Background: Endocytosis has been postulated as a route of HIV entry into cells.
Results: Endocytosed virus led to productive infection of cells, except when cells were cultured with the anti-gp120 antibody IgGb12.
Conclusion: Endocytosis may serve as a virus reservoir capable of inducing trans-infection.
Significance: The route of HIV transmission is important for understanding pathogenesis and drug therapy.
Keywords: Endocytosis, Endosomes, HIV-1, Viral Replication, Virus Entry, Fusion, Transfer, Cell-to-cell
Abstract
Cellular contacts between HIV-1-infected donor cells and uninfected primary CD4+ T lymphocytes lead to virus transfer into endosomes. Recent evidence suggests that HIV particles may fuse with endosomal membranes to initiate a productive infection. To explore the role of endocytosis in the entry and replication of HIV, we evaluated the infectivity of transferred HIV particles in a cell-to-cell culture model of virus transmission. Endocytosed virus led to productive infection of cells, except when cells were cultured in the presence of the anti-gp120 mAb IgGb12, an agent that blocks virus attachment to CD4, suggesting that endocytosed virus was recycled to the outer cell surface. Confocal microscopy confirmed the colocalization of internalized virus antigen and the endosomal marker dynamin. Additionally, virus transfer, fusion, or productive infection was not blocked by dynasore, dynamin-dependent endosome-scission inhibitor, at subtoxic concentrations, suggesting that the early capture of virus into intracellular compartments did not depend on endosomal maturation. Our results suggest that endocytosis is not a mechanism of infection of primary CD4 T cells, but may serve as a reservoir capable of inducing trans-infection of cells after the release of HIV particles to the extracellular environment.
Introduction
Viruses are obligatory intracellular parasites that take advantage of the host cell machinery to replicate and spread from infected to uninfected cells (1–3). Cell-to-cell transmission has been shown to be a highly efficient mechanism of virus spread (4, 5), and its relevance for in vivo dissemination in the active sites of replication, namely, primary and secondary lymphoid tissues, seems probable. HIV may be transferred from infected to uninfected CD4+ cells (6, 7) by a mechanism that requires intimate cell-to-cell contacts involving the HIV envelope glycoprotein gp120 and the CD4 receptor but also accessory cell surface proteins (8). Virus-cell fusion and initiation of a productive infection require engagement to CD4 and to one of the two alternative coreceptors, CCR5 or CXCR4. The various steps in the mechanism of virus entry are considered targets for anti-HIV intervention (9, 10).
Cell-to-cell transfer of HIV particles may be blocked by agents that prevent virus attachment, such as the anti-CD4 monoclonal antibody (mAb) Leu3a, the anti-gp120 mAb IgGb12, or the CD4-IgG2 fusion protein PRO542 (11), but is resistant to HIV entry inhibitors targeting virus coreceptors or gp41-dependent fusion (7, 12), suggesting that virus attachment to CD4 is the sole factor necessary to induce the uptake of HIV particles (13) and that virus capture may occur in the absence of virus fusion and the initiation of a productive infection. Endocytic internalization and endosomal acidification have been shown not to be required to activate HIV entry into the cytoplasm (14–17).
Alternatively, several lines of evidence support clathrin-dependent endocytosis as an infectious pathway (12, 18–21). HIV fusion with endosomal membranes has been observed by electron microscopy (22). Daecke et al. (23) proposed a role for endocytosis in productive entry of HIV-1 by using trans-dominant negative proteins that interfered with specific clathrin-endocytic routes and effectively blocked virus replication. Complete fusion of HIV particles with HeLa cells has been observed to occur within endosome membranes (20), but complete fusion was blocked when endocytosis was inhibited (24). Recent data suggest that after cell-to-cell transfer, virions first need to undergo maturation within endosomes, delaying membrane fusion and reducing sensitivity to patient antisera compared with cell-free virus (25). Thus, the role of endocytosis in HIV replication and whether or not endocytic virus transfer represents an escape mechanism from the immune system or therapeutic agents remain highly controversial (5, 26).
Here, we show that primary CD4+ T lymphocytes take up virus particles into dynamin-containing compartments even in the presence of the endosome-scission inhibitor dynasore. Moreover, purified cells carrying endocytosed virus particles did not become productively infected if cultured in the presence of HIV attachment inhibitors such as the anti-gp120 mAb IgGb12, suggesting that endocytosed virus was recycled to the cell surface to initiate a productive virus infection.
EXPERIMENTAL PROCEDURES
Cells
Peripheral blood mononuclear cells from healthy donors were purified by Ficoll-Hypaque sedimentation. CD4+ T lymphocytes were immediately purified (>95%) from peripheral blood mononuclear cells by negative selection using the CD4+ T cell enrichment kit (Stem Cell Technologies, Vancouver, Canada) and grown in RPMI 1640 l-glutamine medium (Invitrogen) supplemented with 10% (R10) heat-inactivated fetal calf serum (FCS; Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin. When needed, CD4+ T cells were stimulated with phytohemagglutinin (PHA; Sigma) at 4 μg/ml and 6 units/ml interleukin 2 (IL-2; Roche Applied Science). MOLT-4 lymphoid cells (AIDS Reagent Program, National Institutes of Health, Bethesda, MD) were cultured in R10. Chronically HIV-1-infected MOLT cells were generated after the infection of MOLT cells with the NL4-3 X4 HIV-1 (MOLTNL4-3) (23, 24). After the infection peak, the persistently infected culture was grown and characterized for Env expression and virus production. HEK293-T cells (AIDS Reagent Program) were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% heat-inactivated FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Cocultures of Infected and Uninfected Cells
Nonstimulated primary CD4+ T cells (to minimize virus replication) were cocultured with uninfected or HIV-1 persistently infected MOLTNL4-3 cells as previously described (11, 18, 19). Purified CD4+ T cells were first labeled with the cell tracker CMFDA (Molecular Probes) and washed before being mixed with MOLTNL4-3 cells. Briefly, 2.5 × 106 of both infected and target cells (1:1 ratio) were cocultured in 48-well culture plates in a final volume of 1 ml in the absence or presence of the following HIV-1 inhibitors: 80 nm neutralizing anti-gp120 mAb IgGb12 (Polymun Scientific, Wien, Austria); 4 μm reverse transcriptase (RT) inhibitor 3-azido-3-deoxythymidine (AZT)4; 12.5 μm AMD3100 or 80 μm dynamin inhibitor dynasore (all from Sigma-Aldrich). Cocultures were incubated overnight at 37 °C. The capture of CAp24 antigen by primary CD4+ T cells was evaluated by flow cytometry as shown before (10, 11, 25, 27, 28). Prior to staining, cells were trypsinized to eliminate HIV-1 particles bound to the cell surface. For trypsin treatment, cells were washed with phosphate-buffered saline (PBS) and treated for 8 min at room temperature with 0.25% trypsin solution (Invitrogen). Trypsin was stopped by addition of FCS, and cells were then washed with PBS. For intracellular staining, cells were fixed, permeabilized (Fix & Perm; Caltag, Burlingame, CA), and stained with the anti-HIV-CAp24 antigen mAb KC57 (Coulter). Cells were analyzed in a LSRII flow cytometer (BD Bioscience) and identified by morphological parameters and CMFDA staining.
Isolation of Target CD4+ T Cells
CMFDA-loaded target CD4+ T cells were purified (>99% purity) from MOLTNL4-3 cells by fluorescence-activated cell sorting (FACSAria II, BD Biosciences). After separation, contaminating MOLTNL4-3 cells (<1%) were assessed by FSC/SSC parameters using flow cytometry. The possible contribution to infection of persisting MOLTNL4-3 cells (<1%) was evaluated using the coculture performed with the mAb IgGb12, a condition where HIV-1 uptake into CD4+ T cells is blocked, and therefore, infection of purified CD4+ T cells would only come from remaining MOLTNL4-3 cells.
Culture of HIV-1-loaded Cells
Isolated CD4+ T cells from each initial coculture condition were subdivided in three and cultured for 5 days in the following medium conditions: (i) 80 nm mAb IgGb12; (ii) 80 nm mAb IgGb12 and 4 μm RT inhibitor AZT; or (iii) left untreated. CD4+ T cells were activated by adding 4 μg/ml PHA and 6 units/ml IL-2 to the medium. After 5 days, infection in target cells was assessed by enzyme-linked immunosorbent assay (ELISA) for HIV-CAp24 antigen detection in culture supernatants (Genscreen HIV-1 Ag EIA; Bio-Rad Laboratories).
Determination of Anti-HIV Activity in Cell-free Virus Infections and Cell-Cell Transfer
The anti-HIV activity using cell-free virus infections was determined as described before (27). Briefly, PHA-activated CD4+ T lymphocytes (1.5 × 105 cells/well) were incubated with HIV-1NL4-3 (200 TCID50/106 cells) or mock-infected during 7 days at 37 °C, 5% CO2 in the presence of different concentrations of the corresponding test compound. HIV-1 CAp24 antigen production in the supernatant was measured by a commercial ELISA test as described above. To determine cytotoxicity, mock-infected cells were harvested and fixed with 1% formaldehyde. Cell death was quantified by flow cytometry in forward versus side scatter plots. Dead cells showed increased side and reduced forward scatter values compared with those of living cells. Anti-HIV activities were determined in at least three independent experiments, performed in triplicate. To evaluate the anti-HIV activity in cell-cell transfer, overnight cocultures between isolated primary CD4+ T cells (2 × 105) and uninfected or infected MOLTNL4-3 cells (2 × 105) were performed in the presence of serial dilutions of the corresponding test compounds. Virus transfer was measured as described above. The 50% effective concentration (EC50) and the 50% cytotoxic concentration (CC50) were calculated for cell free-virus infections and cell-cell CAp24 antigen transfer. Bafilomycin A1 (BFLA1) and concanamycin A (CON A) were purchased from Sigma.
Infection with Viruses Released from Antigen-loaded Cells
Cocultures between freshly isolated primary CD4+ T cells and uninfected or infected MOLTNL4-3 cells were performed as described above. After 6 h of coculture, to minimize the possibility of CD4+ T cell infection, target cells were sorted (>99% purity) as indicated above, and recovered target cells were cultured (5 × 105 cells/condition) in the presence or the absence of 80 nm IgGb12 to prevent productive infection. After 12 h of culture, the presence of CAp24-antigen was evaluated both in the supernatant and in the purified cells by intracellular CAp24 antigen staining as indicated above. Total viral DNA was also quantified by PCR as indicated below using infected CD4+ T cells as a positive control. For each condition, 20 μl of supernatant was used to infect 3 × 104 MT4 cells for 5 days. Infection of MT4 T cells was evaluated by quantification of supernatant CAp24-antigen content.
Quantitative Real-time PCR for Total HIV-1 DNA Detection
Total DNA was quantified as described before (28, 29). Briefly, purified CD4+ T lymphocytes were centrifuged, supernatant was removed, and pellets were frozen. Total cellular DNA was extracted using QIAamp DNA extraction kit (QIAamp DNA Blood mini kit; Qiagen) as recommended by the manufacturer. Quantitative amplification of LTR for viral entry detection was performed using the following primers and probe (forward, 5′-GACGCAGGACTCGGCTTG-3′; reverse, 5′-ACTGACGCTCTCGCACCC-3′ and probe 5′-TTTGGCGTACTCACCAGTCGCCG-3′ labeled with the fluorophore FAM and the quencher TAMRA). To normalize HIV copy values/cell, amplification of cellular RNaseP gene was performed using TaqMan® RNaseP Control Reagents Kit (Applied Biosystems). DNA extracted from 8E5/LAV cells (harboring one copy of integrated HIV-1/cell) was used to build a standard curve. The PCR was performed in a total volume of 50 μl using 1× TaqMan® Universal PCR Master Mix (Applied Biosystems, Roche), 0.9 μm concentration of the primers, 0.25 μm probe, and 5 μl of the DNA sample. Reactions were analyzed with the ABI PRISM 7000 instrument using SDS 1.1 software (Applied Biosystems). For each condition, the amount of the total viral DNA/cell was normalized to untreated sample with IgGb12, and results are expressed as the relative percent increase.
Virus-Cell Fusion Assay
The quantification of the virus-cell membrane fusion was quantified as described before (30). Briefly, 1 × 105 HEK293-T cells were cotransfected with 0.4 μg of both the NL4-3 HIV provirus plasmid (pNL4-3 from the AIDS Reagents Program) and a plasmid carrying the Vpr gene fused with β-lactamase (Vpr-BlaM; pMM310 from the AIDS Reagents Program). After 48 h, transfected HEK293-T cells were cocultured overnight with primary CD4+ T lymphocytes as described above. Cells were then recovered and loaded with the CCF2-AM loading kit (Invitrogen) following the protocol provided by the manufacturer. Cells were incubated for 1 h at room temperature, then washed and immediately fixed. The change in emission of the cleaved CCF2 generated by the Vpr-BlaM chimera was measured by flow cytometry.
Evaluation of Dynasore Activity
Primary CD4+ T lymphocytes were pretreated with or without different concentrations of dynasore starting at 160 μm, for 30 min at 37 °C. Then, pretreated CD4+ T lymphocytes were cultured in the presence or the absence of phorbol 12-myristate 13-acetate (PMA; Sigma) at 1 μg/ml for 30 min at 37 °C. Cells were fixed with 1% formaldehyde and after washes with PBS, stained for CD4 expression with anti-CD4 mAb conjugated with the fluorochrome FITC (BD Bioscience). Analysis of cells was performed by flow cytometry.
Immunofluorescence, Confocal Microscopy, and Quantification of Colocalization
For immunofluorescence staining, cocultures of primary CD4+ T cells with uninfected or infected MOLTNL4-3 cells were performed as described above. Samples were trypsinized to remove potentially bound viruses into the cell surface and after subsequent washes with PBS cells were fixed, permeabilized (Fix & Perm), and incubated for 1 h at room temperature with the anti-CAp24 mAb KC57-FITC (Coulter) and the CD4-PE (BD Bioscience) or with the goat anti-human-dynamin antibody (clone N-19, Santa Cruz Biotechnology). For dynamin staining, cells were then washed and incubated for 1 h at room temperature with the donkey anti-goat Alexa Fluor 647-conjugated secondary antibody (Molecular Probes, Invitrogen). Cells were adhered onto glass slides using cytospins (Thermo Scientific) and mounted with Prolong Gold antifade reagent (Invitrogen). Images were acquired on a Leica TCS SP5 AOBS confocal microscope (Leica Microsystems CMS GmbH, Mannheim, Germany). Z-sections were acquired at 0.5-μm steps using an Argon 488/458 and HeNe 633 lasers and a plan Apochromat 63 × 1.4 oil objective, supplied with the imaging software LAS AF (Leica Microsystems). Determination of the colocalization coefficient between CAp24 protein and the CD4 receptor or dynamin protein was performed using single Z-stacks and evaluated with LAS AF software.
Statistical Analysis
Student's t test was used to determine statistical significance (**, p < 0.005 or *, p < 0.05) between values.
RESULTS
HIV Transmission during Cell-to-cell Cocultures
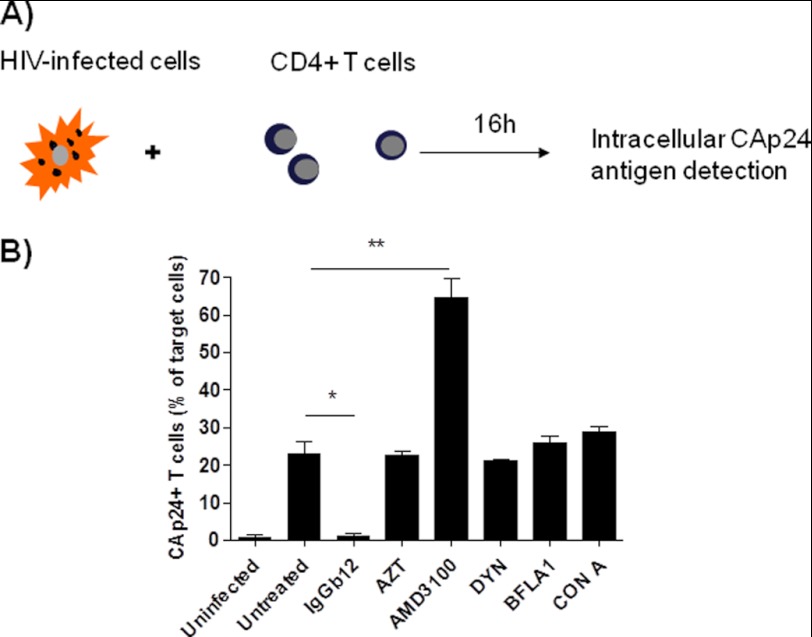
Overnight cocultures of HIV-1 NL4-3 persistently infected MOLT-4/CCR5 cells (MOLTNL4-3) with CMFDA-loaded nonstimulated primary CD4+ T lymphocytes were evaluated by flow cytometry. After overnight coculture, intracellular staining of capsid p24 (CAp24) HIV antigen was detected in 23% of target cells (Fig. 1). The transfer of viral antigens to uninfected cells was clearly blocked by the neutralizing anti-gp120 mAb IgGb12 (95% of inhibition compared with the untreated condition), but was not inhibited by the RT inhibitor AZT or the dynamin-dependent endosome-scission inhibitor dynasore (21 and 22% of p24+ cells, respectively) despite using high drug concentrations (2000-fold higher than the EC50 of AZT under cell-free infection conditions, Table 1). Macrolide antibiotics such as BFLA1 and CON A that prevent endosome and lysosome acidification did not have any effect on virus uptake (Fig. 1B).
FIGURE 1.
CD4-dependent transfer of HIV antigen after cell-to-cell contacts. A, experimental procedure was overnight cocultures of MOLTNL4-3 cells with primary CD4+ T lymphocytes. B, cocultures were performed in the presence of 80 nm anti-gp120 mAb IgGb12; 4 μm RT inhibitor AZT; 12.5 μm CXCR4 antagonist AMD3100; 80 μm dynamin inhibitor dynasore (DYN); 100 nm BFLA1, and 20 nm CON A. Results are represented as the percentage of intracellular CAp24+ target cells, using the coculture between CD4+ T cells and uninfected MOLT cells as a negative control. Results are the mean ± S.D. (error bars) of three independent experiments (**, p < 0.005; *, p < 0.05).
TABLE 1.
Potent postattachment inhibitors of HIV replication do not block cell-to-cell transfer of virus
| Compound | Anti-HIV-1 activity |
Cell-to-cell HIV-1 Transfer |
||
|---|---|---|---|---|
| EC50a | CC50b | EC50c | CC50d | |
| μm | μm | μm | μm | |
| AMD3100 | 0.018 ± 0.0023 | >0.125 | No effect at 62 μm | >62 |
| AZT | 0.0021 ± 0.0003 | >0.4 | No effect at 20 μm | >20 |
| IgGb12 | 0.0003 ± 0.0001 | >0.04 | 0.0006 ± 0.0001 | >0.4 |
| Dynasore | No effect at 40 μm | 40 | No effect at 80 μm | 250 |
a EC50: Effective concentration needed to inhibit 50% replication of the wild-type HIV-1NL4–3 strain in peripheral blood mononuclear cells.
b CC50: Cytotoxic concentration needed to induce 50% death of noninfected cells, evaluated by morphology changes using flow cytometry 7 days after infection.
c EC50: Effective concentration needed to block 50% of HIV-1NL4-3-antigen transfer in CD4+ T cells determined by intracellular CAp24 antigen staining after overnight cocultures between HIV-infected MOLTNL4-3 cells and primary CD4+ T cells.
d CC50 evaluated after overnight cocultures.
Interestingly, in the presence of the coreceptor antagonist AMD3100 the uptake of HIV particles by the target cells increased roughly 3-fold compared with untreated condition (65% of target cells were positive for CAp24 antigen staining) even when cells were cocultured with ∼700-fold higher EC50 (Table 1). Taken together, these results confirmed that cellular contacts between infected lymphoid cells and primary CD4+ T lymphocytes triggered CD4-dependent transmission of high amounts of HIV-1 particles from infected to uninfected cells.
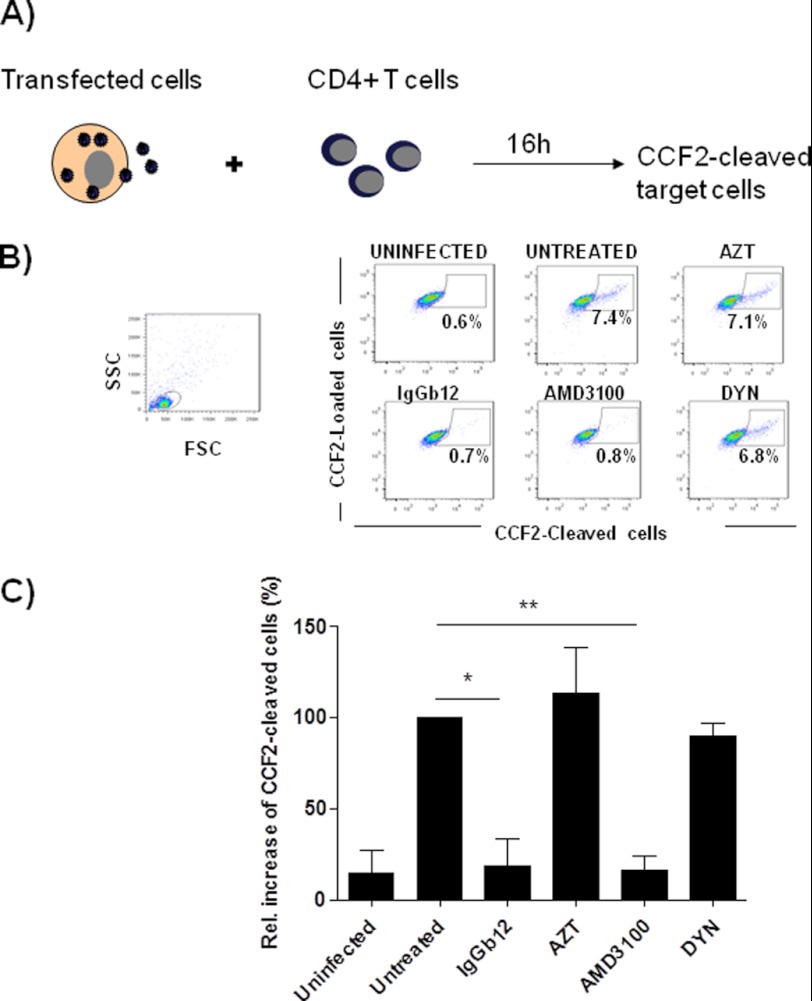
To evaluate virus-cell fusion, HIV-1 NL4-3 transfected Vpr-BlaM+ HEK293-T cells were cocultured with target CD4+ T cells and fusion was measured by detection of cleaved CCF2. As expected, mAb IgGb12 completely blocked virus-dependent fusion similar to the observed inhibition of virus capture (Fig. 2). Conversely, AMD3100 blocked virus-cell fusion (Fig. 2) although it did not block but significantly increased virus transfer (Fig. 1). AZT or dynasore did not prevent cleavage of CCF2, suggesting that virus antigen was passively transferred to CD4+ T cells in the absence of virus cell fusion as noted in the AMD3100-treated cells.
FIGURE 2.
IgGb12 and AMD3100 but not dynasore blocked virus-cell fusion after cell to cell transfer of virus. A, experimental procedure: measurement of viral fusion in cocultures of HEK293-T cells transfected with pNL4-3 and Vpr-BlaM plasmids and primary CD4+ T cells. B, dot plots of CCF2-loaded cells (FITC-labeled) versus CCF2-cleaved cells (Pacific blue-labeled). A representative experiment is shown. C, relative increase of CCF2-cleaved target cells compared with untreated condition. Data are the mean ± S.D. (error bars) of three independent experiments (**, p < 0.005; *, p < 0.05) (DYN, dynasore).
Productive Infection Did Not Occur from within Intracellular Compartments
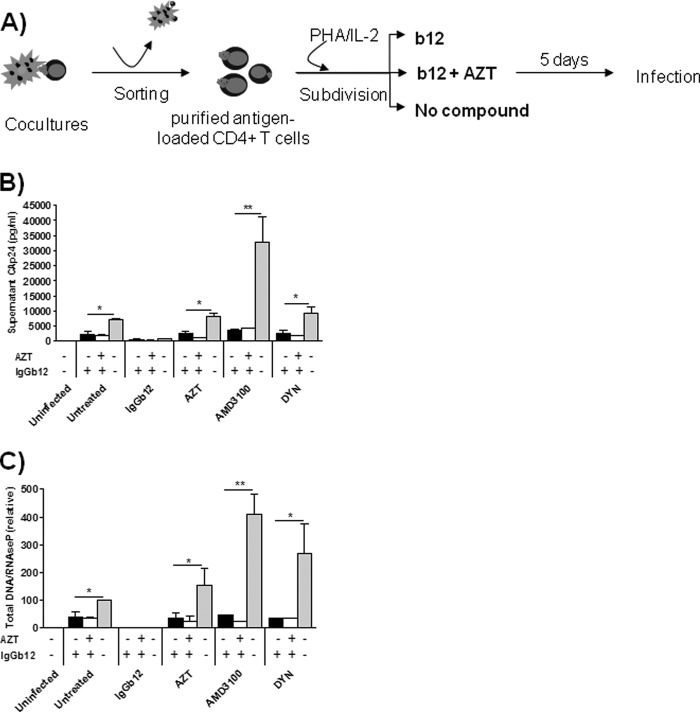
We hypothesize that internalized virus after cell-to-cell transfer could not fuse from within intracellular compartments. To evaluate the fate of internalized HIV-1 particles captured by CD4+ T cells after cell-to-cell transfer, CMFDA-loaded target CD4+ T cells were purified from infected MOLTNL4-3 lymphoid cells by fluorescence-activated cell sorting (>99% purity). Following separation, purified CD4+ T cells were trypsinized to eliminate virus bound to the cell surface. Trypsin treatment dramatically reduced the expression of CD4 in purified T cells; however, CAp24 antigen staining was not significantly reduced (data not shown), suggesting that captured virus resided in intracellular compartments. Immediately after washings, for each initial coculture condition, target cells were subdivided in three and left in culture during 5 days in drug-free medium or in the presence of the mAb IgGb12 or IgGb12+AZT (Fig. 3A). Drug concentrations used clearly ensure complete inhibition of infection (300-fold and 2000-fold higher EC50 for IgGb12 and AZT, respectively, Table 1). Dynasore was not included as it was cytotoxic in long term cultures (data not shown). Virus production is low to undetectable in nonstimulated cells (18, 31, 32); thus, PHA/IL-2 was added to the medium to promote virus replication. After 5 days in culture, CAp24 antigen in cell supernatant (Fig. 3B) and total viral DNA detection by quantitative PCR (Fig. 3C) were evaluated as a measure of virus replication and indicated that antigen-loaded cell cultures became productively infected after PHA/IL-2 activation in the absence of inhibitors in the culture medium (Fig. 3, gray bars). Virus production was in concordance to the amount of virus transferred during the coculture phase (Fig. 1). Thus, in the absence of antigen transfer (IgGb12-treated coculture), no virus production was found. Conversely, the high uptake of CAp24-antigen in the AMD3100-treated cocultures coincided with an increase in virus production in purified cells. The RT inhibitor AZT did not prevent virus transfer or fusion and partially blocked supernatant CAp24 antigen production or total DNA detection as a consequence of being present only during the coculture phase. However, when IgGb12 was present during the purified cell culture phase (Fig. 3, black and white bars), virus replication was significantly blocked irrespective of the condition used during the coculture phase (Fig. 3, x axis, angled labels). Taken together, these results indicate that the conditioned medium with IgGb12 prevented internalized virus particles from initiating a productive infection. Virus needed to reach the extracellular environment to initiate a productive infection, an event that could only occur when the attachment inhibitor, mAb IgGb12, was not present.
FIGURE 3.
Infection of CD4+ T cells by HIV particles captured into trypsin-resistant compartments was inhibited by mAb IgGb12. A, experimental procedure: isolation and culture of CAp24-loaded CD4+ T cells. After 5 days of culture, HIV infection was assessed by supernatant CAp24 antigen production, expressed in pg/ml (B) and quantification of total viral DNA as the copy number of total DNA/RNaseP, expressed relative to the untreated condition (cells untreated during the coculture and culture phase) (C). Results represent the mean ± S.D. (error bars) of three independent experiments (**, p < 0.005; *, p < 0.05) (DYN, dynasore).
Infection by HIV Particles Released from Antigen-loaded Cells
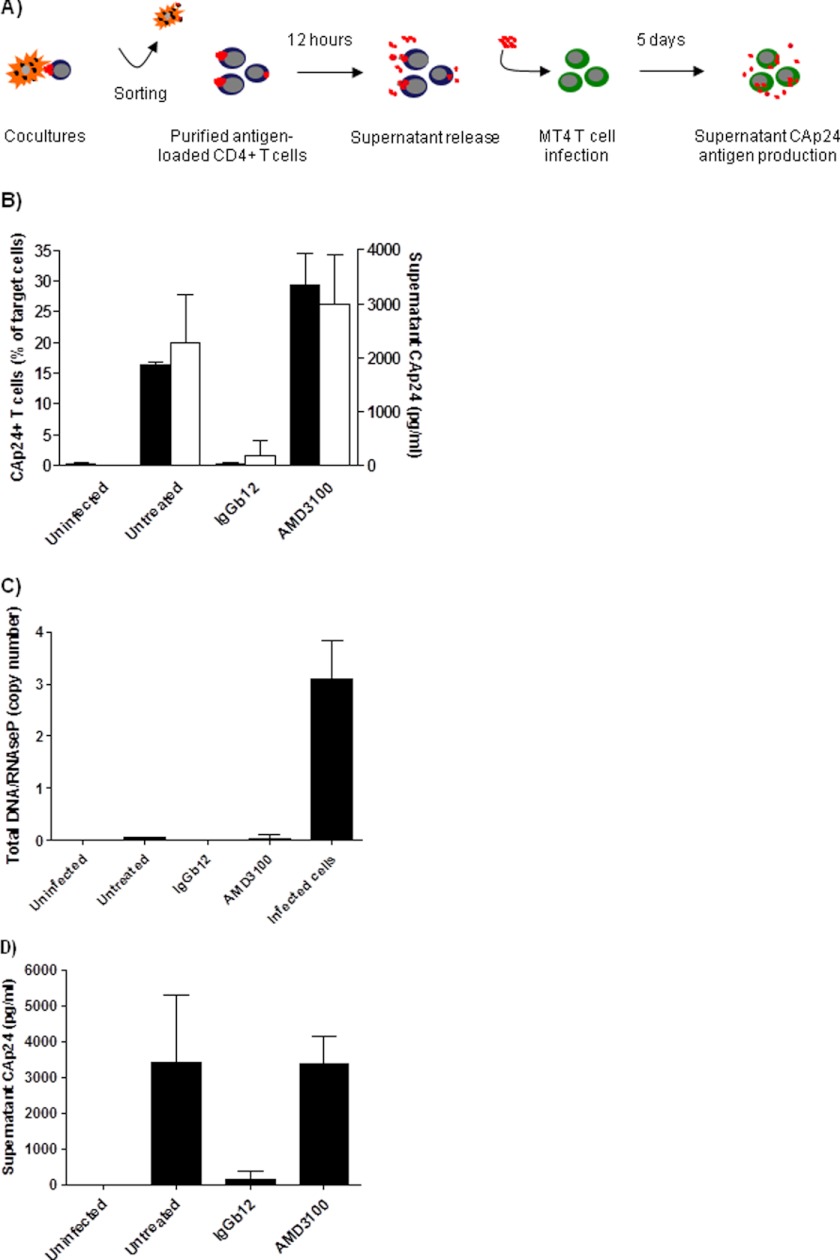
Our results suggest that the inability of virions to infect cells from within endosomal compartments could promote the recycling of HIV particles to the cell surface that could later infect bystander cells. To further explore this hypothesis, antigen-loaded primary CD4+ T lymphocytes were sorted after short term cocultures (6 h) with infected MOLTNL4-3 cells (Fig. 4A). IgGb12-treated coculture, in which CAp24-antigen transfer was completely blocked, was used to control the effect of contaminant MOLTNL4-3 cells (<0.1%). Once purified, antigen-loaded CD4+ T cells were left in culture for 12 h in the presence or the absence of IgGb12 to restrict reinfection events while allowing release of virions in the supernatant. The CAp24-antigen found in the supernatant was concordant with the level of intracellular CAp24-antigen in loaded target cells (Fig. 4B). Total DNA in purified target cells was measured to ensure that antigen-loaded cells did not become infected during the culture (Fig. 4C). Compared with infected control cells, target cells remained negative, suggesting that particles found in the supernatant did not come from new infection events but released from endocytic compartments. Supernatants were collected after 12 h and used to infect lymphoid MT4 T cells (Fig. 4D). The supernatants from untreated and AMD3100-treated cultures were able to establish a productive infection in MT4 cells. Conversely, the supernatant of the IgGb12 condition could not infect target cells, indicating that infection was not generated from contamintant MOLTNL4-3 cells. These results indicate that antigen-loaded cells did not become infected but were able to infect bystander CD4+ cells after recycling of HIV to the cell surface and release to the cell supernatant.
FIGURE 4.
Trans-infection by released HIV viruses from antigen-loaded cells. A, in this experimental procedure, supernatants from cocultures were collected and used to infect MT4 T cells. B, after 12 h of culture, p24-antigen content was evaluated in the supernatant (white bars) and in the purified cells (black bars) by CAp24 ELISA and intracellular CAp24 antigen staining, respectively. C, total viral DNA was also quantified in purified cells by PCR using infected CD4+ T cells as a positive control. Results represent the total viral DNA copy number relative to the cellular control gene RNaseP. D, infection of MT4 T cells by collected supernatants was evaluated at day 5 by supernatant CAp24-antigen content. Data are the mean ± S.D. (error bars) of three independent experiments.
Dynasore Did Not Block Uptake or Infection of CD4+ T Cells
Dynasore (80 μm), a dynamin-dependent endosomal scission inhibitor, has been shown to block the infection of HeLa cells, suggesting that endosomal uptake was a prerequisite for fusion and infection (20). We and others have shown that cell-to-cell transfer of HIV may occur through an endocytic process in which virus antigen is colocalized with clathrin and dynamin (12, 18, 19, 21, 33). However, dynasore did not prevent the CD4-dependent uptake of HIV antigen into target cells (Fig. 1), did not prevent virus replication in antigen-loaded, activated cells after cell-to-cell transfer of virus (Fig. 3), and was devoid of antiviral activity in peripheral blood mononuclear cells at subtoxic concentrations (Table 1). Conversely, dynasore blocked the PMA-induced down-regulation of the CD4 receptor in primary CD4+ lymphocytes (Fig. 5), a process that involves a clathrin-dependent endocytic pathway (34).
FIGURE 5.
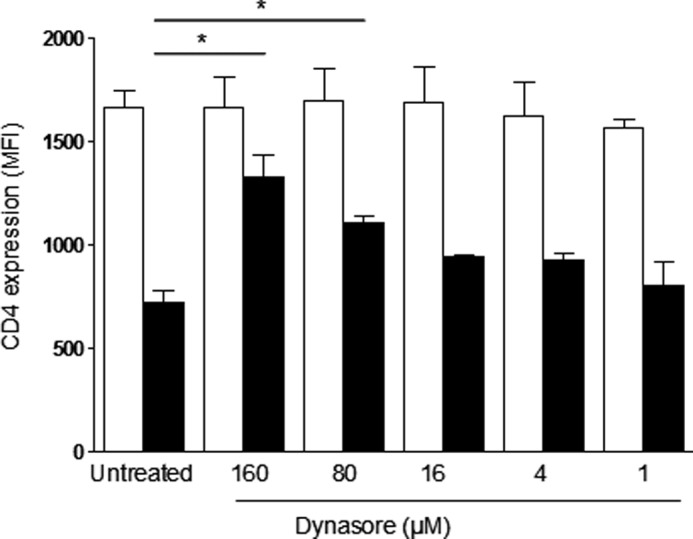
Dynasore prevents PMA-induced down-regulation of CD4 receptor. Primary CD4+ T lymphocytes were pretreated for 30 min with or without 160, 80, 16, 4, and 1 μm of dynasore and then cultured in the absence (white bars) or the presence of PMA at 1 μg/ml (black bars) for an additional 30 min. Then, cells were fixed with 2% of formaldehyde, and surface CD4 expression (mean fluorescence intensity, MFI) was evaluated with an anti-CD4 mAb. Cells were analyzed by flow cytometry and identified by morphology. Dynasore inhibited PMA-induced CD4 down-regulation in a dose-dependent manner. Data are the mean ± S.D. (error bars) of three independent experiments. *, p < 0.05.
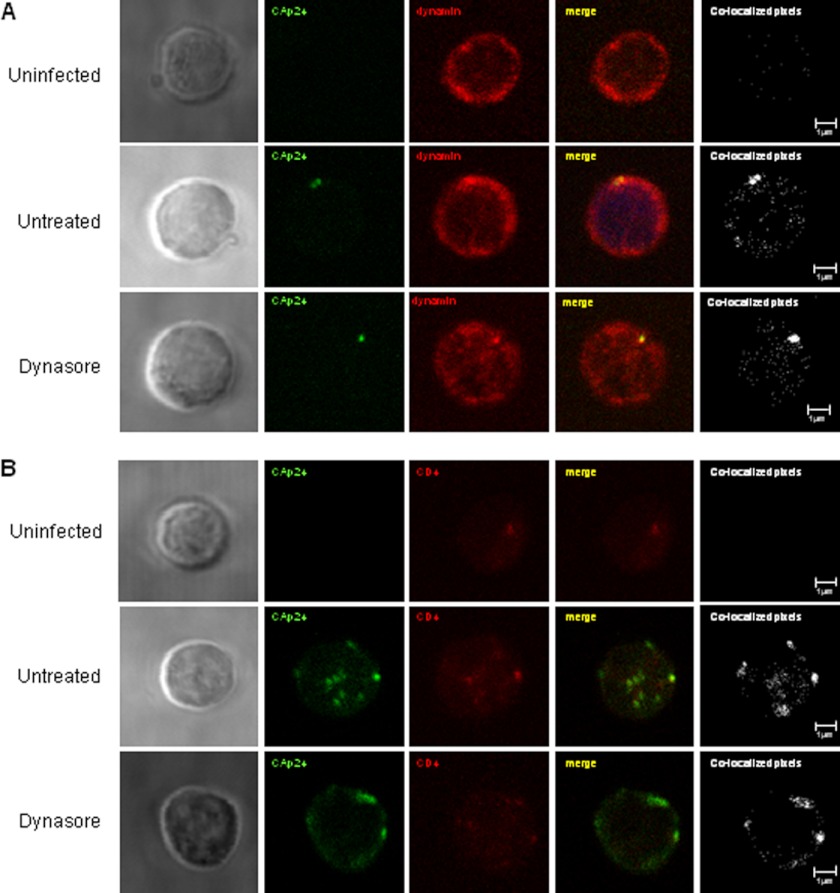
To analyze the effect of dynasore in dynamin function during HIV uptake we performed a colocalization analysis between HIV Gag antigen (CAp24) and dynamin in untreated or dynasore-treated cocultures (Fig. 6A). Colocalization coefficients of 0.73 and 0.75 between CAp24 antigen and dynamin protein were calculated in both untreated and dynasore-treated cocultures respectively, indicating that early compartmentalization of HIV particles was associated with the dynamin endocytic machinery, but could not be blocked by an agent targeting the scission of early formed endosomes. Colocalization between HIV Gag antigen (CAp24) and CD4 receptor in untreated or dynasore-treated cocultures between primary CD4+ T cells and infected MOLTNL4-3 cells showed similar colocalization coefficients (0.78 and 0.84 for untreated and dynasore-treated conditions, respectively) (Fig. 6B).
FIGURE 6.
Uptake of HIV particles into intracellular CD4+ compartments in primary T lymphocytes was not blocked by dynasore. Primary CD4+ T lymphocytes were cocultured overnight with HIV-infected MOLTNL4-3 cells in the presence or the absence of dynasore (80 μm). Recovered cells were trypsinized to remove membrane-bound viruses and immunostained with antibodies against HIV CAp24 antigen, CD4 receptor, and dynamin. Sections of single target CD4+ T cells were viewed and analyzed by confocal microscopy. Colocalization between (A) HIVp24 antigen (green) and dynamin protein (red) or between (B) HIVp24 antigen (green) and CD4 receptor (red) was performed for uninfected (upper panels), untreated (middle panels), and dynasore-treated (lower panels) cocultures. The images show the phase-contrast (left column), the single stainings, the overlay (yellow), and the colocalized pixels (white). A CD4+ T lymphocyte representative of each coculture is shown from at least two independent experiments.
DISCUSSION
Complete fusion of HIV particles within endosomal membranes has been used to indicate that internalization of HIV particles through an endocytic pathway was required for infection (20, 24). Here, we show, using primary CD4 T lymphocytes, that cell-to-cell contacts between HIV-infected and uninfected cells induced the endocytic uptake of viral particles into trypsin-resistant, dynamin-enriched compartments. Only the inhibition of gp120-CD4 interaction (virus attachment to CD4) could block the transfer of HIV particles. Conversely, the addition of the coreceptor inhibitor AMD3100 induced the accumulation of virus particles leading to massive endocytosis into cells in which the virus-cell fusion process was completely arrested (18, 25, 33). Activation of purified antigen-loaded cells initiated a productive infection but only when cells were cultured in the absence of mAb IgGb12, an inhibitor of virus attachment to CD4. IgGb12 should be unable to penetrate the cell surface. However, we cannot completely exclude the possibility of an antibody such as IgGb12 to enter already formed intracellular compartments containing HIV particles.
These results suggest that endocytosed viral particles could not initiate a productive infection from within endosomes in primary CD4+ T cells (i.e. by virus fusion to the endosomal membrane). We hypothesize that endocytosed viruses could only induced infection in trans (trans-infection) because they were required to resurface and reach the extracellular environment and engage CD4 leading to virus-cell fusion and replication, a condition that could only be achieved in the absence of an attachment inhibitor in the cell supernatant. We have shown that antigen-loaded cells may release virus particles (11), and cocultures of antigen-loaded T cell with U87-CD4 target cells may lead to infection of the U87-CD4 cells (18), indicating the possibility of trans-infection. Here, we demonstrate that supernatant from purified antigen-loaded, but viral DNA-negative T cells, released virus to the supernatant that later infected MT4 cells, strongly suggesting that antigen-loaded cells trans-infect bystander CD4+ T cells.
Recent data indicate that prior to membrane fusion, virions may need to undergo maturation after cell-to-cell transfer of HIV-1 (25), a process that might be impaired or further delayed in nonstimulated primary CD4+ T cells, and thus, productive infection was only possible after virus recycling to the cell surface. Moreover, virion maturation may allow the virus to transfer from cell-to-cell in a conformation immunologically distinct that might escape the detection by neutralizing antibodies. However, these findings are in contrast to data showing that anti-gp41 antibodies 4E10 and 2F5 did not block the transfer of HIV particles from infected to target cells but blocked productive infection of target cells (35), suggesting that HIV infection between T cells is transmitted by a neutralization-sensitive mechanism (4, 35). Our results reinforce the idea that endocytosed virus after cell-to-cell contacts may represent an itinerant virus reservoir able to induce the trans-infection of bystander T cells, but not leading to effective virus fusion or replication from within internal endosomal compartments. The contribution of this mechanism in the pathogenesis of HIV in vivo still needs to be completely clarified but should be taken into account when developing new antiviral strategies (36).
Using confocal microscopy, we found clathrin and dynamin proteins colocalized with HIV particles (33) which in turn were colocalized with CD4 (Fig. 6). However, dynasore, a dynamin-dependent endosomal scission inhibitor previously shown to block virus replication in HeLa cells (20, 24), did not prevent virus capture, virus cell fusion, and virus replication after cell-to-cell transfer to primary CD4+ T cells. In concordance, previous observations indicated that VSV-G-pseudotyped HIV infection could not be inhibited after dynasore treatment, suggesting that VSV and HIV envelopes mediate distinct modes of virus entry (37). Moreover, it has been demonstrated that dynasore inhibits clathrin-mediated endocytosis at two different steps. The ultrastructural analysis of the effect of dynasore on clathrin-coated structures shows the appearance of “U” and “O” shape-coated pits associated with the plasma membrane (38). Consequently, internalization of CAp24 antigen into the “initial” coated pits in the presence of dynasore cannot be ruled out. Altogether, it appears that internalization of particles initially required the endocytic machinery, and dynasore might not be able to inhibit the initial formation of these endocytic compartments. Blocking HIV endocytosis (e.g. with dynasore) without preventing virus replication would be the ultimate proof of endocytosis not being necessary for infection. This could not be achieved with dynasore at nontoxic concentrations, and therefore, the hypothesis remains unresolved. However, we clearly show that internalized virus required to resurface to initiate a productive infection, suggesting that endocytosis may not be a route of productive infection.
The development of new small molecule inhibitors of clathrin-coated pit assembly (Pitstop) allowed better characterization of clathrin functions within the endocytic network (39). Pitstop-induced inhibition of clathrin terminal domain interferes with receptor-mediated endocytosis and synaptic vesicle recycling and has been shown to increase the lifetime of clathrin-coated components, including dynamin. These agents were also shown to block HIV entry in HeLa cells, but it remains to be resolved whether inhibition of virus replication was due to preventing virus-endosome fusion or the recycling of HIV particles. Importantly, the antiviral activity of endosome function should be evaluated in primary T cells that better model the interactions between virus and cell functions.
Nef-induced down-regulation of CD4 results in internalization and degradation of surface CD4 in lysosomes (40). Prevention of endosome and lysosome acidification by macrolide antibiotics such as BFLA1 and CON A inhibits degradation of CD4 and consequently promotes accumulation of CD4 in endosomes and lysosomes (41). Moreover, different types of endosome acidification inhibitors increase infectivity of HIV particles presumably by preventing them from degradation in late endosomes and lysosomes (42). Colocalization of HIV particles with CD4 in dynamin-containing endosomes could indicate that CD4 protects virus particles from degradation and helps recycle back HIV to the cell surface. However, in our hands, acidification inhibitors such as BFLA1 and CON A did not prevent or augment virus transfer from infected to uninfected cells. Therefore, our results did not shed light on the protective role of CD4 in endocytic virus degradation.
In conclusion, after cell-to-cell transfer of HIV-1 into target primary CD4+ T cells we observed that cells were only infected if left in culture in the absence of an attachment inhibitor to CD4 (mAb IgGb12), suggesting that virus needed to resurface to begin a productive infection. Moreover, dynasore, an inhibitor of dynamin-dependent endocytosis, did not block virus replication. Endocytosis may not be the primary mechanism of infection by HIV-1 after cell-to-cell contact, but a reservoir able to induce trans-infection of bystander CD4+ T cells.
Acknowledgments
We thank the National Institutes of Health (AIDS Research and Reference Reagent Program) and the EU Programme EVA Centralised Facility for AIDS Reagents, National Institute for Biological Standards and Control, United Kingdom, for reagents. We thank Marco A. Fernández (Instituto Germans Trias i Pujol, Badalona, Spain) for technical assistance.
This work was supported in part by the Spanish Ministerio de Economía y Competitividad Projects BFU2009-06958 and SAF2010-21617-C02, FIPSE 360783-09, and Gala contra la SIDA 2011.
- AZT
- 3-azido-3-deoxythymidine
- BFLA1
- bafilomycin A1
- CMFDA
- 5-chloromethylfluorescein diacetate
- CON A
- concanamycin A
- PHA
- phytohemagglutinin
- PMA
- phorol 12-myristate 13-acetate.
REFERENCES
- 1. Adamson C. S., Freed E. O. (2010) Novel approaches to inhibiting HIV-1 replication. Antiviral Res. 85, 119–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marsh M., Helenius A. (2006) Virus entry: open Sesame. Cell 124, 729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sattentau Q. (2008) Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 6, 815–826 [DOI] [PubMed] [Google Scholar]
- 4. Martin N., Welsch S., Jolly C., Briggs J. A., Vaux D., Sattentau Q. J. (2010) Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J. Virol. 84, 3516–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sigal A., Kim J. T., Balazs A. B., Dekel E., Mayo A., Milo R., Baltimore D. (2011) Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 477, 95–98 [DOI] [PubMed] [Google Scholar]
- 6. Izquierdo-Useros N., Esteban O., Rodriguez-Plata M. T., Erkizia I., Prado J. G., Blanco J., García-Parajo M. F., Martinez-Picado J. (2011) Dynamic imaging of cell-free and cell-associated viral capture in mature dendritic cells. Traffic 12, 1702–1713 [DOI] [PubMed] [Google Scholar]
- 7. Permanyer M., Ballana E., Esté J. A. (2010) Endocytosis of HIV. Trends Microbiol. 18, 543–551 [DOI] [PubMed] [Google Scholar]
- 8. Jolly C., Sattentau Q. J. (2004) Retroviral spread by induction of virological synapses. Traffic 5, 643–650 [DOI] [PubMed] [Google Scholar]
- 9. Esté J. A., Telenti A. (2007) HIV entry inhibitors. Lancet 370, 81–88 [DOI] [PubMed] [Google Scholar]
- 10. Tilton J. C., Doms R. W. (2010) Entry inhibitors in the treatment of HIV-1 infection. Antiviral Res. 85, 91–100 [DOI] [PubMed] [Google Scholar]
- 11. Bosch B., Blanco J., Clotet-Codina I., Pauls E., Armand-Ugón M., Grigorov B., Muriaux D., Clotet B., Darlix J. L., Esté J. A. (2005) Inhibition of coreceptor independent cell-to-cell HIV-1 transmission by a CD4-IgG2 fusion protein. Antimicrob. Agents Chemother. 49, 4296–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hübner W., McNerney G. P., Chen P., Dale B. M., Gordon R. E., Chuang F. Y., Li X. D., Asmuth D. M., Huser T., Chen B. K. (2009) Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323, 1743–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Puigdomènech I., Massanella M., Izquierdo-Useros N., Ruiz-Hernandez R., Curriu M., Bofill M., Martinez-Picado J., Juan M., Clotet B., Blanco J. (2008) HIV transfer between CD4 T cells does not require LFA-1 binding to ICAM-1 and is governed by the interaction of HIV envelope glycoprotein with CD4. Retrovirology 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. (1987) pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49, 659–668 [DOI] [PubMed] [Google Scholar]
- 15. Bedinger P., Moriarty A., von Borstel R. C., 2nd, Donovan N. J., Steimer K. S., Littman D. R. (1988) Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature 334, 162–165 [DOI] [PubMed] [Google Scholar]
- 16. Kielian M., Jungerwirth S. (1990) Mechanisms of enveloped virus entry into cells. Mol. Biol. Med. 7, 17–31 [PubMed] [Google Scholar]
- 17. Skehel J. J., Wiley D. C. (2000) Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69, 531–569 [DOI] [PubMed] [Google Scholar]
- 18. Blanco J., Bosch B., Fernández-Figueras M. T., Barretina J., Clotet B., Esté J. A. (2004) High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J. Biol. Chem. 279, 51305–51314 [DOI] [PubMed] [Google Scholar]
- 19. Clotet-Codina I., Bosch B., Senserrich J., Fernandez-Figueras M. T., Pena R., Ballana E., Bofill M., Clotet B., Este J. A. (2009) HIV endocytosis after dendritic cell to T cell viral transfer leads to productive virus infection. Antiviral Res. 83, 94–98 [DOI] [PubMed] [Google Scholar]
- 20. Miyauchi K., Kim Y., Latinovic O., Morozov V., Melikyan G. B. (2009) HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137, 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen P., Hübner W., Spinelli M. A., Chen B. K. (2007) Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J. Virol. 81, 12582–12595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pauza C. D., Price T. M. (1988) Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J. Cell Biol. 107, 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daecke J., Fackler O. T., Dittmar M. T., Kräusslich H. G. (2005) Involvement of clathrin-mediated endocytosis in human immunodeficiency virus type 1 entry. Journal of Virology 79, 1581–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de la Vega M., Marin M., Kondo N., Miyauchi K., Kim Y., Epand R. F., Epand R. M., Melikyan G. B. (2011) Inhibition of HIV-1 endocytosis allows lipid mixing at the plasma membrane, but not complete fusion. Retrovirology 8, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dale B. M., McNerney G. P., Thompson D. L., Hubner W., de Los Reyes K., Chuang F. Y., Huser T., Chen B. K. (2011) Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. Cell Host Microbe 10, 551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sattentau Q. (2010) Cell-to-cell spread of retroviruses. Viruses 2, 1306–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ballana E., Pauls E., Perron-Sierra F., Clotet B., Tucker G. C., Esté J. A. (2011) β5 integrin is the major contributor to the αv integrin-mediated blockade of HIV-1 replication. J. Immunol. 186, 464–470 [DOI] [PubMed] [Google Scholar]
- 28. Ballana E., Pauls E., Senserrich J., Clotet B., Perron-Sierra F., Tucker G. C., Esté J. A. (2009) Cell adhesion through αv-containing integrins is required for efficient HIV-1 infection in macrophages. Blood 113, 1278–1286 [DOI] [PubMed] [Google Scholar]
- 29. Ballana E., Senserrich J., Pauls E., Faner R., Mercader J. M., Uyttebroeck F., Palou E., Mena M. P., Grau E., Clotet B., Ruiz L., Telenti A., Ciuffi A., Esté J. A. (2010) Zinc ribbon domain-containing 1 (ZNRD1) is a host cellular factor influencing HIV-1 replication and disease progression. Clin. Infect. Dis. 50, 1022–1032 [DOI] [PubMed] [Google Scholar]
- 30. Cavrois M., De Noronha C., Greene W. C. (2002) A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20, 1151–1154 [DOI] [PubMed] [Google Scholar]
- 31. Doitsh G., Cavrois M., Lassen K. G., Zepeda O., Yang Z., Santiago M. L., Hebbeler A. M., Greene W. C. (2010) Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell 143, 789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoder A., Yu D., Dong L., Iyer S. R., Xu X., Kelly J., Liu J., Wang W., Vorster P. J., Agulto L., Stephany D. A., Cooper J. N., Marsh J. W., Wu Y. (2008) HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell 134, 782–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bosch B., Grigorov B., Senserrich J., Clotet B., Darlix J. L., Muriaux D., Esté J. A. (2008) A clathrin-dynamin-dependent endocytic pathway for the uptake of HIV-1 by direct T cell-T cell transmission. Antiviral Res. 80, 185–193 [DOI] [PubMed] [Google Scholar]
- 34. Pelchen-Matthews A., Parsons I. J., Marsh M. (1993) Phorbol ester-induced down-regulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J. Exp. Med. 178, 1209–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Massanella M., Puigdomènech I., Cabrera C., Fernandez-Figueras M. T., Aucher A., Gaibelet G., Hudrisier D., García E., Bofill M., Clotet B., Blanco J. (2009) Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. AIDS 23, 183–188 [DOI] [PubMed] [Google Scholar]
- 36. Permanyer M., Ballana E., Ruiz A., Badia R., Riveira-Munoz E., Gonzalo E., Clotet B., Esté J. A. (2012) Antiretroviral agents effectively block HIV replication after cell to cell transfer. J. Virology 86, 8773–8780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu D., Wang W., Yoder A., Spear M., Wu Y. (2009) The HIV envelope but not VSV glycoprotein is capable of mediating HIV latent infection of resting CD4 T cells. PLoS Pathog. 5, e1000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 39. von Kleist L., Stahlschmidt W., Bulut H., Gromova K., Puchkov D., Robertson M. J., MacGregor K. A., Tomilin N., Tomlin N., Pechstein A., Chau N., Chircop M., Sakoff J., von Kries J. P., Saenger W., Kräusslich H. G., Shupliakov O., Robinson P. J., McCluskey A., Haucke V. (2011) Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146, 471–484 [DOI] [PubMed] [Google Scholar]
- 40. Rhee S. S., Marsh J. W. (1994) Human immunodeficiency virus type 1 Nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J. Virol. 68, 5156–5163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luo T., Anderson S. J., Garcia J. V. (1996) Inhibition of Nef- and phorbol ester-induced CD4 degradation by macrolide antibiotics. J. Virol. 70, 1527–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fredericksen B. L., Wei B. L., Yao J., Luo T., Garcia J. V. (2002) Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 76, 11440–11446 [DOI] [PMC free article] [PubMed] [Google Scholar]