Background: In the fed state, hepatic gluconeogenesis is not completely inhibited by insulin; the mechanism is unknown.
Results: p300 maintains basal glucose production in the liver due to the lack of an insulin phosphorylation site found in CBP.
Conclusion: p300 mediates hepatic glucose production in both post-prandial and fasted states.
Significance: p300 mediates basal glucose production in the fed/post-prandial state may be important for converting gluconeogenic precursors into glucose.
Keywords: CBP, Glucose Metabolism, Liver, p300, Phosphorylation, Gluconeogenesis, Glucose Production
Abstract
A major cause of fasting hyperglycemia in diabetes mellitus is unregulated hepatic glucose production (HGP). Insulin suppresses HGP by phosphorylating CBP and disassembling the CREB-CBP complex from gluconeogenic genes. p300 is closely related to CBP; but in contrast to CBP, p300 binds constitutively to CREB due to the absence of phosphorylation site found in CBP. In a phosphorylation-competent p300(G442S) knock-in mouse model, we demonstrate that HGP is now exquisitely sensitive to insulin suppression. p300(G422S) and hepatic-deleted p300 mice exhibited significant lower blood glucose levels in the fasted and post-prandial states, indicating a role for p300 in maintaining basal HGP.
Introduction
Glucose is a major energy source for mammalian cells and takes a central position in metabolism. It is the sole energy source in some mammalian tissues and cells such as neurons, erythrocytes, and the renal medulla. Lower blood glucose (hypoglycemia) can damage these tissues and cells, even causes death (1, 2). At the other extreme, hyperglycemia can cause serious adverse effects, which are clearly demonstrated in patients with diabetes (3, 4). The maintenance of blood glucose levels within a small, defined range (70∼110 mg/dl, fasting), therefore, is crucial in protecting organisms against hypoglycemia during fasting and postprandial hyperglycemia.
In response to the fasting, glycogenolysis and gluconeogenesis are activated. Glucagon, epinephrine and glucocorticoids increase hepatic glucose production in the liver directly or indirectly through the cAMP-PKA3 signaling pathway (5). The phosphorylation of CREB at Ser-133 by PKA, in turn, recruits co-activators such as CBP, p300, and CRTC2 to CRE containing genes and facilitates hepatic gluconeogenesis. Both glucagon and insulin have been shown to stimulate phosphorylation of CREB at Ser-133 (6), which constitutively occupies the CRE containing promoters in both fasted and fed states (6, 7). Thus, phosphorylation of CREB at Ser-133 is necessary but is not sufficient for modulating hepatic gluconeogenic gene expression. We have previously reported that insulin and metformin inhibit hepatic glucose production (HGP) by phosphorylating CBP at Ser-436, therefore, triggers the disassembly of the CREB-CBP complex (7); furthermore, the phosphorylation of CRTC2 at Ser-171 lead to its nuclear exclusion and degradation (6).
CBP and p300 are closely related proteins that share extensive homology at the amino acid level (∼60%), including three cysteine-histidine rich domains (CH1–3), the CREB binding domain (Kix domain), the bromodomain, the histone acetyltransferase domain, and the SID (steroid receptor co-activator interacting domain) domain (8–10). Through these domains, p300 and CBP interact with basal transcription factors and RNA polymerase II as well as a variety of other transcription factors to function as scaffold proteins facilitating interactions among the basal transcription machinery and upstream transcription factors (8–11). The histone acetyltransferase activity of p300 and CBP modifies histones leading to chromatin remodeling and transcriptional activation; p300 and CBP also acetylate other proteins such as FXR, SREBP-1, CRTC2, and FOXO1, leading to the changes of genes related to glucose and lipid metabolism (12–15). Gene alterations in CBP and p300 cause various diseases such as Rubinstein-Taybi syndrome and leukemia (8, 16–18). Here, we report that the constitutive p300 occupancy on gene promoters is important for gluconeogenesis as it maintains basal hepatic glucose production in the fasted and post-prandial states.
EXPERIMENTAL PROCEDURES
Plasmids and Adenoviruses
The expression vectors for wild type (WT) CBP, CBP mutants, and PKA were described previously (19). The p300(G422S) expression vector was generated using site-directed mutagenesis (Stratagene). CH1 domain mutants linked to the Kix domain of CBP and p300 were cloned into the pCMV-AD vector in the same reading frame as the NF-κB (Rel A) transcriptional activation domain (Stratagene). CRE-luciferase reporter vector contains four tandem consensus CRE sites upstream of the luciferase-coding region. The Pck1 promoter-luciferase reporter was constructed by cloning the proximal promoter of Pck1 (−490 to +1) into the pGL4 luciferase reporter construct, which contains a CRE site (Promega). The BLOCK-iT adenoviral RNAi expression system (Invitrogen) was used to construct adenoviral shRNA for CBP, p300, and scrambled shRNA as we previously described (7).
Cell Cultures
Lipofectamine 2000 (Invitrogen) was used to transfect equal amounts of plasmids into mouse hepatoma cell lines (H2.35 or Hepa1–6). After 48 h of transfection, cells were exposed either to 20 nm insulin for 2–4 h or 2 mm metformin overnight. In the two-hybrid assay, 100 ng of pFR-Luc, 10–20 ng of pFA-CREB (full-length), and 10–20 ng of pCMV-AD vectors and/or 100 ng of PKA were transfected.
Glucose Production Assays
Mouse primary hepatocytes from fed mice (unless otherwise specified) were cultured in six-well plates with William's medium E supplemented with ITS (BD Biosciences) and dexamethasone. Primary hepatocytes were infected 16–24 h after planting with adenoviral shRNAs. After 72 h of incubation, cells were washed twice with PBS. Then, medium was replaced with 1 ml of glucose production buffer consisting of glucose-free DMEM supplemented with 20 mm sodium lactate and 2 mm sodium pyruvate or with 0.2 mm 8-bromo-cAMP. After 5 h of incubation, both medium and cells were collected.
Animal Experiments
All animal protocols were approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University. For generation of p300(G422S) knock-in mice, a point mutation in p300 at Gly-422 was introduced using site-directed mutagenesis into the targeting construct; a self-excising ACN cassette was also inserted into an artificially reconstituted BglII site in intron 5. The linearized and purified targeting vector DNA was electroporated into E14 ES (129) cells that were grown in medium supplemented with G418 to select for those cells in which integration of the targeting vector occurred. Southern blot and the PCR strategy were used to select the ES cells harboring the targeted locus. The targeted embryonic stem cells were injected into blastocysts derived from the mouse strain C57BL/6. The blastocysts were transferred to foster mothers to produce transmitting chimeras. The chimeric male mice with the greatest degree of agouti coat color (∼90%) were used to mate with C57BL/6 mice to generate heterozygous mutant p300 mice. Southern blot and PCR analysis were used to determine the genotype of the generated mouse line. The littermates were used to generate WT and mutant homozygous p300 mice.
For hepatic mutant p300 phosphorylation measurement and tolerance tests, mice were injected intraperitoneally with 1.0 unit/kg insulin, 1.5 g/kg pyruvate, or PBS after fasting for the indicated time. Adenoviral shRNA knockdown experiments were conducted 64–72 h after mice were injected with the adenovirus (7).
Euglycemic-hyperinsulinemic Clamp Experiment
The clamp experiments for p300 mutant mice and their littermates were conducted at the age of 5–6 months. Surgical catheterizations of the jugular vein were performed with PE-10 tubing. After 3 days of recovery, mice were subjected to 5 h of fasting before the clamp procedure as described previously (7). Briefly, a mouse was given 3 μCi of bolus [3H]glucose prime followed by a 0.05 μCi/min continuous infusion, and blood samples were collected at 60, 70, and 80 min through tail vein nick for measuring basal glucose production. Then, we doubled the [3H]glucose infusion rate, and bolus of insulin was given followed by a 5 milliunits/kg/min continuous insulin infusion, and glucose levels were measured every 5 min. An infusion of 30% glucose was adjusted to maintain blood glucose at 100–120 mg/dl. Usually, it took 40–60 min to reach a steady blood glucose level after starting the clamp. After reached the steady blood glucose levels, three blood samples were collected for measuring the [3H]glucose.
Immunoblot and Chromatin Immunoprecipitation
Phospho-Ser-436 CBP antiserum was generated against a phospho-CBP peptide containing amino acids 431–440 of the mouse CBP protein, which contains identical amino acids as those in p300(G422S) mice in the region of the phosphorylation site. The chromatin immunoprecipitation assay was performed as we described previously (7).
Statistical Analyses
Statistical significance was calculated with a Student's t test and analysis of variance test. Significance was accepted at the level of p < 0.05.
RESULTS
The Critical Role of CBP and p300 in Mediating Hepatic Glucose Production and Maintaining Blood Glucose Levels
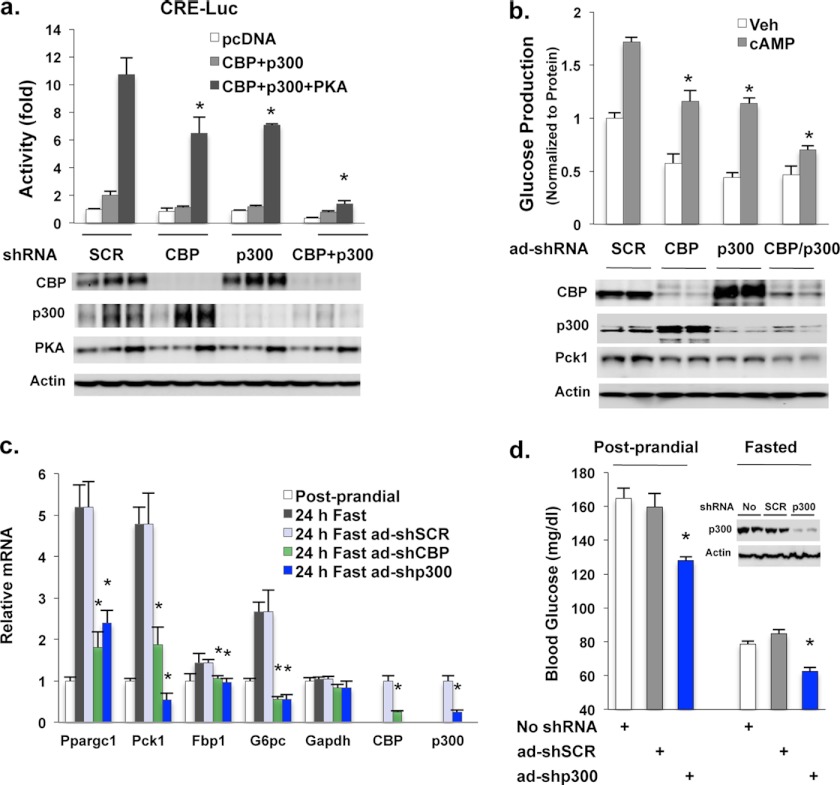
Previous study has shown that the depletion of CBP or p300 significantly decreased Ad-CRE-luciferase reporter activity in fasted mice (20). To examine further whether co-activators CBP and p300 have a similar role in regulating gluconeogenic gene expression, we employed adenoviral shRNAs to knock down CBP and/or p300 in hepatoma Hepa1–6 cells co-transfected with CBP and p300, and conducted CRE-luciferase reporter assays. We found that depletion of either p300 or CBP reduced and that depletion of both proteins abolished PKA-stimulated CRE reporter activity (Fig. 1a). The depletion of CBP protein led to an increase in p300 protein and vice versa. We next performed glucose production assays in primary hepatocytes. Depletion of either CBP or p300 decreased cAMP-stimulated glucose production, whereas depletion of both CBP and p300 reduced glucose production even further (Fig. 1b). The fold induction of PKA or cAMP was still preserved after individual CBP or p300 depletion in these experiments, suggesting that either protein is able to mediate the PKA or cAMP effect (Fig. 1, a and b). Furthermore, the depletion of CBP or p300 resulted in decreased mRNA levels of gluconeogenic genes in the liver of 24-h fasted mice (Fig. 1c). To further assess the role of p300 in mediating HGP, we used adenoviral shRNA through tail vein injection to deplete p300 in the liver (Fig. 1d). Depletion of p300 significantly reduced blood glucose levels in both post-prandial and fasting states (Fig. 1d). These data indicate that p300 functions in maintaining blood glucose levels in both fasted and post-prandial states.
FIGURE 1.
Important role of CBP and p300 in regulating hepatic gluconeogenesis. a, adenoviral shRNAs mediated knockdown of CBP and/or p300 suppressed PKA-stimulated reporter activity in Hepa1–6 cells co-transfected with CBP and p300. Adenoviral shRNAs were added into each well 16 h after the seeding of cells. After 6 h of incubation, plasmids (100 ng of pcDNA, or 50 ng of CBP plus 50 ng of p300 per well) were transfected by using Lipofectamine 2000. b, depletion of CBP and/or p300 decreased glucose production in primary hepatocytes. c, hepatic gluconeogenic enzyme gene expression levels in either WT mice sacrificed at post-prandial state or after 24 h of fasting (n = 5) or in mice with adenoviral shRNAs mediated depletion of CBP or p300 and sacrificed after 24 h fasting (n = 4). d, adenoviral shRNAs mediated depletion of p300 significantly lowered blood glucose levels in post-prandial and/or fasted states (24 h) (n = 4–5). The y-axis has been broken and begins at 40 mg/dl. Veh, vehicle; Ad, adenovirus; Luc, luciferase; SCR, scramble.
Constitutive p300 Gene Promoter Occupancy Maintains Basal HGP Due to the Lack of a Phosphorylation Site Found in CBP
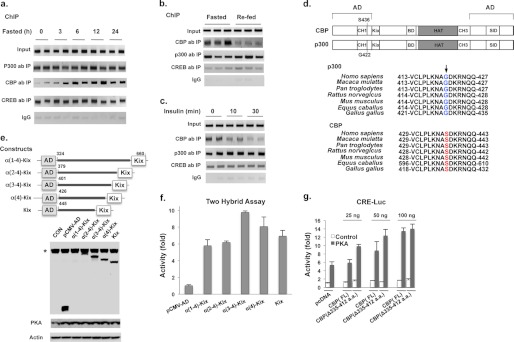
Because depletion of p300 in the liver led to lower blood glucose levels in post-prandial and fasted states (Fig. 1d), we examined the binding of p300 to hepatic CREs. Hepatic protein levels of p300, CBP, and CREB remained unchanged in mice during a 24-h fasting period (supplemental Fig. S2). However, fasting increased CBP binding to the Ppargc-1 gene promoter in a chromatin immunoprecipitation (ChIP) assay, reaching maximal binding by 6 h (Fig. 2a) and consistent with our previous finding that non-phosphorylated CBP reassembles the CREB co-activator complex (7). In contrast, the binding of p300 and CREB to the CRE site of the Ppargc-1 gene promoter was unchanged. We then examined hepatic p300 and CBP gene promoter binding in fasted and refed mice. In agreement with our previous report, CBP was absent from the Ppargc-1 gene promoter in the liver of refed mice (7), whereas p300 constitutively bound to the CRE site independent of the nutritional state (Fig. 2b). Furthermore, intraperitoneal insulin administration decreased binding of CBP to the CRE site but had no effect on p300 binding to the promoter (Fig. 2c). CREB and p300 did not bind to the proximal region of GAPDH promoter in ChIP assay, demonstrating the specificity of this assay (data not shown).
FIGURE 2.
Constitutive p300 binding to CREs due to lack of a phosphorylation found in CBP. a, the binding of CBP, p300, and CREB to the CRE site of the Ppargc-1 promoter in the liver of mice during a 24-h fasting period. b and c, the occupancy of CBP, p300, and CREB on the CRE site in the liver of fasted (16 h) and refed (2 h) mice (b) and in the liver of insulin treated mice (intraperitoneal) (c). d, the schematic structure of CBP and p300 (upper panel). The conserved CBP phosphorylation site at Ser-436 (mouse) does not exist at the corresponding site in p300 (lower panel). e, the protein levels of constructs with serial deletion of α-helix in the CH1 domain of CBP in Hepa1–6 cells; α-(1–4)-Kix helix was masked in endogenous NF-κB Rel A. *, endogenous NF-κB Rel A. f, the effect of α-helices in the CH1 domain of CBP on the binding of Kix domain to CREB in a two-hybrid assay. g, deletion of three α-helices in CH1 domain had no effect on CBP stimulated CRE-luciferase reporter activity in Hepa1–6 cells. Each lane represents an individual mouse sample (a–c). Luc, luciferase; AD, adenovirus; CON, no vector; SID, steroid receptor co-activator interacting domain.
CBP and p300 are highly homologous proteins and have similar functional domains (Fig. 2d, upper panel). Compared with CBP, p300 lacks the insulin phosphorylation site found in CBP due to a one-amino acid difference (Fig. 2d, lower panel). This amino acid difference has also been proposed to affect the length of the α4 helix of the CH1 (also named TAZ1) domain of these proteins (21). To gain insight into how the CH1 domain of CBP may affect binding to CREB via the Kix domain, we fused full-length and truncated CH1-Kix domain fragments downstream of a transcriptional activation domain of NF-κB (Rel A) in an expression vector (Fig. 2e). By using these constructs with Gal4-CREB in a two-hybrid assay in Hepa1–6 cells, we found that the CH1 domain (α[1–4]-helix) was completely dispensable for the binding of the CBP Kix domain to CREB (Fig. 2f). To support this notion, we also deleted the first three α-helices in the CH1 domain of full-length CBP to match a mouse model where CH1 domain deletion was made in the germ line (22). Compared with wild type CBP, the deletion of these α-helices did not affect PKA-stimulated CRE-luciferase reporter activity (Fig. 2g and supplemental Fig. S3a); additionally, insulin treatment decreased reporter activity in cells co-transfected with truncated CBP in the presence or absence of PKA (Fig. 3a). These data do not support the conclusion that the first two α-helices of CH1 domain are important for CREB binding (22).
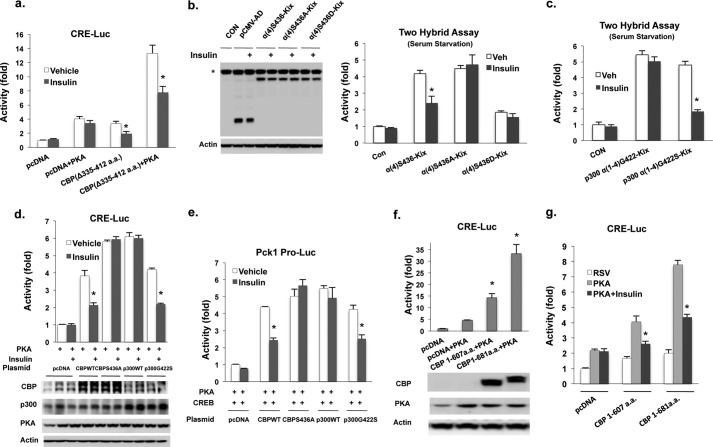
FIGURE 3.
Insulin cannot inhibit p300 stimulated CRE-mediated reporter activity due to lack a corresponding phosphorylation in CBP. a, CBP with the deletion of three α-helices in CH1 domain still responded to insulin treatment in Hepa1–6 cells after 16 h of serum starvation. b, an intact α4-helix in the CH1 domain of CBP is required for the suppression of reporter activity by insulin in Gal4-CREB two-hybrid assay (right panel). Left panel, the protein levels of fusion proteins. *, endogenous NF-κB Rel A. c, insulin had no effect in WT p300-stimulated reporter activity in Gal4-CREB two-hybrid assay. d, insulin failed to suppress PKA-stimulated CRE reporter activity in H2.35 cells co-transfected with CBP(S436A) or WT p300 expression vectors. The protein levels of CBP, p300, and their mutants after 48 h transfection are shown (bottom panel). e, insulin treatment had no effect on PKA-stimulated Pck1 promoter-luciferase (Pro-Luc) reporter activity in Hepa1–6 cells co-transfected with 200 ng of CBP(S436A) or WT p300 plus 100 ng of CREB expression vectors. f, first eight exons of CBP stimulated CRE-luciferase reporter activity in Hepa1–6 cells (upper panel). Protein levels of truncated CBP and PKA are shown (lower panel). g, reporter activity stimulated by the first eight exons of CBP, containing a partial or full Kix domain, was inhibited by insulin in Hepa1–6 cells. a.a., amino acids; RSV, RSV empty vector; CON, no vector.
Because the phosphorylation site of CBP at Ser-436 resides at the end of α4-helix of the CH1 domain (Fig. 2, d and e), we next asked more specifically whether phosphorylation of this site would affect binding of the Kix activation domain construct to CREB. We found that both insulin and metformin treatment decreased Gal4 reporter activity in cells co-transfected with an activation construct containing an intact Ser-436 phosphorylation, but not with a mutated construct (Fig. 3b and supplemental Fig. S3b), supporting our previous finding (7). However, p300 lacks this phosphorylation site found in CBP and constitutively binds to CRE site (Fig. 2, a–c). To determine whether the constitutive binding of p300 to CRE site is due to lack of the corresponding phosphorylation site in CBP, we constructed an activation domain vector of p300 bearing an artificial phosphorylation site at Gly-422. Using the Gal4-CREB in a two-hybrid assay, insulin treatment significantly decreased reporter activity only in cells co-transfected with the construct bearing an artificial phosphorylation site (Fig. 3c). We further compared the effect of co-transfected full-length WT and mutant CBP and p300 expression vectors on either a CRE luciferase reporter in H2.35 cells or a Pck1 promoter in Hepa1–6 cells with or without insulin treatment. Co-transfection of CBP(S436A) and WT p300 led to greater stimulation of reporter activity when compared with co-transfection of either WT CBP or p300(G422S) (Fig. 3, d and e). Insulin treatment decreased reporter activity in cells co-transfected with WT CBP or p300(G422S) but not in cells co-transfected with CBP(S436A) or WT p300. The above data suggest that insulin does not affect WT p300-mediated CREB activation due to the absence of this phosphorylation site.
By using a mutant mouse model containing a putative CBP knock-out allele (23), Bedford et al. (22) showed that these mice had normal glucose metabolism, arguing that CBP is not important in mediating this process. In this mutant mouse model, exon 9 of CBP was floxed; and if this exon is deleted by cell-specific recombination, a frameshift mutation is generated, and a new stop codon is introduced in exon 10. The authors did not formally exclude, however, that the first 8 exons (1–607 amino acids) of CBP are still expressed in this CBP knock-out mouse line. The first eight exons cover the entire N-terminal activation domain and part of Kix domain (Fig. 2, d and e), and strongly activate reporter activity in CRE-luciferase assay (Fig. 3f), and insulin treatment inhibited the reporter activity (Fig. 3g). Thus, the possible expression of first eight exons in their CBP knock-out mice may still be able to mediate gluconeogenic gene expression. Interestingly, their CBP knock-out mice also exhibited increased p300 binding to the CRE site, suggesting that p300 may play a compensatory role (22).
Reconstitution of a Phosphorylation Site in p300(G422S) Knock-in Mice Results in Lower Blood Glucose Levels in Post-prandial State
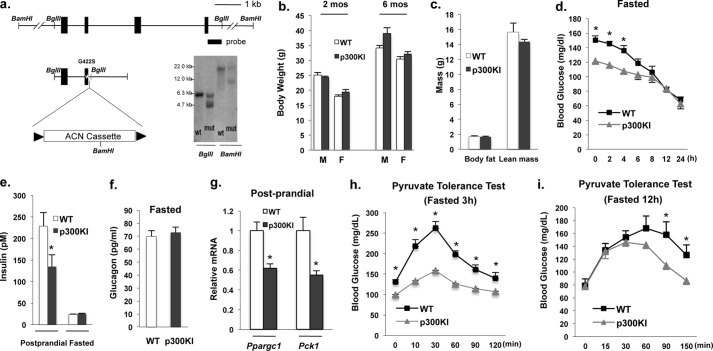
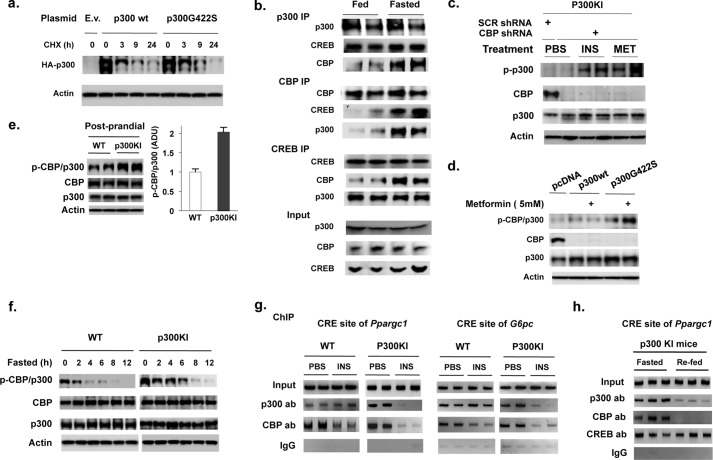
Having seen that p300 is an important mediator of gluconeogenic gene expression and gluconeogenesis (Fig. 1) and that insulin treatment had no effect on reporter activity when co-transfected with WT p300 (Fig. 3, c–e), we generated a p300(G422S) knock-in mouse model bearing a reconstituted a PKC phosphorylation site found in CBP (Fig. 4a). p300(G422S) knock-in mice have similar growth rates, body composition, food consumption levels compared with WT littermates (Fig. 4, b and c, and supplemental Fig. S4a), and hepatic protein levels of CREB, p300, and CBP were not different between WT littermates and p300(G422S) knock-in mice (supplemental Fig. 4b). p300(G422S) knock-in mice, however, displayed lower blood glucose levels and lower serum insulin levels in the post-prandial state when compared with WT littermates (Fig. 4, d and e), but prolonged fasting led to the disappearance of lower blood glucose levels found in p300(G422S) mice (Fig. 4d). This relative hypoglycemia in p300(G422S) knock-in mice was also associated with significantly lower mRNA levels of gluconeogenic enzyme gene in the post-prandial state (Fig. 4g). Even though we examined the mRNA levels of Ppargc1 and Pck1, other enzyme(s) in gluconeogenic pathway may be affected in p300(G422S) knock-in mice as well. p300(G422S) knock-in mice also exhibited decreased glucose production following injection of pyruvate (Fig. 4h,i). In a cycloheximide assay, the introduction of a phosphorylation site in p300 did not affect its protein stability (Fig. 5a). Moreover, the levels of mutant p300 protein did not change in different nutritional states (supplemental Fig. S4b). The phosphopeptide used to generate the phospho-CBP-specific antibody contained the same amino acid sequence found in mutant p300. Therefore, this phospho-CBP antibody should also recognize the mutant p300 site. Because CREB, CBP, and p300 could co-exist in the same complex in the liver of fasted mice (Fig. 5b), we used adenoviral shRNA to deplete CBP in the liver of fasted mice to determine whether p300(G422S) can be phosphorylated. Insulin and metformin treatment phosphorylated hepatic p300(G422S) in the absence of CBP in mutant mice (Fig. 5c). To confirm further that mutant p300(G422S) is phosphorylation-competent, we overexpressed WT or mutant p300 in CBP-depleted hepatoma cells (Hepa1–6) and treated with metformin. Metformin treatment only phosphorylated mutant p300 and not WT p300 (Fig. 5d). The above data clearly demonstrate that mutant p300 is phosphorylation competent. Consistent with this result, we observed increased phosphorylation of CBP and p300 in the liver of p300(G422S) knock-in mice when compared with control mice (Fig. 5e). Additionally, phosphorylation of CBP and p300 persisted for a longer time in the p300(G422S) knock-in mice than in WT controls during a fasting experiment (Fig. 5f). The time course for CBP and p300 dephosphorylation corresponded to the disappearance of lower blood glucose levels in p300(G422S) knock-in versus WT mice (Figs. 4d and 5f).
FIGURE 4.
Characterization of p300(G422S) knock-in mice. a, generation of p300(G422S) knock-in mice. A representative Southern blot is shown. b and c, body weights (b) and body composition (c) of p300(G422S) knock-in mice and littermates (n = 4). d, blood glucose levels of WT and p300(G422S) knock-in mice during the period of 24 h fasting (n = 5–6). e and f, serum insulin levels in post-prandial and fasting states (16 h) mice (e), and fasting glucagon levels (16 h) (f) (n = 5–6). g, p300(G422S) knock-in exhibited lower mRNA levels of Ppargc1 and Pck1 gene in the liver. h and i, pyruvate tolerance test in mice fasted for 3 h (n = 6) or 12 h (n = 4). Asterisk signifies that groups with same treatment are significantly different (p < 0.05). 2 mos, 2 months; mut, mutant; p300KI, P300 knock-in mice.
FIGURE 5.
The introduction of a phosphorylation site in p300 leads to its dissociation from hepatic CREs after insulin treatment. a, after 36 h of transfection of p300 and p300(G422S) plasmids, Hepa1–6 cells were then treated with cycloheximide (CHX; 50 μg/ml) and harvested at the indicated time point. b, co-immunoprecipitation of CREB, p300, and CBP in liver of 24-h fasted and fed mice. c and d, phosphorylation of mutant p300 by insulin and metformin in the liver of fasted p300(G422S) knock-in mice (c), and by metformin in Hepa1–6 cells co-transfected with p300 or p300(G422S) plasmids (d) in the absence of CBP. e, phosphorylated CBP/p300 in liver from mice in the post-prandial state. f, phosphorylated CBP/p300 in liver from mice during fasting (each lane represents a pooled sample of two mice). Because CBP and p300 have a similar molecular weight, they migrate at the same position on Western blot analysis. g, the occupancy of both CBP and p300 on the CRE sites of Ppargc1 and G6pc gene promoter was reduced by insulin treatment in the liver of p300(G422S) knock-in mice. h, The occupancy of CREB co-activators on the CRE site of the Ppargc1 promoter in the liver of 16-h fasted and 4-h refed p300(G422S) mice. E.v., empty vector; ADU, arbitrary density unit; p300KI, P300 knock-in mice; ab, antibody; SCR, scramble.
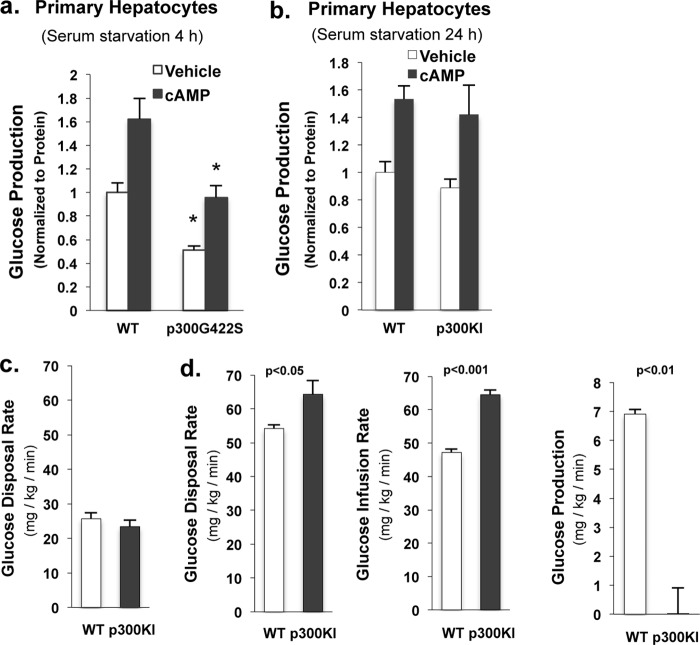
The binding of p300 to the CRE site is independent of the nutritional state (Fig. 2, a–c), and p300 lacks an aPKC phosphorylation site found in CBP, we aimed to determine whether insulin treatment would lead to dissociation of p300 from the CRE sites of the Ppargc1 and Pck1 gene promoter in mutant mice. Indeed, unlike WT p300, mutant p300 was absent from the Ppargc-1 promoter (Fig. 5g, left panel) and Pck1 promoter (data not shown) after insulin administration. We assessed the binding of p300 and CBP on the CRE site of G6pc gene promoter and had a similar pattern of changes as on the CRE sites of Ppargc1 and Pck1 gene promoter (Fig. 5g, right panel). Mutant p300 was absent from the CRE site of the Ppargc-1 promoter in the liver of refed mice (Fig. 5h). To further support the notion that the introduction of a phosphorylation site in p300 resulted in the decreased hepatic glucose production, we conducted glucose production assays in primary hepatocytes. Hepatocytes from p300(G422S) knock-in mice produced significantly less glucose than hepatocytes from control mice in both basal and cAMP treatment groups (Fig. 6a). Primary hepatocytes from 24 h fasted WT and p300(G422S) knock-in mice produced an equal amount of glucose after 24 h of culture in serum starvation conditions (Fig. 6b), which further substantiates the results that prolonged fasting led to the disappearance of the lower blood glucose levels in p300(G422S) knock-in mice. In agreement with the notion that fasting led to the disappearance of lower blood glucose levels in p300(G422S) knock-in mice (Fig. 4d), basal glucose disposal rates in the euglycemic-hyperinsulinemic clamp did not differ between p300 mutant mice and littermate control mice after 5 h of fasting (Fig. 6c). During the clamp experiment, both glucose disposal and glucose infusion rates were significantly increased in p300(G422S) knock-in mice (Fig. 6d), demonstrating enhanced insulin sensitivity in the liver as well as peripheral tissues. A major cause for enhanced sensitivity was hepatic because glucose production was completely suppressed in p300(G422S) knock-in mice but not WT littermate mice at the insulin concentration used in this study (Fig. 6d, right).
FIGURE 6.
p300(G422S) knock-in mice produce significantly less glucose in the liver. a, primary hepatocytes from p300G442S knock-in mouse produced significantly less glucose than hepatocytes from control mouse in the presence or absence cAMP treatment. b, glucose production assays were conducted in primary hepatocytes from 24-h fasted WT and p300 KI mice; cells were subjected to 24 h of serum starvation before the addition of cAMP. c, basal glucose disposal rates in 5-h fasted WT (n = 4) and p300(G422S) (n = 6) mice. d, p300(G422S) mice exhibit increased glucose disposal rates, increased glucose infusion rates, and decreased hepatic glucose production during a clamp experiment (n = 4∼6). Means ± S.E. are shown. p300KI, P300 knock-in mice.
DISCUSSION
An unexplained observation in humans has been that gluconeogenesis is not completely suppressed even in the presence of excess insulin (24–27). We and others (7, 19, 20) have shown that CREB co-activators play critical roles in maintaining fasting blood levels (Fig. 1). In the current study, we found that depletion of hepatic p300 resulted in decreased hepatic mRNA levels of gluconeogenic genes, lower blood glucose levels in the post-prandial state and fasting stages (Fig. 1). These data suggest that p300 is an important co-activator in regulating gluconeogenesis. CBP and p300 are closely related proteins, and one might predict that they would have similar biological function at the CRE site. Alignment of the amino acid sequence of CBP and p300, however, reveals that Ser-436 in CBP corresponds to Gly-422 in p300, indicating that p300 is not phosphorylated at this site (Fig. 2d). As the phosphorylation of CBP at Ser-436 leads to its dissociation from the CRE site of genes important for gluconeogenesis, the lack of phosphorylation at Gly-422 in p300 may lead to constitutive occupancy of p300 on the CRE of target genes. Indeed, p300 constitutively binds to the CRE site such as the Ppargc1 gene promoter in both the fasted and refed states (Fig. 2, a and b). However, mutant p300 is absent from the CRE site in the refed state or after insulin treatment in p300(G422S) knock-in mice (Fig. 5, g and h).
Because insulin phosphorylation of CBP and p300 in p300(G422S) knock-in mice persisted for a longer time in mutant mice (Fig. 5f), we conducted pyruvate tolerance tests at different fasting time points to evaluate the role of this phosphorylation event in regulating gluconeogenesis. In the presence of significant amount of phosphorylated CBP/p300 (3 h of fasting), mutant mice produced less glucose than WT control mice during the entire experiment; in contrast, in the absence of phosphorylated CBP/p300 (12 h fasting), mutant mice produced equal amounts of glucose over the first 60 min of the test (Fig. 4, h and i). We suggest that the reduced glucose production in the latter half of pyruvate tolerance test in p300(G422S) knock-in mice after 12 h of fasting is due to subsequent secretion of insulin and enhanced inhibition of gluconeogenesis in these mice. Moreover, depletion of hepatic p300 led to lower blood glucose levels in the fasted state (Fig. 1d), whereas the lower blood glucose levels disappeared in p300(G422S) knock-in mice after prolonged fasting (Fig. 4d) because of the dephosphorylation of mutant p300 (Fig. 5f). In other words, the phosphorylation of CBP and p300 in p300(G422S) knock-in mice resulted in dissociation of both co-activators from gluconeogenic genes and suppression of gluconeogenic gene expression in the post-prandial state, leading to lower blood glucose levels. Although the p300(G422S) knock-in mice harbor an artificial phosphorylation found in CBP, the characterization of this mouse model proves the importance of non-phosphorylated p300 in driving hepatic gluconeogenesis. Hepatic gluconeogenesis during the post-prandial and fed states has important implications for converting gluconeogenic precursors, such as lactate, fructose, and amino acids delivered from gastrointestinal track and other tissues, into glucose that can be stored as glycogen or released into blood as glucose, especially when consuming a high protein diet.
CREB co-activators have a cooperative effect in regulating HGP. In the fasting state, the dephosphorylation of CBP and CRTC2 by glucagon stimulates the association of these two co-activators to CREB (7, 20). Moreover, the dephosphorylation of p300 at Ser-89 and CBP at Ser-78 in the fasting state results in the activation of their histone acetyltransferase activity (20, 28). The acetylation of CRTC2 by the histone acetyltransferase of p300 and CBP, in turn, increases the protein stability of CRTC2 and inhibits its nuclear exclusion. Cumulatively, p300, CBP, and CRTC2 work together to mediate gluconeogenic gene expression and maintain hepatic gluconeogenesis in the fasting state. In the fed state, insulin suppresses HGP in part through the phosphorylation of CBP at Ser-436. This phosphorylation event leads to the disassembly of CBP and CRTC2 from the CREB complex. Furthermore, the phosphorylation of p300 at Ser-79 and CBP at Ser-78 by insulin decreases their histone acetyltransferase activity (20, 28) as well as the acetylation of CRTC2 and facilitates the nuclear exclusion and degradation of CRTC2 in the cytoplasm (20). We previously reported that the activation of AMP kinase by metformin phosphorylates CBP at Ser-436 via aPKCι/λ (7). A later study showed that the activation of AMP kinase also results in the phosphorylation of p300 at Ser-89 through aPKCι/λ (29). Together, insulin and metformin phosphorylate CBP at Ser-436 and disassemble CBP and CRTC2 from CREB. In addition, the phosphorylation of p300 at Ser-89 and CBP at Ser-78 decreases their histone acetyltransferase activity and increases the degradation of CRTC2. We propose that these phosphorylation events are important for the suppression of HGP.
This work was supported in part by NIDDK, National Institutes of Health Grants K99DK085142 (to L. H.), R00DK085142 (to L. H.), and R01DK063349 (to F. E. W.). This work was also supported by Baltimore Diabetes Research and Training Center Grant P60DK079637 (to F. E. W.).

This article contains supplemental Figs. 1–4.
- PKA
- protein kinase A
- HGP
- hepatic glucose production
- CBP
- CREB-binding protein
- CREB
- cAMP-responsive element-binding protein.
REFERENCES
- 1. Vannucci R. C., Vannucci S. J. (2001) Hypoglycemic brain injury. Semin. Neonatol. 6, 147–155 [DOI] [PubMed] [Google Scholar]
- 2. Desouza C., Salazar H., Cheong B., Murgo J., Fonseca V. (2003) Association ofhypoglycemia and cardiac ischemia: A study based on continuous monitoring. Diabetes Care 26, 1485–1489 [DOI] [PubMed] [Google Scholar]
- 3. Einarsdóttir A. B., Stefánsson E. (2009) Prevention of diabetic retinopathy. Lancet 373, 1316–1318 [DOI] [PubMed] [Google Scholar]
- 4. Bellentani S., Marino M. (2009) Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD). Ann. Hepatol. 8, S4–8 [PubMed] [Google Scholar]
- 5. Krebs E. G. (1989) Role of the cyclic AMP-dependent protein kinase in signal transduction. JAMA 262, 1815–1818 [DOI] [PubMed] [Google Scholar]
- 6. Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 7. He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M. A., Radovick S., Wondisford F. E. (2009) Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137, 635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodman R. H., Smolik S. (2000) CBP/ p300 in cell growth, transformation, and development. Genes Dev. 14, 1553–1577 [PubMed] [Google Scholar]
- 9. Arany Z., Sellers W. R., Livingston D. M., Eckner R. (1994) E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell 77, 799–800 [DOI] [PubMed] [Google Scholar]
- 10. Yao T. P., Oh S. P., Fuchs M., Zhou N. D., Ch'ng L. E., Newsome D., Bronson R. T., Li E., Livingston D. M., Eckner R. (1998) Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93, 361–372 [DOI] [PubMed] [Google Scholar]
- 11. Chan H. M., La Thangue N. B. (2001) p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114, 2363–2373 [DOI] [PubMed] [Google Scholar]
- 12. Giandomenico V., Simonsson M., Grönroos E., Ericsson J. (2003) Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors. Mol. Cell Biol. 23, 2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sundqvist A., Ericsson J. (2003) Transcription-dependent degradation controls the stability of the SREBP family of transcription factors. Proc. Natl. Acad. Sci. 100, 13833–13838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kemper J. K., Xiao Z., Ponugoti B., Miao J., Fang S., Kanamaluru D., Tsang S., Wu S. Y., Chiang C. M., Veenstra T. D. (2009) FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 10, 392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qiang L., Banks A. S., Accili D. (2010) Uncoupling of acetylation from phosphorylation regulates FoxO1 function independent of its subcellular localization. J. Biol. Chem. 285, 27396–27401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Petrij F., Giles R. H., Dauwerse H. G., Saris J. J., Hennekam R. C., Masuno M., Tommerup N., van Ommen G. J., Goodman R. H., Peters D. J. (1995) Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376, 348–351 [DOI] [PubMed] [Google Scholar]
- 17. Wang J., Weaver I. C., Gauthier-Fisher A., Wang H., He L., Yeomans J., Wondisford F., Kaplan D. R., Miller F. D. (2010) CBP histone acetyltransferase activity regulates embryonic neural differentiation in the normal and Rubinstein-Taybi syndrome brain. Dev. Cell 18, 114–125 [DOI] [PubMed] [Google Scholar]
- 18. Iyer N. G., Ozdag H., Caldas C. (2004) p300/CBP and cancer. Oncogene 23, 4225–4231 [DOI] [PubMed] [Google Scholar]
- 19. Zhou X. Y., Shibusawa N., Naik K., Porras D., Temple K., Ou H., Kaihara K., Roe M. W., Brady M. J., Wondisford F. E. (2004) Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat. Med. 10, 633–637 [DOI] [PubMed] [Google Scholar]
- 20. Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S., Milne J., Meyers D. J., Cole P., Yates J., 3rd, Olefsky J., Guarente L., Montminy M. (2008) A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Guzman R. N., Martinez-Yamout M. A., Dyson H. J., Wright P. E. (2004) Interaction of the TAZ1 domain of the CREB-binding protein with the activation domain of CITED2: regulation by competition between intrinsically unstructured ligands for non-identical binding sites. J. Biol. Chem. 279, 3042–3049 [DOI] [PubMed] [Google Scholar]
- 22. Bedford D. C., Kasper L. H., Wang R., Chang Y., Green D. R., Brindle P. K. (2011) Disrupting the CH1 domain structure in the acetyltransferases CBP and p300 results in lean mice with increased metabolic control. Cell Metab. 14, 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang-Decker N., Tong C., Boussouar F., Baker D. J., Xu W., Leontovich A. A., Taylor W. R., Brindle P. K., van Deursen J. M. (2004) Loss of CBP causes T cell lymphomagenesis in synergy with p27(Kip1) insufficiency. Cancer Cell 5, 177–189 [DOI] [PubMed] [Google Scholar]
- 24. Ramnanan C. J., Edgerton D. S., Rivera N., Irimia-Dominguez J., Farmer B., Neal D. W., Lautz M., Donahue E. P., Meyer C. M., Roach P. J., Cherrington A. D. (2010) Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 59, 1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boden G., Cheung P., Stein T. P., Kresge K., Mozzoli M. (2002) FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am. J. Physiol. Endocrinol. Metab. 283, E12-E19 [DOI] [PubMed] [Google Scholar]
- 26. Adkins A., Basu R., Persson M., Dicke B., Shah P., Vella A., Schwenk W. F., Rizza R. (2003) Higher insulin concentrations are required to suppress gluconeogenesis than glycogenolysis in nondiabetic humans. Diabetes 52, 2213–2220 [DOI] [PubMed] [Google Scholar]
- 27. Nuttall F. Q., Ngo A., Gannon M. C. (2008) Regulation of hepatic glucose production and the role of gluconeogenesis in humans: Is the rate of gluconeogenesis constant? Diabetes Metab. Res. Rev. 24, 438–458 [DOI] [PubMed] [Google Scholar]
- 28. Bricambert J., Miranda J., Benhamed F., Girard J., Postic C., Dentin R. (2010) Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J. Clin. Invest. 120, 4316–4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y., Qiu J., Wang X., Zhang Y., Xia M. (2011) AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler. Thromb. Vasc. Biol. 31, 2897–2908 [DOI] [PubMed] [Google Scholar]