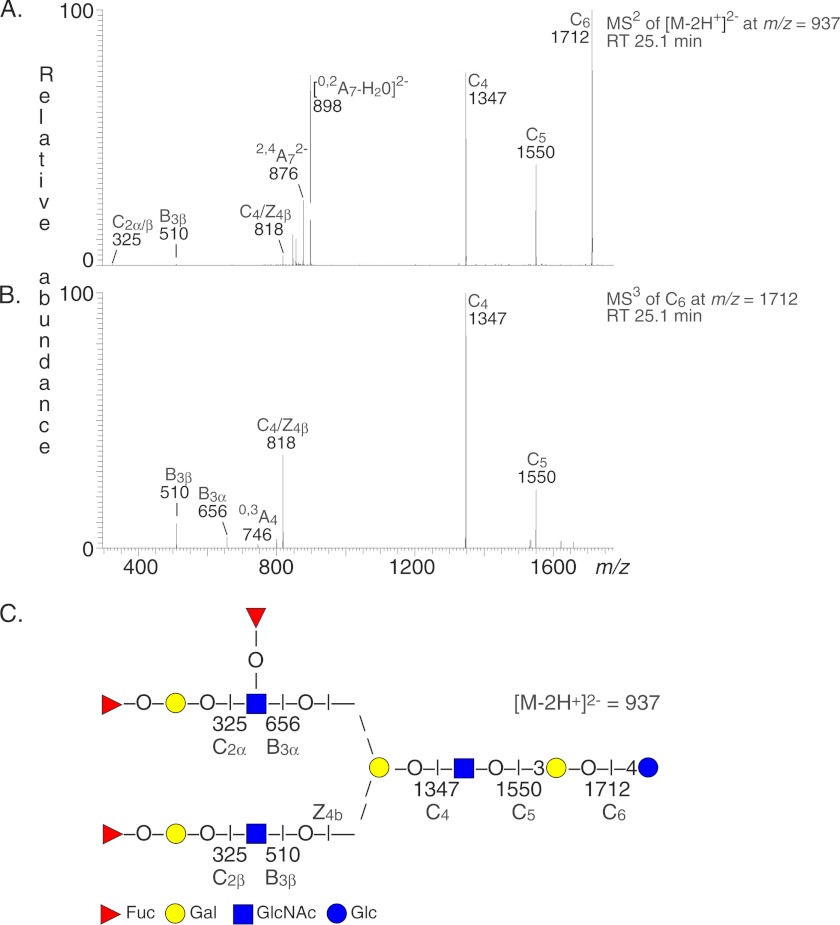
The LC-ESI/MS base peak chromatogram of the oligosaccharides from fraction P-III (supplemental Fig. S2) also had an [M − 2H+]2− ion at m/z 937, corresponding to a molecular ion at m/z 1874, indicating an undecasaccharide with three Fuc, three HexNAc, and five Hex residues. In addition, the MS2 and MS3 spectra obtained had weak lower mass regions (Fig. 5). There was a C2 ion at m/z 325 and a B3 ion at m/z 510, indicating a terminal Fuc-Hex-HexNAc sequence. In addition, the B3 ion at m/z 656 demonstrated a terminal Fuc-Hex-(Fuc-)HexNAc sequence. This was confirmed by the C4/Z4β ion at m/z 818, comprising the 6-linked carbohydrate chain and the core branching Hex residue, and the 0,3A4 cross-ring cleavage ion at m/z 746. Furthermore, both the C4/Z4β ion and the 0,3A4 ion demonstrated that the Fuc-Hex-(Fuc-)HexNAc sequence was carried by the 6-branch (32). The spectra also had a series of prominent C type fragment ions (C4 at m/z 1347, C5 at m/z 1550, and C6 at m/z 1712). Taken all together, MS2 and MS3 indicated a branched undecasaccharide (Fuc-Hex-(Fuc-)HexNAc-(Fuc-Hex-HexNAc-)Hex-HexNAc-Hex-Hex with a Fuc-Hex-(Fuc-)HexNAc sequence on the 6-branch and an H type 1 epitope on the 3-branch.
FIGURE 5.
LC-ESI/MS of the undecasaccharide obtained by hydrolysis of H. pylori-binding fraction P-III with Rhodococcus endoglycoceramidase II. A, MS2 spectrum of the [M − 2H+]2− ion at m/z 937 (retention time (RT), 25.1 min). B, MS3 spectrum of the ion at m/z 1712 (retention time, 25.1 min). C, interpretation formula showing the deduced oligosaccharide sequence.
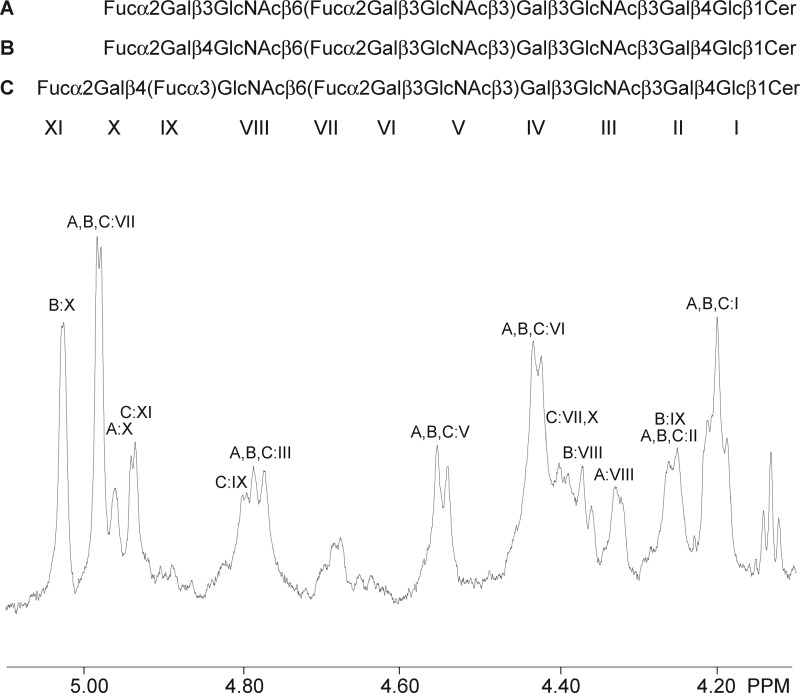
(iv) The anomeric region of the proton NMR spectrum of fraction P-III is shown in Fig. 6. Fraction P-III contains two decaglycosylceramides (Fucα2Galβ4GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer and Fucα2Galβ3GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer) that have been isolated previously from rat (33) and pig intestine (23) and characterized in detail by NMR (using DMSO/D2O (98:2) as solvent). In fraction P-III, the glycosphingolipid with mixed type 1/type 2 branches (Fucα2Galβ4GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer) is the major compound as evidenced by the relative intensities of the Fucα2 signals. The chemical shift data are summarized in Table 1. In addition, a novel glycosphingolipid structure with an Ley determinant on the 6-branch and an H type 1 determinant on the 3-branch (Fucα2Galβ4(Fucα3)GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer) could be characterized as shown in Fig. 6 and Table 1.
FIGURE 6.
Anomeric region of the 600-MHz proton NMR spectrum of the H. pylori-binding fraction P-III from porcine small intestinal epithelium (30 °C). The sample was dissolved in dimethyl sulfoxide/D2O (98:2 by volume) after deuterium exchange.
TABLE 1.
Chemical shifts (ppm) of anomeric resonances of glycosphingolipids in fraction P-III from porcine small intestine dissolved in dimethyl sulfoxide/D2O (98:2 by volume) identified by 600-MHz NMR at 30 °C
| Structure | XI | X | IX | VIII | VII | VI | V | IV | III | II | I | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Fucα2 | Galβ3 | GlcNAcβ6 | (Fucα2) | Galβ3 | (GlcNAcβ3) | Galβ3 | GlcNAβ3 | Galβ4 | Glcβ1 | Cer | |
| 4.962 | 4.41 | 4.36 | 4.982 | 4.427 | 4.547 | 4.20 | 4.779 | 4.26 | 4.19 | |||
| B | Fucα2 | Galβ4 | GlcNAcβ6 | (Fucα2) | Galβ3 | (GlcNAcβ3) | Galβ3 | GlcNAβ3 | Galβ4 | Glcβ1 | Cer | |
| 5.028 | 4.33 | 4.33 | 4.982 | 4.427 | 4.547 | 4.20 | 4.779 | 4.26 | 4.19 | |||
| C | (Fucα2) | Galβ4 | (Fucα3) | GlcNAcβ6) | (Fucα2) | Galβ3 | (GlcNAcβ3) | Galβ3 | GlcNAβ3 | Galβ4 | Glcβ1 | Cer |
| 4.939 | 4.39 | 4.798 | 4.41 | 4.982 | 4.427 | 4.547 | 4.20 | 4.779 | 4.26 | 4.19 | ||
| D | Fucα2 | Galβ4 | (Fucα3) | GlcNAβ3 | Galβ4 | Glcβ1 | Cera | |||||
| 4.962 | 4.398 | 4.852 | 4.680 | 4.261 | 4.218 |
a For comparative purposes the chemical shift values are given for the Ley hexaglycosylceramide from human erythrocytes characterized by Clausen et al. (41) at 30 °C. It is noteworthy that although the Fucα2 signals in the Ley determinants are only separated by 0.020 ppm for structures C and D the corresponding Fucα3 signals are separated by as much as 0.054 ppm due to the fact that the determinant is located on a 6-branch. Ley determinants on a 6-branch have not been described previously. This conclusion is confirmed, however, by examining the H5/H6 correlations in the double quantum-filtered COSY spectrum, which clearly reveals cross-peaks at 4.00/1.08 and 4.68/1.03 ppm originating from the Fucα2 and Fucα3 residues, respectively, of a Ley determinant.
Comparative Glycosphingolipid Binding Assays
Thereafter, the binding of the specialist H. pylori strain S831 and the generalist strain J99 to a number of reference glycosphingolipids was evaluated. The results are summarized in Table 2. When using this set of reference glycosphingolipids, only the Leb hexaglycosylceramide was recognized by the specialist strain S831 (Fig. 7C, lane 1), whereas the generalist H. pylori strain J99 in addition to the Leb hexaglycosylceramide bound to the A type 1 hexaglycosylceramide (GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer; Fig. 7B, lane 4), the B type 1 hexaglycosylceramide (Galα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer; Table 2, Number 9), the A type 1 heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer; Fig. 7B, lane 3), the B type 1 heptaglycosylceramide (Galα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer; Fig. 7B, lane 2), the A type 1 octaglycosylceramide (GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ3GlcNAcβ3Galβ4Glcβ1Cer; Table 2, Number 17), and the repetitive A type 1 nonaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer; Fig. 7B, lane 6). Furthermore, the chromatogram binding assay revealed that the A type 4 heptaglycosylceramide (Globo A; GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer; Fig. 7B, lane 5) was also recognized by the generalist strain.
TABLE 2.
Comparison of glycosphingolipid binding preferences of a generalist H. pylori strain (J99), a specialist H. pylori strain (S831), and a BabA deletion mutant H. pylori strain (J99/BabA−)
| No. | Abbreviation | Structure | H. pylori J99 | H. pylori S831 | H. pylori J99/BabA− | Source (Ref.) |
|---|---|---|---|---|---|---|
| 1 | A-4 | GalNAcα3(Fucα2)Galβ4Glcβ1Cer | Rat intestine (20) | |||
| 2 | Lea-5 | Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | −a | − | − | Human intestine (42) |
| 3 | Lex-5 | Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Dog intestine (42) |
| 4 | H5 type 1 | Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer | + | + | − | Porcine intestine (22) |
| 5 | H5 type 2 | Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Human erythrocytes (43) |
| 6 | H6 type 4 (Globo H) | Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer | + | + | − | Porcine intestineb |
| 7 | Leb-6 | Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | + | + | − | Human intestine (42) |
| 8 | Ley-6 | Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Dog intestine (42) |
| 9 | B6 type 1 | Galα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer | + | − | − | Human intestine (19) |
| 10 | B6 type 2 | Galα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Human erythrocytes (44) |
| 11 | A6 type 1 | GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer | + | − | − | Human intestine (19) |
| 12 | A6 type 2 | GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Human erythrocytes (45) |
| 13 | B7 type 1 | Galα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | + | − | − | Human intestine (19) |
| 14 | A7 type 1 | GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | + | − | − | Human intestine (19) |
| 15 | A7 type 2 | GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Human erythrocytes (45) |
| 16 | A7 type 4 (Globo A) | GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer | + | − | − | Porcine intestine (22) |
| 17 | A8 type 1 | GalNAcα3(Fucα2)Galβ3GlcNAcβGalβ3GlcNAcβ3Galβ4Glcβ1Cer | + | − | − | Porcine intestine (22) |
| 18 | A9 type 1 | GalNAcα3(Fucα2)Galβ3GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer | + | − | − | Porcine intestine (22) |
| 19 | A9 type 2 | GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Cat intestine (21) |
| 20 | A9 type 3 | GalNAcα3(Fucα2)Galβ3GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Human erythrocytes (46) |
| 21 | Dimeric Lea | Galβ3(Fucα4)GlcNAcβ3Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | Human intestinec |
| 22 | Dimeric Lex | Galβ4(Fucα3)GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | d |
| 23 | Fucα2Galβ3GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer | +e | + | − | Pig intestineb | |
| 24 | Fucα2Galβ4GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer | +e | + | − | Pig intestineb | |
| 25 | Fucα2Galβ4(Fucα3)GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer | +e | + | − | Pig intestineb | |
| 26 | NeuGc-nL6 | NeuGcα3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | + | + | + | Rabbit thymus (47) |
a Binding is defined as follows: + denotes a binding when 1 μg of the glycosphingolipid was applied on the thin-layer chromatogram, whereas − denotes no binding even at 4 μg.
b Present study.
c J. Benktander, J. Ångström, H. Karlsson, M. Lebens, and S. Teneberg, submitted manuscript.
d Glycosphingolipid Number 22 was prepared from sialyl-dimeric Lex (10) by mild acid hydrolysis.
e H. pylori binding to glycosphingolipids Numbers 23–25 was determined using a mixture of the three compounds.
FIGURE 7.
Comparison of binding of a generalist and a specialist H. pylori strain to reference glycosphingolipids. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system. The chromatogram in A was stained with anisaldehyde. Duplicate chromatograms were incubated with the 35S-labeled H. pylori generalist strain J99 (B) and the H. pylori specialist strain S831 (C) followed by autoradiography for 12 h as described under “Experimental Procedures.” Lane 1, Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 2, B type 1 heptaglycosylceramide (Galα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 3, A type 1 heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 4, A type 1 hexaglycosylceramide (GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 5, Globo A/A type 4 heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), 2 μg; lane 6, A nonaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 7, A type 2 heptaglycosylceramide (GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg.
However, no type 2 core counterparts of these compounds were recognized such as e.g. the H type 2 pentaglycosylceramide (Fig. 1, lane 2; Table 2, Number 5), the Ley hexaglycosylceramide (Number 8), the A type 2 hexaglycosylceramide (Number 12), the B type 2 hexaglycosylceramide (Number 10), the A type 2 heptaglycosylceramide (Fig. 8B, lane 7; Number 15), and the A type 2 nonaglycosylceramide (Number 19). Furthermore, the A tetraglycosylceramide (Number 1) and the A type 3 nonaglycosylceramide (Number 20) were also non-binding.
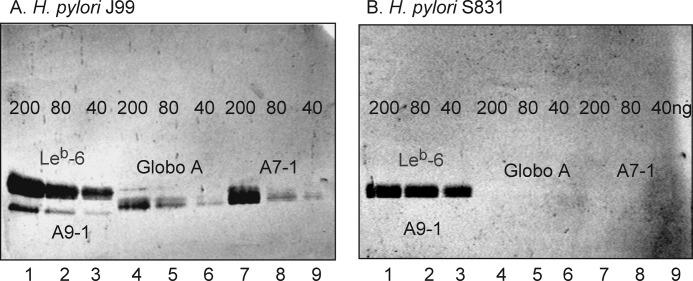
FIGURE 8.
Comparison of binding of a generalist and a specialist H. pylori strain to dilutions of pure glycosphingolipids on thin-layer chromatograms. The glycosphingolipids were separated on aluminum-backed silica gel plates using chloroform/methanol/water (60:35:8 by volume) as the solvent system. The chromatograms were incubated with the 35S-labeled H. pylori generalist strain J99 (A) and the H. pylori specialist strain S831 (B) followed by autoradiography for 12 h as described under “Experimental Procedures.” Lane 1, Leb hexaglycosylceramide (Leb-6; Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 200 ng, and A nonaglycosylceramide (A9-1; GalNAcα3(Fucα2)Galβ3GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 200 ng; lane 2, Leb hexaglycosylceramide, 80 ng, and A nonaglycosylceramide, 80 ng; lane 3, Leb hexaglycosylceramide, 40 ng, and A nonaglycosylceramide, 40 ng; lane 4, Globo A/A type 4 heptaglycosylceramide (Globo A; GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), 200 ng; lane 5, Globo A/A type 4 heptaglycosylceramide, 80 ng; lane 6, Globo A/A type 4 heptaglycosylceramide, 40 ng; lane 7, A type 1 heptaglycosylceramide (A7-1; GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 200 ng; lane 8, A type 1 heptaglycosylceramide, 80 ng; lane 9, A type 1 heptaglycosylceramide, 40 ng.
When the generalist and specialist H. pylori strains were compared with respect to their ability to bind to dilutions of the binding-active glycosphingolipids on thin-layer chromatograms, the Leb hexaglycosylceramide was the preferred ligand of both strains, and two strains bound to this compound with similar detection limits (Fig. 8, lanes 1–3). In addition, the generalist strain J99 bound to the GalNAcα3-substituted Leb (i.e. the A type 1 heptaglycosylceramide), the Globo A heptaglycosylceramide, and the nonaglycosylceramide with repetitive type 1 blood group A determinants in all cases with detection limits at ∼40 ng (Fig. 8A).
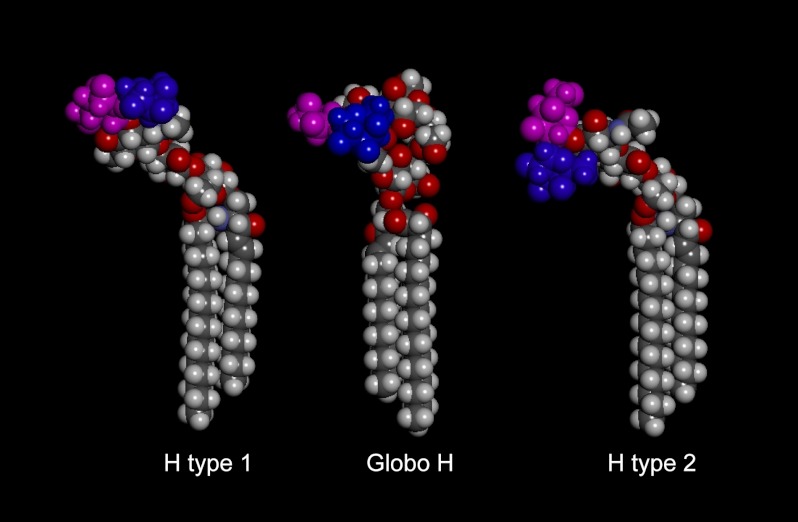
Molecular Modeling
Inspection of the minimum energy models of the H type 1 pentaglycosylceramide and the Globo H hexaglycosylceramide revealed a substantial topographical similarity, which makes it reasonable that these two compounds may be accommodated within the same carbohydrate binding site of BabA (Fig. 9). In contrast, the terminal disaccharide of the non-binding H type 2 pentaglycosylceramide (right) is rotated relative to the same disaccharide in the H type 1 pentaglycosylceramide (left) and the Globo H hexaglycosylceramide (center) by ∼90°, explaining why this compound is non-binding.
FIGURE 9.
Minimum energy models of the H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer) (left), the Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer) (center), and the H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (right). The terminal disaccharides are colored blue (Fucα2) and purple (Galβ3/4). The terminal part of the BabA-binding H type 1 and Globo H glycosphingolipids can be aligned by assuming different Glcβ1Cer torsion angles, whereas the H type 2 terminal disaccharide is rotated ∼90° in comparison, rendering this glycosphingolipid non-binding with respect to BabA.
Binding of Anti-Globo H to Glycosphingolipids from Human Stomach
Having established that H. pylori recognizes the Globo H glycosphingolipid, we next examined whether this glycosphingolipid is present in the target tissue of H. pylori by binding of monoclonal antibodies directed against the Globo H determinant to non-acid glycosphingolipid fractions from human stomach. Thereby, binding in the hexaglycosylceramide region was observed in the non-acid fractions from the stomach of the two individuals tested (Fig. 10B, lanes 1 and 2). Both human stomach samples also contained the Leb hexaglycosylceramide as indicated by the binding of the anti-Leb antibody (Fig. 10C). The anti-H type 1 antibody cross-reacted with the Globo H glycosphingolipid to some extent. However, no binding of the anti-H type 1 antibody to the non-acid glycosphingolipid fractions from human stomach was obtained, although it bound intensely to the pentaglycosylceramide and to the slow migrating glycosphingolipid of blood group O pig intestine (Fig. 10D).
FIGURE 10.
Binding of monoclonal antibodies to human gastric glycosphingolipids. Thin-layer chromatogram after detection with anisaldehyde (A) and autoradiograms obtained by binding of the monoclonal anti-Globo H antibody MBr1 (B), the monoclonal anti-Leb antibody BG-6/T218 (C), and the monoclonal anti-H type 1 antibody 17-206 (D) are shown. The chromatograms were eluted with chloroform/methanol/water (60:35:8 by volume), and the binding assays were done as described under “Experimental Procedures” followed by autoradiography for 12 h. Lane 1, non-acid glycosphingolipids of human stomach (Individual I; blood group O), 40 μg; lane 2, non-acid glycosphingolipids of human stomach (Individual II; blood group A), 40 μg; lane 3, reference Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 4, non-acid glycosphingolipids of the intestinal epithelium of a blood group O pig, 40 μg; lane 5, non-acid glycosphingolipids of the intestinal epithelium of a blood group A pig, 40 μg; lane 6, reference H type 1 pentaglycosylceramide (Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer), 2 μg; lane 7, reference Globo H hexaglycosylceramide (Fucα2Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), 1 μg; lane 8, reference Globo A heptaglycosylceramide (GalNAcα3(Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glcβ1Cer), 4 μg.
DISCUSSION
The binding of microbes to host target cells is crucial to the delivery of virulence factors, and in the case of H. pylori, it was recently shown that BabA-mediated binding of the bacteria to Leb on the epithelium leads to an increased type IV secretion system activity, resulting in the production of proinflammatory cytokines and precancer-related factors (34).
The initial observation that the fucosylated blood group antigens H type 1 and Leb are mediators of H. pylori adhesion to human gastric epithelial cells (5) was followed by a division of BabA-producing H. pylori strains into specialist and generalist strains, depending on their mode of binding to Leb and related carbohydrate sequences (9). The BabA of specialist strains binds only to glycoconjugates with an unsubstituted terminal Fucα2Gal sequence as in the H type 1 and Leb determinants, whereas the generalist BabA tolerates a substitution at 3-position of the Gal with an αGal or αGalNAc as in the A or B type 1 and ALeb or BLeb determinants.
Here, we further explored the structural requirements for carbohydrate recognition by BabA of generalist and specialist H. pylori by isolating and characterizing glycosphingolipids recognized by wild type specialist and/or generalist H. pylori but not by the deletion mutant strain lacking the BabA adhesin. The Leb epitope has only been found in humans, but we initially thought that we had found a porcine Leb glycosphingolipid when an H. pylori BabA-binding glycosphingolipid co-migrating with the Leb hexaglycosylceramide was detected in the non-acid fraction of blood group O pig intestine. However, after isolation, this BabA-binding glycosphingolipid was characterized as the Globo H hexaglycosylceramide. Further comparative binding studies using our glycosphingolipid collection confirmed that the BabA adhesin in addition to blood group determinants on type 1 core chains recognizes blood group O and A determinants on type 4 core chains with binding to Globo H by both strains and Globo A by the generalist strain. The terminal disaccharides (Fucα2Galβ3) of the H type 1 pentaglycosylceramide and the Globo H hexaglycosylceramide adopt conformations very similar to each other, and this is also the case for the terminal trisaccharides (GalNAcα3(Fucα2)Galβ3) of the A type 1 and the Globo A heptaglycosylceramides (18). These conformational similarities thus explain the binding of BabA to the two sets of isoreceptors.
The enzymatic machinery involved in the biosynthesis of Globo H has not yet been fully elucidated. In humans, there are two functional fucosyltransferases, designated FUT1 and FUT2, that catalyze addition of an α2-linked fucose to a terminal galactose to form the blood group H epitope (for a review, see Ref. 35). These two fucosyltransferases are encoded by two distinct genes, FUT1 and FUT2. FUT1 acts preferentially on type 2 chains, whereas type 1 and type 3 chains and to some extent type 2 chains are acceptors for FUT2. Using siRNAs targeting FUT1 and FUT2 in breast cancer stem cells, Chang et al. (36) showed that Globo H may be synthesized by both FUT1 and FUT2.
Non-secretor individuals have an increased risk of peptic ulcer disease (37). In these individuals, the precursor of the Leb sequence, i.e. the H type 1 sequence, is not formed due to lack of a functional FUT2 enzyme. Consequently, non-secretors have low amounts of or no Leb antigens on their epithelial surfaces. However, the Globo H sequence can still be formed by FUT1 and might thus function as an adhesion factor for BabA-expressing H. pylori in non-secretor individuals.
The slow migrating BabA-binding fraction P-III was characterized as a mixture of three complex glycosphingolipids (Fucα2Galβ4GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer, Fucα2Galβ3GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer, and Fucα2Galβ4(Fucα3)GlcNAcβ6(Fucα2Galβ3GlcNAcβ3)Galβ3GlcNAcβ3Galβ4Glcβ1Cer). The undecaglycosylceramide with an Ley epitope on the 6-branch and an H type 1 epitope on the 3-branch are to our knowledge novel glycosphingolipid structures. The three compounds in fraction P-III all had an H type 1 determinant on at least one branch, and thus, all three could be recognized by both specialist and generalist BabA.
The binding of the generalist H. pylori strain to the nonaglycosylceramide with a repetitive blood group A determinant and an internal type 1 core chain (GalNAcα3(Fucα2)Galβ3GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer) is a wild card. We have previously found that generalist H. pylori strains bind to the ganglio-Leb hexaglycosylceramide (Fucα2Galβ3(Fucα4)GalNAcβ4Galβ4Glcβ1Cer) (13). Thus, the binding to this nonaglycosylceramide is most likely due to recognition of the terminal A determinant on the ganglio core by the generalist BabA.
Characterization of the binding specificities of the BabA variants is important for understanding the molecular interactions between H. pylori and the target host cells. The presence of the BabA-binding Globo H glycosphingolipid in the human stomach was here indicated by the binding of monoclonal antibodies directed against the Globo H determinant to human gastric glycosphingolipids. Thus, Globo H may have a role in the BabA-mediated target tissue adherence of H. pylori.
Expression of BabA by H. pylori is associated with severe gastric inflammation and an increased risk of developing peptic ulcer or gastric cancer (38, 39). H. pylori infects more than half of the world's population, and although the prevalence of infection is decreasing in developed countries, the infection rate is still high in developing countries (40). Furthermore, the treatment options in developing countries are currently inadequate. Targeting BabA might be important for the development of novel treatment strategies against H. pylori.
Acknowledgment
The use of the LTQ linear quadrupole ion trap mass spectrometer (obtained by Swedish Research Council Grant 342-2004-4434 to Gunnar Hansson) and the Varian 600-MHz machine at the Swedish NMR Centre, Hasselblad Laboratory, University of Gothenburg, is gratefully acknowledged.
This study was supported by Swedish Research Council Grant 12628, the Swedish Cancer Foundation, and governmental grants (to the Sahlgrenska University Hospital).

This article contains supplemental Figs. S1 and S2.
The glycosphingolipid nomenclature follows the recommendations by the IUPAC-IUB Commission on Biochemical Nomenclature (Chester, M. A. (1998) IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of glycolipids—recommendations 1997. Eur. J. Biochem. 257, 293–298). It is assumed that Gal, Glc, GlcNAc, GalNAc, NeuAc, and NeuGc are of the d configuration; Fuc is of the l configuration; and all sugars are present in the pyranose form.
- Leb
- Lewis b antigen
- BabA
- blood group antigen-binding adhesin
- ESI
- electrospray ionization
- Hex
- hexose
- HexNAc
- N-acetylhexosamine
- Cer
- ceramide
- Ley
- Lewis y antigen
- Lea
- Lewis a antigen
- NeuGc
- N-glycolylneuraminic acid.
REFERENCES
- 1. Karlsson K. A. (1989) Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 58, 309–350 [DOI] [PubMed] [Google Scholar]
- 2. Esko J. D. (1999) Essentials in Glycobiology (Varki A., Cummings R., Esko J., Freeze H., Hart G., Marth J., eds) pp. 429–440, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 3. Pieters R. J. (2011) Carbohydrate mediated bacterial adhesion. Adv. Exp. Med. Biol. 715, 227–240 [DOI] [PubMed] [Google Scholar]
- 4. Stults C. L., Sweeley C. C., Macher B. A. (1989) Glycosphingolipids: structure, biological source and properties. Methods Enzymol. 179, 167–214 [DOI] [PubMed] [Google Scholar]
- 5. Borén T., Falk P., Roth K. A., Larson G., Normark S. (1993) Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262, 1892–1895 [DOI] [PubMed] [Google Scholar]
- 6. Ilver D., Arnqvist A., Ogren J., Frick I. M., Kersulyte D., Incecik E. T., Berg D. E., Covacci A., Engstrand L., Borén T. (1998) Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279, 373–377 [DOI] [PubMed] [Google Scholar]
- 7. Gerhard M., Lehn N., Neumayer N., Borén T., Rad R., Schepp W., Miehlke S., Classen M., Prinz C. (1999) Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. U.S.A. 96, 12778–12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rad R., Gerhard M., Lang R., Schöniger M., Rösch T., Schepp W., Becker I., Wagner H., Prinz C. (2002) The Helicobacter pylori blood group antigen-binding adhesin facilitates bacterial colonization and augments a non-specific immune response. J. Immunol. 168, 3033–3041 [DOI] [PubMed] [Google Scholar]
- 9. Aspholm-Hurtig M., Dailide G., Lahmann M., Kalia A., Ilver D., Roche N., Vikström S., Sjöström R., Lindén S., Bäckström A., Lundberg C., Arnqvist A., Mahdavi J., Nilsson U. J., Velapatiño B., Gilman R. H., Gerhard M., Alarcon T., López-Brea M., Nakazawa T., Fox J. G., Correa P., Dominguez-Bello M. G., Perez-Perez G. I., Blaser M. J., Normark S., Carlstedt I., Oscarson S., Teneberg S., Berg D. E., Borén T. (2004) Functional adaptation of BabA, the Helicobacter pylori blood-group antigen binding adhesin. Science 305, 519–522 [DOI] [PubMed] [Google Scholar]
- 10. Mahdavi J., Sondén B., Hurtig M., Olfat F. O., Forsberg L., Roche N., Angstrom J., Larsson T., Teneberg S., Karlsson K. A., Altraja S., Wadström T., Kersulyte D., Berg D. E., Dubois A., Petersson C., Magnusson K. E., Norberg T., Lindh F., Lundskog B. B., Arnqvist A., Hammarström L., Borén T. (2002) Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297, 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karlsson K. A. (1987) Preparation of total non-acid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 138, 212–220 [DOI] [PubMed] [Google Scholar]
- 12. Waldi D. (1962) in Thin-layer Chromatography (Stahl E., ed) pp. 496–515, Springer-Verlag, Berlin [Google Scholar]
- 13. Fagerberg D., Angström J., Halim A., Hultberg A., Rakhimova L., Hammarström L., Borén T., Teneberg S. (2009) Novel Leb-like Helicobacter pylori-binding glycosphingolipid created by expression of human α-1,3/4-fucosyltransferase in FVB/N mouse stomach. Glycobiology 19, 182–191 [DOI] [PubMed] [Google Scholar]
- 14. Ito M., Yamagata T. (1989) Purification and characterization of glycosphingolipid-specific endoglycosidases (endoglycoceramidases) from a mutant strain of Rhodococcus sp. Evidence for three molecular species of endoglycoceramidase with different specificities. J. Biol. Chem. 264, 9510–9519 [PubMed] [Google Scholar]
- 15. Karlsson H., Halim A., Teneberg S. (2010) Differentiation of glycosphingolipid-derived glycan structural isomers by liquid chromatography-mass spectrometry. Glycobiology 20, 1103–1116 [DOI] [PubMed] [Google Scholar]
- 16. Marion D., Wüthrich K. (1983) Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem. Biophys. Res. Commun. 113, 967–974 [DOI] [PubMed] [Google Scholar]
- 17. Imberty A., Mikros E., Koca J., Mollicone R., Oriol R., Pérez S. (1995) Computer simulation of histo-blood group oligosaccharides: energy maps of all constituting disaccharides and potential energy surfaces of 14 ABH and Lewis carbohydrate antigens. Glycoconj. J. 12, 331–349 [DOI] [PubMed] [Google Scholar]
- 18. Nyholm P. G., Samuelsson B. E., Breimer M., Pascher I. (1989) Conformational analysis of blood group A-active glycosphingolipids using HSEA-calculations. The possible significance of the core oligosaccharide chain for the presentation and recognition of the A-determinant. J. Mol. Recognit. 2, 103–113 [DOI] [PubMed] [Google Scholar]
- 19. Björk S., Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H. (1987) Structures of blood group glycosphingolipids of human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J. Biol. Chem. 262, 6758–6765 [PubMed] [Google Scholar]
- 20. Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H. (1982) Isolation and partial characterization of blood group A and H active glycosphingolipids of rat small intestine. J. Biol. Chem. 257, 906–912 [PubMed] [Google Scholar]
- 21. Angström J., Bäckström M., Berntsson A., Karlsson N., Holmgren J., Karlsson K. A., Lebens M., Teneberg S. (2000) Novel carbohydrate binding site recognizing blood group A and B determinants in a cholera toxin/heat-labile enterotoxin B-subunit hybrid. J. Biol. Chem. 275, 3231–3238 [DOI] [PubMed] [Google Scholar]
- 22. Coddens A., Diswall M., Angström J., Breimer M. E., Goddeeris B., Cox E., Teneberg S. (2009) Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J. Biol. Chem. 284, 9713–9726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diswall M., Angström J., Schuurman H. J., Dor F. J., Rydberg L., Breimer M. E. (2008) Glycolipid studies in small intestine and pancreas of α1,3-galactosyltransferase knockout miniature swine: α1,3GALT-KO animals lack αGAL antigens and contain novel blood group H compounds. Transplant. Proc. 40, 543–546 [DOI] [PubMed] [Google Scholar]
- 24. Kundu S. K. (1993) Glycoconjugates. Composition, Structure and Function (Allen H. J., Kisailus E. E., eds) pp. 203–262, Marcel Dekker Inc., New York [Google Scholar]
- 25. Hakomori S. (2001) Tumor-associated carbohydrate antigens defining tumor malignancy: basis for development of anti-cancer vaccines. Adv. Exp. Med. Biol. 491, 369–402 [DOI] [PubMed] [Google Scholar]
- 26. Teneberg S., Leonardsson I., Karlsson H., Jovall P. A., Angstrom J., Danielsson D., Naslund I., Ljungh A., Wadstrom T., Karlsson K. A. (2002) Lactotetraosylceramide, a novel glycosphingolipid receptor for Helicobacter pylori, present in human gastric epithelium. J. Biol. Chem. 277, 19709–19719 [DOI] [PubMed] [Google Scholar]
- 27. Chai W., Piskarev V., Lawson A. M. (2001) Negative-ion electrospray mass spectrometry of neutral underivatized oligosaccharides. Anal. Chem. 73, 651–657 [DOI] [PubMed] [Google Scholar]
- 28. Robbe C., Capon C., Coddeville B., Michalski J. C. (2004) Diagnostic ions for the rapid analysis by nano-electrospray ionization quadrupole time-of-flight mass spectrometry of O-glycans from human mucins. Rapid Commun. Mass Spectrom. 18, 412–420 [DOI] [PubMed] [Google Scholar]
- 29. Holgersson J., Jovall P. A., Samuelsson B. E., Breimer M. E. (1990) Structural characterization of non-acid glycosphingolipids in kidneys of single blood group O and A pigs. J. Biochem. 108, 766–777 [DOI] [PubMed] [Google Scholar]
- 30. Li Y. T., Chou C. W., Li S. C., Kobayashi U., Ishibashi Y. H., Ito M. (2009) Preparation of homogenous oligosaccharide chains from glycosphingolipids. Glycoconj. J. 26, 929–933 [DOI] [PubMed] [Google Scholar]
- 31. Zhou B., Li S. C., Laine R. A., Huang R. T., Li Y. T. (1989) Isolation and characterization of ceramide glycanase from the leech, Macrobdella decora. J. Biol. Chem. 264, 12272–12277 [PubMed] [Google Scholar]
- 32. Chai W., Piskarev V., Lawson A. M. (2002) Branching pattern and sequence analysis of underivatized oligosaccharides by combined MS/MS of singly and doubly charged molecular ions in negative-ion electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 670–679 [DOI] [PubMed] [Google Scholar]
- 33. Angström J., Larsson T., Hansson G. C., Karlsson K. A., Henry S. (2004) Default biosynthesis pathway for blood group-related glycolipids in human small intestine as defined by structural identification of linear and branched glycosylceramides in a group O Le(a−b−) nonsecretor. Glycobiology 14, 1–12 [DOI] [PubMed] [Google Scholar]
- 34. Ishijima N., Suzuki M., Ashida H., Ichikawa Y., Kanegae Y., Saito I., Borén T., Haas R., Sasakawa C., Mimuro H. (2011) BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J. Biol. Chem. 286, 25256–25264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marionneau S., Cailleau-Thomas A., Rocher J., Le Moullac-Vaidye B., Ruvoën N., Clément M., Le Pendu J. (2001) ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83, 565–573 [DOI] [PubMed] [Google Scholar]
- 36. Chang W. W., Lee C. H., Lee P., Lin J., Hsu C. W., Hung J. T., Lin J. J., Yu J. C., Shao L. E., Yu J., Wong C. H., Yu A. L. (2008) Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc. Natl. Acad. Sci. U.S.A. 105, 11667–11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cederberg A., Varis K., Salmi H. A., Sipponen P., Härkönen M., Sarna S. (1991) Young onset peptic ulcer disease and non-ulcer dyspepsia are separate entities. Scand. J. Gastroenterol. Suppl. 186, 33–44 [DOI] [PubMed] [Google Scholar]
- 38. Fujimoto S., Olaniyi Ojo O., Arnqvist A., Wu J. Y., Odenbreit S., Haas R., Graham D. Y., Yamaoka Y. (2007) Helicobacter pylori BabA expression, gastric mucosal injury, and clinical outcome. Clin. Gastroenterol. Hepatol. 5, 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamaoka Y., Ojo O., Fujimoto S., Odenbreit S., Haas R., Gutierrez O., El-Zimaity H. M., Reddy R., Arnqvist A., Graham D. Y. (2006) Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 55, 775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown L. M. (2000) Helicobacter pylori: epidemiology and routes of transmission. Epidemiol. Rev. 22, 283–297 [DOI] [PubMed] [Google Scholar]
- 41. Clausen H., Levery S. B., McKibbin J. M., Hakomori S. (1985) Blood group A determinants with mono- and difucosyl type 1 chain in human erythrocyte membranes. Biochemistry 24, 3578–3586 [DOI] [PubMed] [Google Scholar]
- 42. McKibbin J. M., Spencer W. A., Smith E. L., Mansson J. E., Karlsson K. A., Samuelsson B. E., Li Y. T., Li S. C. (1982) Lewis blood group fucolipids and their isomers from human and canine intestine. J. Biol. Chem. 257, 755–760 [PubMed] [Google Scholar]
- 43. Stellner K., Watanabe K., Hakomori S. (1973) Isolation and characterization of glycosphingolipids with blood group H specificity from membranes of human erythrocytes. Biochemistry 12, 656–661 [DOI] [PubMed] [Google Scholar]
- 44. Kościelak J., Plasek A., Górniak H., Gardas A., Gregor A. (1973) Structures of fucose-containing glycolipids with H and B blood-group activity and of sialic acid and glucosamine-containing glycolipid of human-erythrocyte membrane. Eur. J. Biochem. 37, 214–225 [DOI] [PubMed] [Google Scholar]
- 45. Koscielak J., Piasek A., Gorniak H. (1970) in Blood and Tissue Antigens (Aminoff D., ed) pp. 163–176, Academic Press, New York [Google Scholar]
- 46. Clausen H., Levery S. B., Nudelman E., Tsuchiya S., Hakomori S. (1985) Repetitive A epitope (type 3 chain A) defined by blood group A1-specific monoclonal antibody TH-1: chemical basis of qualitative A1 and A2 distinction. Proc. Natl. Acad. Sci. U.S.A. 82, 1199–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iwamori M., Nagai Y. (1981) Ganglioside composition of rabbit thymus. Biochim. Biophys. Acta 665, 205–213 [DOI] [PubMed] [Google Scholar]