Background: Ferrous iron (Fe2+) activates p21ras, TAK1, and IKK in hepatic macrophages (HM), but its upstream signaling is unknown.
Results: Fe2+ generates caveosomal superoxide anion (O2˙̄) which in turn reduces protein-tyrosine phosphatase (PTP) activity and activates Src and p21ras. O2˙̄ induces Lys63 TRAF6 polyubiquitination (polyUb) to activate TAK1.
Conclusion: Fe2+-induced oxidative stress in caveosomes activates Src-p21ras via PTP inhibition and TAK1 via TRAF6 polyUb.
Significance: Fe2+-induced upstream signaling is defined.
Keywords: Cytokine, Inflammation, Liver Injury, NF-κB, Signaling, Kupffer Cells, TAK1, Protein-tyrosine Phosphatase, Superoxide Anion
Abstract
Proinflammatory M1 activation of hepatic macrophages (HM) is critical in pathogenesis of hepatitis, but its mechanisms are still elusive. Our earlier work demonstrates the role of ferrous iron (Fe2+) as a pathogen-associated molecular pattern-independent agonist for activation of IκB kinase (IKK) and NF-κB in HM via activation and interaction of p21ras, transforming growth factor β-activated kinase-1 (TAK1), and phosphatidylinositol 3-kinase (PI3K) in caveosomes. However, iron-induced signaling upstream of these kinases is not known. Here we show that Fe2+ induces generation of superoxide anion (O2˙̄) in endosomes, reduces protein-tyrosine phosphatase (PTP) activity, and activates Src at 2∼10 min of Fe2+ addition to rat primary HM culture. Superoxide dismutase (SOD) blocks O2˙̄ generation, PTP inhibition, and Src activation. Fe2+-induced p21ras activity is abrogated with the Src inhibitor PP2 and SOD. Fe2+ stimulates Lys63-linked polyubiquitination (polyUb) of TRAF6 in caveosomes, and a dominant negative K63R mutant of ubiquitin or SOD prevents iron-induced TRAF6 polyUb and TAK1 activation. These results demonstrate that Fe2+-generated O2˙̄ mediates p21ras and TAK1 activation via PTP inhibition and Lys63-polyUb of TRAF6 in caveosomes for proinflammatory M1 activation in HM.
Introduction
Although iron-catalyzed oxidative tissue damage has been known for several decades, the direct signaling role that iron plays for proinflammatory cytokine expression has only recently been appreciated. Chronic inflammation is commonly associated with iron accumulation in inflamed tissues, particularly in macrophages (1–4). Iron loading in macrophages intensifies inflammation in experimental alcoholic liver injury (5, 6). More mechanistically, the intracellular concentration of catalytically active iron increases rapidly and transiently within ∼2 min after LPS or TNFα stimulation to serve as a signaling event for IKK2 and NF-κB activation in cultured rat hepatic macrophages (HM) (7) or phorbol 12-myristate 13-acetate (PMA)-primed human monocytes (6). This signaling is accentuated by increased intracellular iron storage, providing the molecular basis for the proinflammatory effect of iron loading in macrophages (6). This iron signaling is recapitulated by addition of iron which enters macrophages within 2 min (8) and causes immediate activation of IKK in primary HM in a manner dependent on activation of and physical interactions among p21ras, TAK1, and PI3K (9). In this activation pathway, iron increases AKT phosphorylation in a manner dependent on TAK1 and p21ras. Iron causes ERK1/2 activation which is attenuated by inhibition of PI3K or p21ras but not TAK1. Iron-induced TAK1 activation is not affected by the PI3K and mitogen-activated protein kinase kinase-1 (MEK1) inhibitors. These results suggest that TAK1 and p21ras are upstream of PI3K and MEK1 in iron-mediated signaling. Our study also demonstrates that the physical interactions of TAK1, p21ras, and PI3K take place in caveolin-1-containing endosomes and that iron-induced signaling is abrogated by disruption of lipid raft (caveolae) with filipin III (9), suggesting that iron signaling begins at lipid raft and propagates in caveosomes. However, how iron causes TAK1 and p21ras activation in these sites is currently unknown.
The present study reveals that ferrous iron causes immediate endosomal accumulation of superoxide anion (O2˙̄), inhibition of protein-tyrosine phosphate (PTP) activity, activation of Src and p21ras, and Lys63-linked polyubiquitination (polyUb) of TRAF6 known to be critical for TAK1 activation (8) in caveosomes. All of these signaling events are abrogated by treatment with superoxide dismutase (SOD), suggesting that caveosomal oxidant stress mediates the upstream signaling in iron-stimulated HM activation.
EXPERIMENTAL PROCEDURES
Materials
The reagents used in the study were obtained from the following companies: PMA, the selective Src kinase inhibitor PP2, bovine SOD, and catalase were from Sigma-Aldrich. Eeyarestatin I, a blocker of p97-mediated deubiquitination, was from Tocris Bioscience. OxyBURST Green H2HFF (dihydro-2′-4,5,6,7,7′-hexaflurofluorescein)-BSA was from Molecular Probes/Invitrogen. Protease inhibitor mixture with EDTA and phosphatase inhibitor mixture were from Roche Applied Science and Thermo Scientific. Hybond-P membrane and enhanced chemiluminiscent (ECL) substrate kit were from GE Healthcare. Antibodies against phospho-Src (Tyr416/527), TAK1, and phospho-TAK1 (Thr184/187) were from Cell Signaling. Protein A/G-agarose, antibodies against TRAF6, secondary antibodies of rabbit anti-mouse IgG-FITC, and goat anti-rabbit IgG-TR were from Santa Cruz Biotechnology. Antibody against Lys63-linked ubiquitin was from Millipore. Anti-β-actin antibody was from Abcam. Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Vector Laboratories. Plasmids encoding polyhistidine-tagged wild-type (Ub-Wt) and dominant negative mutant (Ub-K63R) ubiquitins under the control of the Ubi-C promoter were generous gifts from Dr. Douglas A. Gray (Ottawa Hospital Research Institute and University of Ottawa).
HM Isolation, Culture, Iron Stimulation, Inhibitor Pretreatment, and Transfection
HM were isolated from male Wistar rats by the Nonparenchymal Liver Core of the Southern California Research Center for ALPD and Cirrhosis as previously published (7–9). Freshly adhered HM on 100-mm plates were cultured in low glucose Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum and penicillin-streptomycin-antimycotic for 3 days with daily media change and then incubated in serum-free DMEM for 4–16 h before treatments. Where indicated, the cells were pretreated with 10 nm PP2, 200 ng/ml SOD, or 10 μm Eeyarestatin I in serum-free DMEM for 1–1.5 h before addition of ferrous sulfate (50 μm) in serum-free DMEM for the indicated time. Stimulated cells were immediately processed for assays described below. For the transient overexpression of Ub-Wt and Ub-K63R, HM were transfected with the respective expression plasmids using the Neon Transfection System (Invitrogen) according to the manufacturer's instructions, at an electroporation setting of 1400 V, 20 ms, and 1 pulse. Electroporated cells were allowed to reattach to culture dishes in DMEM containing 10% bovine serum albumin for 4–5 h, changed to serum-free DMEM for 4 h, pretreated with Eeyarestatin I, stimulated with ferrous sulfate for 5 min, and processed for assays.
Western Blotting, TRAF6 Immunoprecipitation, and Assays for Src Kinase, p21ras, PTP, and TAK1
Treated HM were washed twice with phosphate-buffered saline and lysed by incubation on ice with a lysis buffer (20 mm Tris-Cl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 0.1% Triton X-100, 1 mm phenylmethylsulfonyl fluoride, protease inhibitors, and phosphatase inhibitors) followed by sonication. The lysate was clarified by centrifugation, and its protein concentration was determined by the Bradford method. For direct Western blotting, cell lysate containing 50 μg of protein was resolved by SDS-PAGE, transferred to Hybond-P membrane, probed with the indicated primary antibody, and visualized with HRP-conjugated secondary antibody and ECL reagents. For immunoprecipitation of polyubiquitinated TRAF6, cell lysate containing 500 μg of protein was precleared with protein A/G-agarose and agitated with anti-TRAF6 or anti-Lys63-linked ubiquitin antibody at 4 °C overnight and then with 30 μl of protein A/G-agarose at 4 °C for 2 h. The immune complexes were washed three times with the lysis buffer and eluted from the agarose beads by boiling in 40 μl of SDS-PAGE sample loading buffer for 5 min. The entire immunoprecipitate sample was resolved on an 8% SDS-polyacrylamide gel for Western blot analysis with anti-ubiquitin or anti-TRAF6 antibody. For the Src kinase assay, a Src kinase assay kit (17-131; Millipore) was used to measure the phosphotransferase activity in 10 μg of cell lysate in a 10-min, 30 °C reaction containing [γ-32P]ATP and the Src-specific substrate peptide, KVEKIGEGTYGVVYK, as detailed in the manufacturer's instructions. For Ras activation assay, a Ras activation assay kit (17-218; Millipore) was used according to the manufacturer's instructions. In brief, 500 μl of cell lysate, either untreated or treated with GDP or GTPγS as a negative or positive control, respectively, was subjected to affinity pulldown with Raf-1 RBD-agarose, and the activated Ras bound was resolved by 12% SDS-PAGE and detected by anti-Ras Western blotting. For PTP activity assay, a tyrosine phosphatase assay system (V2471; Promega) was used to measure via molybdate dye color development with the amount of phosphate released, after removal of endogenous phosphate, from a 100-μl reaction containing 2 μg of cell extract and two synthetic PTP-specific phosphopeptide substrates. For TAK1 activation, immunoblotting for anti-p-TAK1 (Thr184/187) was performed.
Immunofluorescence Microscopy
HM were washed with phosphate-buffered saline and fixed in cold methanol for 20 min at −20 °C after cultivation and iron/1,2-dimethyl-3-hydroxypyridin-4-one (L1) treatment. After three washes the cells were permeabilized by incubation with cold 0.1% Triton X-100 in phosphate-buffered saline for 15 min. After treatment with blocking solution containing 5% nonfat milk for 60 min, the staining was performed by incubation of a 1:200 dilution of antibody against TRAF6, Lys63-linked ubiquitin, or caveolin-1 in phosphate-buffered saline with 5% nonfat milk at 4 °C overnight. The cells were washed with phosphate-buffered saline three times for 5 min each. The cells were then incubated with a 1:200 dilution of the secondary antibodies conjugated with FITC or Texas Red for 2 h at room temperature. The nuclei were stained with 4′,6-diamidino-2-phenylindole (0.1 μg/ml) for 10 min after three washes. The cells were viewed using a Nikon microscope equipped with a digital camera. The immunofluorescence-stained HM were examined by confocal laser scanning microscopy using a Zeiss LSM 510 (Cell and Tissue Imaging Core of University of Southern California Research Center for Liver Diseases, P30DK DK48522).
Statistical Analysis
All numerical data are expressed as mean ± S.D., and a significance of a difference (p < 0.05) was determined by two-tailed t test.
RESULTS
Iron Induces M1 Genes in Primary Culture of HM
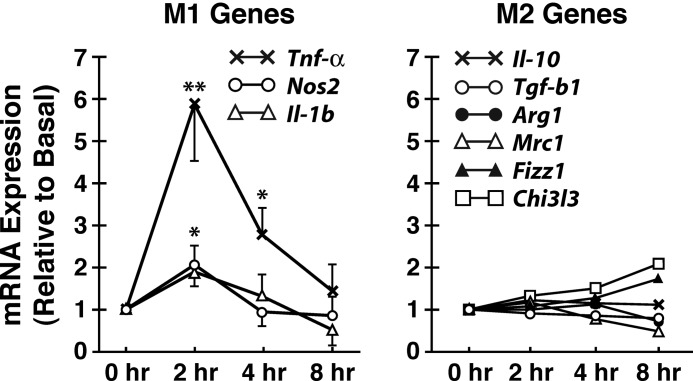
Our previous studies demonstrated iron-stimulated HM expression of TNFα as a prototypical NF-κB-responsive M1 cytokine. However, how iron affects expression of M2 genes has not been examined. Thus, we tested changes in mRNA expression of a panel of M2 genes along with M1 genes in HM treated with ferrous iron at the concentration of 50 μm, which was also used for all subsequent experiments in the present study. Besides Tnfα which is up-regulated 6-fold with iron, other M1 genes such as Nos2 and Il-1β are also induced 2-fold at 2 h after iron treatment followed by a decline to the basal levels between 4 and 8 h (Fig. 1). M2 genes such as Il-10, Tgf-β, Arg1, and Mrc1 are unchanged, but Chi3l3 and Fizz are slightly increased at 8 h (Fig. 1). Thus, these results demonstrate that M1 genes are primary targets of iron and are induced early at 2 h, whereas moderate and late induction of some of the M2 genes occur in the late course of the treatment which could be secondary to induced M1 genes (10).
FIGURE 1.
Ferrous iron induces M1 genes. Treatment of cultured rat hepatic macrophages with FeSO4 (50 μm) causes early induction of M1 genes (Tnfα, Nos2, and Il-1β) while having no effects on anti-inflammatory M2 genes such as Il-10 and Tgf-β1 and other M2 genes (Arg1 and Mrc1). Delayed induction of Fizz1 and Chi3l3 are observed at 8 h. The data shown are from three independent experiments. Standard error bars are shown only for A but removed from B to avoid crowding of the data. *, p < 0.05; **, p < 0.01 compared with the basal expression level.
Iron Activates Src and Inhibits PTP
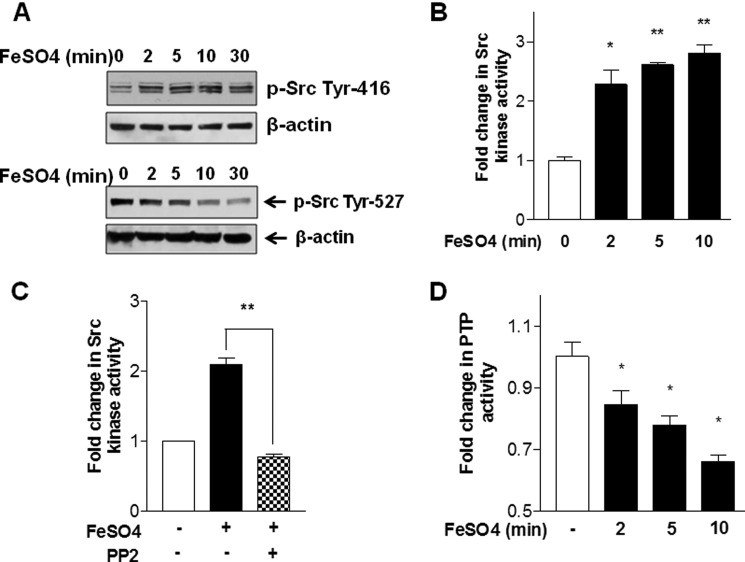
We have previously shown that p21ras is upstream of PI3K and MEK activation in iron-induced IKK activation in HM (9). To investigate upstream mechanisms of p21ras activation caused by iron, we examined the effects on Src, a known effector for p21ras (11, 12). Indeed, the treatment of cultured rat HM with ferrous iron increases Tyr416 phosphorylation (activation marker) and reduces Tyr527 (inhibition marker) of Src from 2 to 30 min (Fig. 2A). These effects are associated with a 2–2.7-fold increase in Src activity (Fig. 2B), and the iron-stimulated Src activity is abrogated by the concomitant treatment of the cells with the Src inhibitor PP2 (Fig. 2C, the data shown are with the treatment for 5 min). Because PTP regulates Src activity via dephosphorylation, we next examined the effects of iron on PTP activity. As shown in Fig. 2D, PTP activity is conversely suppressed from 2 to 10 min subsequent to iron addition, suggesting that iron-mediated suppression of PTP may be responsible for Src activation.
FIGURE 2.
Exogenous ferrous iron activates c-Src and reduces PTP activity in hepatic macrophages. A, treatment of rat hepatic macrophages with FeSO4 (50 μm) increases c-Src phosphorylation at Tyr416 (activation marker) from 2 min onward while reducing phosphorylation at Tyr527 (inactivation marker). B, FeSO4 treatment increases Src kinase activity from 2 to 10 min. C, iron-induced activation of c-Src at 5 min is abrogated by pretreatment with the Src inhibitor PP2. D, PTP activity is conversely reduced by the iron treatment within the same time frame. Three independent experiments were performed for each assay. *, p < 0.05; **, p < 0.01 compared with the basal at time zero. Error bars, S.E.
Iron Causes Endosomal Accumulation of Superoxide Anion
PTP is known to be redox-regulated, and oxidant stress inhibits PTP and consequently activates Src as previously shown in B cells (13). To test this possibility, we assessed endosomal accumulation of O2˙̄ by using O2˙̄-sensitive endosomal florescent probe OxyBURST Green (H2HFF)-BSA. Using primary rat HM preloaded with OxyBURST, iron addition causes robust fluorescence emission at 2 and 5 min, followed by attenuation of the signal at 10 and 20 min (Fig. 3A). We also tested macrophages derived from rat blood monocytes. Monocytes undergo macrophage differentiation by the treatment with PMA and acquire intracellular iron signaling (6). After PMA treatment overnight, the cells were washed and cultured for 6 h in low serum medium prior to loading of the OxyBURST and treatment with ferrous iron. PMA-induced macrophages but not untreated monocytes, generate endosomal O2˙̄ at 2–10 min after iron addition, and this response is abrogated by pretreatment of the cells with SOD but not with catalase (Fig. 3B).
FIGURE 3.
Iron induces endosomal generation of superoxide anion in macrophages. A, FeSO4 treatment of rat hepatic macrophages generates superoxide anion in the endosomal compartment starting at 2 min, as detected by an O2˙̄-sensitive fluorescent probe conjugated with BSA (OxyBURST Green H2HFF-BSA), which is selectively taken up via an endocytic pathway. This iron-induced vesicular ROS production appears to be transient as the fluorescent signal dissipates in 10–20 min. B, similar O2˙̄ generation takes place after iron treatment in rat blood monocytes forced to differentiate into macrophages by PMA treatment but not in monocytes without PMA-induced differentiation. Concomitant treatment with SOD (300 μm) but not catalase eliminates iron-induced ROS fluorescent signal, confirming detection of O2˙̄ but not H2O2 by this probe.
Iron-induced Inhibition of PTP and Src-p21ras Activation Is Prevented by SOD
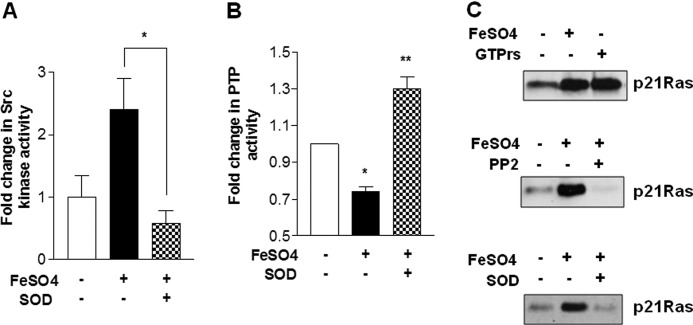
We next tested whether SOD, which abrogates endosomal accumulation of O2˙̄, prevents inhibition of PTP activity and activation of Src and p21ras induced by iron. Indeed, SOD treatment completely abrogates iron-induced Src and p21ras activity (Fig. 4, A and C) and PTP inhibition (Fig. 4B). In fact, SOD not only prevents PTP inhibition but even increases PTP activity above the control level (Fig. 4B), suggesting that the basal oxidant stress may be inhibiting PTP. The Src inhibitor PP2 also blocks iron-induced p21ras activity (Fig. 3C, middle panel), supporting a signaling cascade of iron-induced endosomal oxidant stress causing PTP inhibition and Src activation which then activates p21ras. As we showed previously, p21ras is an integral signaling component of iron-induced IKK activation in HM, the demonstrated O2˙̄-PTP-Src-p21ras pathway is considered a critical upstream mechanism of iron-induced IKK activation.
FIGURE 4.
SOD prevents iron-induced c-Src activation, PTP activity suppression, and p21ras activation. A, pretreatment of rat hepatic macrophages with SOD abrogates iron-induced c-Src activity measured at 5 min. B, iron-mediated suppression of PTP activity at 5 min is also prevented by the SOD treatment. C, iron induces p21ras activity at 5 min (top panel), and this activation is blocked by treatment with the Src inhibitor PP2 (middle panel) or with SOD (bottom panel). Three independent experiments were performed for each assay. *, p < 0.05 compared with no addition; **, p < 0.05 compared with FeSO4 addition. Error bars, S.E.
Iron Induces TRAF6 PolyUb to Activate TAK1 in Oxidant Stress-dependent Manner
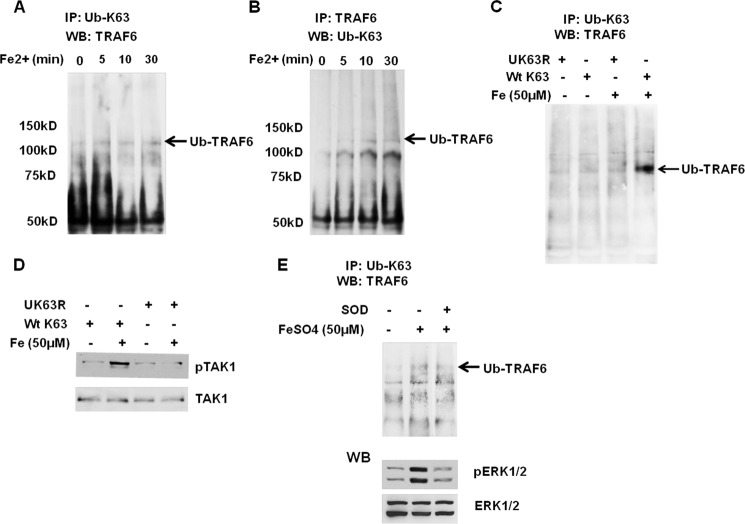
Another arm of iron-mediated IKK activation is TAK1 activation. TRAF6 oligomerization upon IL-1R or TLR ligation activates its own E3 ubiquitin ligase activity and results in Lys63-linked polyUb of TRAF6 itself for activation of TAK1 via TAB2 (14, 15). Thus we speculated that iron also causes TRAF6 polyUb for TAK1 activation in HM. Indeed, increased levels of high molecular mass complexes (>100 kDa) were detected by anti-TRAF6 antibody of protein extract pulled down with anti-Lys63-Ub antibody beginning at 5 min of the iron treatment (Fig. 5A). Reciprocal immunoprecipitation with anti-TRAF6 and immunoblotting with anti-Lys63-Ub also confirm increased polyUb-TRAF6 at 5∼30 min after iron treatment in HM (Fig. 5B). HM transduced with a plasmid expressing wild-type (Ub-Wt) or dominant negative mutant of Ub (Ub-K63R) was also used to validate our finding. Although polyUb-TRAF6 is evident in iron-treated HM expressing Ub-Wt, the cells expressing Ub-K63R fail to show polyUb-TRAF6 after iron treatment, confirming Lys63-polyUb of TRAF6 in iron-treated HM (Fig. 5C). Next, we tested whether this inhibition of Lys63-polyUb of TRAF6 with the mutant prevents TAK1 activation. Indeed, expression of Ub-K63R completely prevents iron-induced TAK1 activation as judged by the absence of increased p-TAK1 (Thr184/187) as opposed to Ub-Wt-expressing cells which show increased p-TAK1 in response to iron (Fig. 5D). These results support the role of polyUb-TRAF6 in iron-induced TAK1 activation. We then asked whether this TAK1 activation mechanism is dependent on O2˙̄ which is required for iron-induced PTP-Src-p21ras pathway. Treatment of the cells with SOD reduces TRAF-polyUb (Fig. 5E), demonstrating that this arm of signaling is also dependent on O2˙̄. Of note is that SOD also suppresses iron-induced increase in p-ERK1/2 (lower panel of Fig. 5E) which we previously showed as a downstream of p21ras activation (9). Collectively, these results suggest that endosomal oxidant stress activates both arms of TRAF6-TAK1 and Src-p21ras pathways. Because caveosomes are shown to be the site of p21ras-TAK1-PI3K interactions in iron-induced IKK activation (9), we asked whether TRAF6-polyUb also occurs in caveosomes. Indeed, our co-immunofluorescent microscopy demonstrates co-localization of TRAF6 and Lys63-Ub as well as that of caveolin-1 and TRAF6 (Fig. 6) in HM treated with iron for 10 min, supporting the notion that TRAF6-polyUb takes place in caveosomes.
FIGURE 5.
Iron induces Lys63-polyUb of TRAF6. A, cell lysates collected from iron-treated hepatic macrophages at different times were immunoprecipitated (IP) with anti-Lys63-Ub and immunoblotted (WB) with anti-TRAF6. An arrow indicates polyubiquitinated TRAF6 (Ub-TRAF6) which increases after iron treatment. B, reciprocally, the cell lysates were immunoprecipitated with anti-TRAF6 and immunoblotted with anti-Lys63-Ub, demonstrating similar results. C, expression of the dominant negative mutant of Ub (Ub-K63R) but not wild-type Ub (Ub-Wt) eliminates the formation of Ub-TRAF6 in response to iron. D, the Ub-K63R also blocks iron-induced activation of TAK1 as assessed by immunoblotting of p-TAK1 (Thr184/187). E, SOD attenuates iron-induced Lys63-Ub of TRAF6 and pERK1/2.
FIGURE 6.
TRAF6 and Lys63-Ub co-localize with caveolin-1 in iron-treated hepatic macrophages. A, iron induces co-localization of TRAF6 and Lys63-Ub in hepatic macrophages. Hepatic macrophages in primary culture were treated with iron sulfate (50 μm) for 10 min and stained for the TRAF6 (green), Lys63-Ub (red), and nuclei (blue). The stained cells were observed by immunofluorescent microscopy. Untreated cells show minimal co-localization. At 10 min, the merged co-localization staining (orange) appears as small punctate dots. B, iron induces co-localization of TRAF6 and caveolin-1 in hepatic macrophages. The cells were treated as indicated in A and stained for TRAF6 (FITC) and caveolin-1 (red). Note co-localization of these two proteins.
DISCUSSION
Compelling evidence supports the pathogen-associated molecular pattern (PAMP)-independent agonist role of reduced iron in proinflammatory gene expression in macrophages (4, 6, 7). Our previous work revealed part of this signaling pathway involving physical interactions of TAK1, p21ras, and PI3K in caveolae, leading to activation of IKK (9). The present study extends this finding and demonstrates that endosomal oxidant stress induced by iron mediates PTP2 inhibition and consequent Src activation as an upstream mechanism of p21ras activation. We also demonstrate that iron induces Lys63-polyUb of TRAF6 which in turn mediates TAK1 activation in a manner dependent on oxidant stress. Further, these upstream events are shown to occur in caveosomes marked by cavelin-1. Combined with the previous findings, we may conclude that reduced iron may enter the cells via lipid raft and generates O2˙̄ in caveosomes to initiate two signaling pathways via Src-p21ras activation and TRAF6-mediated TAK1 activation which converge to IKK activation (Fig. 7). This leads to NF-κB activation and induction of M1 genes such as Tnfα, Nos2, and Il-1β while having no effect on anti-inflammatory M2 genes such as Il-10 and Tgf-β (Fig. 1). This M1-dominant effect of iron may play potentially important roles in chronic inflammatory diseases including chronic venous leg ulcers (4), multiple sclerosis (16), and alcoholic steatohepatitis (5, 6). In artherosclerosis, macrophages in lesions are often loaded with iron (1), and this iron may potentiate M1 activation for aggravation of chronic inflammation in addition to oxidizing low density lipoprotein in lysosomes (17). Iron loading in macrophages also accentuates a transient rise in intracellular labile iron caused by LPS or peroxynitrite which signals to activate IKK-NF-κB (6). Catalytically active iron released in this signaling is exported out of the cells within 5 min.3 Thus, it is possible that released iron may further promote IKK activation via the pathway identified in the present study to perpetuate and sustain an inflammatory response of macrophages in chronic inflammatory diseases. Although we have used the 50 μm concentration of ferrous iron in the present study, we have previously shown stimulation of TNF-α promoter activity and protein release with the concentration as low as 5∼10 μm (18). In tissue damage, iron that is usually compartmentalized into protein-bound forms may also be released transiently into the microenvironment due to oxidative or nitrosative stress, reaching this range of low micromolar concentrations (4, 19, 20).
FIGURE 7.
Schematic diagram of iron-induced upstream signaling. Iron signaling begins with generation of superoxide anion which causes PTP inhibition and consequent Src and p21ras activation while mediating polyUb of TRAF6 leading to TAK1 activation in caveosomes of hepatic macrophages. Our earlier work (9) demonstrates that p21ras, TAK1, and PI3K physically interact in caveosomes and these effectors and p21ras-mediated and pI3K-modulated ERK1/2 activation, all contribute to IKK activation caused by iron.
The previous and present studies both point to the importance of caveosomes as the site of iron-mediated signaling pathways for IKK activation. This is analogous to the finding from an earlier study which revealed endocytic vesicles as the site of ROS-mediated recruitment of TRAF6 to IL-1 receptor and MyD88 in macrophages treated with IL-1 (21). In either scenario, it makes a physiological sense to have an enclosed vesicular compartment for ROS-mediated signaling for both confinement of signaling and protection of other cellular compartments from ROS; the vesicular compartment serves best as a platform for an assembly of a signaling complex via sequential recruitment of participating components and as a mechanism of termination of the signaling by eventual lysosomal degradation.
The emerging role of iron in proinflammatory signaling as supported by the present and other studies sheds important therapeutic implications for chronic inflammatory disease. Whereas iron sequestration by macrophages in inflammation may be largely considered as a bacteriostatic function and M2 macrophage polarization (22), increased macrophage iron storage may need to be considered causal for chronic inflammation via sustained activation of M1 genes. Targeting this iron-mediated proinflammatory signaling at caveosomes may be a potential approach for suppression of M1 activation and chronic inflammation.
Acknowledgment
We acknowledge the technical support of Shigong Xiong.
This work was supported, in whole or in part, by National Institutes of Health Grants P50AA011999 (to H. T.), R24AA012885 (to H. T.), RC2AA019392 (to H. T.), R21AA017288 (to S. Z.), and K01AA020524 (to J. X.), and by the Medical Research Service of the Department of Veterans Affairs (to H. T.) and Suntory Business Expert Ltd.
H. Tsukamoto, unpublished observation.
- IKK
- IκB kinase
- GTPγS
- guanosine 5′-O-(thiotriphosphate)
- H2HFF
- dihydro-2′-4,5,6,7,7′-hexaflurofluorescein
- HM
- hepatic macrophage(s)
- Lys63-polyUb
- lysine 63-linked polyubiquitination
- O2˙̄
- superoxide anion
- PMA
- phorbol 12-myristate 13-acetate
- PTP
- protein-tyrosine phosphatase
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase
- TAK1
- transforming growth factor β-activated kinase-1
- TRAF
- TNF receptor-associated factor
- Ub
- ubiquitin.
REFERENCES
- 1. Yuan X. M. (1999) Apoptotic macrophage-derived foam cells of human atheromas are rich in iron and ferritin, suggesting iron-catalysed reactions to be involved in apoptosis. Free Radic. Res. 30, 221–231 [DOI] [PubMed] [Google Scholar]
- 2. Kennedy J. I., Chandler D. B., Jackson R. M., Fulmer J. D. (1986) Reduction in bleomycin-induced lung hydroxyproline content by an iron-chelating agent. Chest 89, 123S–125S [DOI] [PubMed] [Google Scholar]
- 3. Graham J. M., Paley M. N., Grünewald R. A., Hoggard N., Griffiths P. D. (2000) Brain iron deposition in Parkinson's disease imaged using the PRIME magnetic resonance sequence. Brain 123, 2423–2431 [DOI] [PubMed] [Google Scholar]
- 4. Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., Weiss J. M., Wlaschek M., Sunderkötter C., Scharffetter-Kochanek K. (2011) An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 121, 985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsukamoto H., Lin M., Ohata M., Giulivi C., French S. W., Brittenham G. (1999) Iron primes hepatic macrophages for NF-κB activation in alcoholic liver injury. Am. J. Physiol. 277, G1240–1250 [DOI] [PubMed] [Google Scholar]
- 6. Xiong S., She H., Zhang A. S., Wang J., Mkrtchyan H., Dynnyk A., Gordeuk V. R., French S. W., Enns C. A., Tsukamoto H. (2008) Hepatic macrophage iron aggravates experimental alcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G512–G521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiong S., She H., Takeuchi H., Han B., Engelhardt J. F., Barton C. H., Zandi E., Giulivi C., Tsukamoto H. (2003) Signaling role of intracellular iron in NF-κB activation. J. Biol. Chem. 278, 17646–17654 [DOI] [PubMed] [Google Scholar]
- 8. Atkinson P. G., Barton C. H. (1999) High level expression of Nramp1G169 in RAW264.7 cell transfectants: analysis of intracellular iron transport. Immunology 96, 656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen L., Xiong S., She H., Lin S. W., Wang J., Tsukamoto H. (2007) Iron causes interactions of TAK1, p21ras, and phosphatidylinositol 3-kinase in caveolae to activate IκB kinase in hepatic macrophages. J. Biol. Chem. 282, 5582–5588 [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y. H., Lin J. X., Yip Y. K., Vilcek J. (1988) Enhancement of cAMP levels and of protein kinase activity by tumor necrosis factor and interleukin 1 in human fibroblasts: role in the induction of interleukin 6. Proc. Natl. Acad. Sci. U.S.A. 85, 6802–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Büscher D., Hipskind R. A., Krautwald S., Reimann T., Baccarini M. (1995) Ras-dependent and -independent pathways target the mitogen-activated protein kinase network in macrophages. Mol. Cell. Biol. 15, 466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. David M. D., Cochrane C. L., Duncan S. K., Schrader J. W. (2005) Pure lipopolysaccharide or synthetic lipid A induces activation of p21ras in primary macrophages through a pathway dependent on Src family kinases and PI3K. J. Immunol. 175, 8236–8241 [DOI] [PubMed] [Google Scholar]
- 13. Singh D. K., Kumar D., Siddiqui Z., Basu S. K., Kumar V., Rao K. V. (2005) The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell 121, 281–293 [DOI] [PubMed] [Google Scholar]
- 14. Kanayama A., Seth R. B., Sun L., Ea C. K., Hong M., Shaito A., Chiu Y. H., Deng L., Chen Z. J. (2004) TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 15, 535–548 [DOI] [PubMed] [Google Scholar]
- 15. Sun L., Deng L., Ea C. K., Xia Z. P., Chen Z. J. (2004) The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 [DOI] [PubMed] [Google Scholar]
- 16. Williams R., Buchheit C. L., Berman N. E., LeVine S. M. (2012) Pathogenic implications of iron accumulation in multiple sclerosis. J. Neurochem. 120, 7–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Satchell L., Leake D. S. (2012) Oxidation of low-density lipoprotein by iron at lysosomal pH: implications for atherosclerosis. Biochemistry 51, 3767–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. She H., Xiong S., Lin M., Zandi E., Giulivi C., Tsukamoto H. (2002) Iron activates NF-κB in Kupffer cells. Am. J. Physiol. Gastrointest. Liver Physiol 283, G719–726 [DOI] [PubMed] [Google Scholar]
- 19. Lancaster J. R., Jr., Hibbs J. B., Jr. (1990) EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc. Natl. Acad. Sci. U.S.A. 87, 1223–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tacchini L., Recalcati S., Bernelli-Zazzera A., Cairo G. (1997) Induction of ferritin synthesis in ischemic-reperfused rat liver: analysis of the molecular mechanisms. Gastroenterology 113, 946–953 [DOI] [PubMed] [Google Scholar]
- 21. Li Q., Harraz M. M., Zhou W., Zhang L. N., Ding W., Zhang Y., Eggleston T., Yeaman C., Banfi B., Engelhardt J. F. (2006) Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol. Cell. Biol. 26, 140–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cairo G., Recalcati S., Mantovani A., Locati M. (2011) Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. 32, 241–247 [DOI] [PubMed] [Google Scholar]