Background: ArgIII:26 (Arg3.50) is almost 100% conserved among 7TM receptors.
Results: Alanine substitution of ArgIII:26 was basically silent while providing expected positive and negative effects when applied to its proposed interaction partners.
Conclusion: The ArgIII:26 micro-switch stabilizes both active and inactive receptor conformations but is not directly involved in receptor signaling.
Significance: This work provides clarification of the molecular mechanism of a crucial micro-switch in 7TM receptors.
Keywords: 7-Helix Receptor, Adrenergic Receptor, Arrestin, Computer Modeling, G Protein-coupled Receptors (GPCR), Molecular Dynamics, Molecular Modeling, Mutagenesis Site Specific, Receptor Structure-Function, Signal Transduction
Abstract
Recent high resolution x-ray structures of the β2-adrenergic receptor confirmed a close salt-bridge interaction between the suspected micro-switch residue ArgIII:26 (Arg3.50) and the neighboring AspIII:25 (Asp3.49). However, neither the expected “ionic lock” interactions between ArgIII:26 and GluVI:-06 (Glu6.30) in the inactive conformation nor the interaction with TyrV:24 (Tyr5.58) in the active conformation were observed in the x-ray structures. Here we find through molecular dynamics simulations, after removal of the stabilizing T4 lysozyme, that the expected salt bridge between ArgIII:26 and GluVI:-06 does form relatively easily in the inactive receptor conformation. Moreover, mutational analysis of GluVI:-06 in TM-VI and the neighboring AspIII:25 in TM-III demonstrated that these two residues do function as locks for the inactive receptor conformation as we observed increased Gs signaling, arrestin mobilization, and internalization upon alanine substitutions. Conversely, TyrV:24 appears to play a role in stabilizing the active receptor conformation as loss of function of Gs signaling, arrestin mobilization, and receptor internalization was observed upon alanine substitution of TyrV:24. The loss of function of the TyrV:24 mutant could partly be rescued by alanine substitution of either AspIII:25 or GluVI:-06 in the double mutants. Surprisingly, removal of the side chain of the ArgIII:26 micro-switch itself had no effect on Gs signaling and internalization and only reduced arrestin mobilization slightly. It is suggested that ArgIII:26 is equally important for stabilizing the inactive and the active conformation through interaction with key residues in TM-III, -V, and -VI, but that the ArgIII:26 micro-switch residue itself apparently is not essential for the actual G protein activation.
Introduction
Over the last years, a number of high resolution x-ray structures of not only inactive but also presumably active structures of 7-transmembrane (TM)3 G protein-coupled receptors have been published (1–9). This has revealed an overall activation mechanism involving a major outward movement of the intracellular segment of TM-VI away from TM-III, opening a pocket for the G protein to interact with the receptor, a movement that was expected based on a number of previous biochemical and biophysical studies (10, 11). However, these structures have still left us with a large degree of uncertainty concerning the role of a number of conserved so-called micro-switch regions presumed to display distinct interaction patterns in the inactive versus the active receptor conformations and consequently believed to be important elements of the activation process (12).
The most canonical micro-switch residue is ArgIII:26 (3.50)4 of the so-called DRY motif at the intracellular pole of TM-III, which for decades has been expected to be crucially involved in receptor activation due to the fact that it is conserved as an arginine residue in close to 100% of 7TM receptors (see Fig. 1A) (13). When rhodopsin as the first 7TM receptor was crystallized in its inactive conformation with 11-cis retinal bound (2), ArgIII:26 was found to be stabilized by two salt bridges: one to the neighboring AspIII:25 (3.49) and one to a glutamic acid residue in position VI:-06 (6.30) located a couple of helical turns before TM-VI enters the lipid bilayer (see Fig. 1B). ArgIII:26 and its proposed ionic interaction partners have been subject to mutational analysis in a number of receptors over the years as reviewed by Rovati et al. (14). In a majority of receptors, ArgIII:26 is essential for receptor activation. However, there are receptors where this is not the case. Similarly, mutational analysis of the neighboring Asp or Glu in position III:25 and the proposed interhelical salt-bridge partner GluVI:-06 has somewhat different effects on constitutive activity and agonist-induced signaling in different receptors (14).
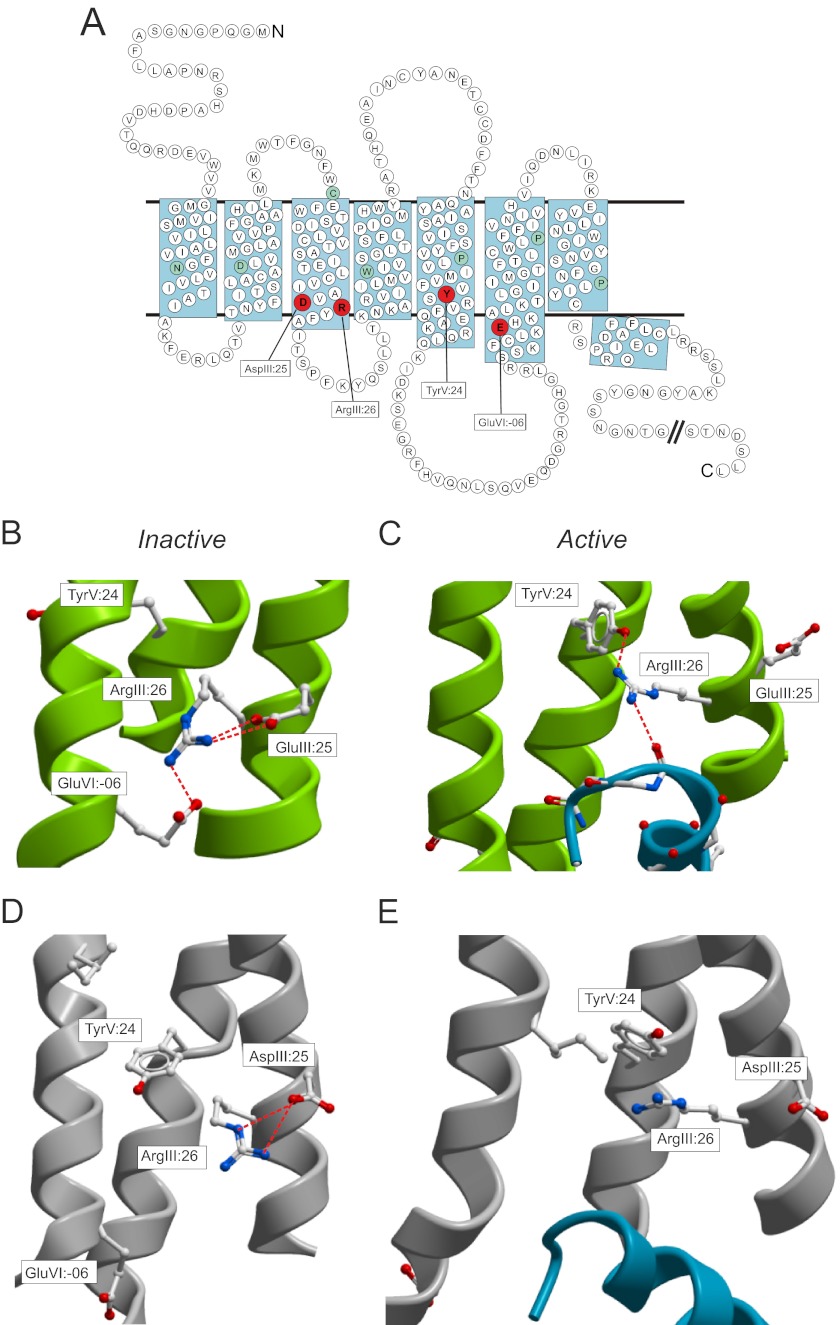
FIGURE 1.
The DRY motif with interaction partners in rhodopsin/opsin and the B2AR. A, serpentine model of the B2AR. The interaction partners for the ArgIII:26 micro-switch for the inactive structure, AspIII:25 and GluVI:-06, and active structure, TyrVI:24, are marked in red. B, inactive structure of rhodopsin (Protein Data Bank (PDB): 1F88) displaying the ionic lock as viewed from TM-VII. Only key residues are indicated in sticks. C, active structure of opsin (PDB: 3DQB) displaying the breakage of the ionic lock and the interaction of the TyrV:24 residue to the ArgIII:26 and again from the ArgIII.26 to the Gα-peptide. D, inactive structure of the B2AR (PDB: 2RH1). E, active structure of B2AR with Gs protein (PDB: 3SN6).
When the first structure of a presumed active conformation of a 7TM receptor, i.e. opsin in complex with the C-terminal peptide fragment of Gα, was published by Scheerer et al. (6) in 2008, it demonstrated that the salt-bridge interactions of ArgIII:26, as expected, were broken and that ArgIII:26 directly interacted with the backbone of the G protein (see Fig. 1C). Interestingly, this structure also revealed an unexpected interaction between a conserved tyrosine residue in TM-V, TyrV:24 (5.58) and ArgIII:26, indicating that TyrV:24 was involved in stabilizing the active conformation of ArgIII:26 and thereby the active conformation of the receptor as such (see Fig. 1C).
The x-ray structures of the β2-adrenergic receptor (B2AR) in its inactive conformation bound to the inverse agonist carazolol confirmed the formation of a salt bridge between ArgIII:26 and the neighboring AspIII:25. However, in contrast to what was found in rhodopsin, no interaction between ArgIII:26 and the proposed “ionic lock” partner GluVI:-06 in TM-VI was observed (3, 8) (see Fig. 1D). In fact, this interhelical salt bridge has not been observed in any 7TM receptor crystal structure besides rhodopsin. Recently, the two active structures of the B2AR both revealed a broken AspIII:25-ArgIII:26 salt bridge but did not demonstrate any direct interaction between ArgIII:26 and the G protein or between ArgIII:26 and TyrV:24 (see Fig. 1E)(4,5). Thus, the various x-ray structures do not clarify the role of ArgIII:26 of the DRY motif and its proposed interaction partners in the active and inactive conformations. Due to the unclear picture concerning the micro-switch residue ArgIII:26 and its proposed interaction partners despite the presence of multiple both active and inactive high resolution x-ray structures, we subjected these residues in the B2AR receptor to mutational analysis and studied them in parallel for their ability to signal through the Gs protein, recruit β-arrestin, and undergo internalization.
EXPERIMENTAL PROCEDURES
Materials
Pindolol and isoproterenol were purchased from Bachem.
Molecular Biology
Receptor cDNAs were cloned into the eukaryotic expression vector pCMV-Tag(2B) (Stratagene). Mutations were constructed by PCR using the QuikChange method. All PCR experiments were performed using Pfu polymerase (Stratagene) according to the instructions from the manufacturer. All mutations were verified by DNA sequence analysis by MWG-Biotech AG (Ebersberg, Germany). For the bioluminescence resonance energy transfer (BRET) assay, the B2AR was tagged C-terminally with Renilla luciferase and cloned into the pcDNA3.1+ vector. β-arrestin2 was cloned into pcDNA3.1+ vector and tagged N-terminally with green fluorescent protein (GFP) as described previously (15, 16).
Tissue Culture and Transfections
COS-7 cells were grown in Dulbecco's modified Eagle's medium 1885 supplemented with 10% fetal calf serum, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were transfected using the calcium phosphate precipitation method with chloroquine addition as described previously (17). The amount of cDNA resulting in maximal basal signaling (20 μg/75 cm2) was used for the dose-response curves. Transfection for the BRET assay was performed using a 1:3 ratio B2AR: β-arrestin2 using a total of 20 μg of DNA/75-cm2 flask. CHO-K1 cells were maintained in HAM F12 medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. CHO-K1 cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Cell Surface Expression (ELISA)
Cells transfected and seeded for cAMP were in parallel seeded for ELISA. The cells were washed twice with PBS, fixed for 10 min with formaldehyde, and incubated in blocking solution (3% dry milk in phosphate-buffered saline (PBS)) for 30 min at room temperature. Subsequently, the cells were incubated 1 h at room temperature with anti-FLAG (M2) (Sigma) antibody diluted 1:1000 with PBS, 3% milk. The cells were washed three times with PBS and incubated 1 h at room temperature with anti-mouse horseradish peroxidase-conjugated antibody (Sigma) diluted 1:1250 in PBS, 3% milk. After three additional washing steps with PBS, immunoreactivity was discovered by the addition of horseradish peroxidase, and after 5 min, the reaction was stopped by the addition of H2SO4. The absorbance was read on the TopCount (PerkinElmer Life Sciences).
cAMP Assay
1 day after transfection, COS-7 cells were plated into poly-d-lysine-coated white 96-well plates (20.00 cells/well). The following day, a cAMP assay was performed using the DiscoveRx HitHunterTM cAMP XS+ kit (Freemont, CA) according to the manufacturer's protocol. For transfected CHO-K1 cells, the cAMP assay was performed as described previously (18).
BRET
The protocol for BRET measurements was developed for the Mithras LB 940 plate reader (Berthold Technologies) as described previously (15, 16). Briefly, 48 h after transfection, the cells were harvested and washed twice in PBS. After counting, the cells were resuspended in PBS (added 1 mg/ml glucose and 36 mg/liter pyruvate) to a density of 1 million cells/ml. After 1 h of recovery, 180 μl of cell suspension/well was added to white 96-well plates. 10 μl of the ligand isoproterenol was added to each well and incubated for 15 min while shaking at room temperature. 10 μl of the substrate coelenterazine (Biotium) was added to each well by the Mithras injector 1, and readings were collected 2 s after the injection. The signals detected at 395 and 515 nm were measured sequentially, and the 515/395 ratios were calculated and expressed as a milliBRET level (BRET ratio × 1000).
Calculations
EC50 values were determined by nonlinear regression using the Prism 3.0 software (GraphPad Software, San Diego). The basal constitutive activity is expressed as percentage of the ligand-induced activation for each mutant construct of the receptors.
Internalization
B2AR constructs were fused to an N-terminal SNAP-tag (New England Biolabs) and inserted into the pcDNA3.1+ vector for expression as stable mixed populations in HEK293 cells. These cells were seeded into poly-d-lysine-coated 96-well imaging plates (Greiner Bio-One Ltd.), and 24 h later, they were labeled with membrane-impermeable SNAP-surface AF488 (0.1 μm in DMEM, New England Biolabs) for 30 min at 37 °C to identify B2ARs initially at the cell surface. Unreacted fluorophore was removed by washing before isoproterenol treatment at 37 °C (30 min) in Hanks' balanced salt solution containing 0.1% BSA and 5 μg/ml Alexa Fluor 633-conjugated transferrin (Invitrogen). Incubations were terminated by fixation (3% paraformaldehyde), and cell nuclei were also labeled (H33342, 2 μg/ml in phosphate-buffered saline). Images at four sites/well were then acquired using the IX Ultra confocal plate reader (Molecular Devices Inc.; 40× ELWD objective), with appropriate excitation and emission filters for nuclei labeling (405 nm excitation), SNAP-surface Alexa Fluor 488-labeled B2ARs (488 nm), and transferrin (633 nm).
Automated translocation analysis of plate reader images (MetaXpress 2.0, Molecular Devices) quantified the fluorescence intensity of labeled B2AR receptors within 3-μm-diameter internal compartments identified by transferrin labeling. For each mutant receptor, individual concentration-response curves performed in duplicate were normalized with respect to the -fold increase over the basal SNAP-B2AR intensity in transferrin compartments. Pooled data were used to obtain EC50 values with GraphPad Prism (sigmoidal fit, nH 0.9–1.0).
Molecular Dynamics Simulation
The inactive x-ray structure of B2AR (2RH1) (8, 19) was used as a starting structure in these simulations where the T4 lysozyme part of the structure was truncated and the intracellular parts of TM-V and TM-VI were covalently connected. Because the structure contained coordinates for residues 29–230 and 263–342, only these residues were considered in the simulation. As it has been seen in the x-ray structure, Cys-341 was also palmitoylated in the simulations. Hydrogens were added using the HBUILD facility in CHARMM (20), and protonation states according to pH = 7 were used for all aspartic acids, except AspII:10 (2.50). The general setup for the molecular dynamics (MD) simulation was as described previously (21).
RESULTS
Molecular Dynamics Simulations of ArgIII:26 Interactions in B2AR
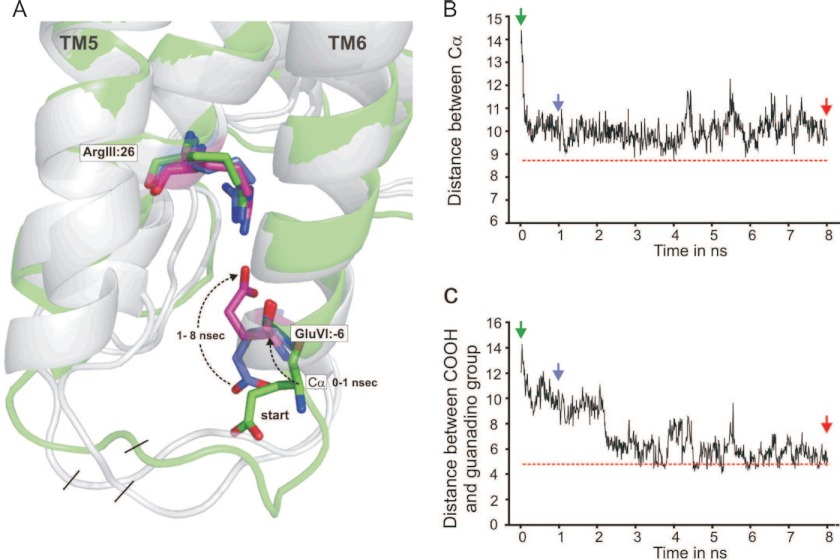
In the x-ray structure of the B2AR fused to T4 lysozyme (T4L) in complex with the inverse agonist carazolol, ArgIII:26 forms a salt-bridge interaction with the neighboring AspIII:25 (8) (Fig. 1D). However, GluVI:-06 forms a salt bridge with an arginine residue, Arg-8, in T4L and is consequently held at a distance from ArgIII:26. To study the potential, free interaction between ArgIII:25 and GluVI:-06, we removed T4L from the structure (see “Experimental Procedures” for details) and subjected this structure to MD simulations. The carboxylic acid group of GluVI:-06 started out being ∼13 Å away from the guanidine group of ArgIII:26 (Fig. 2, green structures); however, during the MD simulations, a salt bridge was nevertheless formed between these two groups. The conformational changes leading to this occurred in a two-step fashion. Initially, i.e. during the first 0.5 ns, the backbone structure around GluVI:-06 folded into a perfect helical conformation as an extension of the TM-VI (Fig. 2A). Consequently, the distance between the α-carbon of GluVI:-06 and the α-carbon of ArgIII:26 decreased from ∼5 to ∼10 Å (Fig. 2B). Thereby the Cα distance became similar to the distance between the Cα of ArgIII:26 and GluVI:-06 in rhodopsin (Fig. 2B, red dotted line). During the continued MD simulation, the side chain of GluVI:-06 probed different conformations in space, and after ∼2 ns, it formed a salt bridge with the guanidine group of ArgIII:26, and this distance is very similar to the distance observed in rhodopsin (Fig. 2, B and C, red lines). Throughout the MD simulations, ArgIII:26 was relatively immobile, conceivably due to its salt-bridge interaction with the neighboring AspIII:25.
FIGURE 2.
Molecular dynamics simulation studies of the salt bridge between ArgIII:26 of the DRY motif at the intracellular end of TM-III and GluVI:-06 at the intracellular extension of TM-VI in B2AR. A, The structure of the intracellular ends of TM-III, -V, and -VI in a molecular model of the B2AR in which T4-lysozyme was removed from the T4L-B2AR structure (2RH1) and the two ends joined. The structure shown in green is the starting structure, and structures after 1- and 8-ns simulation are also shown; in all structures, ArgIII:26, AspIII:25, TyrV:24, and GluVI:-06 are shown as sticks. Note that initially (0–1 ns), the backbone around GluVI:-06, which was in a loop-like structure, swings into a regular α-helical extension of TM-VI, and subsequently (1–8 ns), the side chain of GluVI:-06 swings over toward ArgIII:26 to form the salt bridge. B, the distance between the Cα of ArgIII:26 and GluVI:-06 as a function of simulation time. The horizontal red line indicates the distance between Cα of ArgIII:26 and GluVI:-06 in the rhodopsin structure (1GZM). C, the distance between the carbon atom in the guanidine and carboxylic acid groups of ArgIII:26 and GluVI:-06, respectively, during the molecular dynamics simulation. The horizontal red line indicates the distance between the carbon atom in the guanidine and carboxylic acid groups of ArgIII:26 and GluVI:-0g in the rhodopsin structure (1GZM).
The fact that a salt bridge is formed between ArgIII:26 and GluVI:-06 in B2AR when the T4L fusion partner is removed indicates that this is a preferred interaction in the inactive conformation of the B2AR, where ArgIII:26 in addition makes a similar salt bridge with the neighboring AspIII:25 in TM-III. These two acidic residues could therefore be considered to be “locks for the inactive conformation” of the ArgIII:26 micro-switch.
In the active conformation of B2AR in complex with Gs, the two salt bridges between ArgIII:26 and AspIII:25 and GluVI:-06, respectively, are both broken, and the guanidino group of the ArgIII:26 side chain points toward TM-V and is relatively close to TyrV:24 (5). Although the residues are not close enough to form a strong hydrogen bond in the currently available x-ray structure, we still believe that TyrV:24 could function as a “lock for the active conformation” of the ArgIII:26 micro-switch as it appears to do in the active conformation of rhodopsin/opsin (6, 22).
Effect of Alanine Substitution of ArgIII:26 and Its Potential Interaction Partners on Gs Signaling
The functional importance of the ArgIII:26 micro-switch and its three potential interaction partners was studied by mutating the respective residues and expressing them in COS-7 cells. Introduction of alanine in the respective positions did not affect the surface expression as compared with WT receptor, as judged by ELISA (Fig. 3, A–D, insets).
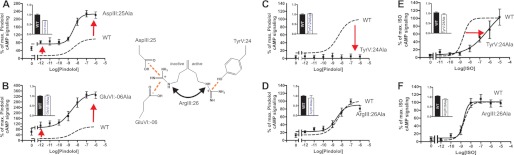
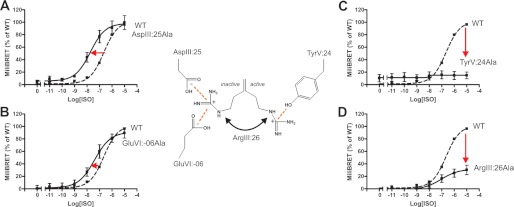
FIGURE 3.
Functional consequence on Gs signaling in the B2AR of Ala substitutions of the ArgIII:26/3.50 micro-switch and its potential interaction partners. A–D, basal and agonist (pindolol)-induced cAMP production in COS-7 cells transiently transfected with either WT β2-AR (dotted line) or mutant forms of the following: AspIII:25Ala (A), GluVI:-06Ala (B), TyrV:24Ala (C), and ArgIII:26Ala (D). % of max., percentage of maximum. E and F, isoproterenol-induced cAMP production in CHO-K1 cells transiently transfected with either WT (dotted line) or mutant forms of the β2-AR: TyrV:24Ala (E) and ArgIII:26Ala (F). Cell surface receptor expression, measured by enzyme-linked immunosorbent assay, is shown in the inserted column diagrams in each panel. Error bars indicate S.E.
Alanine substitution of AspIII:25, one of the two potential locks for the inactive conformation of ArgIII:26, led to a 5-fold increase in basal cAMP levels and a 2-fold increase in the Emax for pindolol without affecting its potency, i.e. the EC50 value (Fig. 3A). Similarly, alanine substitution of GluVI:-06, which we propose also interacts with ArgIII:26 in the inactive conformation of the receptor, led to a similar 5-fold increase in basal cAMP signaling and a 3-fold increase in Emax (Fig. 3B). The potency of the agonist was not affected by the receptor mutagenesis.
Alanine substitution of TyrV:24, which in contrast is suggested to be a lock for the active conformation of ArgIII:26, eliminated the constitutive activity and totally abolished pindolol-induced activation of Gs (Fig. 3C). Importantly, the TyrV:24 mutant form of the receptor was expressed at the surface of the cells as efficiently as the WT receptor (Fig. 3C, inset). Surprisingly, alanine substitution of the proposed micro-switch itself, ArgIII:26, did not affect either the basal cAMP signaling or the pindolol-induced signaling of the B2AR (Fig. 3D).
Pindolol was chosen as agonist for the B2AR as COS-7 cells express an endogenous adrenergic receptor that is activated by isoproterenol but not by pindolol, which consequently provides a clean picture of the effect on the transfected receptors. Nevertheless, due to impressive loss of function upon Ala substitution of TyrV:24 and the surprising lack of effect of Ala substitution of ArgIII:26, we also decided to probe these mutants in CHO-K1 cells, which do not express isoproterenol-sensitive adrenergic receptors. As presented in Fig. 3E, the cAMP response to the full agonist isoproterenol was not totally eliminated by the TyrV:24 to Ala mutation in the CHO-K1 cells, but the dose-response curve was still shifted 2–3 orders of magnitude to the right and displayed a lower Hill coefficient than observed on the wild-type receptor.
Importantly, as observed for pindolol in the COS cells, Ala substitution of ArgIII:26 did not have any effect on either potency or efficacy for isoproterenol with respect to stimulating cAMP production in CHO-K1 cells (Fig. 3F). Thus, although no apparent effect was observed on Gs signaling upon alanine substitution of ArgIII:26 itself, mutation of either of the proposed locks for the inactive conformation of ArgIII:26 led to both increased basal signaling and increased agonist-induced signaling, whereas substitution of the proposed lock for the active conformation of ArgIII:26 eliminated both basal and agonist-induced signaling.
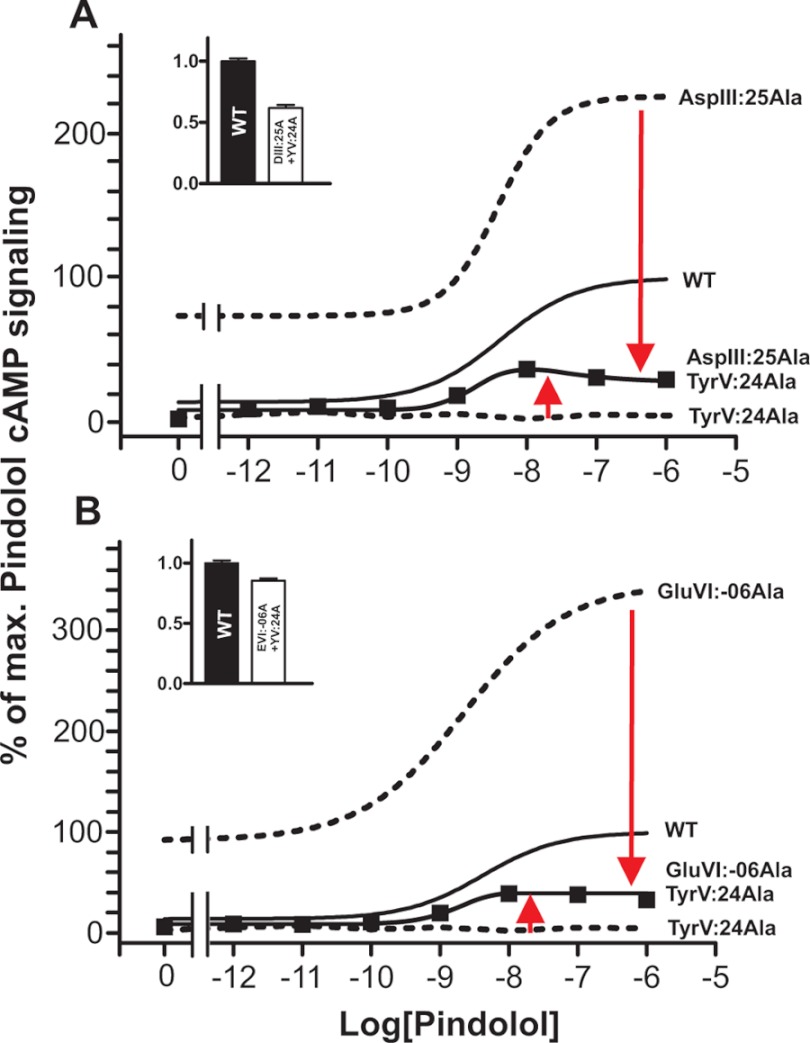
These results would indicate that in the B2AR, the locks for the inactive conformation and the lock for the active conformation are balancing against each other with respect to stabilizing ArgIII:26. This notion was probed by combining the mutants. As shown in Fig. 4A, Ala substitution of AspIII:25 could in fact partly rescue the otherwise total elimination of agonist-induced signaling observed in the TyrV:24 to Ala mutant. The double mutant displayed an Emax for pindolol corresponding to 32% of the Emax observed in the wild-type receptor. Similarly, Ala substitution of GluVI:-06 also partly rescued the TyrV:24 to Ala mutant as this double mutant displayed an Emax for pindolol corresponding to 36% of the Emax observed in the wild-type receptor.
FIGURE 4.
Effect on Gs signaling of combining loss-of-function and gain-of-function mutants of the proposed locks for the ArgIII:26 micro-switch in B2AR. Basal and agonist (pindolol)-induced cAMP production in COS-7 cells transiently transfected with either WT (solid line) or mutant forms of B2AR is shown. A, AspIII:25Ala (dotted line), TyrV:24Ala (dotted line), and the double mutant AspIII:25Ala + TyrV:24Ala as compared with WT (solid line). % of max., percentage of maximum. B, GluVI:-06Ala (dotted line), TyrV:24Ala (dotted line), and GluVI:-06Ala+TyrV:24Ala as compared with WT (solid line). Cell surface receptor expression of the double mutants, measured by enzyme-linked immunosorbent assay, is shown in the inserted column diagrams in each panel. Error bars indicate S.D.
Analysis on β-Arrestin Mobilization in the Receptor Mutants Measured by BRET
The ability of the B2AR receptor mutants to mobilize β-arrestin2 was determined by energy transfer between the two fusion proteins: B2AR C-terminally tagged with Renilla luciferase and β-arrestin2 N-terminally tagged with GFP, measured as BRET (see “Experimental Procedures”). Alanine substitution of AspIII:25 resulted in an improved ability to mobilize β-arrestin2 as compared with WT receptor (Fig. 5A). However, as opposed to what was observed for cAMP accumulation, no increase in maximal efficacy was observed for agonist-induced β-arrestin2 mobilization for AspIII:25Ala, but instead, the dose-response curve was shifted an order of magnitude to the left as compared with WT. A similar, but only 2-fold, shift in the dose-response curve for the agonist was observed for the GluVI:-06 to Ala mutant(Fig. 5B). These results are in agreement with the notion that both AspIII:25 and GluVI:-06 are proposed to stabilize ArgIII:26 and the receptor in the inactive state.
FIGURE 5.
Functional consequence on β-arrestin2 mobilization measured by BRET of Ala substitution of the ArgIII:26/3.50 micro-switch and potential interaction partners. A–D, agonist (isoproterenol)-induced BRET production in COS-7 cells transiently transfected using a 1:3 ratio of either WT B2AR (dotted line)/mutant forms of the receptor and β-arrestin2. The panels show AspIII:25Ala (A), GluVI:-06Ala (B), TyrV:24Ala (C), and ArgIII:26Ala (D). Error bars indicate S.D.
Mutating TyrV:24 to alanine led to an increased basal level of recruitment of β-arrestin2 (Fig. 5C). Importantly, the agonist-induced mobilization of β-arrestin2 was totally eliminated in this mutant. The results are in agreement with the proposed stabilizing role of TyrV:24 for the active conformation of the receptor. When ArgIII:26 itself was substituted by alanine, the ability of the receptor mutant to recruit β-arrestin2 was reduced as compared with WT receptor (Fig. 5D); i.e. although the EC50 value was not significantly affected, the Emax was only ∼30% of that observed in WT receptor.
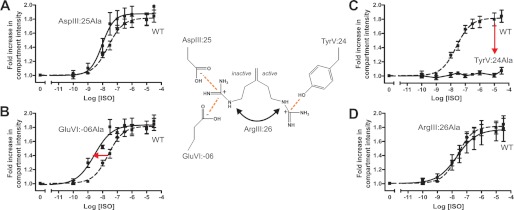
Internalization of the Receptor Mutants in the B2AR
To study receptor internalization, cell surface-expressed SNAP-tagged B2ARs were labeled with a membrane-impermeable fluorophore that specifically reacts with the SNAP enzyme, and the agonist-induced internalization was measured in HEK293 cells by automated confocal microscopy. Automated analysis quantified internal receptor immunofluorescence in transferrin-positive cellular compartments as a measure of wild-type and mutant receptor endocytosis.
All of the mutants were expressed on the cell surface under basal conditions, with little or no constitutive internalization (Fig. 6). Isoproterenol was 2.5 times more potent in stimulating internalization of the AspIII:25Ala mutant as compared with the WT receptor (Fig. 6A). Similarly, alanine substitution of GluVI:-06 increased the potency of isoproterenol-stimulated internalization by 8.8-fold (Fig. 6B); thus, these results are similar to the results concerning both G protein signaling and β-arrestin2 recruitment, all indicating that AspIII:25 and GluVI:-06 are stabilizing the inactive receptor conformation.
FIGURE 6.
Functional consequence on isoproterenol-induced internalization of Ala substitution of the ArgIII:26/3.50 micro-switch and potential interaction partners. A–D, agonist (isoproterenol)-induced internalization in HEK293 cells stably expressing either N-terminal SNAP-tagged WT B2AR (dotted line) or mutant forms of the receptor. The panels show AspIII:25Ala (A), GluVI:-06Ala (B), TyrV:24Ala (C), and ArgIII:26Ala (D). Error bars indicate S.D.
As observed for Gs signaling and for β-arrestin2 mobilization, receptor internalization was totally abolished in the TyrV:24 to Ala mutant (Fig. 6C), indicating that this residue is important for stabilizing the active receptor conformation. The substitution of ArgIII:26 had no effect on receptor internalization, similar to what we observed for the Gs signaling (Fig. 6D).
DISCUSSION
For many years, the highly conserved ArgIII:26 of the DRY motif has been suspected to play a major role in 7TM receptor activation. It has also been the generally accepted dogma that the neighboring AspIII:25 and especially the ionic lock residue, GluVI:-06 located across in TM-VI, would be stabilizing the inactive conformation of the ArgIII:26 micro-switch (14). However, several of the recently published x-ray structures introduced a new player in the game, i.e. TyrV:24, which appears to have a role in stabilizing the active receptor conformation (6, 12). When all of these residues surrounding ArgIII:26 were addressed in parallel in the B2AR, the functional results were in total agreement with their indicated roles in stabilizing either the active or the inactive conformation of the receptor. However, surprisingly, removal of the side chain of the ArgIII:26 micro-switch itself had almost no effect. This could fit a picture where ArgIII:26 is equally important for stabilizing the inactive and the active conformation, at least with respect to Gs signaling, but where the residue in itself is not essential for the actual G protein activation.
AspIII:25 Restrains the B2AR in the Inactive Conformation
An acidic residue has been conserved at position III:25 in close to 90% of the rhodopsin-like 7TM receptors (Asp 67%/Glu 20%) (13). Our results show that alanine substitution of AspIII:25 led to both increased basal activity and an increase in ligand-induced signaling. This is in accordance with previous studies in the B2AR where Rasmussen and co-workers (23, 24) found that mutating AspIII:25 into either Asn or Ala led to an increase in the level of both basal and ligand-induced signaling without affecting the binding affinity of pindolol.
The highly conserved acidic residues in position III:25 have been mutated in a large number of different 7TM receptors over the years, as reviewed by Rovati et al. (14). They concluded that in a major class of receptors, including the B2AR, removal of the acidic side chain led to increased constitutive activity, whereas this was not observed in other receptors, which nevertheless often displayed other evidence of increased activation properties such as increased Emax (14). In rhodopsin, protonation of AspIII:25 is a key determining factor for activation, and it was recently shown that this protonation results in a complete shift in the equilibrium toward the active Meta-II state (25). Thus, the acidic Asp or Glu residue of the DRY motif appears to be generally involved in holding 7TM receptors in their inactive state conceivably by forming a salt bridge to the neighboring ArgIII:26 residue.
The TM-III to TM-VI Ionic Lock
The x-ray structure of rhodopsin clearly displayed the expected ionic lock salt bridge between ArgIII:26 of the DRY motif in TM-III and GluVI:-06 at the intracellular extension of TM-VI (Fig. 1B). The lack of this interaction in the B2AR structures was therefore a mystery, especially since the tools and changes employed to stabilize the B2AR structure such as the insertion of T4L in ICL3 were shown not to interfere with ligand binding or with the conformational changes that occur upon receptor activation (19). In the present study, we find that during MD simulations, the ionic lock is indeed formed in the B2AR, which subsequently rather closely resembles the inactive structure of rhodopsin (Fig. 2). This formation of the interhelical salt bridge has also been observed by Dror et al. (26) using very long timescale MD simulations. Our mutational analysis of GluVI:-06 gave very similar results as observed for AspIII:25 with both increased basal Gs signaling and increased agonist-induced Gs signaling, improved arrestin mobilization, and improved internalization all supporting the notion that GluVI:-06 is important in restraining the inactive state of the B2AR. Similar results, with respect to Gs signaling, have been previously reported for the B2AR (23). However, studies in rhodopsin indicate that the salt bridge between ArgIII:26 and GluVI:-06 is much more easily broken than the comparable intrahelical salt bridge in TM-III between ArgIII:26 and AspIII:25, implying that the breakage of the ionic lock between TM-III and TM-VI is less crucial for activation of rhodopsin (25).
In the majority (75%) of 7TM receptors, the ionic lock between TM-III and TM-VI is not present as there are no acidic residues at the corresponding positions in TM-VI (13). When an ionic lock was engineered into, for example, the human H4 receptor by introducing a Glu residue at position VI:-06, this only decreased the basal activity of this highly constitutively active receptor to a minor degree (27). Nevertheless, although GluVI:-06 is only found in 25% of the receptors, it is apparently involved in stabilizing the inactive conformation of these receptors, conceivably through formation of a salt bridge to ArgIII:26 (14).
TyrV:24 as a Lock for the Active Receptor Conformation
TyrV:24, which is conserved in 77% of the rhodopsin-like receptors, first caught the attention in the supposedly active opsin structure with the bound Gα peptide fragment. Here, a surprising interaction between TyrV:24 and ArgIII:26 was observed that appeared to stabilize the interaction of ArgIII:26 with the backbone of the Gα peptide (Fig. 1C). This was not at all expected from the previous x-ray structures of the inactive form of rhodopsin, where TyrV:24 was pointing almost outward toward the lipid bilayer (Fig. 1B). However, this direct interaction between TyrV:24 and ArgIII:26 was not observed in the recently published active structure of the B2AR in complex with Gs (Fig. 1E) nor in the A2A receptor in complex with an agonist (5, 7). Nevertheless, in the present study, we find that alanine substitution of TyrV:24 eliminates Gs signaling, arrestin mobilization, and internalization of the B2AR, which strongly supports an important role for TyrV:24 in receptor activation.
As opposed to the other residues in this micro-switch region, TyrV:24 has hardly previously been subjected to mutagenesis. However, in the NK1 receptor, Huang et al. (28) found almost 20 years ago that Ala or Glu substitution of TyrV:24 eliminated Gq signaling but did not affect the high affinity binding of Substance P. Furthermore, in rhodopsin, it was recently demonstrated that a TyrV:24 to Ala mutation results in very low signaling activity (22). By use of Fourier transform infrared spectroscopy, it was shown that the TyrV:24 mutation did not change the Meta-I/II ratio and therefore was capable of generating the active Meta-II state. Moreover, these results also indicated that the tyrosine residue was not essential for inducing the breakage of the ionic lock and the subsequent outward movement of TM-VI. However, when TyrV:24 was mutated to Phe, the decay of the Meta-II state of rhodopsin to opsin was increased, confirming a stabilizing role of this residue for the active receptor state. Furthermore, this study revealed that the TyrV:24 mutant was associated with a structural change in ECL2, thereby connecting the extracellular region to the association with the G protein (29). The special, long timescale molecular dynamics simulations showed that although no interaction was observed in the x-ray structures of the active form of B2AR, TyrV:24 can rotate inward to be located between TM-III and TM-VI, which is where it is found in the active opsin structure in complex with the Gα peptide, upon breakage of the TM-III to TM-VI ionic lock (26). Thus, TyrV:24 is apparently involved in stabilizing the active conformation of 7TM receptors as shown in very different receptors such as NK1, B2AR, and rhodopsin.
ArgIII:26 as a Crucial but Balancing Micro-switch
Despite the fact that mutations of its proposed locks for inactive and active conformations all had the expected effects as discussed above, we surprisingly found that mutation of ArgIII:26 itself in the B2AR had no effect on Gs signaling and internalization and only partly reduced β-arrestin2 mobilization. In fact, ArgIII:26 of the B2AR apparently has only been subject to mutagenesis in one previous publication where Seibold et al. (30) found that it could be substituted with multiple amino acids with no effect on Gs coupling.
One explanation could be that ArgIII:26 functions as a balancing micro-switch, which in the B2AR contributes equally as much to the stabilization of the overall, large-scale inactive receptor conformation, through interactions with AspIII:25 and GluVI:-06, as it contributes to the stabilization of the overall, large-scale active conformation, through interactions with TyrV:24. In such a scenario, removal of ArgIII:26 would not shift the balance between the two receptor conformations, which are stabilized by a number of other micro-switch residues such as, for example, TyrVII:20. However, an important part of this explanation would be that ArgIII:26 is not directly involved in the G protein activation process because if that was the case, then we would observe a loss of function upon Ala substitution of ArgIII:26. The notion that ArgIII:26 is a balancing micro-switch is supported by the double mutants where Ala substitution of either AspIII:25 or GluVI:-06 both partly rescues the loss of function observed in the TyrV:24 mutant. The fact that only a partial rescue is observed in both cases is probably a reflection of the fact that the two acidic residues both contribute to the stabilization of the inactive state and one of them is still left in each of the two double mutants.
Rovarti et al. (14) concluded that in a large group of 7TM receptors, mutation of ArgIII:26 seriously affects receptor signaling, whereas this was not the case in other receptors, and that this to some degree was coupled to the effect of mutating the neighboring acidic residues. Although ArgIII:26 is one of the most conserved residues in 7TM receptors, there are receptors that lack this residue. For example, in the highly constitutively active, virally encoded ORF74 receptor, a DTW motif is found instead of a DRY motif. When this motif was modified into a DRY sequence, the constitutive activity of the receptor was significantly decreased, which suggested that the (re)introduced ArgIII:26 residues primarily were involved in stabilization of the inactive conformation in the mutated receptor (31, 32).
Altered β-Arrestin Recruitment Could Potentially Affect Gs Coupling
Several regions in the B2AR have been shown to interact with β-arrestin, including both the C-terminal phosphorylated tail of the receptor and, for example, a stretch of amino acids in ICL2 (33, 34). In principle, mutations that either increase or decrease Gs coupling could do so “indirectly” by having the opposite effect on arrestin recruitment and receptor internalization. This was observed in the α-1b adrenergic receptor where an AspIII:25 to Ala mutant was found to be highly constitutively active with respect to G protein signaling but unable to recruit β-arrestin and internalize, which was interpreted as being the basis for the high constitutive Gs activity of the mutant receptor (35). In the present study, we found that the AspIII:25 to Ala and GluVI:-06 to Ala mutants of the B2AR, which both display increased constitutive Gs activity and increased agonist-induced signaling, had normal or in fact improved ability to recruit arrestin and internalize. These findings are similar to observations in the gonadotropin AspIII:25 to Asn or Glu receptor mutants, which displayed increased G protein signaling along with increased internalization (36). Also, in the case of the TyrV:24 to Ala mutant, we found a parallel effect on Gs signaling, arrestin mobilization, and internalization, i.e. in this case, an elimination of all three functions. However, the ArgIII:26 to alanine mutation of B2AR resulted in a 30% reduced capability to mobilize β-arrestin2 as compared with the WT receptor but did not affect internalization. It could be speculated that the reduced mobilization of β-arrestin2 at least partly could be responsible for the WT-like phenotype observed in the Gs signaling.
In conclusion, we show that residues of the DRY motif along with the identified interaction partners in TM-V and -VI make important contributions to the structural integrity of the B2AR being important for both the inactive as well as the active receptor conformation. This conserved micro-switch region is important not only for signaling through the G protein but more or less in parallel also for arrestin mobilization and internalization. As some of the interactions, which the mutational analysis indicates are functionally very important, are not observed clearly in the presently available x-ray structures of the B2AR, but for example in the rhodopsin structures, our study underlines the well known fact that even high resolution x-ray analysis needs to be complemented by biochemical, biophysical, and other types of studies.
Acknowledgments
We thank a persistent referee for forcing us to make the crucial double mutants. The Novo Nordisk Foundation Center for Basic Metabolic Research is based on an unconditional grant from the Novo Nordisk Foundation.
The Schwartz/Baldwin generic numbering system for 7TM receptor, which is based on the actual location of the residues in each transmembrane helix, is used throughout the article (12).
- TM
- transmembrane
- 7TM
- 7-transmembrane
- B2AR
- β2-adrenergic receptor
- BRET
- bioluminescence resonance energy transfer
- MD
- molecular dynamics.
REFERENCES
- 1. Jaakola V. P., Griffith M. T., Hanson M. A., Cherezov V., Chien E. Y., Lane J. R., Ijzerman A. P., Stevens R. C. (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322, 1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 3. Rasmussen S. G., Choi H. J., Rosenbaum D. M., Kobilka T. S., Thian F. S., Edwards P. C., Burghammer M., Ratnala V. R., Sanishvili R., Fischetti R. F., Schertler G. F., Weis W. I., Kobilka B. K. (2007) Crystal structure of the human β2 adrenergic G protein-coupled receptor. Nature 450, 383–387 [DOI] [PubMed] [Google Scholar]
- 4. Rasmussen S. G., Choi H. J., Fung J. J., Pardon E., Casarosa P., Chae P. S., Devree B. T., Rosenbaum D. M., Thian F. S., Kobilka T. S., Schnapp A., Konetzki I., Sunahara R. K., Gellman S. H., Pautsch A., Steyaert J., Weis W. I., Kobilka B. K. (2011) Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rasmussen S. G., DeVree B. T., Zou Y., Kruse A. C., Chung K. Y., Kobilka T. S., Thian F. S., Chae P. S., Pardon E., Calinski D., Mathiesen J. M., Shah S. T., Lyons J. A., Caffrey M., Gellman S. H., Steyaert J., Skiniotis G., Weis W. I., Sunahara R. K., Kobilka B. K. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheerer P., Park J. H., Hildebrand P. W., Kim Y. J., Krauss N., Choe H. W., Hofmann K. P., Ernst O. P. (2008) Crystal structure of opsin in its G protein-interacting conformation. Nature 455, 497–502 [DOI] [PubMed] [Google Scholar]
- 7. Xu F., Wu H., Katritch V., Han G. W., Jacobson K. A., Gao Z. G., Cherezov V., Stevens R. C. (2011) Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., Stevens R. C. (2007) High resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choe H. W., Kim Y. J., Park J. H., Morizumi T., Pai E. F., Krauss N., Hofmann K. P., Scheerer P., Ernst O. P. (2011) Crystal structure of metarhodopsin II. Nature 471, 651–655 [DOI] [PubMed] [Google Scholar]
- 10. Schwartz T. W., Frimurer T. M., Holst B., Rosenkilde M. M., Elling C. E. (2006) Molecular mechanism of 7TM receptor activation: a global toggle switch model. Annu. Rev. Pharmacol. Toxicol. 46, 481–519 [DOI] [PubMed] [Google Scholar]
- 11. Hubbell W. L., Altenbach C., Hubbell C. M., Khorana H. G. (2003) Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv. Protein Chem. 63, 243–290 [DOI] [PubMed] [Google Scholar]
- 12. Nygaard R., Frimurer T. M., Holst B., Rosenkilde M. M., Schwartz T. W. (2009) Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol. Sci. 30, 249–259 [DOI] [PubMed] [Google Scholar]
- 13. Mirzadegan T., Benkö G., Filipek S., Palczewski K. (2003) Sequence analyses of G protein-coupled receptors: similarities to rhodopsin. Biochemistry 42, 2759–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rovati G. E., Capra V., Neubig R. R. (2007) The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol. Pharmacol. 71, 959–964 [DOI] [PubMed] [Google Scholar]
- 15. Elster L., Elling C., Heding A. (2007) Bioluminescence resonance energy transfer as a screening assay: focus on partial and inverse agonism. J. Biomol. Screen. 12, 41–49 [DOI] [PubMed] [Google Scholar]
- 16. Vrecl M., Jorgensen R., Pogacnik A., Heding A. (2004) Development of a BRET2 screening assay using β-arrestin2 mutants. J. Biomol. Screen. 9, 322–333 [DOI] [PubMed] [Google Scholar]
- 17. Holst B., Zoffmann S., Elling C. E., Hjorth S. A., Schwartz T. W. (1998) Steric hindrance mutagenesis versus alanine scan in mapping of ligand binding sites in the tachykinin NK1 receptor. Mol. Pharmacol. 53, 166–175 [DOI] [PubMed] [Google Scholar]
- 18. Holst B., Nygaard R., Valentin-Hansen L., Bach A., Engelstoft M. S., Petersen P. S., Frimurer T. M., Schwartz T. W. (2010) A conserved aromatic lock for the tryptophan rotameric switch in TM-VI of seven-transmembrane receptors. J. Biol. Chem. 285, 3973–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenbaum D. M., Cherezov V., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Yao X. J., Weis W. I., Stevens R. C., Kobilka B. K. (2007) GPCR engineering yields high resolution structural insights into β2-adrenergic receptor function. Science 318, 1266–1273 [DOI] [PubMed] [Google Scholar]
- 20. MacKerell A. D., Jr., Banavali N., Foloppe N. (2000) Development and current status of the CHARMM force field for nucleic acids. Biopolymers 56, 257–265 [DOI] [PubMed] [Google Scholar]
- 21. Nygaard R., Valentin-Hansen L., Mokrosinski J., Frimurer T. M., Schwartz T. W. (2010) Conserved water-mediated hydrogen bond network between TM-I, -II, -VI, and -VII in 7TM receptor activation. J. Biol. Chem. 285, 19625–19636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elgeti M., Kazmin R., Heck M., Morizumi T., Ritter E., Scheerer P., Ernst O. P., Siebert F., Hofmann K. P., Bartl F. J. (2011) Conserved Tyr-2235.58 plays different roles in the activation and G protein interaction of rhodopsin. J. Am. Chem. Soc. 133, 7159–7165 [DOI] [PubMed] [Google Scholar]
- 23. Ballesteros J. A., Jensen A. D., Liapakis G., Rasmussen S. G., Shi L., Gether U., Javitch J. A. (2001) Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177 [DOI] [PubMed] [Google Scholar]
- 24. Rasmussen S. G., Jensen A. D., Liapakis G., Ghanouni P., Javitch J. A., Gether U. (1999) Mutation of a highly conserved aspartic acid in the β2 adrenergic receptor: constitutive activation, structural instability, and conformational rearrangement of transmembrane segment 6. Mol. Pharmacol. 56, 175–184 [DOI] [PubMed] [Google Scholar]
- 25. Vogel R., Mahalingam M., Lüdeke S., Huber T., Siebert F., Sakmar T. P. (2008) Functional role of the “ionic lock”: an interhelical hydrogen-bond network in family A heptahelical receptors. J. Mol. Biol. 380, 648–655 [DOI] [PubMed] [Google Scholar]
- 26. Dror R. O., Arlow D. H., Borhani D. W., Jensen M. Ø., Piana S., Shaw D. E. (2009) Identification of two distinct inactive conformations of the β2-adrenergic receptor reconciles structural and biochemical observations. Proc. Natl. Acad. Sci. U.S.A. 106, 4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schneider E. H., Schnell D., Strasser A., Dove S., Seifert R. (2010) Impact of the DRY motif and the missing “ionic lock” on constitutive activity and G protein coupling of the human histamine H4 receptor. J. Pharmacol. Exp. Ther. 333, 382–392 [DOI] [PubMed] [Google Scholar]
- 28. Huang R. R., Huang D., Strader C. D., Fong T. M. (1995) Conformational compatibility as a basis of differential affinities of tachykinins for the neurokinin-1 receptor. Biochemistry 34, 16467–16472 [DOI] [PubMed] [Google Scholar]
- 29. Goncalves J. A., South K., Ahuja S., Zaitseva E., Opefi C. A., Eilers M., Vogel R., Reeves P. J., Smith S. O. (2010) Highly conserved tyrosine stabilizes the active state of rhodopsin. Proc. Natl. Acad. Sci. U.S.A. 107, 19861–19866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seibold A., Dagarag M., Birnbaumer M. (1998) Mutations of the DRY motif that preserve β2-adrenoceptor coupling. Receptors. Channels 5, 375–385 [PubMed] [Google Scholar]
- 31. Flanagan C. A. (2005) A GPCR that is not “DRY”. Mol. Pharmacol. 68, 1–3 [DOI] [PubMed] [Google Scholar]
- 32. Rosenkilde M. M., Kledal T. N., Schwartz T. W. (2005) High constitutive activity of a virus-encoded seven-transmembrane receptor in the absence of the conserved DRY motif (Asp-Arg-Tyr) in transmembrane helix 3. Mol. Pharmacol. 68, 11–19 [DOI] [PubMed] [Google Scholar]
- 33. Krasel C., Zabel U., Lorenz K., Reiner S., Al-Sabah S., Lohse M. J. (2008) Dual role of the β2-adrenergic receptor C terminus for the binding of β-arrestin and receptor internalization. J. Biol. Chem. 283, 31840–31848 [DOI] [PubMed] [Google Scholar]
- 34. Marion S., Oakley R. H., Kim K. M., Caron M. G., Barak L. S. (2006) A β-arrestin binding determinant common to the second intracellular loops of rhodopsin family G protein-coupled receptors. J. Biol. Chem. 281, 2932–2938 [DOI] [PubMed] [Google Scholar]
- 35. Mhaouty-Kodja S., Barak L. S., Scheer A., Abuin L., Diviani D., Caron M. G., Cotecchia S. (1999) Constitutively active α-1b adrenergic receptor mutants display different phosphorylation and internalization features. Mol. Pharmacol. 55, 339–347 [DOI] [PubMed] [Google Scholar]
- 36. Arora K. K., Cheng Z., Catt K. J. (1997) Mutations of the conserved DRS motif in the second intracellular loop of the gonadotropin-releasing hormone receptor affect expression, activation, and internalization. Mol. Endocrinol. 11, 1203–1212 [DOI] [PubMed] [Google Scholar]